Abstract
The coronavirus disease-2019 (COVID-19) pandemic has become a major global health problem. COVID-19 is caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) and exhibits pulmonary and extrapulmonary effects, including cardiovascular involvement. There are several attempts to identify drugs that could treat COVID-19. Moreover, many patients infected with COVID-19 have underlying diseases, particularly cardiovascular diseases. These patients are more likely to develop severe illnesses and would require optimized treatment strategies. The current study gathered information from various databases, including relevant studies, reviews, trials, or meta-analyses until April 2022 to identify the impact of SARS-CoV-2 treatment on the cardiovascular system. Studies have shown that the prognosis of patients with underlying cardiovascular disease is worsened by COVID-19, with some COVID-19 medications interfering with the cardiovascular system. The COVID-19 treatment strategy should consider many factors and parameters to avoid medication-induced cardiac injury, mainly in elderly patients. Therefore, this article provides a synthesis of evidence on the impact of different COVID-19 medications on the cardiovascular system and related disease conditions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The World Health Organization (WHO) reported on March 12, 2020, that a new pneumonia-like disease caused by a coronavirus (COVID-19) has become a global pandemic due to its fast transmission, resulting in a substantial political and economic impact globally (World Health Organization 2020). The disease is caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which belongs to a new type of beta-coronavirus characterized by its strong transmission and high pathogenicity (Lancet 2020). Most patients infected with SARS-CoV-2 virus who developed COVID-19 experience respiratory symptoms, manifested as fever, dry cough, and fatigue, accompanied by nasal congestion, runny nose, sore throat, muscle aches, and diarrhea. While some critically infected patients often develop acute respiratory distress and multiorgan failure (Wang et al. 2020a; Huang et al. 2020). Previous studies have shown that the prognosis of patients with underlying cardiovascular disease is worsened by COVID-19, with some patients having myocardial damage related to viral infection (Zhu et al. 2020). In addition, some studies have reported that patients infected with COVID-19 may have various cardiac manifestations, such as arrhythmia and cardiac arrest (Huang et al. 2020; Chen et al. 2020; Wang et al. 2020a; Marfella et al. 2021). Several medications, including antiviral drugs, are essential in managing COVID-19. Therefore, this article provides a synthesis of evidence on the impact of COVID-19 medications on the cardiovascular system and related disease conditions.
COVID-19 and the cardiovascular system
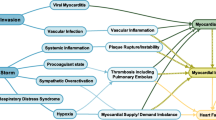
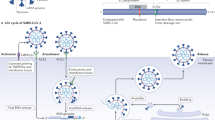
The coronavirus that causes COVID-19 is a positive, single-stranded RNA virus. Whole-genome sequence analysis of SARS-CoV-2 shows 79.6% homology to severe acute respiratory syndrome coronavirus (SARS-CoV) and the ST-elevations encoded by the two. The protein is similar to the angiotensin-converting enzyme 2 (ACE2) receptor domain (Zhou et al. 2020). Studies have shown that after entering the human body, COVID-19 enters cells primarily through ACE2 (Wang et al. 2020b), which in turn increases the level of angiotensin (AngII), leading to multiple organ dysfunctions, including cardiovascular diseases (Liu et al. 2020). Recent data shows that COVID-19 can be complicated by acute myocardial injury, mainly manifested by increased levels of highly sensitive myocardial injury markers of troponin I (cTnI) and creatine kinase isoenzyme (CK-MB) (Xu et al. 2020). In addition, the mean systolic blood pressure of critical patients with COVID-19 is higher than that of non-critical patients, and may be related to decreased expression of ACE2 and increased expression of AngII (Chen et al. 2020). Moreover, studies have shown that 16.7% of patients with COVID-19 have arrhythmia, mainly manifesting as fever-induced tachycardia (Chen et al. 2020). Although patients with COVID-19 suffer mainly from respiratory dysfunction, studies have shown a high proportion of up to 40% of the patients with cardiovascular disease come down with complications (Wang et al. 2020a), Fig. 1. Therefore, the pharmacological treatment of patients with COVID-19 and underlying cardiovascular disease deserves attention.
Therapeutic agents for COVID-19 and cardiovascular system
The accelerated spread of SARS-CoV-2 worldwide and the limited knowledge of its pathogenesis have led to the use of drugs, often empirically, without evidence of their actual efficacy for COVID-19. In addition to invasive or non-invasive ventilatory support, therapeutic choices have been directed toward antivirals and immunomodulators, either alone or in combination. These drugs were already known to treat other rheumatological, infectious and autoimmune diseases. Their use can affect the cardiovascular system, especially in patients with cardiovascular disease and coronary artery disease, either directly or through interaction with other drugs. The aim of this study is to explain the impact of antivirals and immunomodulators on the cardiovascular system, especially in a clinical setting, where cardiac damage may be an integral part of COVID-19.
Antiviral agents
Lopinavir/ritonavir (LR), a combination drug used to treat HIV, inhibits 3-chymotrypsin-like protease and papain-like protease in SARS-CoV-2, thereby blocking viral replication (Mody et al. 2021; Osipiuk et al. 2021). However, in a trial by Cao et al. (2020), the use of LR in adults hospitalized for SARS-CoV-2 infection did not result in any clinical benefit beyond standard care. LR, which belongs to the protease inhibitor group, increases the risk of coronary artery disease. The underlying mechanism is not yet clear, but it is partly due to the increase in total cholesterol and LDL-cholesterol, which underlies atherosclerotic disease (Friis-Møller et al. 2007; Fontas et al. 2004). In addition, Mondy et al. (2011) have shown that ritonavir can cause left ventricular dysfunction, dilation of the left atrium, and pulmonary hypertension through endothelial and pulmonary vascular muscle damage. Finally, LR combination therapy can cause a reversible prolongation of the PR interval, which is a limiting factor for its use (Ou et al. 2021).
