Abstract
Objective
Due to the high side effects of commonly used drugs and according to the pharmacological properties reported for coumaric acid (CA), this study was designed to determine the impact of CA on acetic acid-induced colitis in rats, considering its possible anti-inflammatory, antioxidant, and anti-apoptotic properties.
Materials and methods
Forty-eight male Wistar rats were divided into 6 equal groups (n = 8). Colitis was induced by acetic acid intrarectally. CA in three different doses (50, 100, and 150 mg/kg) was administrated for 5 days. Finally, the macroscopic and histopathological changes in the colon tissue were examined. The expression of inflammatory and apoptotic genes, including NF-κB, TNF-α, INOS, IL-1β, IL-6, TLR4, Caspase-3, Caspase-8, Bax, Bcl-2 was assessed. In addition, changes in the levels of catalase (CAT), superoxide dismutase (SOD), malondialdehyde (MDA), nitrite, and total antioxidant capacity (TAC) were measured in the colon tissue.
Results
Colitis led to a decrease in TAC and the activity levels of CAT and SOD and an increase in the expression of inflammatory and apoptotic genes, MDA, and nitrite levels in the colon. Colitis was also associated with edema and severe damage to the epithelium, infiltration of inflammatory cells, and the presence of ulcers and necrosis in the colon tissue. CA significantly improved the inflammation, oxidative stress, apoptosis, and histopathological indices caused by acetic acid-induced colitis on the colon.
Conclusion
It is concluded that CA probably exerts its positive effects in the management of colitis, through its anti-inflammatory, antioxidant, and anti-apoptotic properties.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Crohn's disease (CD) and ulcerative colitis (UC) are two major types of inflammatory bowel disease (IBD) (Murad et al. 2016; Tekeli et al. 2019). UC, characterized by colonic mucosal inflammation, is associated with symptoms including intestinal bleeding and lower abdominal pain and affects the entire colon and rectum (Wallace et al. 2014). The pathogenesis of this disease is complex and involves the interaction between genetic, environmental factors, and immunology (Wallace et al. 2014; Sales-Campos et al. 2015). Hypersensitivity and abnormalities of the immune system in the mucosal tissue of the intestinal tract activate the innate immune response.
This activation of the innate immune response is followed by the production and accumulation of macrophages, monocytes, neutrophils, and dendritic cells, as well as the secreted mediators of adaptive immune cells such as cytokines and chemokines, that eventually cause inflammation in the colon (Wallace et al. 2014; Sales-Campos et al. 2015). Acetic acid causes clear changes in the colon structure in animals both macroscopically and microscopically (Gupta et al. 2018), which are similar to clinical samples of patients (Peyrin–Biroulet et al. 2014). It has been suggested that morphological changes in the colon tissue in acetic acid-induced animal samples are associated with the cascade of reactions mediated by free radicals (Trivedi and Jena 2013). Studies have shown that administration of acetic acid into the colon of animals causes damage, inflammation, and in acute cases, ulceration and necrosis in the tissue (Murad et al. 2016; Tekeli et al. 2019; Bastaki et al. 2018; Gupta et al. 2018). Due to damage to the colon epithelium in colitis, the uptake of bacterial endotoxins increases. Interaction of LPS (lipopolysaccharide) in the cell wall of bacteria with Toll-like receptor 4 (TLR4) activates the TLR4 signaling cascade. TLR4 pathway is one of the critical signaling pathways involved in the development of colon inflammation. Activation of TLR4 signaling cascade activates NFκB.
NFκB activation leads to the secretion of pro-inflammatory cytokines, including TNF-α, IL-1β, and IL-6, from neutrophils and macrophages. On the other hand, activation of NF-ҡB increases nitrite oxide (NO) production as a potent pro-inflammatory mediator in the inflamed colon tissues through stimulating INOS expression (Shaker et al. 2014). NO, increases the levels of reactive oxygen species (ROS) and reactive nitrogenous species (RNS) in the colon tissue. So, it leads to further colon tissue damage (Fuentes et al. 2015; Andrade et al. 2018).
Common medications used to treat IBD generally have multiple side effects and are temporarily effective, so studies have shifted to using herbal and natural compounds with fewer side effects to cure this disease (Sales-Campos et al. 2015). P-Coumaric acid, 4-hydroxycinnamic acid (CA), is a phenolic acid compound in many fruits, vegetables, and plants from the Gramineae family (Lou et al. 2012). It has various pharmacological effects such as antioxidant, antimicrobial, antiviral, anti-inflammatory, anti-mutagenic, and anti-hyperlipidemia (Shen et al. 2019). However, the anti-inflammatory ability of CA to reduce the expression of pro-inflammatory cytokines has been demonstrated in several studies (Sharma et al. 2018; Rafiee et al. 2020). Also, the antioxidant ability of CA in inhibiting ROS production, in contrast to increased activity of SOD and CAT, has been proven in previous studies (Peng et al. 2018; Shen et al. 2019; Rafiee et al. 2020). Studies have also confirmed the anti-apoptotic properties of CA by reducing the expression of caspases and Bax (Prince and Roy 2013; Peng et al. 2018).
Therefore, the present study aimed to determine the effect of CA on colitis induced by acetic acid in rats considering its possible antioxidant, anti-inflammatory, and anti-apoptotic properties.
Materials and methods
Animals and drugs
Forty-eight healthy adult male Wistar rats (225–240 g) were used for experiments. Rats were obtained from the Pasteur Institute (Tehran, Iran).
The animals were housed in cages under natural laboratory environmental conditions, a constant temperature of 25 ± 1 °C, relative humidity of 45–55%, and in 12 h alternating cycles of daylight and darkness. The rats were fed on commercial feed pellets and water ad libitum. All stages of experimentation were carried out following the regulations of the University and the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. (Ethics code: IR.SKUMS.REC.1399.113) and Guide for the Care and Use of Laboratory Animals (8th edition, National Academies Press). Full efforts were made to diminish the use of animals and to improve their wellbeing.
