Abstract
Arbutus andrachne L. is a medicinal plant that grows in Jordan and has many valuable effects. In the present study, the anti-nociceptive effect of A. andrachne methanolic leaf extract was determined in mice using thermal and chemical tests. Our findings show that different doses of A. andrachne extract reduced the number of writhings significantly compared to control group. The leaf extract also reduced the time of paw licking in the early and late phases of formalin test. In all the conducted tests, 300 mg/kg body wt. was the best effective dose. A peroxisome proliferator-activated receptor alpha (PPARα) antagonist reversed the action of the plant extract in the early phase of formalin test while antagonists of the PPARα, PPAR gamma (PPARγ) and cannabinoid 1 (CB1) receptors were responsible for abolishing its effect in the late phase of this test. Also, the extract administration increased the latency time in hot plate and tail flick, an effect that was reversed by the antagonists of PPARγ, CB1 and transient receptor potential vanilloid 1 (TRPV1). No effect was noticed for α2-adrenergic receptor antagonist in the action of A. andrachne in any of the conducted tests in this study. Furthermore, analysis of the constituents in the methanolic leaf extract using liquid chromatography mass spectrometry (LCMS) showed that the extract is rich in compounds that have anti-nociceptive and/or anti-inflammatory effects such as arbutin, rutin, linalool, linoleic acid, gallic acid, lauric acid, myristic acid, hydroquinone, β-sitosterol, ursolic acid, isoquercetin, quercetin, (+)-gallocatechin, kaempferol, α-tocopherol, myricetin 3-O-rhamnoside and catechin gallate. In conclusion, A. andrachne showed promising anti-nociceptive effects in thermal and chemical models of pain. These findings can open an avenue for natural pain relief.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pain is one of the major health problems that cause serious economic and social burdens (Cazacu et al. 2015). Unfortunately, many of the available analgesics have side effects, (Cazacu et al. 2015). This fact impels the need to find safer therapeutics and explore their mechanism of action. Several medicinal plants have effective analgesic properties and are considered promising drug candidates (de Cássia da Silveira et al. 2017). According to Oran (2014), there are 363 medicinal plants in Jordan. Exploring the pharmacological effects of many of these plants and their constituents can open a gate towards finding powerful analgesics. Arbutus andrachne L. is a flowering tree that belongs to the family Ericaceae (Oran 2015) and to the sub-family Arbutoideae (Tenuta et al. 2018). The plant has other common names including Eastern or Greek strawberry tree and is known as “Qaiqab or Qatlab” in Arabic (Oran 2015). In Jordan, it grows in Jarash, Ajloun and Irbid areas, particularly in the mountains that have 900–1700 m altitude (Oran 2015). It is an edible plant that has many uses in traditional medicine (Oran 2015). Nutritionally, the fruits are rich in many acids and sugars including malic acid, fumaric acid, fructose, glucose and sucrose (Seker and Toplu 2010). Experimentally, it was found that the plant has anti-proliferative (Abu-Dahab and Afifi 2007), anti-inflammatory, antimicrobial (Amro et al. 2013), anti-malarial (Kaisera et al. 2007), antioxidant, antibiotic, anti-cancer and anti-hypertensive effects (Okmen 2015; Baskan et al. 2019). Also, the plant showed hypolipidaemic, hypoglycaemic protective effects in diabetic rats (Abu-zaiton et al. 2019) and immunological defensive effects related to changes in the number of white blood cells (WBCs) in rats (Atrooz et al. 2007). Despite the numerous studies that investigated the effects of A. andrachne, the therapeutic effects of this plant are not fully determined. None of the previous studies, to the best of our knowledge, explored the anti-nociceptive effect of A. andrachne. Therefore, the aim of this study was to assess the anti-nociceptive effect of A. andrachne and the receptors involved in its action in different pain models. The studied receptors included peroxisome proliferator-activated receptors alpha and gamma (PPARα and γ) (Alsalem et al. 2019b), cannabinoid receptor 1 (CB1) (Alsalem et al. 2019a) and transient receptor potential vanilloid 1 (TRPV1) (Julius and Basbaum 2001). The choice of these receptors was based on their involvement in pain relief in different pain models. PPARs are nuclear hormone receptors that have α, β and γ isoforms with different tissue distribution in vertebrates (O’Sullivan 2016). PPARs are involved in adipogenesis, cardiovascular, gastrointestinal, anti-inflammatory, anti-diabetic, analgesic, anti-cancer, metabolic and neuroprotective effects (O’Sullivan 2016; Costa et al. 2008). These receptors exert their effects via the transcription of certain genes (genomic) or through quick non-genomic pathways (Berger and Moller 2002). On the other hand, CB receptors are G protein coupled receptors (GPCRs) and are considered targets for endocannabinoids as well as many analgesic drugs (Donvito et al. 2018). Russo et al. (2007) reported a synergistic effect between endocannabinoids and PPARα receptor in the formalin paw licking test, an effect mediated by the large conductance potassium channel that is activated by PPARα receptor signaling pathway cascade and is important in neuronal excitation (Scholz et al. 1998). Moreover, the activation of PPARs (in particular PPARα and PPARγ) by cannabinoids was reported to be responsible for the analgesic and anti-inflammatory activities of PPARs (O'Sullivan 2016). Another important receptor in pain is TRPV1 which is a non-selective cation channel activated by capsaicin, heat and other mediators that play a key role in the processing of pain (Julius and Basbaum 2001). It has been reported that there is a crosstalk between this channel and many receptors that are involved in pain such as PPARα (Ambrosino et al. 2014), PPARγ and CB1 receptors (Costa et al. 2008) in an indication that these receptors are vital targets for alleviating pain.
Materials and methods
Drugs
GW6471, GW9662, SR141716A, capsazepine, naloxone and yohimbine were purchased from Tocris Bioscience, UK. Diclofenac sodium was from Novartis, Switzerland. Tablets of morphine sulfate were from Napp Pharmaceuticals (Cambridge). The stocks of drugs (except naloxone, yohimbine and morphine) were initially dissolved in absolute ethanol (10 mM capsazepine, 1 mM capsaicin, 1 mM SR141716A, 24 mM GW9662, 20 mM GW6471).
Yohimbine, naloxone and morphine were directly dissolved in saline. All drugs were freshly prepared (from the stock) before use and were diluted in sterile saline.