Atazanavir is also a protease inhibitor that blocks SARS-CoV-2 replication in vitro, for which trials are ongoing to demonstrate efficacy in humans (Fintelman-Rodrigues et al. 2020). Atazanavir can cause PR and QT prolongation and a few cases of torsades de pointes, especially in the presence of congestive heart failure and electrolyte abnormalities (Soliman et al. 2011; Ly and Ruiz 2007). A study of patients with HIV showed that atazanavir reduced the risk of myocardial infarction and stroke compared with darunavir, which is partly explained by changes in serum bilirubin (Li et al. 2020).
Ribavirin is a guanosine analog antiviral approved for HCV and RSV. Currently available data have not shown improved outcomes in patients with COVID-19 (Tong et al. 2020). It has no direct effect on the cardiovascular system, but it can cause anemia and, consequently, myocardial ischemia (type 2 myocardial infarction), especially in patients with known coronary atherosclerosis (Macedo and Ribeiro 1999). An important pharmacokinetic feature of ribavirin is its ability to inhibit the activity of warfarin, which, therefore, requires a dose adjustment of the anticoagulant (Schulman 2002).
Ivermectin is not an antiviral but is an antiparasitic drug approved to treat scabies, strongyloidiasis, and trichuriasis. Although it has been demonstrated to block SARS-CoV-2 replication in-vitro, evidence in humans is lacking (Caly et al. 2020). It has no significant impact on the cardiovascular system, except for cases of hypotension and tachycardia without hemodynamic instability (Sparsa et al. 2006).
Remdesivir is an adenosine analog that inhibits viral RNA polymerase. It can shorten hospitalization times in patients admitted for COVID-19 (Beigel et al. 2020), but further studies are needed to confirm efficacy against SARS-CoV-2 infection. On its effects on the cardiovascular system, a study by Grein et al. (2020) revealed that hypotension was associated with remdesivir administration in patients with COVID-19 (4%) receiving invasive ventilation.
Immunomodulatory drugs
Hydroxychloroquine (HCQ) is widely used to treat malaria and some rheumatological diseases. It can block L-cathepsin activation in SARS-CoV-2, thereby interfering with endosomal acidification and preventing viral entry into the cell. However, despite initial encouraging data, no study has demonstrated the efficacy of HCQ against SARS-CoV-2 infection (Pastick et al. 2020). The cardiotoxicity of HCQ has been known for a long time. In acute cases, this may be due to its “quinidine-like” effects leading to a widened QRS interval and QT prolongation and increasing the risk of torsades de pointes. Other possible electrical complications include atrioventricular block, sick sinus syndrome, and right or left bundle–branch block.
Chronic toxicity results mainly in left ventricular hypertrophy, ventricular hypokinesia, and atrial dilatation. Very rare complications include pulmonary hypertension, valvular regurgitation, and restrictive cardiomyopathy. These effects are attributable to the intracellular accumulation of toxic metabolites, necrosis of cardiomyocytes and mitochondrial damage. Examination by electron microscopy has shown curvilinear bodies and lamellar structures due to the accumulation of glycolipids and glycoproteins (Nadeem et al. 2021; Chen et al. 2006; Chatre et al. 2018).
Tocilizumab (TCZ) is an anti-interleukin-6 monoclonal antibody approved for the treatment of rheumatoid arthritis (Alten 2011). In patients with COVID-19, TCZ can counteract the inflammatory cascade. Nevertheless, in the COVACTA trial (Rosas et al. 2021), TCZ did not improve outcomes in SARS-CoV-2 infection compared with the placebo. Cacciapaglia’s analysis of patients with rheumatoid arthritis demonstrated that treatment with TCZ is associated with an increase in cholesterol and all its fractions. The trend is “bell-shaped,” indicating an increase in the first 6 months of treatment and then a return to baseline by month 12. Despite these changes, TCZ therapy does not increase cardiovascular risk (Cacciapaglia et al. 2018).
Finally, type-I interferons (IFN) are polypeptides involved in inflammation and immune response. Preliminary studies have demonstrated the efficacy of IFN in blocking SARS-CoV-2 replication, either alone or in combination with antiviral drugs (Hensley et al. 2004; Lokugamage et al. 2020). A trial is currently ongoing to determine the efficacy and safety of IFN-α2β in COVID-19 pneumonia (Zhao 2020). Teragawa et al. (1996) concluded that bradyarrhythmias, tachyarrhythmias, and ischemic heart disease frequently occur in patients with hepatitis C treated with IFN. The underlying mechanisms are still unclear, but IFN appears to cause coronary spasm, endothelial damage, increased oxygen demand, and immune-mediated myocardial damage.
Fingolimod is an immune modulator that blocks sphingosine-1-phosphate receptors, thereby interfering with the activation of B and T lymphocytes. It is currently used to treat multiple sclerosis (Chun and Brinkmann 2011), and studies are ongoing to assess its efficacy in patients with COVID-19 (NCT04280588).
Regarding cardiovascular risks, fingolimod may cause bradycardia, which is generally transient, well-tolerated, and rarely fatal (Singer et al. 2011). It confers an atheroprotective effect by reducing atherosclerotic plaque volume (Gräler and Goetzl 2004). Spasm of the retinal artery and retinal vein has been reported, probably due to the increased release of intracellular calcium (Enosawa et al. 1996; English et al. 2000). A similar effect on the systemic circulation causing arterial hypertension has also been observed (Behjati et al. 2014).
Azithromycin is a macrolide widely used in SARS-CoV-2 infection for its immunomodulatory activity. However, it has not demonstrated superiority in efficacy in patients with COVID-19 compared with the prevailing standard of care (Mangkuliguna et al. 2021). It can cause prolongation of the QT interval and, when used in combination with drugs that have the same effect, increases the risk of lethal arrhythmias, such as torsades de pointes (Hancox et al. 2013).
Corticosteroids (CS) are potent anti-inflammatory drugs that can improve outcomes in patients hospitalized for COVID-19. Several studies have shown that they reduce disease progression and mortality rates and increase ventilator-free days (Crisan Dabija et al. 2021). On its cardiovascular effects, CS can cause arterial hypertension by stimulating the renin–angiotensin–aldosterone system, excessive sodium retention, and catecholamine synthesis. Prolonged CS therapy can cause hyperglycemia and hyperlipidemia, thus increasing the cardiovascular risk (Sholter and Armstrong 2000).