CA was purchased from a sigma-aldrich company, (CAS Number: 501-98-4), ≥ 98.0% (HPLC).
Study design
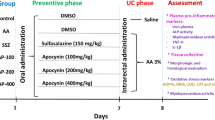
To induce colitis, after fasting for one day, the animals received 0.8 ml of 7% acetic acid intrarectally at 8 cm proximal to the anus for 30 s, after which the rats rested. For the control group, rats similarly received 0.8 ml of saline buffer phosphate instead of acetic acid (Bastaki et al. 2016). Animals were randomly divided into 6 groups (n꞊8), and colitis was induced in all groups except the control group.
The animals were grouped as follows:
Group 1: control group (colitis was not induced in this group, and they were treated with 2 mg/kg/day of saline buffer phosphate). Group II: saline-treated colitis group (colitis induced and treated with saline). Groups III, IV, V, and VI: treatment groups (colitis induced and treated with CA at doses 50, 100, and 150 mg/kg, and dexamethasone (2 mg/kg) as a standard medicine, respectively. One hour after the induction of colitis, treatments were performed and lasted for 5 days. CA was administered to rats by gavage, and dexamethasone by subcutaneous injection.
At the end of the study on the 6th day, rats were euthanized under deep anesthesia (using ketamine: 87 mg/kg and xylazine: 13 mg/kg), and the colon of each rat was cut 8 cm above the anal margin, opened lengthwise, and washed with saline solution. The macroscopic examinations were performed. Then part of the colon tissue was kept in 10% formaldehyde for histopathological studies, and the rest of the colon tissue was used for biochemical tests and assessment of inflammatory and apoptotic genes.
Macroscopic evaluation
Macroscopic lesions were assessed by the Wallace and Keenan scoring system (Wallace and Keenan 1990), which depends on the area of inflammation and the presence or absence of ulcers. In summary, the macroscopic damage assessment criteria are based on a semi-quantitative scoring system whose features are classified as follows:
0: no ulcer, no inflammation, 1: no lesion, localized hyperemia, 2: lesions, no hyperemia, 3: lesions and inflammation in only one area, 4: lesions and inflammation in two or more areas, 5: spread lesions larger than 2 cm (Wallace and Keenan 1990).
Histopathological examination
Colon samples were fixed in 10% formaldehyde in PBS buffer phosphate solution for 1 week and then exposed to freshwater for 2 h. The samples were then dried in graded ethanol and fixed in paraffin wax. The sections were then deparaffinized using xylene, stained with hematoxylin–eosin (H & E), and morphological changes were measured by light microscopy. A scoring system for pathological assessment of colitis was used considering the crypt damage, inflammation extent, inflammation severity, and percentage of involvement, with the scales defined for each indicator as presented in Table 1 (Rees 1998).
Biochemical analysis
The homogenizing of colon tissue was performed in a buffer containing 1.15% KCI with a ratio of 1/10. MDA levels of homogenized tissue were measured by a spectrophotometric method based on the reaction with thiobarbituric acid described by Ohkawa et al. (Ohkawa et al. 1979) and reported as μg/ml. CAT activity levels in homogenized tissue were measured according to the H2O2 decomposition principle at 240 nm described by Aebi (1984) and reported as U/mg protein (Aebi 1984). SOD activity in homogenized tissue was measured according to the method described by Misra and Fridovich 1972 and reported as U/mg protein (Misra and Fridovich 1972). NO levels were estimated by measuring nitrite accumulation in the tissue using the reaction of Griss reagent with sodium nitrate. Nitrite concentration was calculated by comparison with a standard calibration curve with sodium nitrite and reported as μmol/l (Pourkhodadad et al. 2016). Total antioxidant capacity of the colon tissue in different groups was measured by the FRAP assay method, under the method described by Benzie and Strain, using iron chloride and triazine solution, and reported as μg/ml (Benzie and Strain 1996).
Real-time PCR analysis
Based on the manufacturer's instructions, RNA extraction from the colon tissue samples was performed using Trizol (Invitrogen, USA). A nanodrop device evaluated the quality and quantity of RNA. cDNA was prepared from extracted RNA using a cDNA synthesis kit (Thermo Scientific RevertAid First Strand cDNA Synthesis Kit). Examination of mRNA expression levels was performed by real-time polymerase chain reaction (PCR) on a light cycler apparatus (Rotor gene Diagnostics) using the SYBR Premix Ex Taq technology (Takara). The thermal cycling program profile was 95 °C for 30 s and followed by 45 cycles of denaturation for 5 s at 95 °C, an annealing step for 15 s at 60 °C, and extension for 15 s at 72 °C. If the PCR process is a single product for each of the primers, their melting curve was evaluated. All target gene transcription values were normalized to the housekeeping β-actin gene using 2−ΔΔCt relative expression formula (Wang et al. 2017). The primer sequences were listed in Table 2.
Statistical analyses
Statistical analysis was performed using GraphPad Software (San Diego, CA, USA, version 8.4.3). One-way analysis of variance (ANOVA) followed by Tukey’s post hoc test was applied for multiple comparisons. Results were expressed as mean ± standard error mean (SEM). The 95% probability level (P < 0.05) represented significant differences between groups.
Results
CA reduced the expression of inflammatory genes in the colon.