Collection and identification of plant specimens
The leaves of A. andrachne were collected from Jarash, Jordan in March 2019. The plant was authenticated by Prof. Sawsan Oran, a plant taxonomist, Department of Biological Sciences, University of Jordan, Amman, Jordan. A voucher specimen was deposited at the herbarium, Department of Biological Sciences at the University of Jordan.
Preparation of methanolic extract of A. andrachne leaves
The leaves of A. andrachne were washed, dried and ground with a blender. A 100-g sample of the dried leaves was soaked in 1000 ml methanol (10:1 v/w ratio) for 3 days with continuous shaking at room temperature (Pandey and Tripathi 2014). After filtration, a rotary evaporator was used to evaporate the solution at 45 °C under reduced pressure. The methanolic extract was prepared using successive extractions. The extract was weighed to calculate the percentage yield using the following equation: Yield% = (wt. of dry extract/wt. of dry leaves before extraction) × 100%. The extract was kept at − 20 °C in an airtight container.
Liquid chromatography–mass spectrometry (LCMS)
LCMS analysis was done using a gradient of solvents A and B as a mobile phase; solvent A is 0.1% (v/v) formic acid dissolved in water and solvent B is 0.1% (v/v) formic acid dissolved in acetonitrile. The analysis was conducted using the following experimental parameters: Agilent Zorbax Eclipse XDB-C18 column (2.1 × 150 mm × 3.5 μm), 25 °C, 1 μl injection volume and 18 mg sample/ml methanol. SIL-30AC autosampler with cooler, Shimadzu CBM-20A system controller, LC-30AD pump and CTO-30 column oven were used to inject the plant extract into the mass detector. The eluent was monitored under positive ion mode by Shimadzu LC–MS 8030, electrospray ion mass spectrometer (ESI–MS), skimmer 65 V and a fragmentor voltage of 125 V. Nitrogen gas with 99.99% purity and 10 L/min flow rate was used as a drying gas. In addition, 45 lb per square inch (psi) nebulizer and 350 °C capillary temperature were used in this part of the experiments. After that, the eluent was scanned from 100 to 1000 m/z. The results were validated by authentic standard compounds.
Animals
All experiments were conducted in accordance with the guidelines of the International Association for the Study of Pain (IASP) for use of animals in research and were approved by the animal ethics committee at the University of Jordan (approval number 235/2020/19). Male BALB/c mice (25–30 g) were used in all experiments including tail flick, hot plate, formalin-induced paw licking and acetic acid induced-writhing tests. The animals were kept in the animal house at the University of Jordan in an air-controlled room with a cycle of 12 h light and 12 h dark. Food pellets and water were provided ad libitum. The animals were allowed to adapt to the conditions of the experimental room before conducting the experiments.
Acetic acid-induced writhing test
The mice were divided into control and treated groups. Each group had eight animals. Different doses of the plant extract (150, 300 or 600 mg/kg body wt.) were used in the writhing test. The positive control group received 30 mg/kg body wt. diclofenac sodium whereas the control group received normal saline. All injections were intraperitoneal (i.p.). The animals received i.p. injection of 1% acetic acid (10 ml/kg body wt.) 30 min after the injection of vehicle, plant extract or diclofenac sodium. Ten minutes later, the number of writhes was counted and was compared to control group. Abdominal muscle contraction with elongation of the body and the hind limbs was considered a writhe (Jaffal and Abbas 2018).
Tail immersion (tail flick) test
The animals were divided into different groups. The effect of 150, 300 or 600 mg/kg body wt. A. andrachne (i.p.) on tail flick was examined. In the positive control group, the animals received 10 mg/kg body wt. morphine while the control group received normal saline. Another group received 5 mg/kg body wt. naloxone then morphine (30 min after naloxone injection). Tail flick was conducted 1 h after the administration of A. andrachne extract. The test was performed by immersing 5 cm of the tail of every mouse in hot water (55 ± 0.5 °C). The time between immersing the tail and the reflex of withdrawing it was recorded with a stopwatch. A cut-off time of 15 s was used to prevent tissue damage (Jaffal and Abbas 2018). The dose of 300 mg/kg body wt. was chosen to determine the mechanism of action of the plant extract by using different antagonists, namely 0.1 mg/kg body wt. SR141716A (a selective CB1 antagonist), 1 mg/kg body wt. yohimbine (a selective α2-adrenoceptor antagonist), 0.1 mg/kg body wt. capsazepine (a selective TRPV1 antagonist), 1 mg/kg body wt. GW6471 (a selective PPARα antagonist) and 1 mg/kg body wt. GW9662 (a selective PPARγ antagonist). The antagonists were administered (i.p.) 30 min before the treatment with A. andrachne extract.
Hot plate test
The hot plate test was performed in mice divided into different groups. Three doses of A. andrachne (150, 300 or 600 mg/kg body wt., i.p.) were used in the experiment. The control group received normal saline. The positive control group received 10 mg/kg body wt. morphine. Another group received i.p. injection of 5 mg/kg body wt. naloxone then morphine. The effective dose of the leaf extract (300 mg/kg body wt.) was chosen to assess the receptors that are involved in the action of the leaf extract. The animals received i.p. injection of different antagonists. The antagonists, doses and method of treatments were similar to what was used in the previous experimental section. Half an hour later, A. andrachne extract was administered (i.p.). The hot plate test was conducted 60 min after the injection of vehicle or A. andrachne extract. To perform the experiment, the animals were placed individually on electronic hot plate maintained at 55 ± 2.0 °C. The reaction time starting from placing the animal on the hot plate till its jump was recorded. A cut-off time of 60 s was used to prevent tissue damage (Jaffal and Abbas 2018).
Formalin-induced paw licking test
The animals received different doses of A. andrachne extract (150, 300 or 600 mg/kg body wt., i.p.). The control group received normal saline. Positive control groups received 10 mg/kg body wt. morphine or naloxone (5 mg/kg body wt.)/morphine. Mice were treated with different antagonists (as mentioned in the previous experimental sections) 30 min before the administration of 300 mg/kg body wt. A. andrachne extract (Jaffal and Abbas 2018). The formalin test was conducted according to Janssen (1963). In brief, the animals received intraplantar (ipl) injection of 2.5% formalin (20 µl per paw) after different treatments. The time of licking the injected paw (in seconds) was recorded at 0–5 min (early phase) and at 25–30 min (late phase) after formalin injection.