Combined treatment of COVID-19 and cardiovascular complications
A meta-analysis has shown the association between COVID-19 infection and the tendency to develop viral myocarditis and abnormal cardiac biomarkers, including troponin I and creatinine kinase (Lippi et al. 2020). This association explains why the reported mortality rates are higher in COVID-19 patients with pre-existing cardiovascular diseases (Talasaz et al. 2020). Apart from the SARS-CoV-2 virus, which is accountable for causing the COVID-19 disease, the drug–drug interactions that may arise in COVID-19 patients with pre-existing cardiovascular conditions such as heart failure, arrhythmias, and acute ischemic stroke coronary syndrome (ACS) may also complicate the prognosis of the infectious disease (Talasaz et al. 2020). This section of the current review will discuss the drug–drug interactions of concern in COVID-19 patients based on the different types of pre-existing cardiovascular diseases (Dinan et al. 2015).
Heart failure
Considering the possible drug–drug interactions in heart failure patients who acquired COVID-19 infection. Angiotensin-converting enzyme (ACE) inhibitors, angiotensin II receptor blockers (ARBs), angiotensin receptor–neprilysin inhibitor (ARNI), β-blockers, ivabradine, digoxin, diuretics, nitrates are among the commonly used drugs in the treatment of acute and chronic heart failure (Talasaz et al. 2020; McMurray et al. 2012). Most of these drugs have clinically significant interactions with certain medications used to manage COVID-19 (Giguère et al. 2019; Dixon et al. 2020).
Lopinavir/ritonavir from the class of protease inhibitors is considered among the promising drugs in managing COVID-19. However, clinical trials to finalize the efficacy of these drugs in managing COVID-19 are still ongoing (Meini et al. 2020). In COVID-19 patients on lopinavir/ritonavir receiving β-blockers, diligent blood pressure and heart rate monitoring is greatly recommended (Talasaz et al. 2020). This is because ritonavir has been reported to affect the metabolism of certain β-blockers, such as bisoprolol, carvedilol, and metoprolol (Giguère et al. 2019). However, the interaction between lopinavir/ritonavir and bisoprolol is of weak intensity, and these drugs can be administered concurrently with appropriate monitoring.
Meanwhile, concurrent administration of metoprolol or carvedilol with lopinavir/ritonavir was reported to increase the blood levels of these two β-blockers to a moderate extent (Agarwal and Agarwal 2020). Hydroxychloroquine is an antimalarial drug that was earlier used to manage COVID-19 (Singh et al. 2020). This drug may cause bradycardia, which may be worsened by the concomitant use of β-blockers in COVID-19 patients. Meticulous blood pressure and heart rate monitoring are required in COVID-19 patients receiving any β-blockers (Talasaz et al. 2020). However, it should be noted that a recent Cochrane review reports that the use of this antimalarial agent is less likely to be effective in the management of COVID-19 (Singh et al. 2020).
In the class of ARNI, sacubitril’s plasma concentration increases the following coadministration with ritonavir, which warrants close surveillance of serum potassium level, serum creatinine level, and blood pressure (Talasaz et al. 2020). However, this interaction is considered quite controversial as some claim such interaction is unlikely (Hanna et al. 2018). More extensive studies are needed to verify the likelihood of the interaction between sacubitril and ritonavir.
Eplerenone is a mineralocorticoid receptor antagonist classified as a diuretic (Reyes et al. 2005). Concurrently administration of eplerenone with protease inhibitors may cause a fourfold hike in the area under the curve (AUC) of eplerenone, which translates into the delayed clearance of the drug from the body (Talasaz et al. 2020). Delayed clearance of eplerenone due to the said interaction leads to an increased risk of side effects, primarily hyperkalemia (Cordova et al. 2021). Alternatively, the use of spironolactone is recommended in COVID-19 patients on protease inhibitors. Another diuretic that has documented interaction with lopinavir/ritonavir is indapamide. However, this interaction cannot be concluded as a class effect as no drug–drug interaction is known to exist between lopinavir/ritonavir and thiazide diuretics, generally (Talasaz et al. 2020). CYP3A4 enzyme is an extensive metabolizer of indapamide (Sun et al. 2009). Coadministration of indapamide with lopinavir/ritonavir increases the serum concentration of the former. This is due to the nature of lopinavir/ritonavir, which inhibits the CYP3A4 enzyme (Agarwal and Agarwal 2020). Great caution is needed with the concomitant use of indapamide with lopinavir/ritonavir (Talasaz et al. 2020). To minimize the undesirable effects of such interactions, it is recommended to reduce the indapamide dose (Agarwal and Agarwal 2020).
Patients hospitalized for COVID-19, while on digoxin treatment require close monitoring, since there are chances of instability during acute infection. The therapeutic drug monitoring for digoxin is essential due to the changes in its serum concentration when used concomitantly with some drugs used in the treatment of COVID-19, such as ritonavir and hydroxychloroquine. Hydroxychloroquine may increase the serum digoxin concentration via an unknown mechanism. Meanwhile, ritonavir may increase the half-life of digoxin by 43% and may increase the AUC of digoxin by 29% (Dixon et al. 2020). Another study in healthy volunteers reported increased digoxin plasma concentrations when given concomitantly with ritonavir (Penzak et al. 2004). Before initiating lopinavir/ritonavir, it is recommended to reduce the dose of digoxin given to a patient by 50%. Such reduction should be followed by extensive monitoring of the patient’s serum digoxin levels, ECG, and clinical signs and symptoms relevant to the indication of digoxin (Agarwal and Agarwal 2020).
The clinically significant interaction between hydralazine and protease inhibitor is less likely (Giguère et al. 2019; Agarwal and Agarwal 2020). However, COVID-19 patients on concomitant administration of hydralazine or nitrates and protease inhibitors are entitled to dosage adjustments based on their drug response (Talasaz et al. 2020).
