Abstract
Ulcerative colitis is an inflammatory bowel disease with a complex aetiology characterised by abnormal immune responses and oxidative stress–induced tissue injury. Inflammatory cells play an important role in the progression of this pathology through the overproduction of reactive oxygen species (ROS) from various sources including the NADPH oxidases (NOXs). The aim of this study was to investigate the preventive effect of apocynin, a natural antioxidant molecule and a selective inhibitor of NOXs, on acetic acid (AA)-induced ulcerative colitis in rats. Our results first confirmed that apocynin has a high free radical scavenging capacity as well as a potent iron chelating ability. Oral pretreatment of rats with apocynin (200 mg/kg and 400 mg/kg) for 7 days prior to AA-induced colitis suppressed the increase in pro-oxidant markers in colonic homogenates and preserved colonic cytoarchitecture from acetic acid–induced damage. Oral administration of apocynin (200 mg/kg and 400 mg/kg) also reduced several systemic inflammatory markers such as alkaline phosphatase, iron, pro-inflammatory cytokines, C-reactive protein and myeloperoxidase. This study shows that apocynin protects rats from acetic acid–induced colonic inflammation and suggests that apocynin may have a promising beneficial effect in the prevention of ulcerative colitis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Reactive oxygen species (ROS) play a crucial role in physiological and pathological processes [1]. Their overproduction causes oxidative stress, which accelerates cellular dysfunction and leads to several diseases, including atherosclerosis [2], cancer [3], pulmonary inflammation [4] and inflammatory bowel disease (IBD) [5]. Oxidative stress is involved in ulcerative colitis (UC), which is a chronic inflammatory bowel disease [6]. The early stage of this pathology is characterised by an increase in neutrophil and lymphocyte infiltration in the small intestine [7]. Indeed, neutrophilia promotes the accumulation of a large amount of ROS, leading to oxidative stress, activation of proteolytic enzymes [8] and cytokine production of cytokines such as tumour necrosis factor-α (TNF-α) and interleukin-1β (IL-1β) [9]. The generation of ROS is mediated by several enzymatic systems, including the mitochondrial electron transport chain [10], xanthine oxidase [11] and NADPH oxidase [12]. NADPH oxidase (NOX)-derived ROS have been implicated in a variety of pathological conditions associated with oxidative stress [13]. For example, the phagocyte NADPH oxidase NOX2 and its epithelial homologue (NOX1) are the major contributors to superoxide anion production and ROS accumulation in the colon during ulcerative colitis [14]. Furthermore, oxidative stress and a decrease in the free radical scavenging mechanism that controls the formation of ROS and inducible nitric oxide synthase (iNOS) are both major contributors to IBD, such as UC [15]. In fact, the overproduction of ROS and iNOS is the primary cause of an oxidative stress state, which is manifested by a decrease in catalase activity and glutathione level content as well as an increase in lipid peroxidation [16]. UC disease is characterised by an increase in neutrophils, macrophages and leukocyte infiltrates in the colon, which stimulates the production of pro-oxidant markers [17]. The overproduction of free radicals (hydrogen peroxide (H2O2)) in peripheral cells (monocytes and lymphocytes) leads to an increase in plasma CRP [18] and an increase in cytokine production [19]. The dramatic increase in cytokine products activates nuclear factor kappa B (NF-κB), which is implicated in the progression of UC by increasing IL-1β, IL-6 and TNF-α production, mainly through positive regulation of inflammatory genes [20]. Currently, there are a variety of drugs used to treat ulcerative colitis, including corticosteroids and aminosalicylates [21]. However, their therapeutic effect is limited by the maintenance of remission [22]. In addition, there are a number of adverse effects associated with the use of aminosalicylates in the treatment of ulcerative colitis, such as diarrhoea, abdominal pain and nausea [23]. The use of corticosteroids to treat ulcerative colitis [24] has been associated with serious side effects such as glaucoma and adrenaline deficiency [25]. This explains why scientists are investigating NADPH oxidase inhibitors as a potential treatment for inflammatory bowel disease [26]. In addition, natural products are the primary targets of the most recently discovered drugs [27].
Based on these conditions, we could suggest apocynin, a natural compound derived from the Himalayan medicinal plant Picrorhiza kurroa as anti-inflammatory agent. It is an inexpensive molecule and acts as a NADPH oxidase inhibitor by preventing the translocation of p47phox, the cytosolic subunit to the membrane [28], and its expression [29]. Apocynin also has an antioxidant effect as it can react directly with ROS molecules [30]. In an experimental pathological model, apocynin limits the overproduction of ROS in inflammatory, neurological and vascular disorders [31]. Many studies have shown that this compound has anti-inflammatory properties that are useful in the treatment and prevention of chronic inflammatory disorders [32, 33]. Apocynin also has specific antioxidant effects through the metabolic activation of phagocytes and the production of myeloperoxidase (MPO) [34]. The anti-inflammatory effects of this molecule have been observed in vitro, such as the suppression of granulocyte chemotaxis and the reduction of neutrophil oxidative burst [35]. Furthermore, apocynin can act as an inhibitor of NOX2, but it also has several other effects independent of NOX2 inhibition, such as inhibition of the AKT and ERK1/2 pathways activated by granulocyte-macrophage colony-stimulating factor (GM-CSF) [36]. Our research aims to investigate the preventive effects of apocynin on acetic acid–induced ulcerative colitis in rats.
MATERIAL AND METHODS
Reagents
Apocynin (4-hydroxy-3-methoxyacetophenone), dimethylsulfoxide (DMSO), hexadecyltrimethylammonium bromide (HTAB), phosphate-buffered saline (PBS), NaCl, chloramine-T, potassium iodide (KI), acetic acid (AA), sulfasalazine (SSZ), butylated hydroxytoluene (BHT), trichloroacetic acid (TCA), thiobarbituric acid (TBA), hydrochloric acid (HCl), ortho-dianisidine dihydrochloride, Tris buffer, H2O2, 2,2′-azino-bis-3-ethylbenzothiazoline-6-sulfonic acid (ABTS), 1,1-diphenyl-2-picrylhydrazyl (DPPH), ethylenediaminetetraacetic acid (EDTA), epinephrine, bovine catalase and sodium carbonate/bicarbonate were obtained from Sigma-Aldrich (St. Louis, MI, USA). Rat TNF-α enzyme-linked immunosorbent assay (ELISA) kit and rat IL-1β ELISA kit were purchased from PeproTech (Cranbury, NJ, USA). Iron, alkaline phosphatase and C-reactive protein kits were purchased from Biomaghreb (Tunisia).
In Vitro Antioxidant Activity and Iron Chelating Capacity of Apocynin
The antioxidant activity of apocynin was evaluated by using the ABTS and DPPH free radicals, as described respectively by Re et al. [37] and Grzegorczyk et al. [38]. In addition, iron chelating capacity was determined using the method described by Chew et al. [39].
Animal Care
The Ethics Committee of the Tunisian Association of Laboratory Animal Sciences gave its ethical approval (No. 0123/2022 ATSAL) for all studies which were conducted in accordance with the European Community Council Directive of 24 November 1986.
Thirty male Wistar rats weighing 200–220 g were purchased from the Pasteur Institute in Tunis. The rats were maintained in a controlled environment with a 12:12-h light-dark cycle, 65–70% humidity and 22 ± 1 °C temperature, with free access to water and normal rodent chow.
Induction of Ulcerative Colitis
Ulcerative colitis was induced according to a previous study [40] with slight modifications, i.e. rats were given 5 ml/kg of acetic acid, diluted to 3% with 0.9% NaCl using a paediatric catheter via the intrarectal route (a flexible plastic catheter inserted 8 cm into the rectum). Animals were kept in the Trendelenburg position (head-down) for 20 s after receiving AA in order to avoid solution leakage. Twenty-four hours after the induction of colitis, the animals were sacrificed.