Statistical analysis
Data was presented as mean ± SEM. The normality test was conducted for all groups using Shapiro–Wilk test. The statistical significance of difference between groups was assessed by one-way analysis of variance (ANOVA) followed by a suitable post hoc test (Dunnett's test or Fisher’s least significant difference, LSD) using GraphPad Prism version 7. p < 0.05 was considered significant.
Results
In this study, the anti-nociceptive effect of A. andrachne was characterized in thermal (hot plate test, tail flick test) and chemical (formalin paw licking-induced test, acetic acid induced-writhing test) pain models. Different doses of A. andrachne extract were i.p. injected into the animals. Figure 1 shows the number of writhings in the groups that received the three doses of methanolic leaf extract. All three doses reduced the number of writhes significantly compared to control group. A remarkable decrease in the number of writhes occurred in the animals that received 150 and 300 mg/kg body wt. doses of A. andrachne extract. This effect was better than the anti-nociceptive effect exerted by the positive control group treated with 30 mg/kg body wt. diclofenac sodium.
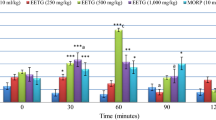
The effect of methanolic leaf extract (at 3 doses) on the early and late phases of formalin test was determined. All doses reduced paw licking in the early phase of the formalin test compared to the vehicle-treated group (Fig. 2). Interestingly, only the dose of 300 mg/kg body wt. A. andrachne extract reduced paw licking significantly compared to control group in the late phase of formalin-induced paw licking test (Fig. 3). The lowest and the highest used doses of A. andrachne extract did not exert significant anti-nociceptive effect in the late phase of this test. Accordingly, 300 mg/kg body wt. A. andrachne extract was chosen to assess its mechanism of action using different antagonists.
Effect of A. andrachne extract on the early phase of formalin test. Data presented as mean ± SEM, n = 8. *Significant compared to control group, #significant compared to A. andrachne (300 mg/kg body wt.), &significant compared to morphine-treated group, p < 0.05. One-way ANOVA followed by LSD post hoc test
Effect of A. andrachne extract on the late phase of formalin test. Data presented as mean ± SEM, n = 8 except the groups of capsazepine/A. andrachne and naloxone/morphine in which n = 7. *Significant compared to control group, #significant compared to A. andrachne (300 mg/kg body wt.), &significant compared to morphine-treated group, p < 0.05. One-way ANOVA followed by LSD post hoc test
The reduction in the time of paw licking exerted by 300 mg/kg body wt. A. andrachne in the early phase of the formalin test was reversed by the use of selective PPARα antagonist (1 mg/kg body wt. GW6471). In the late phase of formalin test, the antagonists of PPARα, PPARγ receptors (1 mg/kg body wt. GW9662) and CB1 receptors (0.1 mg/kg body wt. SR141716A) reduced the effect of 300 mg/kg body wt. A. andrachne. No effect was found for capsazepine (a TRPV1 antagonist) or yohimbine (an α2-adrenergic receptor antagonist) in the action of A. andrachne in either phase of the formalin test.
In thermal tests, the three doses of A. andrachne extract increased the time during which the animal spent on the hot plate (Fig. 4) while only the dose of 300 mg/kg body wt. A. andrachne demonstrated significant effect in the tail flick immersion test (Fig. 5). Our results prove that the i.p. injection of 300 mg/kg body wt. A. andrachne extract showed the best anti-nociceptive effect in thermal tests compared to other doses (150 and 600 mg/kg body wt.). As a result, this dose was used to assess the receptors involved in its action. The action of the leaf extract in hot plate and tail flick tests was reversed by the antagonists of PPARγ, CB1 and TRPV1 receptors, indicating the involvement of these receptors in the mechanism of action of A. andrachne leaf extract in thermal tests. No inhibition was detected for α2-adrenergic receptor or PPARα antagonists in the effect of the plant extract in this part of the experiment.
Effect of A. andrachne extract on hot plate test. Data presented as mean ± SEM, n = 8 except the groups of yohimbine/A. andrachne and SR141716A/A. andrachne in which n = 7. *Significant compared to control group, #significant compared to A. andrachne (300 mg/kg body wt.), &significant compared to morphine-treated group, p < 0.05. One-way ANOVA followed by LSD post hoc test
Effect of A. andrachne extract on tail flick test. Data presented as mean ± SEM, n = 8 except the group of 150 mg/kg A. andrachne in which n = 7. *Significant compared to control group, #significant compared to A. andrachne (300 mg/kg body wt.), &significant compared to morphine-treated group, p < 0.05. One-way ANOVA followed by LSD post hoc test
The active constituents in A. andrachne methanolic leaf extract were identified using LCMS. As shown in Table 1, arbutin is the main constituent and represents 27.8%. α-Tocopherol (a form of vitamin E) is the second most abundant ingredient in A. andrachne leaf extract (representing 9.5%). Other constituents were also detected at different percentages as presented in the table.
Discussion
Medicinal plants are promising sources for finding analgesics with fewer side effects (de Cássia da Silveira et al. 2017). However, the complete value of several plants has not yet been explored. A. andrachne is one of the medicinal plants growing in Jordan. The effect of this plant in pain relief was not studied previously. This study showed a strong anti-nociceptive effect of A. andrachne leaf extract in murine models of pain including hot plate, tail flick, acetic acid induced-writhing and formalin-induced paw licking tests. The plant extract in different tests did not show a dose-related response. The lack of the dose-dependent response can be attributed to the fast clearance of the low dose or its weak affinity to activate the key receptors that are involved in this response (Andersen 1981). On the other hand, the lack of response in the groups treated with the higher dose can be due to a pharmacokinetic mechanism of excretion to avoid toxicity in the body of the animal (Andersen 1981) or to off-target effects.