The formation of nitric oxide from isosorbide dinitrate can be diminished following the coadministration of protease inhibitors. CYP3A4 enzyme, which mediates the biotransformation of nitric oxide, will be inhibited by lopinavir/ritonavir when administered concurrently. Such inhibition of CYP3A4 eventually leads to reduced efficacy of isosorbide dinitrate. However, the clinical relevance of this drug–drug interaction is yet to be established, and monitoring is recommended (Agarwal and Agarwal 2020; Giguère et al. 2019).
The use of ivabradine should ideally be avoided in COVID-19 patients on lopinavir/ritonavir, as the incidence of severe symptomatic bradycardia is likely (Dixon et al. 2020; Romero-León et al. 2016). Lopinavir/ritonavir are potent inhibitors of the CYP3A4 enzyme, which happens to be the metabolizer of ivabradine (Dixon et al. 2020; Chaudhary et al. 2016). Concurrent administration of lopinavir/ritonavir can increase the area under the curve (AUC) of ivabradine by sixfold, which in turn increases the toxicity of ivabradine (Dixon et al. 2020).
Arrhythmia
According to a study conducted in Wuhan, COVID-19 infection itself was found to cause arrhythmia in 44% of patients (Alhazzani et al., 2021). Thus, extra attention is warranted in COVID-19 patients with pre-existing arrhythmia. Possible drugs that may precipitate arrhythmia in COVID-19 patients should be used with caution or avoided. In COVID-19 patients with shock, the use of dopamine as a vasopressor should be avoided as it increases the risk of arrhythmias and mortality. However, the recommendation is derived from clinical trials conducted among critically ill patients, not COVID-19 patients with shock. Thus, more research should be done to conclude the direct of dobutamine on COVID-19 patients with shock (Alhazzani et al. 2021).
In COVID-19 patients, the QTc prolongation risk and torsade de Pointes (TdP) risk are elevated with the simultaneous use of antiarrhythmic agents and chloroquine/hydroxychloroquine. ECG monitoring should be performed before the treatment initiation to manage such risks appropriately. Moreover, it is also essential to maintain the serum potassium level above 4.5 mg/dL and serum magnesium level above 2 mg/dL as a part of the ideal monitoring parameters for the said risks (Baigent et al. 2022; Zeitlinger et al. 2020).
COVID-19 treatment regimens that incorporate the simultaneous use of chloroquine/hydroxychloroquine and lopinavir/ritonavir should be avoided. This is because such coadministration of the drugs may lengthen the QTc interval, which eventually increases the tendency to develop TdP (Zeitlinger et al. 2020; Giudicessi et al. 2020). Moreover, concomitant usage of these drugs is attributed to the atrioventricular block risk. Few forms of alterations in the cardiac function are evident with chloroquine/hydroxychloroquine, which includes ventricular fibrillation, bundle branch block, and ventricular tachycardia (Driggin et al. 2020). The tendency for the incidence of drug-induced sudden cardiac death is increased with the combination of chloroquine/hydroxychloroquine and lopinavir/ritonavir, which is QTc prolonging drugs on their own (Giudicessi et al. 2020).
The effects of antiarrhythmic drugs such as amiodarone, quinidine, and lidocaine can be affected by concurrent administration of protease inhibitors in COVID-19 patients with pre-existing arrhythmia. Thus, ECG surveillance is essential in patients on both drugs, and in some instances, dose reduction may also be considered. If quinidine or lidocaine is used along with amiodarone, regular monitoring of thyroid function, liver function, and blood pressure should be instituted. Concurrent administration of flecainide, a class 1c antiarrhythmic and protease inhibitor, such as ritonavir, is contraindicated (Zeitlinger et al. 2020).
Acute Coronary Syndrome
In the presence of ACS, antiplatelets, anticoagulants, thrombolytics, HMG-CoA reductase inhibitors, β-blockers, angiotensin-converting enzyme inhibitors (ACEIs), and angiotensin receptor blockers (ARBs) are among the commonly used drugs (Talasaz et al. 2020).
In COVID-19 patients with ACS, a drug interaction is likely between ARBs and lopinavir/ritonavir. Losartan and irbesartan may lose their efficacy when used concurrently with ritonavir. Such loss in effectiveness is attributed to the diminished ability of the said ARBs to transform into their active forms, in which their efficacy is optimal (Giguère et al. 2019). COVID-19 patients on ACEIs or ARBs should be closely monitored for their serum potassium level, serum creatinine level, and blood pressure (Dixon et al. 2020).
Another significant drug–drug interaction that should be considered in COVID-19 patients with ACS is the interaction between P2Y12 inhibitors and protease inhibitors. In COVID-19 patients receiving lopinavir/ritonavir, the use of ticagrelor is contraindicated. Lopinavir/ritonavir may also decrease the antiplatelet property of clopidogrel; thus, dosing adjustment for clopidogrel is recommended. Prasugrel is named a better alternative, which can be concurrently administered along with lopinavir/ritonavir in patients without risk of bleeding and coagulopathy (Bikdeli et al. 2020). The use of glycoprotein IIb/IIIa inhibitors should be avoided due to the high tendency to develop acute kidney injury and increased risk of bleeding in COVID-19 patients. Considering the increased risk for the incidence of thrombocytopenia, the use of abciximab should ideally be avoided. Instead, they use eptifibatide, and tirofiban can be regarded when required (Ibanez et al. 2018).
Upon hospitalization, switching to parenteral anticoagulant is preferred in COVID-19 patients on chronic treatment with anticoagulants (Bikdeli et al. 2020). Regarding drug–drug interaction, the use of warfarin and drugs used for COVID-19 is not contraindicated, given that monitoring of the international normalized ratio (INR) is performed periodically. In the context of anticoagulants, apixaban and rivaroxaban have lesser interactions with chloroquine/hydroxychloroquine as compared to edoxaban and dabigatran. However, due to the elevated tendency of bleeding, the simultaneous use of lopinavir/ritonavir and rivaroxaban is contraindicated (Baigent et al. 2022). The administration of edoxaban with lopinavir/ritonavir is not well studied; thus, it is not recommended. The dose of apixaban is recommended to be reduced by half when administering concomitantly with lopinavir/ritonavir. Meanwhile, dabigatran can be given at a conventional dose with a 2-h dosing interval with lopinavir/ritonavir (Dixon et al. 2020; Baigent et al. 2022; Wiggins et al. 2020).