Based on the results, the expression of inflammatory genes significantly increased in the saline-treated colitis group compared to the control group, including INOS (P < 0.001, F.C 16.75), TNF-α (P < 0.01, F.C 4.68), TLR4 (P < 0.01, F.C 7.39), IL-1β (P < 0.05, F.C 15.88), IL-6 (P < 0.001, F.C 14.15), NF-κB (P < 0.05, F.C 2.25) (Fig. 1). (F.C: fold cheng).
The expression of genes related to the inflammation in colon tissue in the experimental groups. All values are reported as mean ± SEM (n = 8). # P < 0.05, ## P < 0.01, ### P < 0.001 compared to the control group. * P < 0.05, ** P < 0.01, *** P < 0.001 compared to the saline-treated colitis group (One-way ANOVA followed by Tukey’s post hoc test). Control control group, colitis saline-treated colitis group, CA 50, CA 100, and CA 150 coumaric acid at a dose of 50, 100, and 150 mg/kg, dexa dexamethasone at a dose of 2 mg/kg
Results showed that administration of CA at doses of 50 (P < 0.001, F.C − 18.51), 100 (P < 0.001, F.C − 135), and 150 mg/kg (P < 0.001, F.C − 7.14) significantly reduced the expression of INOS compared to the saline-treated colitis group. Dexamethasone (2 mg/kg) as a standard medicine also, significantly reduced the expression of INOS compared to the saline-treated colitis group (P < 0.001, F.C − 13.8) (Fig. 1a).
In the case of the gene expression of TNF-α, we found that CA at doses of 50 (P < 0.001, F.C − 30.30), 100 (P < 0.01, F.C − 16.94), and 150 mg/kg (P < 0.01, F.C − 13.51) significantly reduced the expression of TNF-α compared to the saline-treated colitis group. Dexamethasone (2 mg/kg) decreased significantly the expression of TNF-α compared to the saline-treated colitis group (P < 0.05, F.C − 2.5) (Fig. 1b). As for the other studied genes, the results were as follows: CA at doses of 50 (P < 0.001, F.C − 19.60), 100 (P < 0.001, F.C − 24.39), and 150 mg/kg (P < 0.001, F.C − 30.30) significantly reduced the expression of TLR4 compared to the saline-treated colitis group. Dexamethasone (2 mg/kg) also, significantly reduced the expression of TLR4 compared to the saline-treated colitis group (P < 0.01, F.C − 5.5) (Fig. 1c).
CA at doses of 100 (P < 0.05, F.C − 16.66) and 150 (P < 0.01, F.C − 21.27) significantly reduced the expression of IL-1β compared to the saline-treated colitis group. Dexamethasone (2 mg/kg) also, significantly reduced the expression of IL-1β compared to the saline-treated colitis group (P < 0.05, F.C − 11.76) (Fig. 1d).
CA at doses of 50 (P < 0.001, F.C − 166.66), 100 (P < 0.001, F.C − 111), and 150 mg/kg (P < 0.001, F.C − 34.48) significantly reduced the expression of IL-6 compared to the saline-treated colitis group. Dexamethasone (2 mg/kg) also, significantly reduced the expression of IL-6 compared to the saline-treated colitis group (P < 0.001, F.C − 10.30) (Fig. 1e).
CA at doses of 50 (P < 0.001, F.C − 6.6), 100 (P < 0.001, F.C − 9), and 150 mg/kg (P < 0.001, F.C − 8.3) significantly reduced the expression of NF-κB compared to the saline-treated colitis group. Dexamethasone (2 mg/kg) as a standard medicine did not decrease significantly the expression of NF-κB compared to the saline-treated colitis group (Fig. 1f).
CA reduced the expression of apoptotic genes in the colon.
As shown in Fig. 2, the expression of apoptotic genes significantly increased in the saline-treated colitis group compared to the control group including caspase-3 (P < 0.05, F.C 4), Caspase-8 (P < 0.01, F.C 7.15), and Bax (P < 0.001, F.C 6.97).
The expression of genes related to apoptosis in colon tissue in the experimental groups. All values are reported as mean ± SEM (n = 8). # P < 0.05, ## P < 0.01, ### P < 0.001 compared to the control group. * P < 0.05, ** P < 0.01, *** P < 0.001 compared to the saline-treated colitis group (One-way ANOVA followed by Tukey’s post hoc test). Control control group, colitis: saline-treated colitis group, CA 50, CA 100, and CA 150 coumaric acid at a dose of 50, 100, and 150 mg/kg, dexa dexamethasone at a dose of 2 mg/kg
According to the results presented in Fig. 2a, CA at doses of 50 (P < 0.01, F.C − 25), 100 (P < 0.01, F.C − 17.24), and 150 mg/kg (P < 0.01, F.C − 34.48) significantly reduced the expression of Caspase-3 compared to the saline-treated colitis group. Dexamethasone (2 mg/kg) also, significantly reduced the expression of Caspase-3 compared to the saline-treated colitis group (P < 0.05, F.C − 3.84).
CA at doses of 50 (P < 0.01, F.C − 21.27), 100 (P < 0.01, F.C − 30.30), and 150 mg/kg (P < 0.01, F.C − 31.25) significantly reduced the expression of Caspase-8 compared to the saline-treated colitis group. Dexamethasone (2 mg/kg) also, significantly reduced the expression of Caspase-8 compared to the saline-treated colitis group (P < 0.05, F.C − 2.85) (Fig. 2b).
CA at doses of 50 (P < 0.001, F.C − 25), 100 (P < 0.001, F.C − 21.27), and 150 mg/kg (P < 0.001, F.C − 21.73) significantly reduced the expression of Bax compared to the saline-treated colitis group. Dexamethasone (2 mg/kg) also, significantly reduced the expression of Bax compared to the saline-treated colitis group (P < 0.001, F.C − 7.14) (Fig. 2c). The results of the Bcl-2 gene expression were not significantly different in any of the groups (Fig. 2d).