In formalin test, the plant extract showed strong inhibition of paw licking in the early phase and this effect was reversed by GW6471 (a PPARα antagonist). The early phase represents nociceptive pain resulting from the stimulation of peripheral nerve fibres (Julius and Basbaum 2001). In agreement with our results, LoVerme et al. (2006) reported the involvement of PPARα receptor in peripheral pain. Moreover, the i.p. injection of A. andrachne diminished the number of paw lickings in the late phase of the formalin test that represents inflammatory pain (Julius and Basbaum 2001). This effect was abolished by pre-treating the animals with CB1, PPARα and PPARγ antagonists. Notably, PPARγ receptor is widely expressed in dorsal root ganglia (DRGs) and the dorsal horn of the spinal cord (Moreno et al. 2004) and is one of the receptors to which many non-steroidal anti-inflammatory drugs (NSAIDs) bind to (Lehmann et al. 1997). This finding may explain the involvement of PPARγ receptor in the effect of A. andrachne in the late, but not the early phase of the formalin test. Additionally, the role of endocannabinoids in the interaction between inflammatory mediators and cytokines can explain the involvement of CB1 in the mechanism of the plant extract in the late phase (Donvito et al. 2018). In addition to PPARγ receptor, PPARα receptor was involved in the anti-nociceptive effect of ibuprofen (Alsalem et al. 2016). On the other hand, our findings indicate that the anti-nociceptive effect of A. andrachne in thermal tests (hot plate and tail flick tests) was inhibited by pre-injecting the animals with PPARγ, CB1 and TRPV1 antagonists. The reversal of A. andrachne action by the CB1 antagonist (SR141716A) indicates the involvement of this receptor in the action of the plant extract, raising the possibility that the administration of the extract mediates entourage effects (Ben-Shabat et al. 1998). In more detail, the mixture of constituents in the leaf extract may stimulate the release of endocannabinoids such as 2-arachidonoylglycerol (2-AG) or arachidonoylethanolamine (anandamide; AEA). These endocannabinoids are known to stimulate CB1, PPARs and TRPV1 receptors (Donvito et al. 2018). The activation can be direct or indirect through the inhibition or deactivation of certain enzymes responsible for the hydrolysis of endocannabinoids such as fatty acid amide hydrolase (FAAH) and monoacylglycerol lipase (MAGL) (Donvito et al. 2018), leading to increase in the levels of endocannabinoids and their analgesic effects. Accordingly, it is possible that the administration of A. andrachne extract reduces the levels of inflammatory mediators as well as arachidonic acid accompanying the inhibition of MAGL (Donvito et al. 2018) or enhances the anti-inflammatory cytokines associated with cannabinoid production (Cabral and Griffin-Thomas 2009). Starowicz et al. (2013) reported other evidence for this possibility; they showed that the increase in spinal AEA (mediated by FAAH inhibition of its hydrolysis) decreased neuropathic pain in rats by acting at TRPV1 and CB1 receptors. Interestingly, the ingredients identified in A. andrachne can provide a line of evidence that supports this finding. For example, kaempferol is a flavonoid that demonstrated anti-nociceptive and anti-inflammatory effects in writhing and croton oil-induced oedema tests, respectively (De Melo et al. 2009). This flavonoid led to PPARγ activation in a reporter assay and cyclooxygenase inhibition in macrophages (Liang et al. 2001). It also inhibits FAAH and therefore augments the levels of endocannabinoids (Thors et al. 2008). Furthermore, it is worthy to comment that the intracerebroventricular (icv) injection of kaempferol led to pain alleviation in rats in a mechanism that involves TRPV1 receptors (Zarei et al. 2020). Additionally, there is emerging evidence that cannabinoids can activate PPAR receptors (O'Sullivan 2016). Kaczocha et al. (2009) showed that fatty acid binding proteins (FABPs) transport endocannabinoids to PPARα receptor in the nucleus and mediate their effects. The anti-inflammatory and neuroprotective effect of cannabinoids were also dependent on PPARγ receptor (Esposito et al. 2011) while the analgesic effect of palmitoylethanolamide (PEA) was inhibited by using PPARα (LoVerme et al. 2006) and TRPV1 antagonists (Ambrosino et al. 2014). On the other hand, the use of PPARα agonists led to the activation and desensitization of TRPV1, demonstrated by patch clamp in Chinese hamster ovary (CHO) cells (Ambrosino et al. 2014). PPARα and PPARγ receptors were also involved in the anti-nociceptive effect of ibuprofen in hot plate and von Frey tests in rats (Alsalem et al. 2016). A crosstalk between PPARα and TRPV1 receptors was reported by Ambrosino et al. (2014). This interaction was also revealed in a model of complete Freund’s adjuvant (CFA)-induced inflammatory pain and calcium imaging studies (Aldossary et al. 2019).
In DRG neurons, other researches proved that PPARα receptor inhibits the acid-sensing ion channel (ASICs) which is a sensor for extracellular protons, a key activator for TRPV1 (Ambrosino et al. 2014). As an additional evidence for the relation between PPARs and TRPV1 receptors, it was shown that applying tesaglitazar (a dual PPAR agonist) to DRG neurons prior to capsaicin decreased capsaicin-induced cobalt influx (Alsalem et al. 2019b). Moreover, the chemical constituents in A. andrachne were determined using LCMS. Some ingredients were similar to the components that were analysed in A. andrachne leaves collected from Turkey, such as myricetin-3-O-α-l-rhamnosides, quercetin, kaempferol and (+)-gallocatechin (Nahrstedt 1992). Many of the constituents identified in A. andrachne proved to be effective in reducing pain and exert their effects via one or more of the receptors that were assessed in our study: PPARs, CB1 and TRPV1 receptors. Although arbutin is the main constituent in this plant, there is no evidence in the literature to support interaction between arbutin and CB1, PPARs or TRPV1 receptors. However, earlier reports showed several findings regarding the involvement of α-tocopherol in analgesia and inflammation and can justify the effect of A. andrachne in this study. In more detail, α-tocopherol is a naturally occurring antioxidant that showed anti-inflammatory and analgesic effects in neuropathic pain, formalin and writhing tests (Juaira et al. 2018; Chen et al. 1998; Kim et al. 2006). Also, it was reported that it interacts with cannabinoids in rat hippocampus (Crouzin et al. 2011) and with TRPV1 in neuroprotection (Crouzin et al. 2010). Furthermore, it was found that α-tocopherol upregulated the expression of PPARγ mRNA and protein in colon cancer cells (Campbell et al. 2003). In addition, the oral gavage of d-α-tocopherol improved insulin resistance, triglyceride levels and oxidative stress via influencing the expression of PPARγ and α in male mice fed with high fat diet (Kim et al. 2013). Another constituent that was detected in A. andrachne is gallic acid which demonstrated anti-nociceptive activity (Trevisan et al. 2014). Administration of gallic acid extracted from Emblica officinalis showed anti-diabetic potential by increasing the expression of PPARγ (Variya et al. 2019). Also, catechin gallate was listed among the ingredients identified by LCMS. An isomer of catechin gallate (named epicatechin gallate) exhibited an anti-nociceptive effects in hot plate and writhing tests in mice and anti-inflammatory effects in carrageenan-induced paw oedema model (Al-Sayed and Abdel-Daim 2018). In addition, epicatechin gallate activated nitric oxide synthase in endothelial cells through TRPV1 (Guo et al. 2015). Epicatechin gallate extracted from green tea binds to CB receptors (in particular CB1) in the central nervous system (Korte et al. 2009) and PPARγ receptor (Wu et al. 2017). Rutin (also named quercetin-3-rutinoside, sophorin, vitamin P or rutoside) is also a component that was identified in the analysis of A. andrachne leaf extract. Rutin is a flavonoid glycoside that has antioxidative, neuroprotective, analgesic and anti-depressant effects, particularly by the upregulation of CB1 cannabinoid receptor-interacting protein 1 (Ganeshpurkar and Saluja 2017; Su et al. 2014). Linalool is an aromatic monoterpene that exists in many plants, herbs and tea (Peana et al. 2003) and in A. andrachne as shown in Table 1. Linalool demonstrated anti-nociceptive activity in hot plate, formalin and writhing tests in mice as well as anti-inflammatory effect (Peana et al. 2003; Quintans-Júnior et al. 2013). Also, linalool induced fatty acid oxidation in mice through silencing PPARα expression (Jun et al. 2014).