Lopinavir/ritonavir has a likely interaction with rosuvastatin and atorvastatin. Rosuvastatin and atorvastatin belong to the group of HMG–CoA reductase inhibitors. Concerning this drug–drug interaction, it is recommended that the maximum daily dose of rosuvastatin and atorvastatin should be capped at 10 mg and 20 mg, respectively, when used concomitantly with lopinavir/ritonavir (Newman et al. 2019). When used concurrently, tocilizumab may decrease the serum concentrations of the HMG–CoA reductase inhibitors. This is due to the reversal of cytochrome P450 (CYP450) suppression resulting from tocilizumab. The clinical impact of this interaction can be diminished by the short-term use of anti-interleukin-6 receptor treatments (Dixon et al. 2020).
Conclusions
The therapeutic management of COVID-19 should consider cardiovascular disease involvement and adapt the most beneficial and optimized treatment plan. The treatment strategy should consider patient's cardiovascular indices; heart rate, blood pressure, blood lipids, heart function, and ECG changes. To avoid medication-induced cardiac injury, it is important to pay attention to drug interactions. Simultaneously, the monitoring of important signs for the identification of myocardial damage should be bolstered, and the heart function of COVID-19 patients should be assessed using laboratory and imaging data. Currently, the recommendations do not suggest using more than three antiviral medications in combination, and caution should be exercised in elderly patients with COVID-19 and underlying cardiovascular illnesses.
Availability of data and materials
Not applicable.
References
Agarwal S, Agarwal SK (2020) Lopinavir-ritonavir in SARS-CoV-2 infection and drug-drug interactions with cardioactive medications. Cardiovasc Drugs Ther. https://doi.org/10.1007/s10557-020-07070-1
Alhazzani W, Evans L, Alshamsi F, Møller MH, Ostermann M, Prescott HC, Arabi YM, Loeb M, Gong MN, Fan E (2021) Surviving sepsis campaign guidelines on the management of adults with coronavirus disease 2019 (COVID-19) in the ICU: first update. Crit Care Med 49:e219–e234. https://doi.org/10.1097/CCM.0000000000004899
Alten R (2011) Tocilizumab: a novel humanized anti-interleukin 6 receptor antibody for the treatment of patients with rheumatoid arthritis. Ther Adv Musculoskelet Dis 3:133–149. https://doi.org/10.3109/07853890.2013.771986
Baigent C, Windecker S, Andreini D, Arbelo E, Barbato E, Bartorelli AL, Baumbach A, Behr ER, Berti S, Bueno H (2022) ESC guidance for the diagnosis and management of cardiovascular disease during the COVID-19 pandemic: part 2—care pathways, treatment, and follow-up. Eur Heart J 43:1059–1103. https://doi.org/10.1093/eurheartj/ehab697
Behjati M, Etemadifar M, Esfahani MA (2014) Cardiovascular effects of fingolimod: a review article. Iran J Neurol 13:119–126
Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, Hohmann E, Chu HY, Luetkemeyer A, Kline S (2020) Remdesivir for the treatment of Covid-19—preliminary report. N Engl J Med 383:1813–1826. https://doi.org/10.1056/nejmoa2007764
Bikdeli B, Madhavan MV, Jimenez D, Chuich T, Dreyfus I, Driggin E, Nigoghossian CD, Ageno W, Madjid M, Guo Y (2020) COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up: JACC state-of-the-art review. J Am Coll Cardiol 75:2950–2973. https://doi.org/10.1016/j.jacc.2020.04.031
Cacciapaglia F, Anelli MG, Rinaldi A, Fornaro M, Lopalco G, Scioscia C, Lapadula G, Iannone F (2018) Lipids and atherogenic indices fluctuation in rheumatoid arthritis patients on long-term tocilizumab treatment. Mediators Inflamm 2018:1–7. https://doi.org/10.1155/2018/2453265
Caly L, Druce JD, Catton MG, Jans DA, Wagstaff KM (2020) The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antiviral Res 178:104787. https://doi.org/10.1016/j.antiviral.2020.104787
Cao B, Wang Y, Wen D, Liu W, Wang J, Fan G, Ruan L, Song B, Cai Y, Wei M (2020) A trial of lopinavir–ritonavir in adults hospitalized with severe Covid-19. N Engl J Med 382:1787–1799. https://doi.org/10.1056/nejmoa2001282
Chatre C, Roubille F, Vernhet H, Jorgensen C, Pers Y-M (2018) Cardiac complications attributed to chloroquine and hydroxychloroquine: a systematic review of the literature. Drug Saf 41:919–931. https://doi.org/10.1007/s40264-018-0689-4
Chaudhary R, Garg J, Krishnamoorthy P, Shah N, Lanier G, Martinez MW, Freudenberger R (2016) Ivabradine: heart failure and beyond. J Cardiovasc Pharmacol Ther 21:335–343. https://doi.org/10.1177/1074248415624157
Chen C-Y, Wang F-L, Lin C-C (2006) Chronic hydroxychloroquine use associated with QT prolongation and refractory ventricular arrhythmia. Clin Toxicol 44:173–175. https://doi.org/10.1080/15563650500514558
Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Qiu Y, Wang J, Liu Y, Wei Y (2020) Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. The Lancet 395:507–513. https://doi.org/10.1016/s0140-6736(20)30211-7
Chun J, Brinkmann V (2011) A mechanistically novel, first oral therapy for multiple sclerosis: the development of fingolimod (FTY720, Gilenya). Discov Med 12:213
Cordova E, Garibaldi F, Bono L, Rodriguez C (2021) Severe hyperkalaemia due to a potential drug–drug interaction between eplerenone and antiretrovirals in a HIV-positive patient after a myocardial infarction. Int J STD AIDS 32:771–773. https://doi.org/10.1177/0956462420987422
Crisan Dabija R, Antohe I, Trofor A, Antoniu SA (2021) Corticosteroids in SARS-COV2 infection: certainties and uncertainties in clinical practice. Expert Rev Anti Infect Ther 19:1553–1562. https://doi.org/10.1080/14787210.2021.1933437
Dinan MA, Hirsch BR, Lyman GH (2015) Management of chemotherapy-induced neutropenia: measuring quality, cost, and value. J Natl Compr Canc Netw 13:e1–e7. https://doi.org/10.6004/jnccn.2015.0014
Dixon DL, Van Tassell BW, Vecchié A, Bonaventura A, Talasaz AH, Kakavand H, D’ascenzo F, Perciaccante A, Castagno D, Ammirati E (2020) Cardiovascular considerations in treating patients with coronavirus disease 2019 (COVID-19). J Cardiovasc Pharmacol 75:359–367. https://doi.org/10.1097/FJC.0000000000000836
Driggin E, Madhavan MV, Bikdeli B, Chuich T, Laracy J, Biondi-Zoccai G, Brown TS, Der Nigoghossian C, Zidar DA, Haythe J (2020) Cardiovascular considerations for patients, health care workers, and health systems during the COVID-19 pandemic. J Am Coll Cardiol 75:2352–2371. https://doi.org/10.1016/j.jacc.2020.03.031
English D, Welch Z, Kovala AT, Harvey K, Volpert OV, Brindley DN, Garcia JG (2000) Sphingosine 1-phosphate released from platelets during clotting accounts for the potent endothelial cell chemotactic activity of blood serum and provides a novel link between hemostasis and angiogenesis. FASEB J 14:2255–2265. https://doi.org/10.1096/fj.00-0134com
Enosawa S, Suzuki S, Kakefuda T, Li X-K, Amemiya H (1996) Induction of selective cell death targeting on mature T-lymphocytes in rats by a novel immunosuppressant, FTY720. Immunopharmacology 34:171–179. https://doi.org/10.1016/0162-3109(96)00132-4
Fintelman-Rodrigues N, Sacramento CQ, Ribeiro Lima C, Souza Da Silva F, Ferreira AC, Mattos M, De Freitas CS, Cardoso Soares V, Dias DSG, S, Temerozo JR, (2020) Atazanavir, alone or in combination with ritonavir, inhibits SARS-CoV-2 replication and proinflammatory cytokine production. Antimicrob Agents Chemother 64:e00825-e920. https://doi.org/10.1128/AAC.00825-20
Fontas E, Van Leth F, Sabin C, Friis-Møller N, Rickenbach M, D’arminio Monforte A, Kirk O, Dupon M, Morfeldt L, Mateu S (2004) Lipid profiles in HIV-infected patients receiving combination antiretroviral therapy: are different antiretroviral drugs associated with different lipid profiles? J Infect Dis 189:1056–1074. https://doi.org/10.1086/381783
Friis-Møller N, Reiss P, Sabin CA, Weber R, Monforte Ad, El-Sadr W, Thiébaut R, De Wit S, Kirk O, Fontas E, Law MG, Phillips A, Lundgren JD (2007) Class of antiretroviral drugs and the risk of myocardial infarction. N Engl J Med 356:1723–1735. https://doi.org/10.1056/nejmoa062744
Giguère P, Nhean S, Tseng AL, Hughes CA, Angel JB (2019) Getting to the heart of the matter: a review of drug interactions between HIV antiretrovirals and cardiology medications. Can J Cardiol 35:326–340. https://doi.org/10.1016/j.cjca.2018.12.020
Giudicessi JR, Noseworthy PA, Friedman PA, Ackerman MJ (2020) Urgent guidance for navigating and circumventing the QTc-prolonging and torsadogenic potential of possible pharmacotherapies for coronavirus disease 19 (COVID-19). Mayo Clin Proc 95:1213–1221. https://doi.org/10.1016/j.mayocp.2020.03.024
Gräler MH, Goetzl EJ (2004) The immunosuppressant FTY720 down-regulates sphingosine 1-phosphate G protein-coupled receptors. FASEB J 18:551–553. https://doi.org/10.1096/fj.03-0910fje
Grein J, Ohmagari N, Shin D, Diaz G, Asperges E, Castagna A, Feldt T, Green G, Green ML, Lescure F-X (2020) Compassionate use of remdesivir for patients with severe Covid-19. N Engl J Med 382:2327–2336. https://doi.org/10.1056/NEJMoa2007016
Hancox JC, Hasnain M, Vieweg WVR, Crouse ELB, Baranchuk A (2013) Azithromycin, cardiovascular risks, QTc interval prolongation, torsade de pointes, and regulatory issues: a narrative review based on the study of case reports. Ther Adv Infect Dis 1:155–165. https://doi.org/10.1177/2049936113501816
Hanna I, Alexander N, Crouthamel MH, Davis J, Natrillo A, Tran P, Vapurcuyan A, Zhu B (2018) Transport properties of valsartan, sacubitril and its active metabolite (LBQ657) as determinants of disposition. Xenobiotica 48:300–313. https://doi.org/10.1080/00498254.2017.1295171
