Abstract
Purpose
To compare retinal function changes in eyes with proliferative diabetic retinopathy (PDR) after intravitreal ranibizumab (IVR), combined or not with conventional (ETDRS) or multispot laser panretinal (PASCAL) photocoagulation (PRP).
Methods
This study included laser-naive PDR patients that required PRP. Eyes were randomly and prospectively assigned to receive IVR or IVR combined with PASCAL or EDTRS. PRP was performed at baseline in 1 (PASCAL) or 2 (ETDRS) sessions. In eyes with macular edema, macular short pulse grid laser was associated with IVR at baseline and IVR was repeated monthly or quarterly if neovascularization was detected on angiography. Comprehensive ophthalmological evaluations, including SD-OCT, were performed at baseline and every 4 weeks after treatment. Full-field electroretinography (ERG: extended ISCEV standard) was performed at baseline and at 12, 24 and 48 weeks.
Results
IVR = 13, PASCAL = 15 and ETDRS = 15 eyes finished 48-week follow-up. There was a statistically significant BCVA improvement of 0.1–0.3 logMAR in all groups, and fluorescein angiography leakage area (FLA) reduced in 56%, 73%, and 73% from baseline for ETDRS, IVR and PASCAL, respectively, up to 48 weeks without significant differences between groups (p > 0.05). A significant a- and b-wave amplitudes reduction was observed for dark- and light-adapted ERG for ETDRS and PASCAL, but only minor dark-adapted b-wave reduction was found for IVR, up to 48 weeks. As an example, at week 48, combined response b-wave amplitude reduced in 181.5 ± 31.4 µV, 128.0 ± 27.9 µV and 82.4 ± 15.2 µV for ETDRS, PASCAL and IVR (p < 0.05 each group), respectively. No significant difference was observed between ETDRS and PASCAL for any ERG parameter.
Conclusions
IVR combined with single or multiple spot PRP causes similar retinal function impairment during 48 weeks of observation, while IVR alone seems to be similarly effective controlling FLA without changing retinal function.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Diabetic retinopathy (DR) prevalence is estimated in 30% of diabetic subjects [1]. The proliferative form of diabetic retinopathy (PDR) is characterized by the presence of pathological neovascularization, with eventual development of vitreous hemorrhages, retinal detachment and neovascular glaucoma, which are leading causes of severe vision loss in DR [2].
Established treatment of PDR consists in the use of panretinal laser photocoagulation (PRP) [3] that causes tissue destruction, but reduces retinal oxygen consuming, improving inner retina’s oxygenation [4], with consequent regression of retinal new vessels [5]. The standardized laser protocol is the recognized treatment for severe NPDR and PDR since 1991 [6].
Unfortunately, PRP can be associated with pain during application and also undesirable structural and functional retinal changes, such as macular edema [7], delayed dark adaptation [8], visual field loss [9] and impaired color vision [10]. Thus, if possible, efforts should be made to improve or even avoid retinal photocoagulation.
More recently, another laser approach, the pattern scan laser (PASCAL), has become available [11]. PASCAL has the advantage of firing multiple laser shots at once, making the procedure less painful [12] and less time consuming [13]. Several studies compared the effectiveness of both laser strategies in the past years, and overall, conventional PRP and PASCAL showed comparable effectiveness [13], but there are reports about PASCAL being less effective than conventional PRP when looking at regression rates and prevention of neovascularization [14].
Intravitreal anti-vascular endothelial growth factor antibodies (anti-VEGF) have been used to complement PRP in the management of PDR [15]. VEGF is the major factor involved in neovascularization of PDR [16], and elevated levels of VEGF have been found in the vitreous of PDR eyes [17]. There is evidence that laser combined with anti-VEGF is more effective for PDR [18] and, of interest, the association of anti-VEGF to PRP can reduce retinal functional loss due to less extensive PRP, as we previously showed using electroretinography (ERG) [19].
In this context, the aim of this study was to describe ERG changes caused by IVR combined or not with conventional PRP or PASCAL.