Additionally, other compounds were determined in A. andrachne such as kaempferol, lauric acid, myristic acid, ursolic acid, hydroquinone, β-sitosterol, quercetin, and myricetin. Lauric acid and myristic acid are fatty acids that mediate their activities through PPARα transactivation (Popeijus et al. 2014). Ursolic acid is a plant triterpenoid and a PPARα agonist that exhibited anti-nociceptive and anti-inflammatory activities (Tapondjou et al. 2003). It binds strongly to CB1 receptor and has a potent inhibitory activity against TRPV1 (Jia et al. 2011; Milad et al. 2017; Zhang et al. 2011). Moreover, hydroquinone is a sesquiterpene which has analgesic and anti-inflammatory activities (Fawad et al. 2018). Previous studies revealed the binding affinity of hydroquinone, quercetin and β-sitosterol to PPARγ receptor (Ahmed and Alkali 2018; Shashni et al. 2013). In an in vivo study, quercetin alleviated algesia by blocking TRPV1 activity (Gao et al. 2016). Quercetin also upregulated CB1 in a model of induced colon cancer (Tutino et al. 2018). On the other hand, rutin and isoquercetin were the ingredients responsible for the anti-nociceptive action of mulberries (Chen et al. 2018). Isoquercetin was found to enhance the protein expression of PPARα in a rat model of induced nonalcoholic fatty liver disease (Qin et al. 2018). Furthermore, Nirmal et al. (2012) reported the analgesic and anti-inflammatory activities of β-sitosterol isolated from Nyctanthes arbortristis leaves. Additionally, Tong et al. (2009) have shown significant analgesic activity of myricetin isolated from Myrica rubra in writhing and formalin-induced paw licking tests. Patwardhan et al. (2010) found that the metabolites of oxidized linoleic acid (namely 9- and 13-hydroxyoctadecadienoic acid, 9- and 13-HODE) resulting from exposure to noxious heat activate TRPV1 in the skin of mice and rats. Linoleic acid ameliorated colitis through activation of PPARγ receptor in a model of inflammatory bowel disease (Bassaganya-Riera et al. 2004). The effect of linoleic acid on CB receptors was indirect through increasing the levels of 2-AG and anandamide endocannabinoids (Alvheim et al. 2013).
Conclusions
The data obtained from this research prove the strong anti-nociceptive effect of A. andrachne in thermal and chemical pain models. The mode of action of the leaf extract included important receptors in pain pathways such as PPARα, PPARγ, CB1 and TRPV1 receptors. The analysis of the constituents in the leaf extract strongly justified our findings, as the plant is rich in many ingredients that provide pain relief in different tests. Linalool, α-tocopherol, lauric acid, myristic acid, ursolic acid and isoquercetin are known to activate and/or upregulate PPARα receptor while linalool, α-tocopherol, gallic acid, kaempferol, hydroquinone and linoleic acid stimulate and/or upregulate PPARγ receptor. CB1 can be activated/upregulated by α-tocopherol, rutin, ursolic acid, quercetin and linoleic acid. Linalool, α-tocopherol, ursolic acid, quercetin and linoleic acid affect TRPV1. Accordingly, crosstalk between these receptors is expected. These findings are promising and can open a gate towards developing new analgesics.