Hensley LE, Fritz EA, Jahrling PB, Karp C, Huggins JW, Geisbert TW (2004) Interferon-β 1a and SARS coronavirus replication. Emerg Infect Dis 10:317–319. https://doi.org/10.3201/eid1002.030482
Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X (2020) Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The Lancet 395:497–506
Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, Caforio AL, Crea F, Goudevenos JA, Halvorsen S (2018) 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J 39:119–177. https://doi.org/10.1093/eurheartj/ehx393
Lancet T (2020) Emerging understandings of 2019-nCoV. Lancet (london, England) 395:311
Li M, Chan WW, Zucker SD (2020) Association between atazanavir-induced hyperbilirubinemia and cardiovascular disease in patients infected with HIV. J Am Heart Assoc 9:e016310. https://doi.org/10.1161/JAHA.120.016310
Lippi G, Lavie CJ, Sanchis-Gomar F (2020) Cardiac troponin I in patients with coronavirus disease 2019 (COVID-19): evidence from a meta-analysis. Prog Cardiovasc Dis 63:390–391. https://doi.org/10.1016/j.pcad.2020.03.001
Liu Y, Yang Y, Zhang C, Huang F, Wang F, Yuan J, Wang Z, Li J, Li J, Feng C (2020) Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci China Life Sci 63:364–374. https://doi.org/10.1007/s11427-020-1643-8
Lokugamage KG, Hage A, De Vries M, Valero-Jimenez AM, Schindewolf C, Dittmann M, Rajsbaum R, Menachery VD (2020) Type I interferon susceptibility distinguishes SARS-CoV-2 from SARS-CoV. J Virol 94:e01410-e1420. https://doi.org/10.1128/JVI.01410-20
Ly T, Ruiz ME (2007) Prolonged QT interval and torsades de pointes associated with atazanavir therapy. Clin Infect Dis 44:e67–e68. https://doi.org/10.1086/511875
Macedo G, Ribeiro T (1999) Interferon plus ribavirin: a cautionary note. Am J Gastroenterol 94:3087–3088. https://doi.org/10.1111/j.1572-0241.1999.03087.x
Mangkuliguna G, Susanto N, Pramono LA (2021) Efficacy and safety of azithromycin for the treatment of COVID-19: a systematic review and meta-analysis. Tuberc Res Dis 84:299–316. https://doi.org/10.4046/trd.2021.0075
Marfella R, Paolisso P, Sardu C, Palomba L, D’onofrio N, Cesaro A, Barbieri M, Rizzo MR, Sasso FC, Scisciola L (2021) SARS-COV-2 colonizes coronary thrombus and impairs heart microcirculation bed in asymptomatic SARS-CoV-2 positive subjects with acute myocardial infarction. Crit Care 25:1–12. https://doi.org/10.1186/s13054-021-03643-0
Mcmurray JJ, Adamopoulos S, Anker SD, Auricchio A, Böhm M, Dickstein K, Falk V, Filippatos G, Fonseca C (2012) ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the task force for the diagnosis and treatment of acute and chronic heart failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J 33:1787–1847. https://doi.org/10.1093/eurheartj/ehs104
Meini S, Pagotto A, Longo B, Vendramin I, Pecori D, Tascini C (2020) Role of Lopinavir/Ritonavir in the treatment of Covid-19: A review of current evidence, guideline recommendations, and perspectives. J Clin Med 9:2050. https://doi.org/10.3390/jcm9072050
Mody V, Ho J, Wills S, Mawri A, Lawson L, Ebert MC, Fortin GM, Rayalam S, Taval S (2021) Identification of 3-chymotrypsin like protease (3CLPro) inhibitors as potential anti-SARS-CoV-2 agents. Commun Biol 4:1–10. https://doi.org/10.1038/s42003-020-01577-x
Mondy KE, Gottdiener J, Overton ET, Henry K, Bush T, Conley L, Hammer J, Carpenter CC, Kojic E, Patel P (2011) High prevalence of echocardiographic abnormalities among HIV-infected persons in the era of highly active antiretroviral therapy. Clin Infect Dis 52:378–386. https://doi.org/10.1093/cid/ciq066
Nadeem U, Raafey M, Kim G, Treger J, Pytel P, Husain N, A, Schulte JJ, (2021) Chloroquine-and hydroxychloroquine-induced cardiomyopathy: a case report and brief literature review. Am J Clin Pathol 155:793–801. https://doi.org/10.1093/ajcp/aqaa253
Newman CB, Preiss D, Tobert JA, Jacobson TA, Page RL, Goldstein LB, Chin C, Tannock LR, Miller M, Raghuveer G (2019) Statin safety and associated adverse events: a scientific statement from the American Heart Association. Arterioscler Thromb Vasc Biol 39:e38–e81. https://doi.org/10.1161/ATV.0000000000000073
Osipiuk J, Azizi S-A, Dvorkin S, Endres M, Jedrzejczak R, Jones KA, Kang S, Kathayat RS, Kim Y, Lisnyak VG (2021) Structure of papain-like protease from SARS-CoV-2 and its complexes with non-covalent inhibitors. Nat Commun 12:1–9. https://doi.org/10.1038/s41467-021-21060-3
Ou T, Mou H, Zhang L, Ojha A, Choe H, Farzan M (2021) Hydroxychloroquine-mediated inhibition of SARS-CoV-2 entry is attenuated by TMPRSS2. PLoS Pathog 17:e1009212. https://doi.org/10.1371/journal.ppat.1009212
Pastick KA, Okafor EC, Wang F, Lofgren SM, Skipper CP, Nicol MR, Pullen MF, Rajasingham R, Mcdonald EG, Lee TC (2020). Hydroxychloroquine and chloroquine for treatment of SARS-CoV-2 (COVID-19). Open Forum Infect Dis, 7, ofaa130. https://doi.org/10.1093/ofid/ofaa130.