Methods
Patient eligibility and evaluation
A total of 45 eyes (of 34 patients) with PDR according to the guidelines of the Early Treatment Diabetic Retinopathy Study [6] were included. Further inclusion criteria were: age (18 years, or older), visual acuity better than 20/800, no earlier laser treatment and the presence of active neovascularization with immediate treatment indication. Exclusion criteria consisted of the presence of intravitreal hemorrhages or tractional retinal detachment involving the macula, injection of intravitreal drugs 6 months prior to study enrollment, major opacity of cornea, crystalline lens or intraocular lens, cataract surgery 3 months prior to study enrollment, posterior vitrectomy or retinopexia with scleral introflexion, acute ocular infection, allergy to fluorescein, other ocular pathology such as glaucoma, or any medical or psychological condition at baseline examination that would not allow conclusion of study.
Ophthalmological evaluation
Ophthalmological evaluation was performed monthly, including assessment of LogMAR best corrected visual acuity (BCVA), slit-lamp and fundus examination, and spectral-domain optical coherence tomography (SD-OCT—Heidelberg Engineering) to assess macular thickness. Fluorescein angiography was performed monthly to detect new vessels in the first 3 months, and quarterly afterward.
ERG protocol
Full-field ERG was performed at baseline, and 12, 24 and 48 weeks after treatment (ColorDome and Espion E2—Diagnosys LLC, Middleton, MA, USA). ERG was executed in accordance with ISCEV standard [20] using DTL as positive electrodes. Skin electrodes (Red-Dot—3M) were placed on each temporal orbital rim to serve as references, and on forehead as ground. A- and b-wave amplitudes and implicit time were evaluated.
After 30-min dark adaptation, a series of flashes with increasing luminance was used as light stimuli: 0.003, 0.01 (rod ERG), 0.03, 0.1, 0.3, 1.0, 3.0 (combined rod-cone ERG) and 10 cd s/m2. Oscillatory potentials were filtered out of combined rod-cone ERG, using an off-line fast-Fourier algorithm set as a band-pass frequency filter (75–300 Hz) as previously described [21], and area under the curve (OP-AUC) between a- and b-wave implicit times was calculated.
Thereafter, patients were light-adapted for 10 min, and photopic ERG measurements were also taken a series of increasing stimuli luminance: 0.1, 0.3, 1.0, 3.0 (cone ERG), 10.0 and 30.0 cd s/m2, followed by the 30 Hz flicker (background during photopic stimulation = 30 cd/m2).
Group treatment assignment
Eyes (n = 45) were randomized and assigned into three different treatment groups (n = 15):
-
EDTRS + IVR PRP with conventional single spot laser (Purepoint, Alcon, Fort Worth, Texas) at two sessions (baseline and, week 2), associated with single intravitreal injection of 0.05 ml (0.5 mg) ranibizumab after first laser session;
-
PASCAL + IVR patient underwent PRP with multiple spot laser (PASCAL (OptiMedica, Santa Clara, California) at baseline in single session, associated with intravitreal injection of 0.05 ml (0.5 mg) ranibizumab;
-
IVR patient received intravitreal injection of 0.05 ml (0.5 mg) ranibizumab at baseline. In eyes with macular edema, macular shortpulse grid laser was associated with IVR at baseline. IVR was repeated monthly if central subfield thickness (CSFT) measured with spectral-domain optic coherence tomography was higher than 300 µm, or quarterly if neovascularization was detected by angiography.
After week 12, IVR was applied monthly if macular edema was detected, or every 12 weeks if neovascularization was detected.
Fluorescein leakage area (FLA) measurement
Digital red-free fundus photography and fluorescein angiography were performed using a certified fundus camera system (Spectralis HRA, Heidelberg, Germany, using a 50 degree of field of view), and fluorescein leakage area (FLA) was measured using the system built-in software in pictures taken around 2 min after dye infusion.
Statistical analysis
Baseline data were compared with one-way analysis of variance followed by Tukey–Kramer test for multiple mean comparisons, while group comparisons during follow-up were performed using analysis of covariance by means of a mixed-effects model, to consider intraindividual correlation, using the terms “group,” “time” and “group cross time” as effects, and a random effect was attributed to the patients’ ID followed by Tukey HSD test.
Correlations between continuous variables were investigated by calculating Pearsons’ coefficient. Calculations were performed using JMP 10.0 (SAS).
Results
From the 45 eyes included, 43 (33 patients) were followed for 48 weeks: 15 eyes from group ETDRS (10 patients) and PASCAL (12 patients), and 13 eyes in IVR (11 patients). Patients’ demographic data are shown in Table 1.