Experimental Design
Rats were divided into six groups: the control and acetic acid groups received daily oral DMSO. One group received a daily oral dose of SSZ at 150 mg/kg for 7 days, while the other three groups received different concentrations of apocynin (100 mg/kg, 200 mg/kg and 400 mg/kg) for 7 days. Following the pretreatment phase, all animals received intrarectal acetic acid, except the control group, which received intrarectal NaCl 0.9% (Fig. 1). Twenty-four hours after the induction of colitis (day 8), the animals were sacrificed.
Summary of the experimental design used in this study. Rats were divided into six groups: the control and acetic acid groups received daily oral DMSO. One group received a daily oral dose of SSZ at 150 mg/kg, while the other three groups received different concentrations of apocynin (100 mg/kg, 200 mg/kg and 400 mg/kg) for 7 days. Following the pretreatment phase, all animals received intrarectal acetic acid, except the control group, which received intrarectal NaCl 0.9%. Twenty-four hours after the induction of colitis (day 8), the animals were sacrificed and samples were collected for the assessment of inflammation and oxidative stress.
Disease Activity Index (DAI) Measurement
During the study, the body weight of the rats was recorded daily using an electronic weighing machine. Feeding behaviour was also monitored. In addition, we looked for signs of animal stress, such as diarrhoea and a decrease in activity. Twenty-four hours after the induction of colitis (day 8), the animals were sacrificed. As previously described, plasma and organs were carefully collected, and their relative weights measured. Disease activity index [41], weight (W) and length (L) of the colon (from the cecum to the rectum) of each rat were determined to evaluate the wet colon sample (W (g)/L (cm)) ratio.
Protein Determination
Colon homogenate was rinsed in ice-cold NaCl 0.9% (w/v) and homogenised in a 0.1 M phosphate-buffered solution (pH 7.4). Homogenates were centrifuged at 4000 rpm for 20 min at 4 °C. Total tissue protein content was determined by the Lowry method [42].
Advanced Oxidation of Protein Products
Advanced oxidation of protein products (AOPPs) in the colon were assessed using the technique described by Witko-Sarsat et al. [43]. This procedure is based on a standard curve prepared with acetic acid, KI and chloramine-T (10 mM). The level of AOPPs is determined by measuring the absorbance at 340 nm with a reference absorbance at 490 nm. The AOPP levels were expressed as µM/mg of colon protein according to the chloramine standard curve.
Malondialdehyde (MDA) Content of Lipid Peroxidation
The MDA level in colonic homogenate was estimated according to the method described by Yagi [44]. Aliquots of colonic homogenates were mixed with a BHT-TCA solution containing 1% BHT (w/v) dissolved in 20% TCA (w/v), then centrifuged at 1000 g for 5 min at 4 °C. The supernatant was mixed with HCl (0.5 N), 120 mM TBA buffered in a 26 mM Tris solution, and heated at 80 °C for 10 min. Absorbance was measured at 532 nm using a UV spectrophotometer (Agilent Cary 3500). MDA levels were calculated using an extinction molar coefficient of 1.56 × 105 M/cm of the MDA-TBA complex, and results are expressed as nmol/mg of protein.
Catalase Activity
H2O2 detoxification was evaluated by the method of Aebi [45], which is based on the evaluation of the rate constant of H2O2 decomposed by the catalase enzyme at 240 nm by using a UV-visible spectrophotometer (Agilent Cary 3500) for 3 min. The enzymatic activity was calculated from the extinction coefficient of H2O2, and then the results are expressed as mmol of H2O2/min/mg of protein.
Superoxide Dismutase Activity
Superoxide dismutase (SOD) activity was determined by using an epinephrine assay described by Misra and Fridovich [46]. Briefly, the supernatant was added to 2 ml of a reaction solution containing bovine catalase, epinephrine and a buffer solution of sodium carbonate/bicarbonate (pH = 10.2). Enzymatic activity was measured spectrophotometrically at 480 nm for 3 min using a UV-visible spectrophotometer (Agilent Cary 3500).
Myeloperoxidase Activity
A spectrophotometric method described by Bradley et al. [47] was used to determine the index of neutrophil infiltration in plasma and colonic tissue. The colon was briefly centrifuged, and the supernatant was mixed with PBS containing 0.5% HTAB and sonicated. The supernatant was mixed with PBS containing 0.167 mg/ml ortho-dianisidine dichloride and 0.0005% H2O2. The rate of absorbance change was measured at 460 nm for 10 min to evaluate this activity. The results are given as 1 unit of MPO = mM of H2O2/min/mg protein.
Determination of Reduced Glutathione (GSH) Content
Colonic GSH levels were measured according to the method described by Ellman [48]. According to the 5,5′-dithiobis-(2-nitrobenzoic acid) (DTNB) reduction, the yellow colour produced depends on the GSH concentration, and the optical density was measured at 412 nm using a UV-visible spectrophotometer (Agilent Cary 3500).
Assessment of Inflammatory Mediators
For the assessment of inflammatory mediators in rat plasma, TNF-α (catalogue N°900-M73, lot N°0521073-M) and IL-1β (catalogue N°900-M91, lot N°0319091-M) were measured using ELISA kits from PeproTech according to the manufacturer’s instructions and plasma C-reactive protein was determined using the colorimetric method as described in the Biomaghreb kit (reference 41012). In addition, alkaline phosphatase activity and plasma iron levels were determined using Biomaghreb kits (references 13026 and 20061).
Histological Study
Intestinal tissue was collected and stored overnight in 10% formol. Colon samples were processed in graded ethanol solutions and embedded in paraffin. Histological sections were cut at 5 µm and stained with haematoxylin and eosin (H&E), and the morphological evaluation was performed by light microscopy (Carl Zeiss, Jena, Germany).
Statistical Analysis
Results are presented as mean ± SEM. Variation between groups was analysed by one-way ANOVA, followed by Tukey’s multiple comparison post-test, using GraphPad Prism 7 software (GraphPad Software, San Diego, CA, USA) with a p value ≤ 0.05 which was considered statistically significant.
RESULTS
Effect of Apocynin on Macroscopic and Histological Changes of the Colon in AA-Induced Colitis in Rats
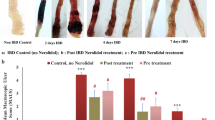
The aim of this study was to investigate the effect of apocynin, an antioxidant and NADPH oxidase inhibitor, on AA-induced colitis in rats. The results show that AA induced a dramatic increase in the disease activity index (DAI), an indicator of the degree of inflammation determined by the examining each animal’s body weight, diarrhoea and faecal blood (Fig. 2a). The ulcerative colitis group (AA group) showed a significant increase (p < 0.05) in the DAI as compared to the control group. Furthermore, SSZ, used as a positive control, significantly decreased (p < 0.05) the DAI compared to the AA group. Interestingly, pretreatment of rats with apocynin at doses of 200 mg/kg and 400 mg/kg significantly (p < 0.05) reduced this index compared to the AA group (Fig. 2a).
Effect of apocynin on macroscopic changes of the colon in AA-induced colitis in rats. Rats were treated as described in the experimental design scheme, colons were isolated and disease activity index (DAI) (a), macroscopic state (b) and wet colon weight (c) were determined. Results are presented as mean ± SEM (n = 5) with *p < 0.05 compared to the control group; #p < 0.05, ##p < 0.01 and ###p < 0.001 compared to the AA group; and $$p < 0.01 compared to the SSZ group.
Macroscopic examination of the colon showed that the colon of the AA group was characterised by a reduction in length, signs of inflammation and redness, and that SSZ and apocynin protected it from this inflammation (Fig. 2b). In addition, the wet colon weight in the AA group was significantly higher (p < 0.05) than that in the control group, and apocynin dramatically reduced this AA-induced effect compared to the AA group. Apocynin concentrations of 200 mg/kg and 400 mg/kg were more effective (p < 0.01) than SSZ (Fig. 2c).
