Abstract
The goal of this study is to evaluate the chemical compounds, the anti-bacterial/fungal activity, and the cytotoxicity of the essential oil of three species of lamiaceae in Iran. After the extraction of the essential oil implementing the hydrodistillation method, the analysis and identification of the compounds were carried out with a chromatograph coupled with a mass spectrometer. For the evaluation of the anti-bacterial/fungal activity of the essential oils, the measurement of the diameter of inhibition halo, the minimum inhibitory concentration (MIC), bactericidal and fungicidal concentrations (MBC/MFC) were utilized; and for the evaluation of the cytotoxic activity of the essential oils, the 3-(4,5-dimethylthiazolyl)-2,5-diphenyltetrazolium bromide (MTT) method was used. The results show that the dominant compounds in the Perovskia abrotanoides Kar essential oil were camphor (21.68%), 1,8-cineole (14.26%), and α-pinene (7.23%); moreover, the dominant compounds in the Salvia reuteriana Boiss. Essential oil were benzyl benzoate (27.10%), linalool (13.27%), and sclareol (7.75%); in addition, the dominant compounds in the Ziziphora clinopodioides subsp. rigida (Boiss.) Rech.f. were cyclofenchene (25.29%), pulegone (14.14%), and menthol (7.70%). The largest halo diameter of inhibition halo (~ 22 mm) was against Streptococcus pyogenes and the strongest inhibiting and killing activity was against Candida albicans (MIC and MFC = 125 μg/mL) shown by the S. reuteriana essential oil which, respectively, matched the control antibiotics rifampin and nystatin. The analysis of the MTT test results showed that the Z. clinopodioides subsp. rigida essential oil (with IC50 value of ~ 144.2500) had the strongest cytotoxic activity against human ovarian cancer cells (OVCAR-3). On the whole, the results show that the essential oil of the Lamiaceae family plants is a source for various compounds with potential biological activities which can serve as a possible alternative to produce herbal medicine which are effective on some microorganisms and cancer cell lines.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Infectious diseases caused by various microorganisms are very common all over the world (Razavi et al. 2016). Despite all the efforts that have been made to control infectious diseases, their impact remains a major concern. In fact, the use of antimicrobials is inadequate and widespread, leading to the emergence of several drug-resistant strains that severely impair the anti-microbial effect (World Health Organization WHO 2002). Pathogenic microorganisms are resistant to antibiotics by gene mutations, creating strains that cannot be fought. One of the most important of these factors is the arbitrary or excessive use of antibiotics (Nazer and Darvishi 2017). In the face of this worrying situation, alternatives must be created for the natural, safe and more anti-microbial development of natural products such as essential oils. Essential oils have historically proven their value as molecular sources with anti-microbial potential and may have the potential for topical or future systemic administration in bacterial and fungal infections (Brahim et al. 2015; Elhidar et al. 2019). The nature of essential oils as a complex chemical mixture, with varying amounts of biologically active components, makes them resistant to any microbial resistant mechanism (Napoli and Vito 2021).
Aromatic plants have many benefits for human nutrition and health due to their rich contents of bioactive compounds (Costa et al. 2015). The Lamiaceae family include many popular spicy or aromatic plants and are one of the largest sources of cooking and medicinal plants in the world whose aroma, taste, and medical properties have advantages for humans (Hazrati et al. 2020; Mohammadhosseini 2017). The plants of this family are mostly known for having essential oils rich in monoterpenoids (Grayer et al. 2003).
From this family, the Perovskia genus consists of seven species of aromatic shrubs, found in the dry habitats of Central Asia. Three of these are native to Iran, among which Perovskia abrotanoides Kar, called “Barazambal” in Farsi, has the most dispersal (Ghahraman 1994; Mozaffarian 1996). The anti-oxidant (Iraji et al. 2021), anti-bacterial (Hasan et al. 1978), and anti-fungal (Inouye et al. 2001) properties of the essential oil are reported accordingly. In previous studies, various compounds such as α-pinene, 1,8-cineole, camphor, and δ-3-carene have been recorded as the principal compounds of the essential oil of this species (Ghaffari et al. 2019; Pourhosseini et al. 2018; Salimpour et al. 2014).
The Salvia genus of the Lamiaceae family consists of about 700–900 species all over the world, growing mostly in tropical and mild climates (Villalta et al. 2021). This genus is known in Iran with Farsi names of “Maryami”, “Maryam Goli” and “Salvi” and consists of 58 species of herbaceous perennials, and annuals, 17 of which are native and exclusive to Iran (Chalchat et al. 1998). One of these exclusive aromatic species is Salvia reuteriana Boiss. with the Farsi name “Esfahan Maryam Goli” which grows in the mountains of Central and Western Iran (Akhani 2006). Above-ground parts of this plant are used as tranquilizers and anti-stress in Iranian traditional medicine (Bahmani et al. 2014). Anti-oxidant (Aminzadeh et al. 2015; Panahi et al. 2020) and anti-fungal (Esmaeili et al. 2008; Karamian et al. 2013) effects are reported for the essential oil of this species. In the previous studies on this essential oil, compounds such as germacrene D, β-caryophyllene, bicyclogermacrene, linalool, α-gurjunene, β-elemene, and sclareol have been approved as the main compounds (Aminzadeh et al. 2015; Fattahi et al. 2016; Norouzi and Norouzi 2018).
The Ziziphora genus from the mint family, called Mountain Ziziphora (“Kakouty”) or narrow-leaf thyme, has four native species in the flora of Iran, distributed all over the country (Mohammadhosseini 2017; Shahbazi 2015). Ziziphora clinopodioides Lam. is a native species of Iran, called “Moshk-E-Taramoshk” and “Sa’tar” in Farsi traditional medicine texts. In Iranian popular culture, the dried above-ground parts of the plant are usually used to treat common cold, cough, wounds and as antiseptic (Zargari 1995). Moreover, it is used as a spice and additive in cooking soups, meat products, and dairy products such as milk, cheese, yogurt, or doogh, which is the Iranian yogurt drink (Mohammadhosseini 2017). The studies on the essential oil of this species have proven the existence of various anti-oxidant (Hazrati et al. 2020; Aliakbarlu and Shameli 2013; Salehi et al. 2005; Sharifzadeh et al. 2016), anti-bacterial (Hazrati et al. 2020; Shahbazi 2015, 2020; Shahbazi and Shavisi 2016), and anti-fungal (Sharifzadeh et al. 2016; Mahboubi et al. 2018; Shokri et al. 2012) bioactivities. This species consists of nine sub-species in Iran among which Z. clinopodioides subsp. rigida (Boiss.) Rech.f. as a wild native sub-species grows in Iran, Afghanistan, Iraq, and Talish. The most important compounds of the essential oil of this sub-species are reported to be pulegone, 1,8-cineole, piperitenone, and p-menth-3-en-8-ol (Salehi et al. 2005; Dehghan et al. 2014, 2010).
Research on the medicinal properties and biological effects of the essential oils is continuously carried out and their use in the manufacturing of various drugs, food, agriculture, and cosmetics industries is studied. The medicinal and aromatic plants of the Lamiaceae family are considered to be among the most important plant genetic resources, because of their high ecologic flexibility in various climates, and possessing diverse aromatic compounds. They have many applications in drug, cosmetics and health industries which makes them appropriate choices for these types of studies. Considering the traditional applications and pharmacologic effects of P. abrotanoides, S. reuteriana, and Z. clinopodioides subsp. rigida, this research was designed and carried out to study and compare the type and percentage of the constituent compounds and some biologic activities (anti-bacterial, anti-fungal, cytotoxicity) of the essential oil of these three species (collected from a natural habitat) for the first time.
Materials and methods
Plant
Collection
The flowering twigs of P. abrotanoides, S. reuteriana, and Z. clinopodioides subsp. rigida were collected from three random spots from 50 different bushes in May 2019 when the flowers emerge, in Sheikh Bahaei Dam area in Kashan, Iran (E 51° 27′ 20ʺ and N 33° 42′ 25ʺ and an altitude of 1995 from sea level). The twigs were put in different envelops in fresh form and brought to the laboratory.