There were no statistically significant differences between groups regarding number of IVR injections (mean ± SE: 4.2 ± 0.2, 5.5 ± 0.5 and 4.6 ± 0.5 for ETDRS, PASCAL and IVR, respectively; p = 0.1059). No difference was observed between groups regarding the frequency of diabetic macular edema detection or the presence of active retinal new vessels at angiography (p > 0.05).
Best corrected visual acuity (BCVA)
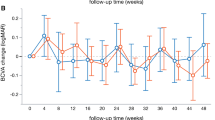
Mean baseline BCVA (logMAR) was 0.53 ± 0.07 (20/68); 0.45 ± 0.09 (20/56); and 0.53 ± 0.11 (20/68) for ETDRS, PASCAL and IVR, respectively (p = 0.7246). There was statistically significant within-group BCVA improvement of 0.1–0.3 logMAR in all groups during follow-up (p < 0.05), without statistically significant difference between groups (p > 0.05). Table 2 shows best corrected visual acuity (BCVA, mean ± SE), best central subfield thickness (CSFT, in µm) and fluorescein leakage area (FLA in mm2) for all groups at all study visits.
Central subfield thickness (CSFT) and fluorescein leakage area (FLA)
There was no significant difference between groups’ baseline CSFT; also a similar number of eyes showing macular edema (CSFT > 300 µm) were observed across groups ETDRS (8/15), IVR (6/13) and PASCAL (11/15) (p = 0.3018; likelihood ratio).
Although no significant between-groups difference was found on CSFT changes throughout follow-up (p > 0.05), a small, but statistically significant decrease in CSFT was observed overtime for groups ETDRS (r = 0.288; p < 0.001; slope = 0.405 µm/week) and PASCAL (r = 0.698; p < 0.001; slope = 0.946 µm/week, but not for group IVR (r = 0.679; p < 0.351) (Table 2).
FLA reduced in 55.9% ± 9.8%, 73.1% ± 14.5% and 73.3% ± 11.5% from baseline for ETDRS, IVR and PASCAL, respectively (p < 0.05), without significant between-groups differences. An increase in FLA was then observed up to week 12, with stabilization from week 24 onwards, but kept below baseline levels during entire follow-up for the 3 groups (Table 2).
Dark-adapted ERG
After PRP, a significant reduction in dark-adapted a- and b-wave amplitudes was observed in all ERG responses for dark-adapted stimuli (from 0.003 to 10.0 cd s/m2), from 12, up to 48-week follow-up.
Although a-, b-wave implicit times were increased if compared to normal subjects, there was no significant changes during follow-up.
As expected, OP-AUC was massively reduced at baseline for all groups, compared to normal subjects (Fig. 1), and they were further reduced after PRP in ETDRS and PASCAL groups, from 12 up to 48 weeks, but no significant changes were observed for IVR during follow-up (Table 3).
Mean baseline rod-response b-wave amplitude was 175.4 ± 20.0 µV, 169.0 ± 14.1 µV and 164.8 ± 17.8 µV (p > 0.05), and there was a statistically significant reduction of − 96.8 ± 15.7 µV (p < 0.05), and − 59.9 ± 14.9 µV for ETDRS and PASCAL groups, but the reduction was not significant for group IVR − 26.8 ± 13.70 µV (p > 0.05) up to week 24 (between groups: p = 0.003). Nevertheless, at week 48, rod b-wave amplitude also reduced in group IVR in − 42.6 ± 10.7 µV (p < 0.05) and kept significantly below baseline levels for ETDRS and PASCAL groups (Table 3; Figs. 1 and 2).
Similar picture was found for combined response a- and b-wave and oscillatory potential amplitude, but no significant changes were found for a- or b-wave implicit time (Table 3; Figs. 1 and 2).
No significant correlations were observed between a- or b-wave amplitudes or implicit times and BCVA, CSFT or FLA (r < 0.250 and p > 0.05 for all possible pairwise combinations, or multivariate analyses).
Light-adapted ERG
Similar to dark-adapted results, cone-driven responses also showed reduced b-wave amplitudes after PRP during follow-up (Fig. 2; Table 3), but no changes on implicit times (Table 2). There was a significant amplitude reduction for groups PASCAL and ETDRS from 12 weeks on, but no changes were found for IVR.