CA increased TAC
As shown in Fig. 3a, colitis significantly reduced TAC in the colon tissue compared to the control group (P < 0.05). Results showed that administration of CA at doses of 50 (P < 0.01), 100 (P < 0.05), and 150 mg/kg (P < 0.001) significantly increased TAC in the colon tissue compared to the saline-treated colitis group. Dexamethasone (2 mg/kg) as a standard medicine did not increase TAC significantly in the colon tissue compared to the saline-treated colitis group.
TAC, MDA, CAT, SOD, NO levels in colon tissue in the experimental groups. All values are reported as mean ± SEM (n = 8). # P < 0.05, ## P < 0.01, ### P < 0.001 compared to the control group. * P < 0.05, ** P < 0.01, *** P < 0.001 compared to the saline-treated colitis group (One-way ANOVA followed by Tukey’s post hoc test). Control control group, colitis saline-treated colitis group, CA 50, CA 100, and CA 150 coumaric acid at a dose of 50, 100, and 150 mg/kg, dexa dexamethasone at a dose of 2 mg/kg. TAC total antioxidant capacity, MDA malondialdehyde, CAT catalase, SOD superoxide dismutase, NO Nitrite oxide
CA decreased the MDA in the colon tissue
The results showed that colitis significantly increased the level of MDA in the colon tissue compared to the control group (P < 0.001). Findings showed that administration of CA at doses of 50 (P < 0.01), 100 (P < 0.001), and 150 mg/kg (P < 0.001) significantly reduced the levels of MDA in the colon tissue compared to the saline-treated colitis group. Dexamethasone (2 mg/kg) also significantly reduced the level of MDA in the colon tissue compared to the saline-treated colitis group (P < 0.01) (Fig. 3b).
CA decreased the NO in the colon tissue
Effects of CA on NO levels are shown in Fig. 3c. Colitis significantly increased NO levels (P < 0.001) compared to the control group. NO levels were markedly decreased after treatment with CA at doses of 100 (P < 0.05) and 150 mg/kg (P < 0.05) in the colon tissue compared to the saline-treated colitis group. Also, treatment with CA at doses of 50 and dexamethasone (2 mg/kg) did not significantly reduce the NO levels compared to the saline-treated colitis group.
CA increased the activity of CAT and SOD in the colon tissue
The results showed that the activity of CAT and SOD was significantly lower in the saline-treated colitis group compared to the control group (P < 0.05), (P < 0.01), respectively (Fig. 3d,e). The activity of CAT was significantly higher in the CA-treated group at the dose of 100 mg/kg (P < 0.05) compared to the saline-treated colitis group (Fig. 3d). The activity of SOD was significantly higher in the CA-treated group in the dose of 150 mg/kg (P < 0.05) compared to the saline-treated colitis group (Fig. 3e). CA at the dose of 50 and as well as, dexamethasone could not cause a significant difference in CAT and SOD activity compared to the saline-treated colitis group.
CA reduced the macroscopic lesions in the colon.
The severe macroscopic lesions (mucosal edema, bleeding, erosions, and necrosis) were seen in the colon tissue of the saline-treated colitis group (Fig. 4). Observations showed that scoring of the lesions in the colon of the saline-treated colitis group had a significant increase compared to the control group (P < 0.001) (Table 3). CA at doses of 50 (P < 0.001), 100 (P < 0.001), and 150 mg/kg (P < 0.001), caused a significant decrease in the mean macroscopic ulcer score compared to the control group. Dexamethasone (2 mg/kg) also significantly reduced the mean macroscopic ulcer score compared to the saline-treated colitis group (P < 0.001). The results showed that all three doses of CA were able to significantly reduce the rate of ulcers, hyperemia, and inflammation caused by colitis induction (Table 3).
CA mitigated the histopathological changes in the colitis group
Images of histopathological changes are shown in Fig. 5. As seen in Fig. 5a, the colon tissue in the control group had a completely normal microscopic appearance. In contrast, severe damage to the epithelium was observed in the colon tissue in the saline-treated colitis group. Furthermore, cryptic abscess formations, severe ulceration, and necrosis were observed in the mucosa. Also, infiltration of inflammatory cells was visible in all layers (Fig. 5b). Histopathological changes in the groups treated with CA were as follows:
In the colon tissue in the treatment group receiving CA at 50 mg/kg, edema, neutrophil infiltration, lymphatic infiltration, and lymphoid hyperplasia were still found in all layers. Still, the rate was lower than in the saline-treated colitis group. Cryptic abscess formations were observed in the mucosa. The ulcer was not found; only necrosis was detected in the mucosa (Fig. 5c). As shown in Fig. 5d&e, damage to the epithelium in the treatment groups receiving CA at 100 and 150 mg/kg was much less. Edema, neutrophil infiltration, lymphatic infiltration, and lymphoid hyperplasia were much less in the colon tissue than in the saline-treated colitis group. The ulcer was not found, and cryptic abscess formations and necrosis were detected in the mucosa on a much smaller scale than in the other colitis groups. In the receiving group of dexamethasone (2 mg/kg) (Fig. 5f), inflammation and edema were observed in the mucosa and submucosa, much less than in the saline-treated colitis group. Ulcer, necrosis, and cryptic abscess formations were detected in the mucosa. Neutrophil infiltration and lymphatic hyperplasia were present in the mucosa and submucosa much less than in the saline-treated colitis group.