Drying and identification
The flowering twigs of the plants were washed with distilled water and spread on clean flat paper sheets in the laboratory environment and dried after being one week at 20 °C. P. abrotanoides, S. reuteriana, and Z. clinopodioides subsp. rigida were identified and approved by a botanist, with the help of the Flora of Iran (Jamzad 2012). They are registered and kept under the codes 1014, 1013, and 1015 in the herbarium of the Faculty of Natural Resources and Earth Sciences in the University of Kashan, Iran.
Essential oil
Extraction
For each species, essential oil extraction was carried out using 100 g of dried and powdered plant parts, implementing the hydrodistillation method by the Clevenger apparatus (the British Pharmacopoeia model) for 120 min in three repetitions. This was the maximum time for essential oil extraction since there was no increase in the volume or weight of the essential oil after investing more time. The extracted essential oils, after dehydration by sodium sulfate, were kept in small, dark, sealed containers in a refrigerator (4 °C) until further tests (Ghavam 2021).
Yield determination
By calculating the moisture percentage, the essential oil yield of each species was determined as per the dried weight w/w and reported as the mean ± standard deviation (Azarnivand et al. 2010).
Determining the compounds
The chromatograph in the University of Kashan was a model 6890, coupled with a mass spectrometer model N5973 made in Agilent Company of the USA. The capillary column of HP-5MS with the stationary phase of 5% methyl phenyl siloxane with 30 m length, the internal diameter of 0.25 mm, and stationary layer thickness of 0.25 μm was utilized. The column temperature was increased from 60 to 246 °C with a speed of 3 °C per minute. The temperature of the injection room was 250 °C. The carrier gas was Helium with the flow velocity of 1.5 mL/min and the mass spectrometer was operated with the ionization energy of 70 eV. To calculate the retention index of the compounds, normal alkanes C8–C28 were injected. The identification of the compounds was carried out by the study of mass spectrums and comparison with the mass spectrums of standard compounds, using the information available in the library and with the help of the retention indexes of the compounds of their comparison to the standard retention indexes published in various sources (Adams 2007).
Biologic activity
Anti-bacterial and anti-fungal activity
Bacteria and fungi strains
Four Gram-positive bacteria namely Bacillus subtilis (ATCC 6633), Staphylococcus aureus (ATCC 29737), Staphylococcus epidermidis (CIP 81.55), and Streptococcus pyogenes (ATCC 19615); in addition to five Gram-negative bacteria, namely Escherichia coli (ATCC 25922), Klebsiella pneumonia (ATCC 10031), Pseudomonas aeruginosa (ATCC 27853), Salmonella paratyphi-A serotype (ATCC 5702), and Shigella dysenteriae (PTCC 1188) were inoculated overnight at 37 °C in nutrient culture medium and three fungus strains of Aspergillus brasiliensis (ATCC 16404), Aspergillus niger (ATCC 9029), and Candida albicans (ATCC 16404) were inoculated overnight at 30 °C in the Sabouraud Dextrose Agar culture medium (Ghavam 2021). The microorganisms were obtained from the Iranian Research Organization for Science and Technology (IROST).
Determining the inhibition halo diameter
Determining microbial sensitivity was carried out using the Agar well diffusion method as per standard instructions (Clinical Institute LS 2012). For this purpose, first, wells with a diameter of 6 mm were created in equal distances in the culture medium by a sterile Pasteur pipette, specific for creating wells. 100 μL of microbial suspensions (1.5 × 108 CFU/mL of bacteria, 104 spore/mL of fungi, or 106 CFU/mL of yeast) with 0.5 McFarland turbidity were separately cultured, respectively, in Mueller–Hinton Agar medium (Merck, Germany) and Dextrose Agar medium (Merck, Germany) using the Bacterial Lawn method. The essential oil of each plant sample was produced with a concentration of 300 μg/mL and 10 μL were inoculated into each well. After that, the plates were placed in an incubator for 24 h at 37 °C for the bacteria and 48 h for the C. albicans, and 72 h for the A. brasiliensis and A. niger in 30 °C. Dimethyl sulfoxide was used as negative control, and for positive control, the antibiotics gentamicin (10 μg/disc) and rifampin (5 μg/disc) were used for the bacteria and nystatin (100,000 units/mL) for the fungi to compare their inhibiting power with the essential oils. For each essential oil sample, the test was repeated three times and the anti-microbial activity was determined by a precise measurement of the inhibition halo diameter (in mL) as mean ± standard deviation.
Determining the minimum inhibitory concentration and the minimum bactericidal/fungicidal concentration
Minimum inhibitory concentration (MIC) for various microorganisms was carried out by implementing the dilution method in broth media (Clinical Institute LS 2012). Various dilutions of the essential oil were prepared first. A certain volume of the essential oil was weighed, dissolved in the culture medium and DMSO at a suitable ratio to prepare the initial stock at a concentration of 4 mg/mL. This stock was used to prepare the 2, 1, 0.25, 0.125, and 0.0625 mg/mL dilutions. Sterile 96-well microplates were procured; 95 μL of Brain Heart infusion (BHI; Merck, Germany) liquid medium, 5 μL of bacterial/fungal suspension (1.5 × 108 CFU/mL of bacteria, 104 spore/mL of fungi, or 106 CFU/mL of yeast), with 0.5 McFarland turbidity and 100 μL of one of the various essential oil dilutions (4, 2, 1, 0.5, 0.25, 0.125, and 0.0625 mg/mL) were all added to each well of the microplate. The negative control wells contained 195 μL of Brain Heart infusion liquid medium and 5 μL of microbial suspension with 0.5 McFarland turbidity. To create the positive control, gentamicin (10 μg/disc) and rifampin (5 μg/disc) antibiotic powders were used for the bacteria and nystatin (100,000 units/mL) for the fungi. The positive control wells contained 195 μL of Brain Heart infusion liquid medium and 5 μL of microbial suspension with 0.5 McFarland turbidity and 100 μL of one of the antibiotic concentrations (0.0625–4 mg/mL). After that, the microplates which were injected with bacteria and fungi strains were incubated, respectively, at 37 °C for 24 h and 30 °C for 48 and 72 h in an incubator. After being taken out of the incubator, the first concentration without a red hue formed inside was designated as the MIC. The MIC was the lowest concentration of an anti-microbial substance that inhibited visible growth (absence of turbidity). To determine the minimum bactericidal/fungicidal concentration (MBC/MFC), 5 μL of the well contents that the bacteria had not cultured in and did not manifest red hue was injected into the nutrient Agar medium and was incubated for 24 h at 37 °C temperature in the incubator. The first concentration of each essential oil that did not show any culture in its plate was designated as the MBC/MFC. MBC/MFC refers to the minimal concentration of the essential oil that kills 99.9% of the inoculated bacteria (Clinical Institute LS 2012). These tests were repeated for the essential oil of each plant species three times.
Cytotoxicity
Cell preparation and culture
The ovarian cancer cell line (OVCAR-3) was purchased from the National Cell Bank of Iran in Pasteur Institute of Iran. To culture the cells, the culture medium RPMI-1640 (Gibco, Invitrogen, USA), 10% fetal bovine serum (FBS) (Biochrom AG, Berlin, Germany), 2 mM l-glutamine (Sigma Aldrich, USA), 100 U/mL Penicillin, and 100 μg/mL of Streptomycin were utilized. The cells were cultured in a 25 cm3 flask at 37 °C with 5% CO2 pressure and 95% humidity (Sylvester 2011). After cell propagation and reaching 80% abundance, the culture medium was evacuated and the cells were separated from the bottom of the flask using Trypsin–EDTA (Gibco, USA) solution and were centrifuged at 1000 rpm for 5 min by a centrifuge device (Hermle, Germany). The resulting cellular deposit was washed into suspension in a 1 cc culture medium and the living cells were counted.