No correlation was found between light-adapted ERG amplitude or amplitude reduction and BCVA, FLA or CSFT (r < 0.250 and p > 0.05 for all possible pairwise combinations, or multivariate analyses).
Discussion
These data indicate that multiple spot (PASCAL) or single (ETDRS) panretinal photocoagulation (PRP), in association with intravitreal ranibizumab (IVR), or IVR alone, show similar effectivity controlling fluorescein angiography leakage (FLA), and improving visual acuity in patients with proliferative diabetic retinopathy, up to 48 weeks. Furthermore, this work reports electroretinographic changes due to ETDRS and PASCAL and investigates if IVR alone could avoid this functional loss.
Many studies suggest that ERG implicit time increase is sensitive parameters to detect the functional changes in DM patients [19, 22], and as expected, it was notably changed in our cohort from the baseline on. However, they were not changed after anti-VEGF treatment, combined or not with retinal photocoagulation and therefore were not used for group comparisons. In addition, it is also known that eyes with proliferative DR is associated with even marked ERG changes, particularly lower dark-adapted b-wave amplitude and that retinal photocoagulation causes further ERG amplitude reduction [19].
During treatment of PDR, the goal is to inhibit angiogenesis, which is mainly controlled by expression of vascular endothelial growth factor (VEGF) that is regulated by availability of oxygen. Considering dark adaptation as an important process causing retinal hypoxia [23], it seems reasonable to perform photocoagulation to intentionally destroy retinal structures intrinsically associated with dark adaptation, namely the rods.
Indeed, it has been shown that photocoagulation reduces retinal O2 consumption [4] and decreases final retinal dark-adapted sensitivity by 1.1 log units [8]. In this scenario, reductions in dark-adapted ERG amplitude are very likely and the ERG has been even suggested as an objective assessment of the degree of adequacy of panretinal photocoagulation [24].
In this perspective, as laser applications target posterior retinal structures (retinal pigment epithelium and photoreceptors) on the peripheral retina, one could expect that ERG components generated by the posterior retina would be more affected than inner-retinal signals. However, the ERG changes found after retinal photocoagulation—slightly greater b- than a-wave amplitude reduction—might indicate that the treatment might not only destroy the retinal areas directly illuminated by the laser beam, but also affect the functional integrity of adjacent areas, as previously hypothesized [19, 25], as far as in the macula [26]. These observations could also explain reduction in cone-driven ERG responses after PDR [19] and are certainly undesirable side effects of the laser treatment.
Of interest, data and other reports [13] suggest that PASCAL is as effective as conventional PRP in the treatment of PDR, and it has been suggested that PASCAL laser burns cause less inner-retinal destruction [25] and minor retinal sensitivity loss, with consequent only mild visual field changes detected at 6 months after treatment [26]. However, although ERG changes found on group PASCAL were slightly milder than on group ETDRS, difference between groups was not statistically significant, probably due to the small sample.
As for the best of authors knowledge, this is the first time that ERG responses after IVR treatment (without combination with retinal photocoagulation) for PDR is reported. The hypothesis was that ERG changes due to laser burns would be avoided, or any improvement could be observed. As a matter of fact, ERG amplitude or implicit time did not improve, for dark or light-adapted a-, or b-wave, OPs or whatsoever, but they also did not worsen up to 24 weeks, indicating that inhibiting VEGF permits controlling angiogenesis without reduction in oxygen demand caused by laser retinal damage. More about, a significant dark-adapted b-wave amplitude reduction was detected after 48 weeks, which is probably due to earlier damage and subsequent loss of retinal function, or associated with eventual macular edema treatment over time.
These observations should be interpreted considering the limitations of our work. For instance, the study was designed, allowing the inclusion of the two eyes from one patient into a group, 5 of 10 patients from group ETDRS, 3 of 12 from PASCAL and 2 of 11 from IVR. Obviously, our analysis should have been clearer if only one eye per patient was included, considering eventual changes on diabetic control or overall systemic variations that would directly affect both eyes of one subject. However, the main reasons that justify this study design was: (1) The progress of proliferative diabetic retinopathy might be fairly asymmetric on both eyes; (2) these patients have oftentimes other health complications, such as cardiovascular and kidney diseases, which limit the inclusion of large number of patients that are able to perform long-time examinations; and (3) ERGs are routinely performed bilaterally, so that the data are automatically available. To minimize this potential bias, we compared the data using a mixed model with a random attribute to the subject (not the eye).