Microscopic scores of the groups are presented in Table 3 concerning microscopic scores (inflammation severity, inflammation extent, crypt damage, and percent of involvement). The difference in scoring between saline-treated colitis and control groups was significant in all 4 cases (P < 0.001). Inflammation severity was significantly different in the groups treated with CA at doses of 100 (P < 0.01), and 150 mg/kg (P < 0.01), and also, dexamethasone (2 mg/kg) (P < 0.05) compared to the saline-treated colitis group. Inflammation extent was not significantly different in therapeutic groups compared to the saline-treated colitis group. Crypt damage was significantly different in all groups, the groups treated with CA were at doses of 50 (P < 0.001), 100 (P < 0.001), and 150 mg/kg (P < 0.001). Dexamethasone (2 mg/kg) did not significantly reduce crypt damage compared to the saline-treated colitis group. Regarding the percent of involvement, a significant difference was observed between the saline-treated colitis group compared to the groups treated with CA at doses of 100 (P < 0.01), 150 mg/kg (P < 0.01), and also dexamethasone (2 mg/kg) (P < 0.01).
Discussion
In this study, it was shown that colitis is associated with the increase in the expression of inflammatory and apoptotic genes as well as the level of oxidative stress index factors. Furthermore, it was determined that the activity of antioxidant enzymes, including SOD and CAT, and TAC significantly decreased following the induction of colitis. Histopathologic evaluation showed the damage to the epithelium of the colon tissue as the presence of an ulcer, edema, and the infiltration of inflammatory cells in the colon tissue.
As a consequence of the treatment with CA, the adverse effects of the following colitis were significantly reversed. Results showed that the expression of inflammatory and apoptotic genes, the level of oxidative stress markers, and histopathologic damages significantly decreased. At the same time, the activity of antioxidant enzymes and TAC significantly increased the consequence of treatment with CA.
Because colitis is an ulcer-inflammatory disease, it leads to mucosal destruction and possibly ulceration in the colon (Robbins and Cotran 1979; Omidi-Ardali et al. 2019). Various studies have reported that activation of caspases leads to increased apoptosis of colon epithelial cells and simultaneous severe epithelial damage of the colon tissue (Ramachandran et al. 2000; Mahmoud et al. 2021). In many intestinal diseases associated with immunodeficiency, including colitis, we mainly encounter accelerated epithelial cell turnover. It leads to apoptosis in the crypts of the affected areas and adjacent areas, resulting in increased intestinal barrier permeability and facilitating the invasion of pathogenic microorganisms (Ramachandran et al. 2000). Caspase-3 and Caspase-8 are critical factors in the process of apoptosis (Ramachandran et al. 2000; Mahmoud et al. 2021). Bcl-2 is an anti-apoptotic gene, and Bax is a pro-apoptotic gene. So the expression level of Bax/Bcl2 determines whether the cell survives or not (Scorrano and Korsmeyer 2003). On the other hand, studies have shown that ROS plays an important role in inducing apoptosis (Ramachandran et al. 2000).
In line with the studies mentioned above, we showed that colitis is associated with edema and severe damage to the epithelium, infiltration of inflammatory cells, and ulcer and necrosis in the colon tissue. Also, we showed that induction of colitis significantly increased the apoptosis of epithelial cells of the colon tissue by increasing the expression of Caspase-3, Caspase-8, and Bax in the colon tissue. In general, oxidative stress and inflammation are essential factors in the pathogenesis of colitis (Wallace et al. 2014). Colitis leads to damage to the intestinal epithelium and results in an increase in the uptake of bacterial endotoxins. Subsequent interaction between TLR4 and LPS in the cell wall of bacteria leads to the activation of NFκB (Mahmoud et al. 2021). NF-κB is a transcription factor in the cytoplasm as an inactive complex that, after activation, can translocate into the nucleus and activate transcriptions of pro-inflammatory cytokine genes, including TNF-α, IL-6, and IL-1β (Liu et al. 2018). Pro-inflammatory cytokines, including TNF-α, IL-6, and IL-1β, are crucial inflammatory mediators whose levels in the colon tissue of patients with colitis are significantly increased (Liu et al. 2018; Liu and Wang 2011; Haj-Mirzaian et al. 2017). As the NF-κB has a unique role in maintaining homeostasis and mucosal barrier functions as well as intestinal integrity, its abnormal activation in the intestinal mucosal leads to colitis in animals and humans (Tekeli et al. 2019). The TLR4/NF-κB pathway is one of the most important signaling pathways in colon inflammation.
On the other hand, activation of NF-ҡB increases NO production by stimulating INOS expression (Mahmoud et al. 2021). Other studies have reported that increased levels of NO as a potent pro-inflammatory mediator in inflamed colon tissues are directly related to the increased release of pro-inflammatory cytokines (Kim et al. 2017; Fuentes et al. 2015). In addition, NO increases ROS and RNS in the colon tissue, leading to further damage to the colon tissue (Andrade et al. 2018). Therefore, it could be concluded that NF-κB/INOS/NO signaling pathway is another important signaling pathway involved in inflammation and oxidative stress in the colon tissue (Cheng et al. 2014).
In fact, with the onset of immune responses associated with colitis, the production of inflammatory agents such as cytokines, eicosanoids, and reactive oxygen species, increases in the colon tissue (Wallace et al. 2014). The presence of several reactive products in the inflamed mucosa, such as superoxides and peroxides secreted by active leukocytes, exacerbates the conditions of oxidative stress in this tissue (Balmus et al. 2016; Zhu and Li 2012). Superoxides are converted to highly reactive hydroxyl radicals and interact with fatty acids in the intestinal cell membranes. Lipid peroxidation occurs, resulting in increased MDA production and decreased activity of antioxidant enzymes such as SOD and CAT (Alavala et al. 2019). SOD and CAT protect tissue against the high reactive hydroxyl radicals that levels of both of these enzymes are reduced in colitis (Eissa et al. 2018; Abdelkader et al. 2016). Therefore, inflammatory responses begin with the induction of colitis and exacerbate colitis. On the other hand, inflammation leads to increased levels of oxidative stress markers and oxidative agents, further exacerbating inflammation in the tissues.