Treatment of cells
To determine the cytotoxicity of essential oils, the 3-(4,5-dimethylthiazolyl)-2,5-diphenyltetrazolium bromide (MTT; Sigma–Aldrich, USA) method was implemented (Mosmann 1983). MTT is a water-soluble yellow tetrazolium salt that reduces into purple color water-insoluble formazan by the action of mitochondrial succinate hydrogenase enzyme in living cells. This phenomenon occurs because of tetrazolium ring being broken which shows the natural performance of the mitochondria and the cell being alive (Lau et al. 2004). To perform this test, the cells with the density of 104 cells/mL were seeded into the 96-well plate and after 24 h, the cells filled the bottom of the wells. The cell culture medium was removed under a laminar hood. Then, a stock with a concentration of 400 μg/mL essential oil in culture medium was prepared and added to the wells in serial dilutions (400, 200.100, 50, 25, 12.5 and 6.25 μg/mL) with at least 5 repetitions.
After 24 h, first, the DMEM culture medium (Gibco, USA) containing the essential oils were evacuated and 20 μL of MTT solution (with 0.5 mg/mL concentration in PBS) was added. After 3 h of incubation with 5% CO2 at 37 °C in the incubator (Memmert, Germany), the culture medium was evacuated. To solve more of the purple-colored turbidity, 200 μL of dimethyl-sulfoxide (DMSO) solvent was added to each well. After complete dissolution, the solution absorbance intensity of each well was read in 570 nm wavelength by a microplate reader (BMG Labtech, Germany). After that, by a comparison between the average optical absorbance in wells of various concentrations and the optical absorbance of the control cells (no essential oil was added to the culture medium cells), the cell viability percentage was calculated as per the following equation (Oueslati et al. 2020):
The nonlinear regression curve on the inhibition percentage calculated for the concentration of each essential oil was drawn using GraphPad Prism 6. The concentration yielding up to 50% of cell growth inhibition was designated as per IC50. The test was repeated three times for each essential oil.
Statistical analysis
The evaluation of the statistical significance of differences (essential oil chemical compounds and bioactivities) was carried out implementing the univariate analysis of variance and ANOVA one-way variance analysis in SPSS. Duncan’s multiple range test (P < 0.01) was implemented to compare the means.
Results and discussion
Essential oil yield
The results of the variance showed that the effect of the species on the essential oil yield were significant at the error probability level of 1% (P ≤ 0.01). The effect of the species on the essential oil yield has been confirmed by Salimpour et al. (2011) for various salvia species, Zarezadeh et al. (2017) for various species of Satureja, Giatropoulos et al. (2018) for 14 different species of the Lamiaceae, and by Ghavam (2021) for 3 species of the Asteraceae family. The highest yield (2.0001% ± 0.0002) with a significant difference from other species belonged to the white-colored essential oil extracted from P. abrotanoides. Similarly, the yield of this essential oil has been reported to be 2% by Sajjadi et al. (2005) in Khorasan province, 1.55% by Rustaiyan et al. (2006) in Kerman province, 2.3% by Pourhosseini et al. (2018) and Salimpour et al. (2014) in Northern Khorasan province, and 2.66% by Ghaffari et al. (2019) in Khorasan Razavi province which are in accordance with the present results. The Z. clinopodioides subsp. rigida essential oil had a white color and a yield of (1.6001% ± 0.0001). This agrees with the results reported by Mirjalili et al. (2020) in Yazd province which indicated a percentage of 1.7, yet contradicts with the results reached by Salehi et al. (2005) in Tabriz province (1.0%), and Dehghan et al. (2014) in Hamedan province (0.1–37). The least yield (0.2286% ± 0.00006) belonged to the yellow-colored essential oil of S. reuteriana which was in accordance with the findings of Norouzi and Norouzi (2018) in Ahar city (2%). The highest recorded yield for S. reuteriana has been about 0.8% in Lorestan province and Tehran (Salimpour et al. 2011; Amiri et al. 2006). Differences in habitat characteristics such as height, slope, slope direction, coverage percentage, and other climate conditions have a substantial impact on the essential oil yield (Omara et al. 2021; Mirjalili et al. 2020).
Chemical composition of the essential oils
Eighty-nine different compounds (98.54–100%) were identified in the essential oils under study (Table 1 and Figs. 1, 2, 3). Regarding the type and number of compounds, P. abrotanoides, and Z. clinopodioides subsp. rigida essential oils, with 39 compounds and oxygenated monoterpene dominance had a significant difference with the S. reuteriana essential oil (26 compounds and nonterpenoid dominance). By a comparison between the present data about P. abrotanoides and Z. clinopodioides subsp. rigida essential oils, it can be seen that oxygenated monoterpenes have always been the dominant group of compounds in these essential oils (Salehi et al. 2005; Rustaiyan et al. 2006). Various studies on S. reuteriana have shown hydrocarbon and oxygenated sesquiterpenes (Esmaeili et al. 2008; Fattahi et al. 2016; Amiri et al. 2006; Rajabi et al. 2014). Moreover, in other studies, monoterpenes (Karamian et al. 2013) were the dominant part of the essential oil which contradict our results. The changes in the essential oil compounds might be a result of environmental (climate, seasons, geography) and genetic differences (Ghavam et al. 2021a; Russo et al. 2016).
Regarding the P. abrotanoides essential oil, the main compound was camphor (21.68%) along with 1,8-cineole (14.26%), α-pinene (7.23%), β-myrcene (4.90%), bornyl ester, acetic acid (4.34%), ledol (4.34%), β-caryophyllene (4.16%), and δ-cadinene (4.05%). Similarly, Ghaffari et al. (2019) found camphor (4.8–29.52%) as the main compound of this species which agrees with our results. There have been several studies about P. abrotanoides which report 1,8-cineole (27.1–28.0%) as the main compound of its essential oil (Sajjadi et al. 2005). There has been no report of bornyl ester, acetic acid, and ledol in previous studies; thus, our study is the first to report the presence of these compounds in this essential oil. Moreover, in previous studies, δ-cadinene was either not reported or reported in very low amounts (0.4–1.1%) which is in contradiction with the results of the current study (Sajjadi et al. 2005; Rustaiyan et al. 2006). Despite numerous reports which have recorded δ-3-carene as the main compound of the P. abrotanoides essential oil (Inouye et al. 2001; Ghaffari et al. 2019; Pourhosseini et al. 2018; Sajjadi et al. 2005; Salimpour et al. 2014), our study found (+)-3-carene as a stereoisomer of this compound present at the amount of 3.24% in this essential oil. The ecologic conditions of various habitats have affected the biosynthesis routes of the essential oil of this species; therefore, different compounds are synthesized in different environments (Azarnivand et al. 2010; Ghavam et al. 2021b).
The analysis of the chemical compounds of the S. reuteriana essential oil showed benzyl benzoate (27.10%), linalool (13.27%), sclareol (7.75%), hexyl ester, benzoic acid (6.29%), 3-methyl, benzoate, 1-butanol (4.92%), and γ-selinene (4.32%) to be the dominant and main compounds, respectively. Benzyl benzoate, hexyl ester, benzoic acid, benzoate, 3-methyl, and 1-butanol were detected in the essential oil of S. reuteriana for the first time in the present study; therefore, this can be considered as a special feature. However, in contrast to the present results, other studies have reported linalool to have an insignificant amount of 0.1% (Aminzadeh et al. 2015) and 0.17% (Fattahi et al. 2016) in this essential oil. Despite the present results, in previous studies, γ-selinene was only found in the area of Anjileh in the small amount of 1.28% (Fattahi et al. 2016). The compound germacrene D has been reported to be the first dominant compound for the S. reuteriana essential oil in various amounts (11.17–32.50%) by many studies (Esmaeili et al. 2008; Salimpour et al. 2011; Amiri et al. 2006; Mazooji et al. 2010); whereas, the present findings indicated that this compound existed at 1.12% and was not dominant. The results indicate that these compounds vary in accordance with the ecologic features of a species population. Therefore, various geographical regions are an important factor in the variety of chemical compounds in the essential oil of a species population; in addition, some chemical compounds are specific to a particular species and thus designated as a taxonomic factor (Mazooji et al. 2010).