In addition, the use of anti-VEGF agents in individualized discontinuous-variable posology also called “as-needed” or “pro re nata” (PRN) for diabetic macular edema leads to an irregular distribution of intravitreal injections during study visits. As an example, on week 44, 7, 2 and 1, patients received intravitreal ranibizumab for CSFT > 300 µm in PASCAL, IVR and ETDRS groups, respectively. This may have influenced diabetic macular edema, and consequently ERG responses at week 48. This is always a limitation when a PRN regimen for anti-VEGF treatment is employed. In this context, we also observed a continuous small CSFT reduction overtime on 2 out of the 3 groups (0.405 µm/week for ETDRS and 0.946 µm/week for PASCAL), which might be related to the tendency of chronic macular atrophy observed on patients with DME treated with anti-VEGF [27].
In summary, conventional PRP and multiple spot PRP associated with IVR or IVR alone are similarly effective controlling PDR progression up to 1 year. The two laser strategies cause similar retinal functional changes, while retinal function was mostly preserved for eyes treated with IVR without laser.
References
Yau JW, Rogers SL, Kawasaki R, Lamoureux EL, Kowalski JW, Bek T, Chen SJ, Dekker JM, Fletcher A, Grauslund J, Haffner S, Hamman RF, Ikram MK, Kayama T, Klein BE, Klein R, Krishnaiah S, Mayurasakorn K, O’Hare JP, Orchard TJ, Porta M, Rema M, Roy MS, Sharma T, Shaw J, Taylor H, Tielsch JM, Varma R, Wang JJ, Wang N, West S, Xu L, Yasuda M, Zhang X, Mitchell P, Wong TY, Meta-Analysis for Eye Disease Study G (2012) Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care 35:556–564
Engerman RL (1989) Pathogenesis of diabetic retinopathy. Diabetes 38:1203–1206
Group TDRSR (1976) Preliminary report on effects of photocoagulation therapy. The Diabetic Retinopathy Study Research Group. Am J Ophthalmol 81:383–396
Landers MB 3rd, Stefansson E, Wolbarsht ML (1982) Panretinal photocoagulation and retinal oxygenation. Retina 2:167–175
Vander JF, Duker JS, Benson WE, Brown GC, McNamara JA, Rosenstein RB (1991) Long-term stability and visual outcome after favorable initial response of proliferative diabetic retinopathy to panretinal photocoagulation. Ophthalmology 98:1575–1579
ETDRS (1991) Early photocoagulation for diabetic retinopathy. ETDRS report number 9. Early Treatment Diabetic Retinopathy Study Research Group. Ophthalmology 98:766–785
McDonald HR, Schatz H (1985) Macular edema following panretinal photocoagulation. Retina 5:5–10
Pender PM, Benson WE, Compton H, Cox GB (1981) The effects of panretinal photocoagulation on dark adaptation in diabetics with proliferative retinopathy. Ophthalmology 88:635–638
Henricsson M, Heijl A (1994) The effect of panretinal laser photocoagulation on visual acuity, visual fields and on subjective visual impairment in preproliferative and early proliferative diabetic retinopathy. Acta Ophthalmol (Copenh) 72:570–575
Khosla PK, Rao V, Tewari HK, Kumar A (1994) Contrast sensitivity in diabetic retinopathy after panretinal photocoagulation. Ophthalmic surgery 25:516–520
Blumenkranz MS, Yellachich D, Andersen DE, Wiltberger MW, Mordaunt D, Marcellino GR, Palanker D (2006) Semiautomated patterned scanning laser for retinal photocoagulation. Retina 26:370–376
Muqit MM, Marcellino GR, Gray JC, McLauchlan R, Henson DB, Young LB, Patton N, Charles SJ, Turner GS, Stanga PE (2010) Pain responses of Pascal 20 ms multi-spot and 100 ms single-spot panretinal photocoagulation: Manchester Pascal Study, MAPASS report 2. Br J Ophthalmol 94:1493–1498
Muqit MM, Marcellino GR, Henson DB, Young LB, Patton N, Charles SJ, Turner GS, Stanga PE (2010) Single-session vs multiple-session pattern scanning laser panretinal photocoagulation in proliferative diabetic retinopathy: The Manchester Pascal Study. Arch Ophthalmol 128:525–533