In this study, in agreement with previous studies, we showed that with the induction of colitis in rats, the expression of inflammatory cytokines (NF-κB, TNF-α, INOS, IL-1β, IL-6, TLR4) and the levels of factors related to oxidative stress (MDA, NO) significantly increased. In contrast, the levels of defense mechanisms such as antioxidant enzymes (SOD, CAT) and TAC significantly decreased.
Responses to common medications used in colitis treatment are varied in different patients, often short-term and with side effects. Following the effort to find novel and more effective treatments, herbal therapy has had outstanding and considerable results in various studies (MacPherson and Pfeiffer 1978; Gilardi et al. 2014; Triantafillidis et al. 2016), including alpha-ketoglutarate (Tian et al. 2021), nerolidol from terpenes (Bastaki et al. 2021), and caffeic acid from polyphenols (Khan et al. 2018). CA is a polyphenolic compound in many fruits, vegetables, and plants of the Gramineae family (Lou et al. 2012). It has various pharmacological effects such as antioxidant, antimicrobial, antiviral, anti-inflammatory, anti-cancer, anti-mutagenic, anti-diabetic, and hyperlipidemic effects (Shen et al. 2019). Studies have shown that CA significantly reduces inflammation by reducing the expression of inflammatory cytokines, including IL-6 and TNF-α (Sharma et al. 2018). Other studies have shown that CA is a powerful antioxidant that reduces free radicals and increases serum CAT levels and TAC (Shen et al. 2019). Also, previous studies showed that CA pretreatment has protective effects on apoptosis in isoproterenol-induced myocardial infarcted rats by reducing the expression of Bax, Caspase-8, Caspase-9, and Fas genes. This effect of CA is linked to its antioxidant power (Prince and Roy 2013). Another study showed that pretreatment with CA was able to protect against H2O2-induced cell death by downregulating the expressions of the Caspase-3 gene (Peng et al. 2018).
However, the exact mechanisms of CA in the treatment of colitis have not been understood; thus, in this study, we evaluated the effect of CA in the treatment of colitis, considering its possible anti-inflammatory, antioxidant and anti-apoptotic properties. Secondary metabolites of plants, including polyphenols and flavonoids, are among the natural compounds of plant origin that have shown positive results in improving colitis in vitro and in vivo (Gupta et al. 2018). The proposed mechanism for the function of these compounds is the inhibitory power of free radicals (Gupta et al. 2018), which leads to a reduction in lipid peroxidation and subsequent inhibition of MDA production, in contrast to increasing levels of antioxidant enzymes such as SOD and CAT (Khan et al. 2020). It is reported that flavonoids and polyphenols interact with TLR4 (Ravindran et al. 2019), and inhibit the TLR4/NF-κB signaling cascade in the intestinal mucosa (Magrone et al. 2020), and thereby inhibit the expression of IL-6, IL-1β, TNF-α, also reducing the production of inflammatory cytokines and subsequent inflammation. On the other hand, inhibition of NF-κB also reduces INOS expression and NO levels in tissues (Lee et al. 2019).
So, it can be concluded that the anti-inflammatory and antioxidant properties of CA be attributed to the inhibitory of TLR4/NF-κB signaling cascade as well as the NF-κB/INOS/NO signaling pathway, which leads to down-regulation of TLR4, NF-κB, TNF-α, IL-1β, IL-6, INOS, also its power of free radical scavenging like other polyphenols. As a result, the healing effects of CA against histopathological damage in the colon tissue can be related to its antioxidant properties as well as the ability of this compound to inhibit apoptosis and reduce apoptotic factors such as caspases and Bax.
The present study showed that CA significantly improved complications related to inflammation, oxidative stress, and apoptosis, as well as histopathological indices. In this study, the expression of inflammatory and apoptotic genes (TLR4, NF-κB, TNF-α, INOS, IL-1β, IL-6, Caspase-3, Caspase-8, and Bax) and the level of factors related to oxidative stress (MDA, NO) as well as histopathological damage in the colon tissue, was significantly reduced, and in the opposite, the level of defense mechanisms such as antioxidant enzymes (SOD, CAT) and TAC significantly increased in colitis rats treated with CA.
Considering that the results showed that dexamethasone could not have a significant effect in reducing the expression of NF-κB, on the other hand, dexamethasone reduced the expression of TNF-α, IL-6, and IL-1β regarding the mechanism of dexamethasone's effect in reducing The expression of TNF-α, IL-6, and IL-1β can be concluded that dexamethasone was effective through pathways other than the NF-κB pathway.
Conclusion
It is concluded that CA probably exerts its positive effects in managing colitis through its anti-inflammatory, antioxidant, and anti-apoptotic properties.
Data availability
Data regarding the present study are available at the Medical Plants Research Center, Shahrekord University of Medical Sciences.