Cyclofenchene (25.29%), pulegone (14.14%), menthol (7.70%), p-menth-3-en-8-ol (7.20%), borneol (5.30%), and piperitenone (4.51%) were, respectively, the main components of the Z. clinopodioides subsp. rigida essential oil. cyclofenchene has never been reported for this sub-species in the area even in small amounts. Pulegone has been reported as the first main component of the sub-species (5.2–60.4%) in previous studies carried out in various areas and the variations in its amount were deemed to be associated with the effects of habitat conditions (Dehghan et al. 2014, 2010; Salehi et al. 2005; Mirjalili et al. 2020). One significant point about the essential oil of this sub-species in the present study was the absence of 1,8-cineole and the dominance of menthol instead. A review of the literature revealed that menthol has never been detected in this sub-species (Dehghan et al. 2014, 2010; Mirjalili et al. 2020) or was recorded in very small amounts (0.1%) (Salehi et al. 2005). Similarly, borneol (3.9%) was reported by Mirjalili et al. (2020) as the fifth dominant compound which is an exact match with our findings. The presence or absence of some compounds indicates the unique characteristics of this sub-species in the area chosen by this study. These variations result from the different environmental conditions of the chosen habitat compared to the previous studies and can imply a Chemotype of this sub-species (Ghavam et al. 2021c).
Bactericidal/fungicidal activity
The results of determining the inhibition halo diameter, implementing the Agar well diffusion, showed that only the S. reuteriana essential oil formed an inhibition halo (~ 22 mm) against the Gram-positive bacteria Streptococcus pyogenes which was significant (P ≤ 0.01) compared to the inhibition halos of the positive controls rifampin (~ 21 mm) and gentamicin (~ 32 mm) (Table2). This indicates the strong bactericidal activity of this essential oil and might be a potential natural alternative agent against S. pyogenes. Moreover, the findings on the minimum inhibitory concentration and inhibition by dilution indicated that the value of MIC and MBC of the S. reuteran essential oil against S. pyogenes was 62.50 μg/mL which compared to the positive controls rifampin and gentamicin (MIC = 0.975 μg/mL) had a relatively good effect. The Gram-positive bacteria show more susceptibility to essential oils. There is mucopeptide in their cell wall and the major part of the wall structure contains lipoprotein and lipopolysaccharide (Tabatabaei et al. 2014). A review of the literature shows no previous reports of the effect of the S. reuteran essential oil and extract against S. pyogenes. On the other hand, there are reports about the strong effects (MIC = 125 μg/mL and MBC = 250 μg/mL) of some Salvia species such as Salvia officinalis L. against this bacterium (Wijesundara and Rupasinghe 2019, 2018). Streptococcal pharyngitis is one of the most common upper respiratory infections in children and young adults from 5 to 15 years old (Shaikh et al. 2010). The major bacterial causes of streptococcal pharyngitis are Streptococcus pyogenes (Abachi et al. 2016). Studies show that S. pyogenes has become resistant to antibiotics in many countries and resistant variants are rapidly spreading around the world (Wijesundara and Rupasinghe 2019; Richter et al. 2005). Therefore, the present study is of great significance being the first to report the inhibitory strength of S. reuteriana essential oil against this bacterium. It seems that the dominance of non-terpene compounds such as venzonate, sclareol, hexyl ester, benzoic acid, benzoate, 3-methyl-, and 1-butanol and also the oxygenated monoterpene linalool and the sesquiterpene γ-selinene are the major factors contributing to this strong bactericidal activity. Linalool is a key ingredient in the production of many home products, cosmetics, and chemical aromatic goods; moreover, it is an organic compound in the synthesis of vitamins A and E (Herman et al. 2016; Kamatou and Viljoen 2008). There have been reports of the bactericidal effect of the essential oils against S. pyogenes with linalool as their main compound (Sfeir et al. 2013). The oxygenated monoterpenes are lipophile compounds that act in the cell membrane and cause many morphologic damages eventually leading to changes in the membrane permeability and diffusion of cell contents; thus, they manifest significant anti-microbial qualities (Moosavy et al. 2008). The anti-microbial effect of the essential oils is not only due to their main compounds. Regarding this essential oil, the compounds that exist in smaller amounts such as monoterpenes 1,8-cineole, β-ocimene, and sesquiterpenes β-elemene, germacrene D, α-gurjunene, and β-cadinene can also have a part in the anti-microbial activity. In fact, there is the possibility of a synergistic effect between the compounds of a lower percentage and the effective and active compounds (Bassolé and Juliani 2012; Younessi et al. 2019). On the other hand, this essential oil did not form any inhibition halo against other Gram-positive bacteria. Esmaeili et al. (2008) reported inhibition halos against Staphylococcus aureus (about 35 mm) and Staphylococcus epidermidis (about 20 mm). Karamian et al. (2013) reported an inhibition halo diameter against Bacillus subtilis (about 11 mm) which is not in accordance with the present study. Moreover, this essential oil acted two, three, and six times weaker than rifampin against S. aureus, B. subtilis, and S. epidermidis, respectively. It seems that the higher inhibition against S. aureus is due to the dominance of linalool. The inhibition effect of linalool against S. aureus (about 9–63 mm) has been reported (Herman et al. 2016; Yi et al. 2019).
The minimum inhibitory and bactericidal/fungicidal concentration value of P. abrotanoides and Z. clinopodioides subsp. rigida varied from 2000 to 4000 μg/mL against various microorganisms. This shows a significant difference from the minimum inhibitory and bactericidal/fungicidal concentration value of S. reuteriana and positive controls (P ≤ 0.01) (Table 3). In fact, the effect of the P. abrotanoides and Z. clinopodioides subsp. rigida essential oils against various variations under study were similar and very weak. It seems that the similarity in the chemical profile of the essential oil of these two plants regarding oxygenated monoterpenes could be a possible cause for this similar anti-microbial activity.
Although the S. reuteriana essential oil did not form an inhibitory halo against the variations of Gram-negative bacteria and fungi, it was effective in their inhibition at different concentrations. The minimum inhibitory and bactericidal/fungicidal concentration against some variations was significant compared to antibiotic controls. The strongest inhibition activity of this essential oil was against Candida albicans (MIC and MFC 125 μg/mL). The inhibitory strength was equal to nystatin (125 μg/mL). There has been no study recorded about the effect of the essential oil and extract of S. reuteriana against C. albicans which makes this study the first report to introduce a potent and promising possible natural alternative against this yeast. Candida albicans is an opportunistic fungus which is the cause of epithelial and systemic infections in individuals suffering from immune system deficiency and diabetes (Dehghani et al. 2015). In similar studies, there have been reports of strong anti-yeast activity against C. albicans by Sookto et al. (2013) for Salvia officinalis L., (Potente et al. 2020) for Salvia mirzayanii Rech.f. & Esfand., and Ghavam et al. (Ghavam et al. 2020) for Salvia hydrangea DC. ex Benth. It seems that the dominance of linalool in the S. reuteriana essential oil is a contributing factor for this activity. The strong inhibitory effects of linalool against C. albicans have been proven (Kunduhoglu 2017). Regarding its effects on other fungi variants, the S. reuteriana essential oil had very little effect on the inhibition of A. brasiliensis and A. sniger (> 2000 μg/mL).
Another of S. reuteran essential oil’s strong activities was against the Gram-negative bacterium Pseudomonas aeruginosa (MIC = 62.50 μg/mL and MBC = 500 μg/mL). Similarly, the bactericidal activity of S. reuteran essential oil against P. aeruginosa has been reported with the formation of an inhibition halo diameter (13–16 mm) (Esmaeili et al. 2008). Pseudomonas aeruginosa is an opportunistic and important pathogen resistant to most antibiotics (Harris et al. 2002). Therefore, the treatment of infections caused by this bacterium has always been challenging (Akhani 2006). This bacterium is one of the main causes of death among people suffering from immune deficiency, patients affected by severe burns, and people suffering from Cystic Fibrosis (Roshani et al. 2016; Ryall et al. 2008). Ghavam et al. (2020) have attributed the strong anti-bacterial activity of the S. hydrangea essential oil against P. aeruginosa bacterium to the existence of oxygenated monoterpenes such as 1,8-cineole. In the present study the oxygenated monoterpenes as dominant components and 1,8-cineole in a slight way are the factors contributing to the strong activity against P. aeruginosa as well. Herman et al. (2016) have reported that adding linalool to the Syzygium aromaticum (L.) Merr. & L.M.Perry essential oil synergistically increased the anti-microbial activity against P. aeruginosa. Regarding the mechanism of action for this compound against this bacterium, it has been mentioned that linalool first affects the cell membrane and causes damage to the structure and performance of the membrane and the diffusion of macromolecules inside the cell. Moreover, it seems that linalool affects some enzymes inside the cell by the inhibition of enzyme activity related to the TCA cycle and glycolysis pathway (Guo et al. 2021).