Penzak SR, Shen JM, Alfaro RM, Remaley AT, Natarajan V, Falloon J (2004) Ritonavir decreases the nonrenal clearance of digoxin in healthy volunteers with known MDR1 genotypes. Ther Drug Monit 26:322–330. https://doi.org/10.1097/00007691-200406000-00018
Reyes AJ, Leary WP, Crippa G, Maranhão MF, Hernández-Hernández R (2005) The aldosterone antagonist and facultative diuretic eplerenone: A critical review. Eur J Intern Med 16:3–11. https://doi.org/10.1016/j.ejim.2004.10.007
Romero-León JM, Gálvez-Contreras MC, Díez-García LF (2016) Symptomatic bradycardia and heart failure triggered by ivabradine in a patient receiving antiretroviral therapy. Revista Espa Ola De Cardiolog a (english Edition) 5:529–530. https://doi.org/10.1016/j.rec.2016.02.005
Rosas IO, Bräu N, Waters M, Go RC, Hunter BD, Bhagani S, Skiest D, Aziz MS, Cooper N, Douglas IS (2021) Tocilizumab in hospitalized patients with severe Covid-19 pneumonia. N Engl J Med 384:1503–1516. https://doi.org/10.1007/s11427-020-1643-8
Schulman S (2002) Inhibition of warfarin activity by ribavirin. Ann Pharmacother 36:72–74. https://doi.org/10.1345/aph.1A181
Sholter DE, Armstrong PW (2000) Adverse effects of corticosteroids on the cardiovascular system. Can J Cardiol 16:505–511
Singer B, Ross A, Tobias K (2011) Oral fingolimod for the treatment of patients with relapsing forms of multiple sclerosis. Int J Clin Pract 65:887–895. https://doi.org/10.1586/ern.13.52
Singh B, Ryan H, Kredo T, Chaplin M, Fletcher T (2020) Chloroquine or hydroxychloroquine for prevention and treatment of COVID-19. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD013587.pub2
Soliman EZ, Lundgren JD, Roediger MP, Duprez DA, Temesgen Z, Bickel M, Shlay JC, Somboonwit C, Reiss P, Stein JH (2011) Boosted protease inhibitors and the electrocardiographic measures of QT and PR durations. AIDS (london, England) 25:367. https://doi.org/10.1097/QAD.0b013e328341dcc0
Sparsa A, Bonnetblanc J-M, Peyrot I, Loustaud-Ratti V, Vidal E, Bédane C (2006) Effets secondaires de l’ivermectine utilisée dans le traitement de la gale. Ann Dermatol Venereol 133:784–787. https://doi.org/10.1016/S0151-9638(06)71044-4
Sun H, Moore C, Dansette PM, Kumar S, Halpert JR, Yost GS (2009) Dehydrogenation of the indoline-containing drug 4-chloro-N-(2-methyl-1-indolinyl)-3-sulfamoylbenzamide (indapamide) by CYP3A4: correlation with in silico predictions. Drug Metab Dispos 37:672–684. https://doi.org/10.1124/dmd.108.022707
Talasaz AH, Kakavand H, Van Tassell B, Aghakouchakzadeh M, Sadeghipour P, Dunn S, Geraiely B (2020) Cardiovascular complications of COVID-19: Pharmacotherapy perspective. Cardiovasc Drugs Ther. https://doi.org/10.1007/s10557-020-07037-2
Teragawa H, Hondo T, Amano H, Hino F, Ohbayashi M (1996) Adverse effects of interferon on the cardiovascular system in patients with chronic hepatitis C. Jpn Heart J 37:905–915. https://doi.org/10.1536/ihj.37.905
Tong S, Su Y, Yu Y, Wu C, Chen J, Wang S, Jiang J (2020) Ribavirin therapy for severe COVID-19: A retrospective cohort study. Int J Antimicrob Agents 56:106114. https://doi.org/10.1016/j.ijantimicag.2020.106114
Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y (2020a) Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA 323:1061–1069. https://doi.org/10.1001/jama.2020.1585
Wang Q, Zhang Y, Wu L, Niu S, Song C, Zhang Z, Lu G, Qiao C, Hu Y, Yuen K-Y (2020b) Structural and functional basis of SARS-CoV-2 entry by using human ACE2. Cell 181:894–904. https://doi.org/10.1016/j.cell.2020.03.045
Wiggins BS, Dixon DL, Neyens RR, Page RL, Gluckman TJ (2020) Select drug-drug interactions with direct oral anticoagulants: JACC review topic of the week. J Am Coll Cardiol 75:1341–1350. https://doi.org/10.1016/j.jacc.2019.12.068
World Health Organization (2020). Novel Coronavirus (2019-nCoV): situation report, 11. https://apps.who.int/iris/handle/10665/330776. Accessed 1 Apr 2022
Xu X, Chen P, Wang J, Feng J, Zhou H, Li X, Zhong W, Hao P (2020) Evolution of the novel coronavirus from the ongoing Wuhan outbreak and modeling of its spike protein for risk of human transmission. Sci China Life Sci 63:457–460. https://doi.org/10.1007/s11427-020-1637-5
Zeitlinger M, Koch BC, Bruggemann R, De Cock P, Felton T, Hites M, Le J, Luque S, Macgowan AP, Marriott DJ (2020) Pharmacokinetics/pharmacodynamics of antiviral agents used to treat SARS-CoV-2 and their potential interaction with drugs and other supportive measures: A comprehensive review by the PK/PD of Anti-Infectives Study Group of the European Society of Antimicrobial Agents. Clin Pharmacokinet 43:1–22. https://doi.org/10.1007/s40262-020-00924-9
Zhao J (2020) Efficacy and safety of IFN-α2β in the treatment of novel Coronavirus patients.ClinicalTrials.gov Identifier: NCT04293887 [Internet]. https://clinicaltrials.gov/ct2/show/NCT04293887. Accessed 1 Apr 2022
Zhou P, Yang X-L, Wang X-G, Hu B, Zhang L, Zhang W, Si H-R, Zhu Y, Li B, Huang C-L (2020) A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 579:270–273. https://doi.org/10.1038/s41586-020-2012-7
Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, Huang B, Shi W, Lu R (2020) A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. https://doi.org/10.1056/NEJMoa2001017
Acknowledgements
This work was supported by the Discipline of Clinical Pharmacy, School of Pharmaceutical Science, Universiti Sains Malaysia.
Funding
This research received no external funding.
Author information
Authors and Affiliations
Contributions
MAA, AC, GSG, MAM and AV: Conceptualization, literature review, and writing the manuscript; BI, AS, IY, AIJ, MM, AASJ and MASAK: manuscript revision,.; supervision, BI and AS.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate.
Not applicable.
Consent for publication
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Akkaif, M.A., Sha’aban, A., Cesaro, A. et al. The impact of SARS-CoV-2 treatment on the cardiovascular system: an updated review. Inflammopharmacol 30, 1143–1151 (2022). https://doi.org/10.1007/s10787-022-01009-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10787-022-01009-8