The results of the histological examination of the colon (Fig. 3) showed that the colonic architecture of the control group appeared normal (magnification × 4), with intact epithelium (black arrow) (magnifications × 10 and × 20). A microstructure was clearly visualised: external mucosa (EM), submucosa (SM), muscularis mucosa (MM) and intact lamina (LM) (magnification × 20) with the presence of goblet cells (magnification × 40) (green arrow). For the AA group, histological analysis showed that acetic acid caused extensive ulceration and deterioration of tissue architecture (red arrow), erosion of mucosa (blue arrows) (× 4 magnification) with mucosal haemorrhage, loss of epithelial and goblet cells (purple arrow), oedema (red stars) and neutrophil infiltration in the submucosal layers (yellow stars) (× 40 magnification). Pretreatment with sulfasalazine protected the microstructure of the colon (magnification × 4) and attenuated mucosal ulceration and neutrophil infiltration in the submucosal layers (× 10 and × 20 magnifications). Furthermore, examination of the colon shows the presence of goblet cells without neutrophil infiltration (magnification × 40). Our results also showed that apocynin at 100 mg/kg prevented the erosion of colonic structure and ulceration induced by acetic acid (magnification × 4) and reduced the accumulation of inflammatory cells to some extent (magnifications × 10 and × 20), with the presence of oedema in the submucosal layers (magnification × 40) (Fig. 3). Pretreatment with apocynin at doses of 200 mg/kg and 400 mg/kg protected the structural integrity of the colon (magnification × 4) with a similar length of villus to the control and SSZ groups (magnification × 10). In addition, there was no ulceration or accumulation of neutrophils in these two groups (× 20 magnification). Higher magnification also shows the presence of goblet cells (× 40 magnification).
Histological examination of colon structure after apocynin pretreatment and induction of ulcerative colitis by glacial acetic acid. Rats were treated as described in the experimental design scheme, and colons were isolated, fixed and embedded in paraffin. Histological sections were stained with haematoxylin and eosin (H&E), and the morphological evaluation was performed by light microscopy at different magnifications (× 4, × 10, × 20 and × 40). “Control” are colons from untreated rats, “AA” are colons from acetic acid–treated rats, “SSZ+AA” are colons from SSZ-treated and acetic acid–treated rats and “AP-100+AA”, “AP-200+AA” and “AP-400+AA” are colons from rats treated with apocynin at 100 mg/kg, 200 mg/kg and 400 mg/kg, respectively, and acetic acid. Mucosa (M), external mucosa (EM), submucosa (SM), muscularis mucosa (MM), intact lamina (LM), goblet cells (green arrow), deterioration of tissue architecture (red arrow), loss of epithelial cells (purple arrow), mucosal erosions (blue arrows), oedema (red stars) and infiltration at the submucosal layers (yellow stars). Representative images from six different rats.
Apocynin Exerts Antioxidant and Iron Chelating Properties In Vitro and Prevents Oxidative Stress in the Colon
To test the involvement of an oxidative stress in AA-induced colitis and the effect of apocynin on this process, we first evaluated the antioxidant activities of apocynin in vitro using the ABTS and DPPH tests, which revealed that apocynin had a potent antioxidant activity by scavenging free radicals with a half-maximal inhibitory concentration (IC50) of 124.12 ± 3.79 µg/ml for the ABTS test compared to that of Trolox (IC50 = 66.29 ± 1.03 µg/ml). In addition, our results showed that apocynin also scavenges DPPH free radicals (IC50 = 31.56 ± 0.21 µg/ml) compared to the BHT test (IC50 = 12.73 ± 4.01 µg/ml). Furthermore, our results indicate that apocynin has a potent iron chelating ability (IC50 = 8.23 ± 0.151 µg/ml) close to that of EDTA (IC50 = 5.44 ± 0.205 µg/ml) (Table 1).
We then investigated the effect of apocynin on oxidative stress markers such as protein and lipid oxidation and MPO in vivo in the rat colon. Measurement of AOPP levels, a marker of protein damage in colonic homogenate, showed that intrarectal administration of acetic acid caused a significant increase (p < 0.001) in the AOPPs as compared to the control group (Fig. 4a). Furthermore, pretreatment of rats with different doses of apocynin and SSZ significantly reduced AOPP levels in the colon as compared to the AA group. Furthermore, our results showed that there was no significant difference between SSZ and apocynin (AP) at 400 mg/kg (Fig. 4a).
Effect of apocynin on colonic pro-oxidant markers in AA-induced colitis in rats. Rats were treated as described in the experimental plan, colons were isolated and AOPP (a), MDA (b) and MPO activity (c) were determined. Results are presented as mean ± SEM (n = 5) with ***p < 0.001 compared to the control group, ##p < 0.01 and ###p < 0.001 compared to the AA group and $p < 0.05, $$p < 0.01 and $$$p < 0.001 compared to the SSZ group.
Evaluation of lipid peroxidation status by the determination of MDA levels in colonic homogenate showed that acetic acid significantly increased the MDA levels in the AA group compared to the control group (p < 0.001). Our analysis showed that pretreatment of rats with apocynin exerted a significant preventive effect (p < 0.001 vs. the AA group) on the increase in MDA content induced by acetic acid. Based on our results, there is no significant difference between SSZ and AP at 400 mg/kg (Fig. 4b).
MPO is a pro-oxidant enzyme mainly found in neutrophils, and its activity has been used as an index of neutrophil infiltration and activation in the colon. Our results showed that intrarectal administration of AA significantly (p < 0.001) increased MPO activity in the colon as compared to the control group. SSZ pretreatment significantly reduced (p < 0.001) the MPO activity by 55% compared to the AA group. In addition, the different doses of apocynin significantly reduced MPO activity (p < 0.001) in colonic homogenate as compared to the AA group. Therefore, there is no significant difference in myeloperoxidase activity between the SSZ and apocynin (400 mg/kg) groups (Fig. 4c). These redox changes in the colonic tissue were prevented by pretreatment with apocynin at doses of 200 mg/kg and 400 mg/kg.
We then investigated the effect of apocynin on antioxidative stress markers such as SOD and catalase in vivo in the rat colon. Determination of SOD activity in colonic homogenate shows that induction of ulcerative colitis with an AA group significantly reduced (p < 0.001) SOD activity compared to the control group (Fig. 5a). Our results showed that apocynin at 100 mg/kg for 7 days significantly (p < 0.05) prevented the decrease in SOD activity in colonic homogenate as compared to the AA group. This increase is more effective (p < 0.01) at 200 mg/kg and 400 mg/kg. Furthermore, there was no significant difference in SOD levels between the SSZ and the 400 mg/kg apocynin groups.
Effect of apocynin on colonic antioxidant markers in the colon in AA-induced colitis in rats. Rats were treated as described in the experimental design scheme, colons were isolated and SOD (a), catalase activity (b) and GSH (c) were determined. Results are presented as mean ± SEM (n = 5) with ***p < 0.001 compared to the control group and #p < 0.05, ##p < 0.01 and ###p < 0.001 compared to the AA group.
Determination of catalase activity in colonic homogenates shows that the AA group leads to a significant reduction (p < 0.001) of this enzymatic activity as compared to the control group (Fig. 5b). Our analysis showed that SSZ significantly increased (p < 0.001) the catalase activity as compared to the AA group and apocynin treatment at 200 mg/kg significantly improved (p < 0.05) the catalase activity compared to the AA group. This increase is more significant (p < 0.001) in the apocynin (400 mg/kg) group than in the AA group. Furthermore, our analysis revealed no significant variation in catalase levels between the SSZ and apocynin (400 mg/kg) groups (Fig. 5b).
Evaluation of the GSH content in colonic homogenate showed that acetic acid induced a significant decrease (p < 0.001) in this antioxidant marker as compared to the control group (Fig. 5c). Pretreatment with SSZ also had no effect on the GSH levels compared to the AA group. Interestingly, pretreatment with apocynin at different doses indicated that apocynin at 400 mg/kg significantly (p < 0.01) increased the GSH content in colonic homogenate as compared to the AA group. Furthermore, our analysis showed no significant variation between SSZ and apocynin (400 mg/kg) (Fig. 5c).