The results showed that the S. reuteriana essential oil had an acceptable effect against the Gram-negative bacteria Klebsiella pneumonia (MIC = 62.50 μg/mL and MBC = 500 μg/mL) compared to rifampin (MIC = 31.25 μg/mL). Klebsiella pneumonia is an opportunistic pathogen and a cause of infection in humans and animals. Antibiotic resistance is increasing in the isolates of Klebsiella pneumonia (Estabraghi et al. 2018). Although there is no report about the effect of S. reuteran against this bacterium, some reports exist about the effect of some Salvia species. For instance, strong activity against K. pneumonia has been recorded by Fournomiti et al. (2015) for the S. officinalis essential oil, and by Ghavam et al. (2020) for the S. hydrangea essential oil.
The inhibitory effects of S. reuteriana essential oil on the Gram-negative bacteria Escherichia coli (MIC = 62.50 μg/mL and MBC = 125 μg/mL) were not significant with four times weaker activity compared to the positive controls (MIC = 3.90 μg/mL). Escherichia coli (E. coli), the saprotrophic micro-organism in the human intestine, can cause serious infections resistant to anti-microbial treatments (Mendoza-Palomar et al. 2017). Esmaeili et al. (2008) have recorded the inhibitory strength of the S. reuteriana essential oil against this bacterium with the identification of the inhibition halo diameter (13–16 mm). The effect of linalool against the E. coli has been proven (Herman et al. 2016). Moreover, in other studies link, the strong activity of the essential oils against this bacterium to germacrene D dominance (Liolios et al. 2007) or β-elemene dominance (Adebayo et al. 2014). It seems that for the present species, the slight presence of germacrene D and β-elemene are among the other contributing factors for this anti-bacterial activity. On the other hand, the S. reuteriana essential oil had the same effect against the Gram-negative bacteria Salmonella paratyphi-A serotype and Shigella dysenteriae (MIC and MBC = 125 μg/mL) which was extremely weak compared to rifampin (MIC = 3.90 μg/mL) and relatively weak compared to gentamicin (MIC = 15.36 μg/mL). Contrary to our findings, Ghavam et al. (2020) reported strong activity against Sh. dysenteriae for S. hydrangea essential oil. The type and amount of the essential oil components determine the anti-microbial activity. The difference in anti-microbial activity of various essential oils of the same species could be attributed to differences in chemical profiles (Popović-Djordjević et al. 2019).
Cytotoxicity
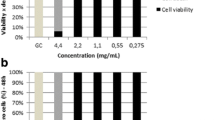
The cytotoxic activity results of various essential oils using the MTT method after 24 h of treatment with the ovarian cancer cell line (OVCAR-3) are illustrated in Figs. 4, 5, and 6. The results indicated that the ovarian cancer cell survival depended on the concentration; the more concentrated the essential oil, the less the survival of the cancer cells. This shows a significant difference from the control (P ≤ 0.01). The 6.25, 12.50, and 25 μg/mL concentrations of P. abrotanoides and S. reuteriana essential oils had no effect on the survival of the cells; whereas, the 125 and 250 μg/mL concentrations of these two essential oils lead to the death of the OVCAR-3 cells in 24 h. The 6.25 μg/mL concentration of the Z. clinopodioides subsp. rigida essential oil had the least impact and the 100, 200 and 400 μg/mL concentrations had significant increasing effects on the death of OVCAR-3 cells in 24 h. The studies that have been carried out on the subject of inhibition and cytotoxic activities of the essential oils of the Lamiaceae family against different cancers show that the lethality of these plants depend on the cell type and vary for different cell lines (Hajebi et al. 2017; Shakhseniaie et al. 2020; Wang et al. 2012). The MTT analysis showed that the Ziziphora clinopodioides subsp. rigida essential oil with the IC50 value of 144.2500 ± 2.8723 μg/mL had the strongest activity against human ovarian cancer cells, having a significant difference from the two other essential oils (P ≤ 0.01). There has been no report of the cytotoxicity of the essential oil and extract of this sub-species; nevertheless, in other studies of this nature exist about the Ziziphora clinopodioides Lam species. Similarly, Ghazanfari et al. (2013) reported the effect of Z. clinopodioides essential oil against Adenocarcinoma (AGS) gastric cancer cell line with IC50 value of 2.356 mg/mL. Yousefbeyk et al. (2016) recorded the IC50 values of Z. clinopodioides essential oil on intestine cancer (HT-29), leukemia (K562), and breast cancer (T-47D) cell lines at > 1000, 319.48 ± 11.2, and 633.29 ± 3.1 μg/mL, respectively. They attributed this activity to the dominance of pulegone, menthol, and menthone compounds. Ahmadi et al. (2021) reported a noticeable decrease in the survival of human lymphocytes for the methanolic extract of Z. clinopodioides from 52 to 100 implementing the MTT method. Various cell lines could have diverse sensitivities to various essential oils; therefore, this difference is most probably due to the diversity in the essential oil compounds and the various mutual reactions for essential oil components with various cell lines (Bardaweel et al. 2014; Silva et al. 2020). The anticancer activity of each of the compounds present in the essential oils in the cancer cell lines under study has not been evaluated individually; therefore, it cannot be claimed with certainty that which compounds are responsible for the observed effects. Nevertheless, it seems that in the Ziziphora clinopodioides subsp. rigida essential oil, the dominance of monoterpenes such as cyclofenchene, pulegone, menthol, p-menth-3-en-8-ol, borneol, and piperitenone are the contributing factors to this cytotoxic activity. The cytotoxic activity of pulegone, piperitenone, menthofuran, and menthone against the urothelial cells of mice (MYP-3) and humans (1T1) has been confirmed (Rocha et al. 2012). Previous research has shown that dependent on concentration, menthol causes cytotoxicity against blood cancer cells WEHI-3 in vitro; furthermore, due to the mobilization of Ca2+ from the endoplasmic reticulum, it causes apoptosis in human leukemia cells HL-60 (Lu et al. 2007). Moreover, it has been proven that menthol inhibits the DNA topoisomerase I, II alpha, and beta and has increased the expression of NF-kappa B in human stomach cancer cells SNU-5 (Yousefbeyk et al. 2014). In addition, the minor compounds of the essential oil can also synergize with the major compounds and impact this activity (Cardile et al. 2009). Therefore, compounds such as γ-terpinene, Linalool, Piperitone, Thymol, β-elemene, α-Humulene, and germacrene D have also been effective on the cytotoxic activity of this essential oil. For instance, Russo et al. (2016) have reported that the thymol present in Salvia judaica Boiss. and Salvia viscosa Jacq essential oils inhibits growth in cancer cells such as HL-60, MCF-7, PC-3, A-549, and MDAMB-231 and can cause apoptosis in HL-60 cells.