References
Abu-Dahab R, Afifi F (2007) Antiproliferative activity of selected medicinal plants of Jordan against a breast adenocarcinoma cell line (MCF7). Sci Pharm 75:121–136
Abu-zaiton A, Abu-Samak M, Oran S, Yousef I, Abu-Zaitoon Y, Algaramseh A (2019) Hypoglycemic, hypolipidimic and protective effects of Arbutus andrachne extract in streptozotocin induced diabetic rats. Res J Biol Sci 14(3):56–60
Ahmed HA, Alkali IY (2018) In silico molecular docking studies of some phytochemicals against peroxisome-proliferator activated receptor gamma (PPAR-γ). GSC Biol Pharm Sci 5:001–005
Aldossary SA, Alsalem M, Kalbouneh H, Haddad M, Azab B, Al-Shboul O, Mustafa AG, Obiedat S, El-Salem K (2019) The role of transient receptor potential vanilloid receptor 1 and peroxisome proliferator-activated receptors-α in mediating the antinociceptive effects of palmitoylethanolamine in rats. NeuroReport 30(1):32–37
Alsalem M, Altarifi A, Kalbouneh H, Al-Zer H, Azab B, El-Salem K (2016) Role of PPARα and PPARγ in mediating the analgesic properties of ibuprofen in vivo and the effects of dual PPARα/γ activation in inflammatory pain model in the rat. Int J Pharmacol 12(8):812–820
Alsalem M, Haddad M, Aldossary SA, Kalbouneh H, Altarifi A, Jaffal SM, Abbas MA, Aldaoud N, El-Salem K (2019a) Role of cannabinoid receptor 1 and the peroxisome proliferator-activated receptor α in mediating anti-nociceptive effects of synthetic cannabinoids and a cannabinoid-like compound. Inflammopharmacology 27(6):1131–1142
Alsalem M, Haddad M, Aldossary SA, Kalbouneh H, Azab B, Dweik A, Imraish A, El-Salem K (2019b) Effects of dual peroxisome proliferator-activated receptors and activation in two rat models of neuropathic pain. PPAR Res 2019:1–9
Al-Sayed E, Abdel-Daim MM (2018) Analgesic and anti-inflammatory activities of epicatechin gallate from Bauhinia hookeri. Drug Dev Res 79(4):157–164
Alvheim A, Torstensen B, Lin Y, Lillefosse H, Lock EJ, Madsen L, Frøyland L, Hibbeln J, Kjellevold M (2013) Dietary linoleic acid elevates the endocannabinoids 2-AG and anandamide and promotes weight gain in mice fed a low fat diet. Lipids 49(1):59–69
Ambrosino P, Soldovieri MV, De Maria M, Russo C, Taglialatela M (2014) Functional and biochemical interaction between PPARα receptors and TRPV1 channels: potential role in PPARα agonists-mediated analgesia. Pharmacol Res 87:113–122
Amro B, Haddadin R, Tawaha K, Mohammad M, Mashallah S, Assaf A (2013) In vitro antimicrobial and anti-inflammatory activity of Jordanian plant extracts, a potential target therapy for Acne vulgaris. Afr J Pharm Pharmacol 7:2087–2099
Andersen ME (1981) Saturable metabolism and its relationship to toxicity. Crit Rev Toxicol 9(2):105–150
Atrooz O, Omary A, Khamas W, Shaikhly A, Salim M (2007) The effects of alcoholic extract of Arbutus andrachne on some biochemical parameters and visceral organs in rats. Int J Food Sci Tech 42:1046–1051
Baskan C, Kılıc DD, Sırıken B, Tanrikulu G, Gül M, Ertürk O (2019) In vitro study on antioxidant, antibacterial and DNA interaction activities of extracts from Arbutus andrachne L. EJFS 7(3):293–300
Bassaganya-Riera J, Reynolds K, Martino-Catt S, Cui Y, Hennighausen L, Gonzalez F, Rohrer J, Benninghoff AU, Hontecillas R (2004) Activation of PPAR gamma and delta by conjugated linoleic acid mediates protection from experimental inflammatory bowel disease. Gastroenterology 127(3):777–791
Ben-Shabat S, Fride E, Sheskin T, Tamiri T, Rhee M, Vogel Z, Bisogno T, De Petrocellis L, Di Marzo V, Mechoulam R (1998) An entourage effect: inactive endogenous fatty acid glycerol esters enhance 2-arachidonoyl-glycerol cannabinoid activity. Eur J Pharmacol 353:23–31
Berger J, Moller DE (2002) The mechanisms of action of PPARs. Annu Rev Med 53:409–435
Cabral GA, Griffin-Thomas L (2009) Emerging role of the cannabinoid receptor CB2 in immune regulation: therapeutic prospects for neuroinflammation. Exp Rev Mol Med 11:e3–e33
Campbell SE, Stone WL, Whaley SG, Qui M, Krishnan K (2003) Gamma (gamma) tocopherol upregulates peroxisome proliferator activated receptor (PPAR) gamma (gamma) expression in SW 480 human colon cancer cell lines. BMC Cancer 3:25–37
Cazacu I, Mogosan C, Loghin F (2015) Safety issues of current analgesics: an update. Clujul Med 88(2):128–136
Chen Z, Ma C, Song B (1998) The analgesic effect of vitamine E and its mechanism. Chin Pharm J 33:285–288
Chen H, Yu W, Chen G, Meng S, Xiang Z, He N (2018) Antinociceptive and antibacterial properties of anthocyanins and flavonols from fruits of black and non-black mulberries. Molecules (Basel, Switzerland) 23(4):1–13
Costa B, Comelli F, Bettoni I, Colleoni M, Giagnoni G (2008) The endogenous fatty acid amide, palmitoylethanolamide, has anti-allodynic and anti-hyperalgesic effects in a murine model of neuropathic pain: involvement of CB1, TRPV1 and PPARγ receptors and neurotrophic factors. Pain 139(3):541–550
Crouzin N, Ferreira MC, Cohen-Solal C, Barbanel G, Guiramand J, Vignes M (2010) Neuroprotection induced by vitamin E against oxidative stress in hippocampal neurons: involvement of TRPV1 channels. Mol Nutr Food Res 54(4):496–505
Crouzin N, Ferreira MCJ, Cohen-Solal C, M’kadmi C, Bernad N, Martinez J, Barbanel G, Vignes M, Guiramand J (2011) α-Tocopherol and α-tocopheryl phosphate interact with the cannabinoid system in the rodent hippocampus. Free Radic Biol Med 51(9):1643–1655
de Cássia da Silveira E Sá R, Lima TC, da Nóbrega FR, de Brito AEM, de Sousa DP (2017) Analgesic-like activity of essential oil constituents: an update. Int J Mol Sci 18(12):2392–2431
De Melo GO, Malvar Ddo C, Vanderlinde FA, Rocha FF, Pires PA, Costa EA, de Matos LG, Kaiser CR, Costa SS (2009) Antinociceptive and anti-inflammatory kaempferol glycosides from Sedum dendroideum. J Ethnopharmacol 124(2):228–232