Effect of Apocynin on Pro-inflammatory Mediators in Rat Plasma
Evaluation of plasma alkaline phosphatase activity in plasma shows a significant increase (p < 0.001) in this activity in the AA group compared with the control group. Furthermore, according to our results, pretreatment with sulfasalazine for 7 days significantly reduced (p < 0.001) the increase in alkaline phosphatase (ALP) activity as compared to the AA group. Furthermore, apocynin at 200 mg/kg and 400 mg/kg significantly prevented (p < 0.001) the increase in ALP compared to the AA group. Also, according to our results, there is no significant difference between the SSZ and apocynin groups treated with 400 mg/kg (Fig. 6a).
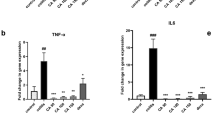
Effect of apocynin on plasma inflammatory markers in AA-induced colitis in rats. Rats were treated as described in the experimental design scheme, plasmas were isolated and the enzymatic activities of alkaline phosphatase (a), iron concentration (b), CRP level (c), myeloperoxidase activity (d), TNF-α (e) and IL-1β (f) were determined. Results are presented as mean ± SEM (n = 5) with **p < 0.01 and ***p < 0.001 compared to the control group, ##p < 0.01 and ###p < 0.001 compared to the AA group and $p < 0.05 and $$$p < 0.001 compared to the SSZ group.
According to the biochemical analysis of iron in plasma, ulcerative colitis exacerbated by acetic acid was characterised by a significant increase (p < 0.001) in this parameter as compared to the control group. Our results showed that sulfasalazine at 150 mg/kg for 7 days significantly reduced (p < 0.001) the increase in plasma iron content compared to the AA group. However, pretreatment with apocynin at a dose of 100 mg/kg had no effect on the level of plasma iron. On the other hand, apocynin at 200 mg/kg and 400 mg/kg significantly (p < 0.001) decreased the plasma iron content compared to the AA group (Fig. 6b).
A typical sign of inflammation produced by the liver is C-reactive protein, and our results show that acetic acid significantly increased (p < 0.01) this plasmatic marker compared to a control group. Furthermore, SSZ at 150 mg/kg significantly (p < 0.001) reduced CRP levels, as well as those of the AA group. Our analysis also showed that apocynin at different doses reduced CRP levels; this reduction was more significant at 200 mg/kg and 400 mg/kg of apocynin pretreatment than at 100 mg/kg. Our study also found no statistically significant difference between the 400 mg/kg apocynin and SSZ groups (Fig. 6c).
Assessment of myeloperoxidase activity showed that acetic acid significantly increased (p < 0.001) the MPO in rat plasma compared with the control group. Pretreatment with sulfasalazine significantly reduced (p < 0.001) MPO activity compared to the AA group. Interestingly, apocynin at different doses significantly reduced the increase of this marker as compared to the AA group. According to our results, pretreatment with apocynin at 200 mg/kg and 400 mg/kg is more effective (p < 0.001) in reducing MPO activity compared to the AA group. Our results showed no significant difference between the SSZ and apocynin groups treated with apocynin 400 mg/kg (Fig. 6d).
Enzyme-linked immunosorbent assay analysis of pro-inflammatory mediators in rat plasma clearly showed that acetic acid caused a significant increase (p < 0.001) in TNF-α (4.258 ± 0.325 ng/ml) compared with the control group (0.586 ± 0.115 ng/ml). Pretreatment with SSZ (150 mg/kg) significantly (p < 0.001) reduced this pro-inflammatory marker (1.806 ± 0.165 ng/ml) compared to the control group. Our research showed that apocynin, at doses of 100 mg/kg, 200 mg/kg and 400 mg/kg, significantly reduced the production of TNF-α; this prevention is more significant (p < 0.001) at doses of 200 mg/kg and 400 mg/kg as compared to the AA group. Furthermore, our analysis shows that there is a significant difference (p < 0.05) in TNF-α levels between the SSZ group and the group pretreated with apocynin at 400 mg/kg (Fig. 6e). The determination of IL-1β showed a significant increase (p < 0.01) of this pro-inflammatory marker in the AA group (3.515 ± 0.192 ng/ml) as compared to the control group (1.633 ± 0.127 ng/ml). Our analysis shows that SSZ at 150 mg/kg significantly (p < 0.001) reduced the level of this marker (1.26 ± 0.199 ng/ml) as compared to the AA group. In addition, our results indicate that apocynin at 200 mg/kg and 400 mg/kg prevented significantly more overproduction of IL-1β compared to the AA group. Furthermore, our results showed that there was no significant difference in IL-1β levels between the SSZ group and the apocynin group at 400 mg/kg (Fig. 6f).
DISCUSSION
Oxidative stress has become a major focus of basic and clinical research. It is defined as the imbalance between ROS production and their clearance resulting in excessive ROS production, which is involved in the progression of many inflammatory diseases such as ulcerative colitis [49]. Finding new strategies to inhibit oxidative stress could be a novel anti-inflammatory approach. In this study, we used apocynin, a molecule which is known to inhibit NADPH oxidase NOX2 and to scavenge ROS, to investigate its effect in acetic acid–induced colitis, an inflammatory model associated with high oxidative stress. Our results show that apocynin protected rats against AA-induced colitis and inhibited key oxidative and inflammatory markers.
Histopathological analysis revealed an altered colonic cytoarchitecture and neutrophil infiltration on the colonic epithelial surface in this model of colitis. Our findings are in agreement with a previous study [50] which showed that AA-induced ulcerative colitis is characterised by inflammatory cell infiltration into the intestinal tissue, necrosis and ulceration [51]. According to our results, AA-induced colitis promotes a dramatic increase in colonic pro-oxidant markers with a significant reduction of antioxidant markers. Furthermore, AA-induced colitis leads to a dramatic increase in plasma iron. Iron accumulation and excessive ROS generation may be involved in the Fenton and Haber–Weiss reactions. Indeed, the increase in labile iron may activate the NF-κB pathway and increase pro-inflammatory cytokine production [52]. Our data are supported by previous studies indicating the important role of TNF-α and cytokines in IBD [53]. Currently, the use of anti-inflammatory drugs for the treatment of IBD is associated with several side effects. For example, corticosteroids cause a number of adverse effects [54]. Long-term corticosteroid therapy may be associated with an increased risk of death and may be corticosteroid dependent [55].
Given this adverse effect, there is an urgent need to develop inexpensive and effective anti-inflammatory drugs. Recently, research has focused on reducing ROS production [56]. Due to its low toxicity, apocynin could be an interesting candidate, as it inhibits NADPH oxidase activity [57] and has an antioxidant and anti-inflammatory properties [58, 59]. Evaluation of apocynin’s antioxidant effect indicates that it can scavenge free radicals [60] with a potent iron chelating ability. Furthermore, pretreatment with apocynin reduces plasma iron levels in ulcerative colitis. In fact, apocynin has a high ferrous chelating ability explaining its potent anti-inflammatory effect as the use of iron chelators was tested in the treatment of inflammation disorders [61].
Our research showed that pretreatment with apocynin reduced the (W/L) colon index and the macroscopic damage to the colon. Our results are in agreement with the study of Marín et al. [32], who showed that apocynin has a protective role in DSS murine colitis. This protective effect is more effective at higher apocynin doses (similar to sulfasalazine). Some in vivo studies involving apocynin in the treatment of ulcerative colitis support our findings [62]. According to the histological analysis of the colon, pretreatment with apocynin at high doses preserved the cytoarchitecture of the colon and reduced inflammatory cells in the epithelial layers. Our findings are supported by those of Komiya et al. [63], who showed that apocynin in drinking water reduced the aberrant crypt in the colon. Our results also showed that pretreatment with apocynin prevented AOPPs and MDA levels initially exacerbated by colitis, justifying the antioxidant key role of this natural molecule [64].
Furthermore, our results showed that apocynin restored the SOD and catalase activities and stabilised the GSH levels in colonic tissue, which is consistent with previous research showing that apocynin prevents the oxidative stress state [65, 66]. Our results also demonstrated the key role of apocynin in inhibiting NF-κB, which is one of the major targets of oxidative stress [67]. Furthermore, Hamilton et al. [68] showed that apocynin inhibits the production of superoxide anions in human arteries and veins. Pretreatment with apocynin maintained the activity of the antioxidant enzymes SOD and catalase in colonic homogenate, which is in agreement with the study by Rosa et al. [69], and protected against the increase of ALP activity in ulcerative colitis which is consistent with previous research [70]. In addition, apocynin preserves the level of plasma iron in ulcerative colitis. Previous studies have shown that apocynin was effective in maintaining iron homeostasis [71].