The results indicated that the IC50 value of the S. reuteriana essential oil against human ovarian cancer cells (OVCAR-3) was 183.5000 ± 0.5774 μg/mL which shows a significantly weaker performance compared to Ziziphora clinopodioides subsp. rigida essential oil (P ≤ 0.01). The concentration-dependent cytotoxic activity of S. reuteriana essential oil against HeLa (human cervical cancer), Raji (Burkitt’s lymphoma), Fen (bladder cancer), K562 (myelogenous leukemia), and Jurkat (T cell leukemia) has been reported to have IC50 values of 156 ± 5, 21 ± 0.8, > 200, > 200, and 158 ± 6 μg/mL, respectively (Lu et al. 2007). Similarly, Don et al. (2006) reported the cytotoxic activity of Salvia miltiorrhiza Bunge against OVCAR-3. The anticancer activity of plant essential oils is mainly attributed to terpenoids (Langhasova et al. 2014). By the influx of terpenes to the lipid compositions of the cell membrane, the cell walls are damaged, which leads to cytoplasm decay, and the denaturation of peptide compounds leads to apoptosis (D'Ambro et al. 2017). Although the non-terpene compounds constitute the major portion of S. reuteriana essential oil compounds, it seems that linalool dominance as an oxygenated monoterpene is the main cause for this activity. The antimicrotubule effects of pulegone and linalool have been proven. These molecular compounds accelerate the formation of microtubules from tubulin dimers which leads to the inhibition of microtubule depolymerization (Nasiri Tarzejani and Nasri 2020). This inhibits microtubule stability in cells, eventually leading to the demolition of flexible cell skeleton inside the cell which is crucial to vital cycles such as mitosis. Such cells which encounter growth and division suspension face deficiency and apoptosis (Triantaphyllou and Boskou 2001). Nevertheless, as previously mentioned, there is the possibility of the minor molecules regulating the activity of the essential oil compounds and acting synergically as active compounds (Bakkali et al. 2008). Therefore, monoterpenes 1,8-cineole, and β-ocimene, and sesquiterpenes β-elemene, germacrene D, α-gurjunene, and β-cadinene can also be effective in this activity. The cytotoxic activity of the 1,8-cineole against the cell line SF-9 by the increase of p53 acetylation expression has been confirmed (Mobarakian et al. 2021). Numerous studies have shown that β-elemene inhibits the growth of human lung and ovarian cancer cells (Li et al. 2009, 2005; Wang et al. 2005; Zhao et al. 2007). Salvador et al. (2011) attributed the cytotoxic activity of the Casearia lasiophylla Eichler essential oil against the human ovarian cancer cell line (OVCAR-3) to germacrene D dominance. The inhibition strength of Jatropha ribifolia (Pohl) Baill (GI50 = 8.0 μg/mL) essential oil against human ovarian cancer (OVCAR-3) was attributed by Silva et al. (2015) to the oxygenated sesquiterpenes. In other studies, the anticancer activity of various Salvia species is attributed to the presence of diterpenoids with diverse skeletons such as abietanes, labdanes, clerodanes, pimaranes, and icetexanes (Akaberi et al. 2015).
Based on the results, P. abrotanoides essential oil with IC50 value of 277.0000 ± 0.8665 μg/mL was effective against human ovarian cancer cells (OVCAR-3). Similarly, Iraji et al. (2021) have reported that the hydroalcoholic essential oil of P. abrotanoides has significant cytotoxic activity against human stomach adenocarcinoma cancer cells MKN-45 (IC50 = 10 μg/mL), where the cell division decrease was dependent on concentration. Geryani et al. (2016) have confirmed that the cytotoxic activity of P. abrotanoides essential oil against the MCF-7 (500–1000 μg/mL) and Hela (1000 μg/mL) cell lines is dependent on concentration. Zaker et al. (2017) have reported the IC50 value of the P. abrotanoides root extract against MCF-7 and Hela cell lines to be 87.69 μg/mL and 24.83 μg/mL respectively. Based on the literature review, there is a hypothesis that the cytotoxic effects shown by P. abrotanoides essential oil can be related to monoterpenes present in this essential oil in significant amounts compared to other compounds. It seems that the dominant monoterpenes camphor, 1,8-cineole, α-pinene, and β-myrcene along with the other minor monoterpenes such as camphene, β-pinene, and γ-terpinene are the main reasons for the cytotoxic activity of this essential oil. The cytotoxic activity of α-pinene and γ-terpinene against Jurkat (T cell leukemia) cell lines, J774A.1 (mice macrophage tumor), and HeLa (cervical cancer) has been confirmed (Döll-Boscardin et al. 2012). Studies have shown that α-pinene is capable of inducing apoptosis and is effective in treating malignant melanoma and curing metastatic melanoma (Matsuo et al. 2011). Based on the literature review, β-pinene possesses strong antiproliferative effects against A-549 (IC50 = 85.0 μM) (Bourgou et al. 2010), and has average inhibitory activity against human Erythroleukemia K562 cells (Lampronti et al. 2006). As another example, the cytotoxic activity of Salvia officinalis essential oil with dominant compounds thujone, β-pinene, and 1,8-cineole has been reported against the oral cavity (UMSCC1) cell line (Sertel et al. 2011). Moreover, dominant sesquiterpenes ledol, β-caryophyllene, and δ-cadinene, as well as minor ones such as caryophyllene oxide and α-cadinol, are thought to be among the possible contributing factors for this activity. Studies have shown that β-caryophyllene and cadinol exhibit cytotoxic activity against mouth, liver, lung, large intestine, melanoma, and blood cancer cells (Russo et al. 2013). It has been reported in experimental studies that caryophyllene oxide is capable of inhibiting life in a wide variety of tumor cells by apoptosis (Gautam et al. 2014; Kim et al. 2014; Sain et al. 2014). Previous studies have confirmed cytotoxic effects of various species of Salvia containing caryophyllene oxide against melanoma cells (Russo et al. 2013, 2015; Cardile et al. 2009). The effects of α-humulene on the inhibition of liver cancer CaCo-2 cells have been reported (Ambrož et al. 2015).
Numerous studies have confirmed that cyclooxygenase enzyme and oxidative stress cause disruption in various cellular cycles such as growth and division which result in disorder and cancer (Kim et al. 2008). For instance, cyclooxygenase enzyme inhibits apoptosis in cancer cells by overactivity (Hajebi et al. 2017; Nasiri Tarzejani and Nasri 2020). It is clear that the plants having the capability of inhibiting these factors can play a vital role in the prevention and spread of cancer. Various studies have proven the presence of factors in the Lamiaceae family that enhance oxidative capacity (Triantaphyllou and Boskou 2001). Impacting the morphology of cancer cells is one of the mechanisms that the Lamiaceae family affect these cells. The cells are dependent on the surface, but the essential oil of the plants of this family affect the permeability of the cell membrane, causing circularization and release from media which causes death and inhibits growth (Surmaghi et al. 1992).
Conclusion
The P. abrotanoides and Ziziphora clinopodioides subsp. rigida essential oils dominated by oxygenated monoterpenes differed in the phytochemical to S. reuteriana essential oil which contain nonterpenoids as the major constituent. The dominant compounds in these three essential oils were camphor, cyclofenchene, benzyl benzoate in order, which suggest that this may be a new chemotype for these species with different dominant chemical compounds. In this study, the difference in essential oil compounds leads to the exhibition of diverse and significant bioactivities. On the whole, the S. reuteriana essential oil showed strong bactericidal/fungicidal effects against S. pyogenes and C. albicans, and Ziziphora clinopodioides subsp. rigida had strong cytotoxicity against the human ovarian cancer cell line. Therefore, it can be deduced that these essential oils as natural medicine can be strong potential alternatives to anti-microbial and anticancer chemical drugs. The anticancer effects have not been shown outside cell culture work. The matter which calls for clinical research in the future.
Data availability
Enquiries about data availability should be directed to the authors.