Donvito G, Nass SR, Wilkerson JL, Curry ZA, Schurman LD, Kinsey SG, Lichtman AH (2018) The endogenous cannabinoid system: a budding source of targets for treating inflammatory and neuropathic pain. Neuropsychopharmacology 43(1):52–79
Esposito G, Scuderi C, Valenza M, Togna GI, Latina V, De Filippis D, Cipriano M, Carratù MR, Iuvone T, Steardo L, Linden R (2011) Cannabidiol reduces Aβ-induced neuroinflammation and promotes hippocampal neurogenesis through PPARγ involvement. PLoS ONE 6(12):e28668
Fawad K, Islam NU, Subhan F, Shahid M, Ali G, Rahman F, Mahmood W, Ahmad N (2018) Novel hydroquinone derivatives alleviate algesia, inflammation and pyrexia in the absence of gastric ulcerogenicity. Trop J Pharm Res 17(1):53–63
Ganeshpurkar A, Saluja AK (2017) The pharmacological potential of rutin. Saudi Pharm J 25(2):149–164
Gao W, Zan Y, Wang ZJ, Hu XY, Huang F (2016) Quercetin ameliorates paclitaxel-induced neuropathic pain by stabilizing mast cells, and subsequently blocking PKCε-dependent activation of TRPV1. Acta Pharmacol Sin 37(9):1166–1177
Guo BC, Wei J, Su KH, Chiang AN, Zhao JF, Chen HY, Shyue SK, Lee TS (2015) Transient receptor potential vanilloid type 1 is vital for (−)-epigallocatechin-3-gallate mediated activation of endothelial nitric oxide synthase. Mol Nutr Food Res 59(4):646–657
Jaffal SM, Abbas MA (2018) Antinociceptive action of Achillea biebersteinii methanolic flower extract is mediated by interaction with cholinergic receptor in mouse pain models. Inflammopharmacology 27(5):961–968
Janssen PA (1963) The inhibitory effect of fentanyl and other morphine-like analgesics on the warm water induced tactile withdrawal reflex in rat. Arzneimittelforschung 13:502–507
Jia Y, Bhuiyan MJH, Jun HJ, Lee JH, Hoang MH, Lee HJ, Kim N, Lee D, Hwang KY, Hwang BY, Choi D, Lee S (2011) Ursolic acid is a PPAR-α agonist that regulates hepatic lipid metabolism. Bioorg Medl Chem Lett 21(19):5876–5880
Juaira T, Begum N, Yusuf MA, Patwary MA (2018) Effect of αlfa-tocopherol on pain and inflammation of rats. J Curr Adv Med Res 5(1):15–18
Julius D, Basbaum AL (2001) Molecular mechanisms of nociception. Nature 413:203–210
Jun HJ, Lee JH, Kim J, Jia Y, Kim K, Hwang KY, Yun E, Do KR, Lee SJ (2014) Linalool is a PPAR ligand that reduces plasma TG levels and rewires the hepatic transcriptome and plasma metabolome. J Lipid Res 55(6):1098–1110
Kaczocha M, Glaser ST, Deutsch DG (2009) Identification of intracellular carriers for the endocannabinoid anandamide. Proc Natl Acad Sci USA 106:6375–6380
Kaiser J, Yassin M, Prakash S, Safi N, Agami M, Lauw S, Ostrozhenkova E, Bacher A, Rohdich F, Eisenreich W, Safi J (2007) Anti-malarial drug targets: screening for inhibitors of 2C-methyl-d-erythritol 4-phosphate synthase (IspC protein) in Mediterranean plants. Phytomedicine 14(4):242–249
Kim HK, Kim JH, Jun-Li Zhou XG, Lee I, Chung K, Chung JM (2006) Analgesic effect of vitamin E is mediated by reducing central sensitization in neuropathic pain. Pain 122(1–2):53–62
Kim DY, Kim J, Ham HJ, Choue R (2013) Effects of d-alpha-tocopherol supplements on lipid metabolism in a high-fat diet-fed animal model. Nutr Res Pract 7(6):481–487
Korte G, Dreiseitel A, Schreier P, Oehme A, Locher S, Geiger S, Heilmann J, Philipp GS (2009) Tea catechins' affinity for human cannabinoid receptors. Phytomedicine 17:19–22
Lehmann JM, Lenhard JM, Oliver BB, Ringold GW, Kliewer SA (1997) Peroxisome proliferator-activated receptors α and γ are activated by indomethacin and other non-steroidal anti-inflammatory drugs. J Biol Chem 272:3406–3410
Liang YC, Tsai SH, Tsai DC, Lin-Shiau SY, Lin JK (2001) Suppression of inducible cyclooxygenase and nitric oxide synthase through activation of peroxisome proliferator-activated receptor-γ by flavonoids in mouse macrophages. FEBS Lett 496:12–18
LoVerme J, Russo R, La Rana G, Fu J, Farthing J, Mattace-Raso G, Meli R, Hohmann A, Calignano A, Piomelli D (2006) Rapid broad-spectrum analgesia through activation of peroxisome proliferator-activated receptor-α. J Pharmacol Exp Ther 319(3):1051–1061
Milad R, Svs R, Youssef F, Ross S (2017) Secondary metabolites isolated from Pinus roxburghii and interpretation of their cannabinoid and opioid binding properties by virtual screening and in vitro studies. Saudi Pharm J 26(3):437–444
Moreno SS, Farioli-Vecchioli S, Ceru MP (2004) Immunolocalization of peroxisome proliferator-activated receptors and retinoid X receptors in the adult rat CNS. Neuroscience 123:131–145
Nahrstedt A (1992) Flavonoids of Arbutus andrachne L. leaves. Turk J Pharm 2:17–23
Nirmal SA, Pal SC, Mandal SC, Patil AN (2012) Analgesic and anti-inflammatory activity of β-sitosterol isolated from Nyctanthes arbortristis leaves. Inflammopharmacology 20(4):219–224
Okmen AS (2015) Antioxidant and antibacterial activities of different plants extracts against Staphylococcus aureus isolated from soccer player’s shoes and knowledge and applications about foot hygiene of the soccer players. Afr J Tradit Complement Altern Med 12(3):143–149
Oran SA (2014) The status of medicinal plants in Jordan. J Agric Sci Tech 4:46–467
Oran SA (2015) Flora of Bader Al-Jadida County, western high mountains of Amman city/Jordan. Int J Herb Med 3:49–59
O'Sullivan S (2016) An update on peroxisome proliferator-activated receptor (PPAR) activation by cannabinoids. Br J Pharmacol 173:1899–1910
Pandey A, Tripathi SM (2014) Concept of standardization, extraction and pre phytochemical screening strategies for herbal drug. J Pharmacogn Phytochem 2(5):115–119