Chappelow AV, Tan K, Waheed NK, Kaiser PK (2012) Panretinal photocoagulation for proliferative diabetic retinopathy: pattern scan laser versus argon laser. Am J Ophthalmol 153(137–142):e132
Jorge R, Costa RA, Calucci D, Cintra LP, Scott IU (2006) Intravitreal bevacizumab (Avastin) for persistent new vessels in diabetic retinopathy (IBEPE study). Retina 26:1006–1013
Witmer AN, Vrensen GF, Van Noorden CJ, Schlingemann RO (2003) Vascular endothelial growth factors and angiogenesis in eye disease. Prog Retin Eye Res 22:1–29
Aiello LP, Avery RL, Arrigg PG, Keyt BA, Jampel HD, Shah ST, Pasquale LR, Thieme H, Iwamoto MA, Park JE et al (1994) Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med 331:1480–1487
Tonello PS, Rosa AH, Abreu CH Jr, Menegario AA (2007) Use of diffusive gradients in thin films and tangential flow ultrafiltration for fractionation of Al(III) and Cu(II) in organic-rich river waters. Anal Chim Acta 598:162–168
Messias A, Filho JA, Messias K, Almeida FP, Costa RA, Scott IU, Gekeler F, Jorge R (2012) Electroretinographic findings associated with panretinal photocoagulation (PRP) versus PRP plus intravitreal ranibizumab treatment for high-risk proliferative diabetic retinopathy. Doc Ophthalmol 124:225–236
McCulloch DL, Marmor MF, Brigell MG, Hamilton R, Holder GE, Tzekov R, Bach M (2015) ISCEV Standard for full-field clinical electroretinography (2015 update). Doc Ophthalmol 130:1–12
Messias A, Jaegle H, Gekeler F, Zrenner E (2008) Software for evaluation of electroretinograms XLVI annual symposium of the international society for clinical electrophysiology of vision (ISCEV), Morgantown—USA
Tzekov R, Arden GB (1999) The electroretinogram in diabetic retinopathy. Surv Ophthalmol 44:53–60
Arden GB, Sidman RL, Arap W, Schlingemann RO (2005) Spare the rod and spoil the eye. Br J Ophthalmol 89:764–769
Moschos M (1982) ERG and VER findings after laser photocoagulation of the retina. Metab Pediatr Syst Ophthalmol 6:101–105
Schuele G, Rumohr M, Huettmann G, Brinkmann R (2005) RPE damage thresholds and mechanisms for laser exposure in the microsecond-to-millisecond time regimen. Invest Ophthalmol Vis Sci 46:714–719
Subash M, Comyn O, Samy A, Qatarneh D, Antonakis S, Mehat M, Tee J, Mansour T, Xing W, Bunce C, Viswanathan A, Rubin G, Weleber R, Peto T, Wickham L, Michaelides M (2016) The effect of multispot laser panretinal photocoagulation on retinal sensitivity and driving eligibility in patients with diabetic retinopathy. JAMA Ophthalmol 134:666–672
Willmann G, Nepomuceno AB, Messias K, Barroso L, Scott IU, Messias A, Jorge R (2017) Foveal thickness reduction after anti-vascular endothelial growth factor treatment in chronic diabetic macular edema. Int J Ophthalmol 10:760–764
Funding
FAPESP Grant Numbers: 2012/16265-0, and 2013/02169-2. Rodrigo Jorge received travel support from Novartis to attend the 2015 American Society of Retina Specialists (ASRS) meeting. The sponsor had no role in the design or conduct of this research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Informed consent
All participants gave written informed consent before entering the study.
Statement of human rights
All procedures were in accordance with the ethical standards of the institutional research committee (Comitê de Ética em Pesquisa do Hospital das Clínicas da Faculdade de Medicina de Ribeirão Preto—USP, Protocol Number 11685/2012) and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Statement on the welfare of animals
No animals were used in this research.
Rights and permissions
About this article
Cite this article
Messias, K., Barroso, R.M., Jorge, R. et al. Retinal function in eyes with proliferative diabetic retinopathy treated with intravitreal ranibizumab and multispot laser panretinal photocoagulation. Doc Ophthalmol 137, 121–129 (2018). https://doi.org/10.1007/s10633-018-9655-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10633-018-9655-9