References
Abdelkader NF, Safar MM, Salem HA (2016) Ursodeoxycholic acid ameliorates apoptotic cascade in the rotenone model of Parkinson’s disease: modulation of mitochondrial perturbations. Mol Neurobiol 53:810–817
Aebi H (1984) [13] Catalase in vitro. Methods Enzymol 105:121–126
Alavala S, Sangaraju R, Nalban N, Sahu BD, Jerald MK, Kilari EK, Sistla R (2019) Stevioside, a diterpenoid glycoside, shows anti-inflammatory property against Dextran Sulphate Sodium-induced ulcerative colitis in mice. Eur J Pharmacol 855:192–201
Andrade AWL, Machado KDC, Machado KDC, Figueiredo DDR, David JM, Islam MT, Uddin SJ, Shilpi JA, Costa JP (2018) In vitro antioxidant properties of the biflavonoid agathisflavone. Chem Cent J 12:1–9
Balmus IM, Ciobica A, Trifan A, Stanciu C (2016) The implications of oxidative stress and antioxidant therapies in inflammatory bowel disease: clinical aspects and animal models. Saudi Journal of Gastroenterology: Official Journal of the Saudi Gastroenterology Association 22:3
Bastaki SM, Amir N, Adeghate E, Ojha S (2021) Nerolidol, a sesquiterpene, attenuates oxidative stress and inflammation in acetic acid-induced colitis in rats. Mol Cell Biochem 476(9):3497–3512. https://doi.org/10.1007/s11010-021-04094-5
Bastaki SM, Al Ahmed MM, Al Zaabi A, Amir N, Adeghate E (2016) Effect of turmeric on colon histology, body weight, ulcer, IL-23, MPO and glutathione in acetic-acid-induced inflammatory bowel disease in rats. BMC Complement Altern Med 16:1–14
Benzie IF, Strain JJ (1996) The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem 239:70–76
Cheng G, Zhao Y, Li H, Wu Y, Li X, Han Q, Dai C, Li Y (2014) Forsythiaside attenuates lipopolysaccharide-induced inflammatory responses in the bursa of Fabricius of chickens by downregulating the NF-κB signaling pathway. Exp Ther Med 7:179–184
Eissa N, Hussein H, Kermarrec L, Ali AY, Marshall A, Metz-Boutigue M-H, Hendy GN, Bernstein CN, Ghia J-E (2018) Chromogranin-A regulates macrophage function and the apoptotic pathway in murine DSS colitis. J Mol Med 96:183–198
Fuentes F, Paredes-Gonzalez X, Kong A-NT (2015) Dietary glucosinolates sulforaphane, phenethyl isothiocyanate, indole-3-carbinol/3, 3′-diindolylmethane: Antioxidative stress/inflammation, Nrf2, epigenetics/epigenomics and in vivo cancer chemopreventive efficacy. Curr Pharmacol Rep 1:179–196
Gilardi D, Fiorino G, Genua M, Allocca M, Danese S (2014) Complementary and alternative medicine in inflammatory bowel diseases: what is the future in the field of herbal medicine? Expert Rev Gastroenterol Hepatol 8:835–846
Gupta RA, Motiwala MN, Mahajan UN, Sabre SG (2018) Protective effect of Sesbania grandiflora on acetic acid induced ulcerative colitis in mice by inhibition of TNF-α and IL-6. J Ethnopharmacol 219:222–232
Haj-Mirzaian A, Amiri S, Amini-Khoei H, Hosseini M-J, Haj-Mirzaian A, Momeny M, Rahimi-Balaei M, Dehpour AR (2017) Anxiety-and depressive-like behaviors are associated with altered hippocampal energy and inflammatory status in a mouse model of Crohn’s disease. Neuroscience 366:124–137
Khan MN, Lane ME, McCarron PA, Tambuwala MM (2018) Caffeic acid phenethyl ester is protective in experimental ulcerative colitis via reduction in levels of pro-inflammatory mediators and enhancement of epithelial barrier function. Inflammopharmacology 26:561–569
Khan M, Liu H, Wang J, Sun B (2020) Inhibitory effect of phenolic compounds and plant extracts on the formation of advance glycation end products: a comprehensive review. Food Res Int 130:108933
Kim Y, Wu AG, Jaja-Chimedza A, Graf BL, Waterman C, Verzi MP, Raskin I (2017) Isothiocyanate-enriched moringa seed extract alleviates ulcerative colitis symptoms in mice. PLoS ONE 12:e0184709
Lee W, Yang S, Lee C, Park EK, Kim K-M, Ku S-K, Bae J-S (2019) Aloin reduces inflammatory gene iNOS via inhibition activity and p-STAT-1 and NF-κB. Food Chem Toxicol 126:67–71
Liu X, Wang JM (2011) Iridoid glycosides fraction of Folium syringae leaves modulates NF-κ B signal pathway and intestinal epithelial cells apoptosis in experimental colitis. PLoS ONE 6:e24740
Liu D-M, Zhou C-Y, Meng X-L, Wang P, Li W (2018) Thymol exerts anti-inflammatory effect in dextran sulfate sodium-induced experimental murine colitis. Trop J Pharm Res 17:1803–1810
Lou Z, Wang H, Rao S, Sun J, Ma C, Li J (2012) p-Coumaric acid kills bacteria through dual damage mechanisms. Food Control 25:550–554
Macpherson B, Pfeiffer C (1978) Experimental production of diffuse colitis in rats. Digestion 17:135–150
Magrone T, Magrone M, Russo MA, Jirillo E (2020) Recent advances on the anti-inflammatory and antioxidant properties of red grape polyphenols: In vitro and in vivo studies. Antioxidants 9:35
Mahmoud TN, El-Maadawy WH, Kandil ZA, Khalil H, El-Fiky NM, el Alfy TSM (2021) Canna x generalis LH Bailey rhizome extract ameliorates dextran sulfate sodium-induced colitis via modulating intestinal mucosal dysfunction, oxidative stress, inflammation, and TLR4/NF-ҡB and NLRP3 inflammasome pathways. J Ethnopharmacol 269:113670
Misra HP, Fridovich I (1972) The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem 247:3170–3175
Murad HA, Abdallah HM, Ali SS (2016) Mentha longifolia protects against acetic-acid induced colitis in rats. J Ethnopharmacol 190:354–361
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358