Patwardhan AM, Akopian AN, Ruparel NB, Diogenes A, Weintraub ST, Uhlson C, Murphy RC, Hargreaves KM (2010) Heat generates oxidized linoleic acid metabolites that activate TRPV1 and produce pain in rodent. J Clin Invest 120(5):1617–1626
Peana A, D'Aquila PS, Chessa M, Moretti L, Serra G, Pippia P (2003) (−)-Linalool produces antinociception in two experimental models of pain. Eur J Pharmacol 460:37–41
Popeijus HE, van Otterdijk SD, van der Krieken SE, Konings M, Serbonij K, Plat J, Mensink RP (2014) Fatty acid chain length and saturation influences PPARα transcriptional activation and repression in HepG2 cells. Mol Nutr Food Res 58(12):2342–2349
Qin G, Ma J, Huang Q, Yin H, Han J, Li M, Deng Y, Wang B, Hassan W, Shang J (2018) Isoquercetin improves hepatic lipid accumulation by activating AMPK pathway and suppressing TGF-β signaling on an HFD-induced nonalcoholic fatty liver disease rat model. Int J Mol Sci 19(12):4126–4140
Quintans-Júnior LJ, Barreto RSS, Menezes PP, Almeida JRGS, Viana AFSC, Oliveira RCM, Oliveira AP, Gelain DP, de Lucca Júnior W, Araújo AAS (2013) β-Cyclodextrin-complexed ( )-linalool produces antinociceptive effect superior to that of ( )-linalool in experimental pain protocols. Basic Clin Pharmacol Toxicol 113(3):167–172
Russo R, LoVerme J, La Rana G, D'Agostino G, Sasso O, Calignano A, Piomelli D (2007) Synergistic antinociception by the cannabinoid receptor agonist anandamide and the PPAR-α receptor agonist GW7647. Eur J Pharmacol 566(1–3):117–119
Scholz A, Gruß M, Vogel W (1998) Properties and functions of calcium-activated K+ channels in small neurones of rat dorsal root ganglion studied in a thin slice preparation. J Physiol 513(1):55–69
Seker M, Toplu C (2010) Determination and comparison of chemical characteristics of Arbutus unedo L. and Arbutus andrachnae L. (family Ericaceae) fruits. J Med Food 13(4):1013–1018
Shashni B, Sharma K, Singh R, Sakharkar K, Dhillon SK, Nagasaki Y, Sakharkar M (2013) Coffee component hydroxyl hydroquinone (HHQ) as a putative ligand for PPAR gamma and implications in breast cancer. BMC Genomics 14(5):S6
Starowicz K, Makuch W, Korostynski M, Malek N, Slezak M, Zychowska M, Petrosino S, De Petrocellis L, Cristino L, Przewlocka B, Di Marzo V (2013) Full inhibition of spinal FAAH leads to TRPV1-mediated analgesic effects in neuropathic rats and possible lipoxygenase-mediated remodeling of Anandamide metabolism. PLoS One 8(4):e60040
Su KY, Yu CY, Chen YW, Huang YT, Chen CT, Wu HF, Chen YL (2014) Rutin, a flavonoid and principal component of Saussurea involucrata, attenuates physical fatigue in a forced swimming mouse model. Int J Med Sci 11(5):528–537
Tapondjou A, Lontsi D, Sondengam B, Choi J, Lee KT, Jung HJ, Park HJ (2003) In vivo anti-nociceptive and anti-inflammatory effect of the two triterpenes, ursolic acid and 23-hydroxyursolic acid, from Cussonia bancoensis. Arch Pharm Res 26:143–146
Tenuta M, Tundis R, Xiao J, Loizzo M, Dugay A, Deguin B (2018) Arbutus species (Ericaceae) as source of valuable bioactive products. Crit Rev Food Sci Nutr 59:1–18
Thors L, Belghiti ME, Fowler CJ (2008) Inhibition of fatty acid amide hydrolase by kaempferol and related naturally occurring flavonoids. Br J Pharmacol 155:244–252
Tong Y, Zhou XM, Wang SJ, Yang Y, Cao YL (2009) Analgesic activity of myricetin isolated from Myrica rubra Sieb. et Zucc Leaves. Arch Pharm Res 32:527–533
Trevisan G, Rossato MF, Tonello R, Hoffmeister C, Klafke JZ, Rosa FW, Pinheiro K, Pinheiro FD, Boligon AA, Athayde ML, Ferreira J (2014) Gallic acid functions as a TRPA1 antagonist with relevant antinociceptive and antiedematogenic effects in mice. N-S Arch Pharmacol 387:679–689
Tutino V, De Nunzio V, Tafaro A, Bianco G, Gigante I, Scavo MP, D’Alessandro R, Refolo MG, Messa C, Caruso MG, Notarnicola M (2018) Cannabinoid receptor-1 up-regulation in azoxymethane (AOM)-treated mice after dietary treatment with quercetin. Anticancer Res 38(8):4485–4491
Variya BC, Bakrania AK, Patel SS (2019) Antidiabetic potential of gallic acid from Emblica officinalis: improved glucose transporters and insulin sensitivity through PPAR-γ and Akt signaling. Phytomedicine 2019:152906–152916
Wu M, Liu D, Zeng R, Xian T, Lu Y, Zeng G, Sun Z, Huang B, Huang Q (2017) Epigallocatechin-3-gallate inhibits adipogenesis through down-regulation of PPARγ and FAS expression mediated by PI3K-AKT signaling in 3T3-L1 cells. Eur J Pharmacol 795:134–142
Zarei M, Izadi Dastenaei Z, Jabbari S (2020) Study of the effect of intracerebroventricular injection of kaempferol and its intraction with the transient receptor potential vanilloid 1 on pain in male rats. PSJ 18(2):67–74
Zhang Y, Sreekrishna K, Lin Y, Huang L, Eickhoff D, Degenhardt D, Xu T (2011) Modulation of transient receptor potential (TRP) channels by Chinese herbal extracts. Phytother Res 25(11):1666–1670
Acknowledgements
The authors acknowledge the Deanship of Scientific Research, The University of Jordan for the financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interests
The authors have no conflict of interest.
Ethics approval
All experiments were approved by the animal ethics committee at the University of Jordan (approval number 235/2020/19).
Consent to participate
All authors consent to participate in this research and manuscript publication.
Consent for publication
All authors consent to publish and agree on the contents.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jaffal, S.M., Oran, S.A. & Alsalem, M. Anti-nociceptive effect of Arbutus andrachne L. methanolic leaf extract mediated by CB1, TRPV1 and PPARs in mouse pain models. Inflammopharmacol 28, 1567–1577 (2020). https://doi.org/10.1007/s10787-020-00746-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10787-020-00746-y