CRP is the most common indicator of inflammation produced by the liver [72]. Its significant increase shows that ulcerative colitis is associated with extra-intestinal symptoms that alter liver metabolism [73, 74]. Based on our findings, pretreatment with apocynin reduces the plasmatic level of this pro-inflammatory markers and suppresses the increase in plasma cytokines. Apocynin is a natural anti-inflammatory agent that reduces cytokines and pro-inflammatory factors [67]. During the degranulation process, myeloperoxidase is released into the neutrophil phagosome, which then releases hypochlorous acid (HOCl) and impairs healthy tissue function [75]. Our results show that apocynin reduces the increase in MPO activity during ulcerative colitis. In fact, this natural molecule contributes to the reduction of HOCl produced by phagocytic cells [76]. MPO is also involved in the oxidation of apocynin to a dimer, which is more efficient than apocynin itself [27].
CONCLUSION
In conclusion, our results clearly show that pretreatment of rats with apocynin prevents acetic acid–induced ulcerative colitis. This natural molecule protects the colon from damage caused by oxidative stress and acts as a prodrug by reducing the levels of TNF-α, IL-1β and CRP. Taking apocynin may be beneficial in the prevention of ulcerative colitis.
DATA AVAILABILITY
All data generated during this study are included in this published version; for further details on data availability, please contact the corresponding author.
Abbreviations
- AA:
-
Acetic acid
- ABTS:
-
2,2′-Azino-bis-3-ethylbenzothiazoline-6-sulfonic acid
- ALP:
-
Alkaline phosphatase
- AOPPs:
-
Advanced oxidation protein products
- AP:
-
Apocynin
- BHT:
-
Butylated hydroxytoluene
- CRP:
-
C-reactive protein
- DAI:
-
Disease activity index
- DMSO:
-
Dimethylsulfoxide
- DPPH:
-
1,1-Diphenyl-2-picrylhydrazyl
- DTNB:
-
5,5′-Dithiobis-(2-nitrobenzoic acid)
- EDTA:
-
Ethylenediaminetetraacetic acid
- ELISA:
-
Enzyme-linked immunosorbent assay
- EM:
-
External mucosa
- GM-CSF:
-
Granulocyte-macrophage colony-stimulating factor
- GSH:
-
Glutathione
- H2O2 :
-
Hydrogen peroxide
- HTAB:
-
Hexadecyltrimethylammonium bromide
- IBD:
-
Inflammatory bowel disease
- IC50 :
-
Half-maximal inhibitory concentration
- IL-1β:
-
Interleukin-1β
- iNOS:
-
Inducible nitric oxide synthase
- L:
-
Length
- LM:
-
Lamina
- MDA:
-
Malondialdehyde
- MM:
-
Muscularis mucosa
- MPO:
-
Myeloperoxidase
- NADPH:
-
Nicotinamide adenine dinucleotide phosphate oxidase
- NOX:
-
NADPH oxidase
- PBS:
-
Phosphate-buffered saline
- ROS:
-
Reactive oxygen species
- SM:
-
Submucosa
- SOD:
-
Superoxide dismutase
- SSZ:
-
Sulfasalazine
- TBA:
-
Thiobarbituric acid
- TCA:
-
Trichloroacetic acid
- TNF-α:
-
Tumour necrosis factor-α
- UC:
-
Ulcerative colitis
- W:
-
Weight
REFERENCES
Hamanaka, Robert B., and Navdeep S. Chandel. 2010. Mitochondrial reactive oxygen species regulate cellular signaling and dictate biological outcomes. Trends in Biochemical Sciences 35 (9): 505–513. https://doi.org/10.1016/j.tibs.2010.04.002.
Ohashi, Masuo, Marschall S. Runge, Frank M. Faraci, and Donald D. Heistad. 2006. MnSOD deficiency increases endothelial dysfunction in ApoE-deficient mice. Arteriosclerosis, Thrombosis, and Vascular Biology 26 (10): 2331–2336. https://doi.org/10.1161/01.ATV.0000238347.77590.c9.
Franco, Rodrigo, Onard Schoneveld, Alexandros G. Georgakilas, and Mihalis I. Panayiotidis. 2008. Oxidative stress, DNA methylation and carcinogenesis. Cancer Letters 266 (1): 6–11. https://doi.org/10.1016/j.canlet.2008.02.026.
Kouki, Ahmed, Wafa Ferjani, Néziha. Ghanem-Boughanmi, Mossadok Ben-Attia, Pham My-Chan. Dang, Abdelaziz Souli, and Jamel El-Benna. 2023. The NADPH oxidase inhibitors apocynin and diphenyleneiodonium protect rats from LPS-induced pulmonary inflammation. Antioxidants (Basel, Switzerland) 12 (3): 770. https://doi.org/10.3390/antiox12030770.
Zhu, Hong, and Y. Robert Li. 2012. Oxidative stress and redox signaling mechanisms of inflammatory bowel disease: Updated experimental and clinical evidence. Experimental biology and Medicine (Maywood, N.J.) 237(5): 474–480. https://doi.org/10.1258/ebm.2011.011358.
Okur, Hamit, Mustafa Küçükaydin, Kader Köse, Olgun Kontaş, Pakize Doǧan, and Ahmet Kazez. 1995. Hypoxia-induced necrotizing enterocolitis in the immature rat: The role of lipid peroxidation and management by vitamin E. Journal of Pediatric Surgery 30 (10): 1416–1419. https://doi.org/10.1016/0022-3468(95)90395-x.
Bitton, Alain, Mark A. Peppercorn, Donald A. Antonioli, John L. Niles, Samir Shah, Athos Bousvaros, Bernard Ransil, Bernard Wild, Gary Cohen, Albert Deb Edwardes, Michael D. Stevens, and C. Anthony. 2001. Clinical, biological, and histologic parameters as predictors of relapse in ulcerative colitis. Gastroenterology 120 (1): 13–20. https://doi.org/10.1053/gast.2001.20912.
Oshitani, Nobuhide, Yoshinori Sawa, Junichi Hara, Kenji Adachi, Shiro Nakamura, Takayuki Matsumoto, Tetsuo Arakawa, and Tetsuo Kuroki. 1997. Functional and phenotypical activation of leucocytes in inflamed human colonic mucosa. Journal of Gastroenterology and Hepatology 12 (12): 809–814. https://doi.org/10.1111/j.1440-1746.1997.tb00376.x.
Guan, Qingdong, and Jiguo Zhang. 2017. Recent advances: The imbalance of cytokines in the pathogenesis of inflammatory bowel disease. Mediators of Inflammation 2017: 4810258. https://doi.org/10.1155/2017/4810258.
Fridovich, Irwin. 2004. Mitochondria: Are they the seat of senescence? Aging Cell 3 (1): 13–16. https://doi.org/10.1046/j.1474-9728.2003.00075.x.
Spiekermann, Stephan, Ulf Landmesser, Sergey Dikalov, Martin Bredt, Graciela Gamez, Helma Tatge, Nina Reepschläger, Burkhard Hornig, Helmut Drexler, and David G. Harrison. 2003. Electron spin resonance characterization of vascular xanthine and NAD(P)H oxidase activity in patients with coronary artery disease: Relation to endothelium-dependent vasodilation. Circulation 107 (10): 1383–1389. https://doi.org/10.1161/01.cir.0000056762.69302.46.
Bedard, Karen, and Karl-Heinz. Krause. 2007. The NOX family of ROS-generating NADPH oxidases: Physiology and pathophysiology. Physiological Reviews 87 (1): 245–313. https://doi.org/10.1152/physrev.00044.2005.
Lambeth, J. David. 2007. Nox enzymes, ROS, and chronic disease: An example of antagonistic pleiotropy. Free radical Biology & Medicine 43 (3): 332–347. https://doi.org/10.1016/j.freeradbiomed.2007.03.027.