References
Abachi S, Lee S, Rupasinghe H (2016) Molecular mechanisms of inhibition of Streptococcus species by phytochemicals. Molecules 21(2):215
Adams RP (2007) Identification of essential oil components by gas chromatography/mass spectrometry, vol 456. Allured publishing corporation, Carol Stream
Adebayo MA, Lawal OA, Sikiru AA, Ogunwande IA, Avoseh ON (2014) Chemical constituents and antimicrobial activity of essential oil of Senna podocarpa (Guill. Et Perr.) lock. Am J Plant Sci 5 (15):2448–2453
Ahmadi A, Gandomi H, Derakhshandeh A, Misaghi A, Noori N (2021) Phytochemical composition and in vitro safety evaluation of Ziziphora clinopodioides Lam. ethanolic extract: cytotoxicity, genotoxicity and mutagenicity assessment. J Ethnopharmacol 266:113428
Akaberi M, Mehri S, Iranshahi M (2015) Multiple pro-apoptotic targets of abietane diterpenoids from Salvia species. Fitoterapia 100:118–132
Akhani H (2006) Flora Iranica: facts and figures and a list of publications by KH Rechinger on Iran and adjacent areas. Rostaniha 7(Suppl 2):19–61
Aliakbarlu J, Shameli F (2013) In vitro antioxidant and antibacterial properties and total phenolic contents of essential oils from Thymus vulgaris, T. kotschyanus, Ziziphora tenuior and Z. clinopodioides. Turk J Biochem 38(4):425–431
Ambrož M, Boušová I, Skarka A, Hanušová V, Králová V, Matoušková P, Szotáková B, Skálová L (2015) The influence of sesquiterpenes from Myrica rubra on the antiproliferative and pro-oxidative effects of doxorubicin and its accumulation in cancer cells. Molecules 20(8):15343–15358
Aminzadeh M, Jamshidi A, Mortazavimoghadam F, Azarnivand H, Naghavi M, Sarvestani R (2015) Evaluation of phytochemical and compare the yield of antioxidant essential oils and extracts of Salvia reuteriana Boiss. from Damavand region. Ecophytochem J Med Plants 3(3):1–9
Amiri H, Meshkat AM, Lari YH, Goudarzi A (2006) Essential oil composition of Salvia reuteriana Boiss. 22(3):270–275
Azarnivand H, Ghavam Arabani M, Sefidkon F, Tavili A (2010) The effect of ecological characteristics on quality and quantity of the essential oils of Achillea millefolium L. subsp Millefolium. Iran J Med Aromat Plants Res 25(4):556–571
Bahmani M, Zargaran A, Rafieian-Kopaei M (2014) Identification of medicinal plants of Urmia for treatment of gastrointestinal disorders. Rev Bras 24(4):468–480
Bakkali F, Averbeck S, Averbeck D, Idaomar M (2008) Biological effects of essential oils–a review. Food Chem Toxicol 46(2):446–475
Bardaweel SK, Tawaha KA, Hudaib MM (2014) Antioxidant, antimicrobial and antiproliferative activities of Anthemis palestina essential oil. BMC Complement Altern Med 14(1):1–8
Bassolé IHN, Juliani HR (2012) Essential oils in combination and their antimicrobial properties. Molecules 17(4):3989–4006
Bourgou S, Pichette A, Marzouk B, Legault J (2010) Bioactivities of black cumin essential oil and its main terpenes from Tunisia. S Afr J Bot 76(2):210–216
Brahim MA, Fadli M, Hassani L, Boulay B, Markouk M, Bekkouche K, Abbad A, Ali MA, Larhsini M (2015) Chenopodium ambrosioides var. ambrosioides used in Moroccan traditional medicine can enhance the antimicrobial activity of conventional antibiotics. Ind Crop Prod 71:37–43
Cardile V, Russo A, Formisano C, Rigano D, Senatore F, Arnold NA, Piozzi F (2009) Essential oils of Salvia bracteata and Salvia rubifolia from Lebanon: chemical composition, antimicrobial activity and inhibitory effect on human melanoma cells. J Ethnopharmacol 126(2):265–272
Chalchat J, Michet A, Pasquier B (1998) Study of clones of Salvia officinalis L. yields and chemical composition of essential oil. Flavour Fragr J 13(1):68–70
Clinical Institute LS (2012) Performance standards for antimicrobial disk susceptibility tests; approved standard. Clinical and Laboratory Standards Institute, Wayne
Costa DC, Costa H, Albuquerque TG, Ramos F, Castilho MC, Sanches-Silva A (2015) Advances in phenolic compounds analysis of aromatic plants and their potential applications. Trends Food Sci Technol 45(2):336–354
Da Rocha MS, Dodmane PR, Arnold LL, Pennington KL, Anwar MM, Adams BR, Taylor SV, Wermes C, Adams TB, Cohen SM (2012) Mode of action of pulegone on the urinary bladder of F344 rats. Toxicol Sci 128(1):1–8
da Silva CE, Minguzzi S, da Silva RC, Matos MF, Tofoli D, Carvalho JE, Ruiz AL, Costa WF, Simionatto E (2015) Chemical composition and cytotoxic activity of the root essential oil from Jatropha ribifolia (Pohl) Baill (Euphorbiaceae). J Braz Chem Soc 26:233–238
D'Ambro E, Schobesberger S, Lopez-Hilfiker F, Shilling JE, Lee BH, Thornton JA (2017) Molecular characterization and volatility evolution of α-pinene ozonolysis SOA during isothermal evaporations. AGU Fall Meeting Abstracts 2017: A12G-02
Dehghan Z, Sefidkon F, Khaniki G, Kalvandi R (2010) Effects of some ecological factors on essential oil content and composition of Ziziphora clinopodioides Lam. Subsp. rigida (Boiss.). Iran J Med Aromat Plants 26(1):49–63
Dehghan Z, Sefidkon F, Emami S, Kalvandi R (2014) The effects of ecological factors on essential oil yield and composition of Ziziphora clinopodioides lam. subsp. Rigida (Boiss) Rech. F J Plant Res 27(1):61–71
Dehghani E, Eidi M, Noorbakhsh F (2015) Antifungal effect of hydro-methanolic extract of Salvia officinalis leaves on growth of Candida albicans in diabetic male rats. Iran J Biol Sci 10(1):13–19
Döll-Boscardin PM, Sartoratto A, Sales Maia BHLN, Padilha de Paula J, Nakashima T, Farago PV, Kanunfre CC (2012) In vitro cytotoxic potential of essential oils of Eucalyptus benthamii and its related terpenes on tumor cell lines. Evid Based Complement Altern Medicine 2012:342652
Don M-J, Shen C-C, Syu W-J, Ding Y-H, Sun C-M (2006) Cytotoxic and aromatic constituents from Salvia miltiorrhiza. Phytochemistry 67(5):497–503
Elhidar N, Nafis A, Kasrati A, Goehler A, Bohnert JA, Abbad A, Hassani L, Mezrioui NE (2019) Chemical composition, antimicrobial activities and synergistic effects of essential oil from Senecio anteuphorbium, a Moroccan endemic plant. Ind Crops Prod 130:310–315
Esmaeili A, Rustaiyan A, Nadimi M, Larijani K, Nadjafi F, Tabrizi L, Chalabian F, Amiri H (2008) Chemical composition and antibacterial activity of essential oils from leaves, stems and flowers of Salvia reuteriana Boiss. grown in Iran. Nat Prod Res 22(6):516–520
Estabraghi E, Zahraei Salehi T, Amini K, Jamshidian M (2018) Survey genotyping of animal and human Klebsiella pneumoniae isolates using ERIC-PCR and evaluation of antibiotic sensitivity pattern. J Comp Pathobiol 15(2):2469–2478
Fattahi B, Nazeri V, Kalantari S, Bonfill M, Fattahi M (2016) Essential oil variation in wild-growing populations of Salvia reuteriana Boiss. collected from Iran: using GC–MS and multivariate analysis. Ind Crops Prod 81:180–190
Fournomiti M, Kimbaris A, Mantzourani I, Plessas S, Theodoridou I, Papaemmanouil V, Kapsiotis I, Panopoulou M, Stavropoulou E, Bezirtzoglou EE (2015) Antimicrobial activity of essential oils of cultivated oregano (Origanum vulgare), sage (Salvia officinalis), and thyme (Thymus vulgaris) against clinical isolates of Escherichia coli, Klebsiella oxytoca, and Klebsiella pneumoniae. Microb Ecol Health Dis 26(1):23289