Omidi-Ardali H, Lorigooini Z, Soltani A, Balali-Dehkordi S, Amini-Khoei H (2019) Inflammatory responses bridge comorbid cardiac disorder in experimental model of IBD induced by DSS: protective effect of the trigonelline. Inflammopharmacology 27:1265–1273
Peng J, Zheng T-T, Liang Y, Duan L-F, Zhang Y-D, Wang L-J, He G-M, Xiao H-T (2018) p-Coumaric acid protects human lens epithelial cells against oxidative stress-induced apoptosis by MAPK signaling, Oxidative Medicine and Cellular Longevity, vol 2018. p 8549052. https://doi.org/10.1155/2018/8549052
Pourkhodadad S, Alirezaei M, Moghaddasi M, Ahmadvand H, Karami M, Delfan B, Khanipour Z (2016) Neuroprotective effects of oleuropein against cognitive dysfunction induced by colchicine in hippocampal CA1 area in rats. J Physiol Sci 66:397–405
Prince PSM, Roy AJ (2013) p-Coumaric acid attenuates apoptosis in isoproterenol-induced myocardial infarcted rats by inhibiting oxidative stress. Int J Cardiol 168:3259–3266
Rafiee Z, Moaiedi MZ, Gorji AV, Mansouri E (2020) p-Coumaric acid mitigates doxorubicin-induced nephrotoxicity through suppression of oxidative stress, inflammation and apoptosis. Arch Med Res 51:32–40
Ramachandran A, Madesh M, Balasubramanian KA (2000) Apoptosis in the intestinal epithelium: its relevance in normal and pathophysiological conditions. J Gastroenterol Hepatol 15:109–120
Ravindran R, Swamy MK, Jaganathan R (2019) Therapeutic potential of plant polyphenolics and their mechanistic action against various diseases. Springer, Natural Bio-Active Compounds
Rees V (1998) Chronic experimental colitis induced by dextran sulphate sodium (DSS) is characterized by Th1 and Th2 cytokines. Clin Exp Immunol 114:385–391
Robbins SL, Cotran RS (1979) Pathologic basis of disease. Saunders
Sales-Campos H, Basso P, Alves V, Fonseca M, Bonfá G, Nardini V, Cardoso C (2015) Classical and recent advances in the treatment of inflammatory bowel diseases. Braz J Med Biol Res 48:96–107
Scorrano L, Korsmeyer SJ (2003) Mechanisms of cytochrome c release by proapoptotic BCL-2 family members. Biochem Biophys Res Commun 304:437–444
Shaker ME, Ashamallah SA, Houssen ME (2014) Celastrol ameliorates murine colitis via modulating oxidative stress, inflammatory cytokines and intestinal homeostasis. Chem Biol Interact 210:26–33
Sharma SH, Rajamanickam V, Nagarajan S (2018) Antiproliferative effect of p-Coumaric acid targets UPR activation by downregulating Grp78 in colon cancer. Chem Biol Interact 291:16–28
Shen Y, Song X, Li L, Sun J, Jaiswal Y, Huang J, Liu C, Yang W, Williams L, Zhang H (2019) Protective effects of p-coumaric acid against oxidant and hyperlipidemia-an in vitro and in vivo evaluation. Biomed Pharmacother 111:579–587
Tekeli İO, Ateşşahin A, Sakin F, Aslan A, ÇERIBAŞı, S. & YIPEL, M. (2019) Protective effects of conventional and colon-targeted lycopene and linalool on ulcerative colitis induced by acetic acid in rats. Inflammopharmacology 27:313–322
Tian Q, Bravo Iniguez A, Sun Q, Wang H, Du M, Zhu MJ (2021) Dietary Alpha-Ketoglutarate promotes epithelial metabolic transition and protects against DSS-induced colitis. Mol Nutr Food Res 65:2000936
Triantafillidis JK, Triantafyllidi A, Vagianos C, Papalois A (2016) Favorable results from the use of herbal and plant products in inflammatory bowel disease: evidence from experimental animal studies. Ann Gastroenterol Q Publ Hell Soc Gastroenterol 29:268
Wallace JL, Keenan CM (1990) An orally active inhibitor of leukotriene synthesis accelerates healing in a rat model of colitis. Am J Physiol Gastrointest Liver Physiol 258:G527–G534
Wallace KL, Zheng L-B, Kanazawa Y, Shih DQ (2014) Immunopathology of inflammatory bowel disease. World J Gastroenterol: WJG 20:6
Wang L, Liu H, Zhang L, Wang G, Zhang M, Yu Y (2017) Neuroprotection of dexmedetomidine against cerebral ischemia-reperfusion injury in rats: involved in inhibition of NF-κB and inflammation response. Biomol Ther 25:383
Zhu H, Li YR (2012) Oxidative stress and redox signaling mechanisms of inflammatory bowel disease: updated experimental and clinical evidence. Exp Biol Med 237:474–480
Acknowledgements
The authors thank the Medical Plants Research Center, Shahrekord University of Medical Sciences, Shahrekord, Iran, for supporting this study.
Funding
This study was supported by a research grant (5186) from Shahrekord University of Medical Sciences, Shahrekord, Iran.
Author information
Authors and Affiliations
Contributions
All the authors were involved in the design, Material preparation, data collection and analysis, manuscript writing, and editing. All the authors read and approved the final draft of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare regarding the study described in this article and the preparation of the manuscript.
Ethical approval
All procedures were carried out under the regulations of the University and the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health (ethical code: IR.SKUMS.REC.1399.113) and Guide for the Care and Use of Laboratory Animals (8th edition, National Academies Press).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ghasemi-Dehnoo, M., Amini-Khoei, H., Lorigooini, Z. et al. Coumaric acid ameliorates experimental colitis in rats through attenuation of oxidative stress, inflammatory response and apoptosis. Inflammopharmacol 30, 2359–2371 (2022). https://doi.org/10.1007/s10787-022-01074-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10787-022-01074-z