Bhattacharyya, Asima, Ranajoy Chattopadhyay, Sankar Mitra, and Sheila E. Crowe. 2014. Oxidative stress: An essential factor in the pathogenesis of gastrointestinal mucosal diseases. Physiological Reviews 94 (2): 329–354. https://doi.org/10.1152/physrev.00040.2012.
Baumgart, Daniel C., and William J. Sandborn. 2007. Inflammatory bowel disease: Clinical aspects and established and evolving therapies. Lancet (London, England) 369 (9573): 1641–1657. https://doi.org/10.1016/S0140-6736(07)60751-X.
Hamouda, Hala E., Soha S. Zakaria, Saber A. Ismail, Mahmoud A. Khedr, and Wael W. Mayah. 2011. p53 antibodies, metallothioneins, and oxidative stress markers in chronic ulcerative colitis with dysplasia. World Journal of Gastroenterology 17 (19): 2417–2423. https://doi.org/10.3748/wjg.v17.i19.2417.
Wu, Chia-Chao, Jin-Shuen Chen, Wen-Mein Wu, Tung-Nan Liao, Pauling Chu, Shih-Hua Lin, Chien-Huei Chuang, and Yuh-Feng Lin. 2005. Myeloperoxidase serves as a marker of oxidative stress during single haemodialysis session using two different biocompatible dialysis membranes. Nephrology, Dialysis, Transplantation 20 (6): 1134–1139. https://doi.org/10.1093/ndt/gfh764.
Beltran, Belen, Pilar Nos, Francisco Dasí, Marisa Iborra, Guillermo Bastida, Marcial Martínez, José-Enrique. O’Connor, Guillermo Sáez, Inés. Moret, and Julio Ponce. 2010. Mitochondrial dysfunction, persistent oxidative damage, and catalase inhibition in immune cells of naïve and treated Crohn’s disease. Inflammatory Bowel Diseases 16 (1): 76–86. https://doi.org/10.1002/ibd.21027.
Neurath, Markus F., Ivan Fuss, Guido Schürmann, Sven Pettersson, H.E.L.M.U.T. Karl Arnold, Warren Strober Müller-Lobeck, Christian Herfarth, and Karl-Hermann Meyer Zum. Büschenfelde. 1998. Cytokine gene transcription by NF-kappa B family members in patients with inflammatory bowel disease. Annals of the New York Academy of Sciences 859: 149–159. https://doi.org/10.1111/j.1749-6632.1998.tb11119.x.
Altenhöfer, Sebastian, Kim A. Radermacher, Pamela WM. Kleikers, Kirstin Wingler, and Harald HHW. Schmidt. 2015. Evolution of NADPH oxidase inhibitors: Selectivity and mechanisms for target engagement. Antioxidants & Redox Signaling 23 (5): 406–427. https://doi.org/10.1089/ars.2013.5814.
Probert, Chris. 2013. Steroids and 5-aminosalicylic acids in moderate ulcerative colitis: Addressing the dilemma. Therapeutic Advances in Gastroenterology 6 (1): 33–38. https://doi.org/10.1177/1756283X12461395.
Roselli, Marianna, and Alberto Finamore. 2020. Use of synbiotics for ulcerative colitis treatment. Current Clinical Pharmacology 15 (3): 174–182. https://doi.org/10.2174/1574884715666191226120322.
Feagan, Brian G., and John K. MacDonald. 2012. Oral 5-aminosalicylic acid for induction of remission in ulcerative colitis. The Cochrane Database of Systematic Reviews 10: CD000543. https://doi.org/10.1002/14651858.CD000543.pub3.
Ford, A.C., C.N. Bernstein, K.J. Khan, M.T. Abreu, J.K. Marshall, N.J. Talley, and P. Moayyedi. 2011. Glucocorticosteroid therapy in inflammatory bowel disease: Systematic review and meta-analysis. The American Journal of Gastroenterology 106 (4): 590–600. https://doi.org/10.1038/ajg.2011.70.
Singh, Amandeep, Kirandeep Kaur, Veerpal Kaur, Gurmeet Singh, Uttam Kumar Mandal, Neeraj Mishra, and Raj Kumar Narnag. 2019. Importance of nanocarriers and probiotics in the treatment of ulcerative colitis. Journal of Drug Delivery and Therapeutics 9: 216–228.
Newman, David J., and Gordon M. Cragg. 2020. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. Journal of Natural Products 83 (3): 770–803. https://doi.org/10.1021/acs.jnatprod.9b01285.
Johnson, David K., Kurt J. Schillinger, David M. Kwait, Chambers V. Hughes, Erin J. McNamara, Fauod Ishmael, Robert W. O'Donnell, Ming-Mei Chang, Michael G. Hogg, Jonathan S. Dordick, Lakshmi Santhanam, Linda M. Ziegler and James A. Holland. 2002. Inhibition of NADPH oxidase activation in endothelial cells by ortho-methoxy-substituted catechols. Endothelium: Journal of Endothelial Cell Research 9 (3): 191–203. https://doi.org/10.1080/10623320213638.
Vejražka, Martin, Radan Míček, and Stanislav Štípek. 2005. Apocynin inhibits NADPH oxidase in phagocytes but stimulates ROS production in non-phagocytic cells. Biochimica et biophysica acta 1722 (2): 143–147. https://doi.org/10.1016/j.bbagen.2004.12.008.
Hur, Jinyoung, Pyeongjae Lee, Mi Jung Kim, Younghoon Kim, and Young-Wuk Cho. 2010. Ischemia-activated microglia induces neuronal injury via activation of gp91phox NADPH oxidase. Biochemical and Biophysical Research Communications 391 (3): 1526–1530. https://doi.org/10.1016/j.bbrc.2009.12.114.
Muijsers, R.B.R., E. van Den Worm, G. Folkerts, C.J. Beukelman, A.S. Koster, D.S. Postma, and F.P. Nijkamp. 2000. Apocynin inhibits peroxynitrite formation by murine macrophages. British Journal of Pharmacology 130 (4): 932–936. https://doi.org/10.1038/sj.bjp.0703401.
Hougee, Sander, Anita Hartog, Annemarie Sanders, Yvo MF. Graus, Maarten A. Hoijer, Johan Garssen, Wim B. van den Berg, Henk M. van Beuningen, and H. Friso Smit. 2006. Oral administration of the NADPH-oxidase inhibitor apocynin partially restores diminished cartilage proteoglycan synthesis and reduces inflammation in mice. European Journal of Pharmacology 531 (1–3): 264–269. https://doi.org/10.1016/j.ejphar.2005.11.061.
Marín, Marta, Rosa María Giner, José-Luis. Ríos, and María del Carmen Recio. 2013. Protective effect of apocynin in a mouse model of chemically-induced colitis. Planta Medica 79 (15): 1392–1400. https://doi.org/10.1055/s-0033-1350710.
Stefanska, J., and R. Pawliczak. 2008. Apocynin: Molecular aptitudes. Mediators of Inflammation 2008: 106507. https://doi.org/10.1155/2008/106507.
Simons, Jos M., Bert A't Hart, Theo RAM Ip Vai Ching, Hans Van Dijk, and Rudi P. Labadie. 1990. Metabolic activation of natural phenols into selective oxidative burst agonists by activated human neutrophils. Free Radical Biology & Medicine 8 (3): 251–258. https://doi.org/10.1016/0891-5849(90)90070-y.
Müller, Andreas A., Susanne A. Reiter, Karin G. Heider, and Hildebert Wagner. 1999. Plant-derived acetophenones with antiasthmatic and anti-inflammatory properties: Inhibitory effects on chemotaxis, right angle light scatter and actin polymerization of polymorphonuclear granulocytes. Planta Medica 65 (7): 590–594. https://doi.org/10.1055/s-1999-14029.
Pintard, Coralie, Marwa Ben Khemis, Dan Liu, Pham My-Chan. Dang, Margarita Hurtado-Nedelec, and Jamel El-Benna. 2020. Apocynin prevents GM-CSF-induced-ERK1/2 activation and -neutrophil survival independently of its inhibitory effect on the phagocyte NADPH oxidase NOX2. Biochemical Pharmacology 177: 113950. https://doi.org/10.1016/j.bcp.2020.113950.
Re, Roberta, Nicoletta Pellegrini, Anna Proteggente, Ananth Pannala, Min Yang, and Catherine Rice-Evans. 1999. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Biology & Medicine 26 (9–10): 1231–1237. https://doi.org/10.1016/s0891-5849(98)00315-3.
Grzegorczyk, I., A. Matkowski, and H.J.F.C. Wysokińska. 2007. Antioxidant activity of extracts from in vitro cultures of Salvia officinalis L. Food Chemistry 104: 536–541. https://doi.org/10.1016/j.foodchem.2006.12.003.
Chew, Yik-Ling, Joo-Kheng Goh, and Yau-Yan Lim. 2009. Assessment of in-vitro antioxidant capacity and polyphenolic composition of selected medicinal herbs from Leguminosae family in Peninsular Malaysia. Food Chemistry 116: 13–18. https://doi.org/10.1016/j.foodchem.2009.01.091.
Ghasemi-Pirbaluti, M., E. Motaghi, A. Najafi, and M.J. Hosseini. 2017. The effect of theophylline on acetic acid induced ulcerative colitis in rats. Biomedicine & Pharmacotherapy 90: 153–159. https://doi.org/10.1016/j.biopha.2017.03.038.
Han, F.H., H. Zhang, X. Xia, H. Xiong, D. Song, X. Zong, and Y. Wang. 2015. Porcine β-defensin 2 attenuates inflammation and mucosal lesions in dextran sodium sulfate-induced colitis. Journal of Immunology (Baltimore, Md. 1950) 194 (4): 1882–1893. https://doi.org/10.4049/jimmunol.1402300.
Classics Lowry, O., N. Rosebrough, A. Farr, and R. Randall. 1951. Protein measurement with the Folin phenol reagent. The Journal of Biological Chemistry 193 (1): 265–275. https://doi.org/10.1016/S0021-9258(19)52451-6.
Witko-Sarsat, Véronique., Miriam Friedlander, Chantal Capeillère-Blandin, Thao Nguyen-Khoa, Anh Thu Nguyen, Johanna Zingraff, Paul Jungers, and Béatrice. Descamps-Latscha. 1996. Advanced oxidation protein products as a novel marker of oxidative stress in uremia. Kidney International 49 (5): 1304–1313. https://doi.org/10.1038/ki.1996.186.
Yagi, Kunio. 1976. A simple fluorometric assay for lipoperoxide in blood plasma. Biochemical Medicine 15 (2): 212–216. https://doi.org/10.1016/0006-2944(76)90049-1.
Aebi, Hugo. 1984. [13] Catalase in vitro. Meth Enzymol 105: 121–126. https://doi.org/10.1016/s0076-6879(84)05016-3.
Misra, Hara P., and Irwin Fridovich. 1972. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. The Journal of Biological Chemistry 247 (10): 3170–3175. https://doi.org/10.1016/s0021-9258(19)45228-9.
Bradley, Peter P., Dennis A. Priebat, Robert D. Christensen, and Gerald Rothstein. 1982. Measurement of cutaneous inflammation: Estimation of neutrophil content with an enzyme marker. The Journal of Investigative Dermatology 78 (3): 206–209. https://doi.org/10.1111/1523-1747.ep12506462.
Ellman, George L. 1959. Tissue sulfhydryl groups. Archives of Biochemistry and Biophysics 82 (1): 70–77. https://doi.org/10.1016/0003-9861(59)90090-6.
Torres, Joana, Vincent Billioud, David B. Sachar, Laurent Peyrin-Biroulet, and Jean-Frédéric. Colombel. 2012. Ulcerative colitis as a progressive disease: The forgotten evidence. Inflammatory Bowel Diseases 18 (7): 1356–1363. https://doi.org/10.1002/ibd.22839.
Ali, Azza Abdelfattah, Ekram Nemr Abd Al. Haleem, Sahar Abdel-Hafeez. Khaleel, and Amany Said Sallam. 2017. Protective effect of cardamonin against acetic acid-induced ulcerative colitis in rats. Pharmacological Reports PR 69 (2): 268–275. https://doi.org/10.1016/j.pharep.2016.11.002.
Randhawa, Puneet Kaur, Kavinder Singh, Nirmal Singh, and Amteshwar Singh Jaggi. 2014. A review on chemical-induced inflammatory bowel disease models in rodents. The Korean Journal of Physiology & Pharmacology 18 (4): 279–288. https://doi.org/10.4196/kjpp.2014.18.4.279.
Xiong, Shigang, Hongyun She, Chin K. Sung, and Hidekazu Tsukamoto. 2003. Iron-dependent activation of NF-kappaB in Kupffer cells: A priming mechanism for alcoholic liver disease. Alcohol (Fayetteville, N.Y.) 30 (2): 107–113. https://doi.org/10.1016/s07418329(03)00100-9.
Nemeth, Zoltan H., Dorian A. Bogdanovski, Patricia Barratt-Stopper, Samantha R. Paglinco, Luca Antonioli, and Rolando H. Rolandelli. 2017. Crohn’s disease and ulcerative colitis show unique cytokine profiles. Cureus 9 (4): e1177. https://doi.org/10.7759/cureus.1177.
Volk, Neil, and Corey A. Siegel. 2019. Defining failure of medical therapy for inflammatory bowel disease. Inflammatory Bowel Diseases 25 (1): 74–77. https://doi.org/10.1093/ibd/izy238.
Kornbluth, Asher, David B. Sachar, and Practice Parameters Committee of the American College of Gastroenterology. 2010. Ulcerative colitis practice guidelines in adults: American College of Gastroenterology, Practice Parameters Committee. The American Journal of Gastroenterology 105 (3): 501–524. https://doi.org/10.1038/ajg.2009.727.
Mileo, Anna Maria, and Stefania Miccadei. 2016. Polyphenols as modulator of oxidative stress in cancer disease: New therapeutic strategies. Oxidative Medicine and Cellular Longevity 2016: 6475624. https://doi.org/10.1155/2016/6475624.
Radecki, A. 1981. Analysis of an industrial smoke preparation. Part VI. Acute toxicity, and bactericidal and antioxidative activities of some phenolic fraction components of industrial smoke preparation. Bromatologia i Chemia Toksykologiczna 14 (3): 301–306.
Nam, Yoon Jeong, Arum Kim, Dong Suep Sohn, and Chung Soo Lee. 2016. Apocynin inhibits Toll-like receptor-4-mediated activation of NF-κB by suppressing the Akt and mTOR pathways. Naunyn-Schmiedeberg’s Archives of Pharmacology 389 (12): 1267–1277. https://doi.org/10.1007/s00210-016-1288-5.
Mouzaoui, Souad, Bahia Djerdjouri, Nesrine Makhezer, Yolande Kroviarski, Jamel El-Benna, and Pham My-Chan. Dang. 2014. Tumor necrosis factor-α-induced colitis increases NADPH oxidase 1 expression, oxidative stress, and neutrophil recruitment in the colon: Preventive effect of apocynin. Mediators of Inflammation 2014: 312484. https://doi.org/10.1155/2014/312484.
Heumüller, Sabine, Sven Wind, Eduardo Barbosa-Sicard, Harald HHW Schmidt, Rudi Busse, Katrin Schröder, and Ralf P. Brandes. 2008. Apocynin is not an inhibitor of vascular NADPH oxidases but an antioxidant. Hypertension (Dallas, Tex. 1979) 51 (2): 211–217. https://doi.org/10.1161/HYPERTENSIONAHA.107.100214.
Lehmann, C., S. Islam, S. Jarosch, J. Zhou, D. Hoskin, A. Greenshields, N. Al-Banna, N. Sharawy, A. Sczcesniak, M. Kelly, K. Wafa, W. Cheliak, and B. Holbein. 2015. The utility of iron chelators in the management of inflammatory disorders. Mediators of Inflammation 2015: 516740. https://doi.org/10.1155/2015/516740.
Hwang, Young-Jae., Seung-Joo. Nam, Wanjoo Chun, Song In Kim, Sung Chul Park, Chang Don Kang, and Sung Joon Lee. 2019. Anti-inflammatory effects of apocynin on dextran sulfate sodium-induced mouse colitis model. PLoS ONE 14 (5): e0217642. https://doi.org/10.1371/journal.pone.0217642.
Komiya, Masami, Gen Fujii, Shingo Miyamoto, Mami Takahashi, Rikako Ishigamori, Wakana Onuma, Kousuke Ishino, Yukari Totsuka, Kyoko Fujimoto, and Michihiro Mutoh. 2015. Suppressive effects of the NADPH oxidase inhibitor apocynin on intestinal tumorigenesis in obese KK-A(y) and Apc mutant Min mice. Cancer Science 106 (11): 1499–1505. https://doi.org/10.1111/cas.12801.
Paracatu, Luana C., Maria L. Zeraik, Luiza de Carvalho Bertozo, Aloisio de Andrade Bartolomeu, Luiz C. da Silva Filho, Luiz M. da Fonseca, and Valdecir F. Ximenes. 2016. Synthesis, antioxidant and anti-inflammatory properties of an apocynin- derived dihydrocoumarin. Medicinal Chemistry (Shariqah (United Arab Emirates)) 13 (1): 93–100. https://doi.org/10.2174/1573406412666160610093216.
Impellizzeri, Daniela, Emanuela Mazzon, Emanuela Esposito, Irene Paterniti, Placido Bramanti, and Salvatore Cuzzocrea. 2011. Effect of apocynin, an inhibitor of NADPH oxidase, in the inflammatory process induced by an experimental model of spinal cord injury. Free Radical Research 45 (2): 221–236. https://doi.org/10.3109/10715762.2010.526604.
Sun, Yijun, Futai Gong, Jichao Yin, Xiaoyan Wang, Xiangyang Wang, Qing Sun, Zhiqiang Zhu, Su Xiaoqiang, Jie Zheng, Li Liu, Yang Li, Hu Xinglv, and Jia Li. 2017. Therapeutic effect of apocynin through antioxidant activity and suppression of apoptosis and inflammation after spinal cord injury. Experimental and Therapeutic Medicine 13 (3): 952–960. https://doi.org/10.3892/etm.2017.4090.
Barbieri, Silvia S., Viviana Cavalca, Sonia Eligini, Marta Brambilla, Alessia Caiani, Elena Tremoli, and Susanna Colli. 2004. Apocynin prevents cyclooxygenase 2 expression in human monocytes through NADPH oxidase and glutathione redox-dependent mechanisms. Free Radical Biology & Medicine 37 (2): 156–165. https://doi.org/10.1016/j.freeradbiomed.2004.04.020.
Hamilton, Carlene A., Julia M. Brosnan, Sammy Al-Benna, Geoffrey Berg, and Anna F. Dominiczak. 2002. NAD(P)H oxidase inhibition improves endothelial function in rat and human blood vessels. Hypertension (Dallas, Tex. 1979) 40 (5): 755–762. https://doi.org/10.1161/01.hyp.0000037063.90643.0b.
Rosa, C.M., R. Gimenes, D.H.S. Campos, G.N. Guirado, C. Gimenes, A.A.H. Fernandes, A.C. Cicogna, R.M. Queiroz, I. Falcão-Pires, D. Miranda-Silva, P. Rodrigues, F.R. Laurindo, D.C. Fernandes, C.R. Correa, M.P. Okoshi, and K. Okoshi. 2016. Apocynin influence on oxidative stress and cardiac remodeling of spontaneously hypertensive rats with diabetes mellitus. Cardiovascular Diabetology 15 (1): 126. https://doi.org/10.1186/s12933-016-0442-1.
Mizanur, Rahman, Md., Awale Yousuf Muse, D.M. Isha Olive Khan, Ismaile Hussein Ahmed, Nusrat Subhan, Hasan Mahmud Reza, Md Ashraful Alam, Lutfun Nahar, and Satyajit Dey Sarker. 2017. Apocynin prevented inflammation and oxidative stress in carbon tetra chloride induced hepatic dysfunction in rats. Biomedicine & Pharmacotherapy 92: 421–428. https://doi.org/10.1016/j.biopha.2017.05.101.
Kapoor, Monika, Sheetal Sharma, Rajat Sandhir, and Bimla Nehru. 2021. Maintenance of iron homeostasis by apocynin during states of global ischemia in rat brain and retina. Advances in Redox Research 3: 100012. https://doi.org/10.1016/j.arres.2021.100012.
Schrödl, Wieland, Rita Büchler, Sindy Wendler, Petra Reinhold, Petra Muckova, Johanna Reindl, and Heidrun Rhode. 2016. Acute phase proteins as promising biomarkers: Perspectives and limitations for human and veterinary medicine. Proteomics Clinical Applications 10 (11): 1077–1092. https://doi.org/10.1002/prca.201600028.
Vavricka, Stephan R., Alain Schoepfer, Michael Scharl, Peter L. Lakatos, Alexander Navarini, and Gerhard Rogler. 2015. Extraintestinal manifestations of inflammatory bowel disease. Inflammatory Bowel Diseases 21 (8): 1982–1992. https://doi.org/10.1097/MIB.0000000000000392.
Serada, Satoshi, Minoru Fujimoto, Fumitaka Terabe, Hideki Iijima, Shinichiro Shinzaki, Shinya Matsuzaki, Tomoharu Ohkawara Tomoharu, Riichiro Nakajima Nezu, Sachiko Kobayashi, Taku Plevy, Scott Eric Takehara, and Tetsuo and Naka Tetsuji. 2012. Serum leucine-rich alpha-2 glycoprotein is a disease activity biomarker in ulcerative colitis. Inflammatory Bowel Diseases 18 (11): 2169–2179. https://doi.org/10.1002/ibd.22936.
Wagner, Michael, Christer GB. Peterson, Peter Ridefelt, Per Sangfelt, and Marie Carlson. 2008. Fecal markers of inflammation used as surrogate markers for treatment outcome in relapsing inflammatory bowel disease. World Journal of Gastroenterology 14 (36): 5584–5588. https://doi.org/10.3748/wjg.14.5584.
de Almeida, Ana, Otávio Cabral Carolina, Christina Arslanian Marques, Antonio Condino-Neto, and Valdecir F. Ximenes. 2011. 4-Fluoro-2-methoxyphenol, an apocynin analog with enhanced inhibitory effect on leukocyte oxidant production and phagocytosis. European Journal of Pharmacology 660 (2–3): 445–453. https://doi.org/10.1016/j.ejphar.2011.03.043.
ACKNOWLEDGEMENTS
The authors would like to thank the INSERM, the CNRS, the University of Paris-cité, the LabEx Inflamex and the Tunisian DGRST for providing laboratory facilities during this study.
Funding
The research was funded by the INSERM, the CNRS, the City University of Paris and the DGRST of the Tunisian Ministry of Higher Education, Scientific Research and Technology.
Author information
Authors and Affiliations
Contributions
A. K. contributed to the methodology, statistical analysis, animal experiments and drafting of the manuscript. W. F., P. M.-C. D., N. G.-B. and A. S. contributed to the data curation, formal analysis, critical revision, visualisation and validation. All authors read and approved the manuscript. J. E.-B. and M. B.-A. contributed to the study design, methodology, drafting, critical revision, editing and validation.
Corresponding author
Ethics declarations
Ethical Approval and Consent to Participate
The experimental protocols (No. 0123/2022 ATSAL) were approved by the Ethics Committee of the Tunisian Association of Laboratory Animal Sciences and were carried out in accordance with the European Community Council Directive of 24 November 1986. Consent to participate is not applicable.
Competing Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kouki, A., Ferjani, W., Dang, P.MC. et al. Preventive Anti-inflammatory Effects of Apocynin on Acetic Acid–Induced Colitis in Rats. Inflammation 47, 438–453 (2024). https://doi.org/10.1007/s10753-023-01920-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-023-01920-4