Gautam N, Mantha AK, Mittal S (2014) Essential oils and their constituents as anticancer agents: a mechanistic view. BioMed Res Int 2014:154106
Geryani MA, Mahdian D, Mousavi SH, Hosseini A (2016) Ctotoxic and apoptogenic effects of Perovskia abrotanoides flower extract on MCF-7 and HeLa cell lines. Avicenna J Phytomed 6(4):410
Ghaffari Z, Rahimmalek M, Sabzalian MR (2019) Variation in the primary and secondary metabolites derived from the isoprenoid pathway in the Perovskia species in response to different wavelengths generated by light emitting diodes (LEDs). Ind Crops Prod 140:111592
Ghahraman A (1994) Iran cormqphit (plant systematic). Tehran University Publication Center, Tehran, pp 294–295
Ghava M (2021) Tripleurospermum disciforme (CA Mey.) Sch. Bip., Tanacetum parthenium (L.) Sch. Bip, and Achillea biebersteinii Afan.: efficiency, chemical profile, and biological properties of essential oil. Chem Biol Technol Agric 8:45
Ghavam M, Manca ML, Manconi M, Bacchetta G (2020) Chemical composition and antimicrobial activity of essential oils obtained from leaves and flowers of Salvia hydrangea DC. ex Benth. Sci Rep 10(1):1–10
Ghavam M, Manconi M, Manca ML, Bacchetta G (2021a) Extraction of essential oil from Dracocephalum kotschyi Boiss. (Lamiaceae), identification of two active compounds and evaluation of the antimicrobial properties. J Ethnopharmacol 267:113513
Ghavam M, Afzali A, Manconi M et al (2021b) Variability in chemical composition and antimicrobial activity of essential oil of Rosa × damascena Herrm. from mountainous regions of Iran. Chem Biol Technol Agric 8:22
Ghavam M, Afzali A, Manca ML (2021c) Chemotype of damask rose with oleic acid (9 octadecenoic acid) and its antimicrobial effectiveness. Sci Rep 11:8027
Ghazanfari T, Yaraee R, Shams J, Rahmati B, Radjabian T, Hakimzadeh H (2013) Cytotoxic effect of four herbal medicines on gastric cancer (AGS) cell line. Food Hydrocolloids 24(1):1–7
Giatropoulos A, Kimbaris A, Michaelakis Α, Papachristos DP, Polissiou MG, Emmanouel N (2018) Chemical composition and assessment of larvicidal and repellent capacity of 14 Lamiaceae essential oils against Aedes albopictus. Parasitol Res 117(6):1953–1964
Grayer RJ, Eckert MR, Veitch NC, Kite GC, Marin PD, Kokubun T, Simmonds MS, Paton AJ (2003) The chemotaxonomic significance of two bioactive caffeic acid esters, nepetoidins A and B, in the Lamiaceae. Phytochemistry 64(2):519–528
Guo F, Chen Q, Liang Q, Zhang M, Chen W, Chen H, Yun Y, Zhong Q, Chen W (2021) Antimicrobial activity and proposed action mechanism of linalool against Pseudomonas fluorescens. Front Microbiol 12:49
Hajebi S, Chehregani Rad A, Lariyazdi H (2017) Study of cytotoxic effect of essential oil of Tymus vulgaris L by MTT and Trypan blue assay on breast cancer cell line MCF-7. Dev Biol 9(4):1–12
Harris AD, Smith D, Johnson JA, Bradham DD, Roghmann M-C (2002) Risk factors for imipenem-resistant Pseudomonas aeruginosa among hospitalized patients. Clin Infect Dis 34(3):340–345
Hasan M, Iqbal R, Ullah I, Inam U (1978) Antibacterial activity of the essential oils of Perovskia abrotanoides. Islamabad J Sci 5:22–25
Hazrati S, Govahi M, Sedaghat M, Kashkooli AB (2020) A comparative study of essential oil profile, antibacterial and antioxidant activities of two cultivated Ziziphora species (Z. clinopodioides and Z. tenuior). Ind Crops Prod 157:112942
Herman A, Tambor K, Herman A (2016) Linalool affects the antimicrobial efficacy of essential oils. Curr Microbiol 72(2):165–172
Inouye S, Uchida K, Yamaguchi H, Miyara T, Gomi S, Amano M (2001) Volatile aroma constituents of three Labiatae herbs growing wild in the Karakoram-Himalaya district and their antifungal activity by vapor contact. J Essent Oil Res 13(1):68–72
Iraji M, Sadegh M, Khaleghian A (2021) Anticancer effects of Perovskia abrotanoides hydroalcoholic extracts on MKN45 gastric cancer cells. Koomesh 23(2):291–300
Jamzad Z (2012) Flora of Iran, vol 76. Lamiaceae
Kamatou GP, Viljoen AM (2008) Linalool—a review of a biologically active compound of commercial importance. Nat Prod Commun 3(7):727
Karamian R, Asadbegy M, Pakzad R (2013) Essential oil compositions and in vitro antioxidant and antibacterial activities of the methanol extracts of two Salvia species (Lamiaceae) from Iran. Int J Agric Crop Sci 5(11):1171
Kim H-J, Chung J-I, Lee SH, Jung Y-S, Moon C-H, Baik EJ (2008) Involvement of endogenous prostaglandin F2α on kainic acid-induced seizure activity through FP receptor: the mechanism of proconvulsant effects of COX-2 inhibitors. Brain Res 1193:153–161
Kim C, Cho SK, Kapoor S, Kumar A, Vali S, Abbasi T, Kim SH, Sethi G, Ahn KS (2014) β-caryophyllene oxide inhibits constitutive and inducible STAT3 signaling pathway through induction of the SHP-1 protein tyrosine phosphatase. Mol Carcinog 53(10):793–806
Kunduhoglu B (2017) Anti-yeast activity of cinnamaldehyde, eugenol and linalool. World J Res Rev 5(5):32–34
Lampronti I, Saab AM, Gambari R (2006) Antiproliferative activity of essential oils derived from plants belonging to the Magnoliophyta division. Int J Oncol 29(4):989–995
Langhasova L, Hanusova V, Rezek J, Stohanslova B, Ambroz M, Kralova V, Vanek T, Lou JD, Yun ZL, Yang J (2014) Essential oil from Myrica rubra leaves inhibits cancer cell proliferation and induces apoptosis in several human intestinal lines. Ind Crops Prod 59:20–26
Lau C, Ho C, Kim C, Leung K, Fung K, Tse T, Chan H, Chow M (2004) Cytotoxic activities of Coriolus versicolor (Yunzhi) extract on human leukemia and lymphoma cells by induction of apoptosis. Life Sci 75(7):797–808
Li X, Wang G, Zhao J, Ding H, Cunningham C, Chen F, Flynn D, Reed E, Li Q (2005) Antiproliferative effect of β-elemene in chemoresistant ovarian carcinoma cells is mediated through arrest of the cell cycle at the G2-M phase. Cell Mol Life Sci CMLS 62(7):894–904
Li QQ, Wang G, Zhang M, Cuff CF, Huang L, Reed E (2009) β-Elemene, a novel plant-derived antineoplastic agent, increases cisplatin chemosensitivity of lung tumor cells by triggering apoptosis. Oncol Rep 22(1):161–170
Liolios C, Laouer H, Boulaacheb N, Gortzi O, Chinou I (2007) Chemical composition and antimicrobial activity of the essential oil of Algerian Phlomis bovei De Noé subsp. bovei. Molecules 12(4):772–781
Lu H-F, Liu J-Y, Hsueh S-C, Yang Y-Y, Yang J-S, Tan T-W, Kok L-F, Lu C-C, Lan S-H, Wu S-Y (2007) (–)-Menthol inhibits WEHI-3 leukemia cells in vitro and in vivo. In Vivo 21(2):285–289
Mahboubi M, Tabar RH, Mahdizadeh E (2018) Chemical composition and antifungal activities of Ziziphora tenuior and Z. clinopodioides essential oils against dermatophytes. Herba Polon 64(2)
Matsuo AL, Figueiredo CR, Arruda DC, Pereira FV, Scutti JAB, Massaoka MH, Travassos LR, Sartorelli P, Lago JH (2011) α-Pinene isolated from Schinus terebinthifolius Raddi (Anacardiaceae) induces apoptosis and confers antimetastatic protection in a melanoma model. Biochem Biophys Res Commun 411(2):449–454
Mazooji A, Salimpour F, Jabbari Moghaddam N (2010) Chemotaxonomic comparison and identification of chemical compositions of essential oil in two species of Salvia L. (S. reuteriana Boiss. & S. macrosiphon Boiss.). Iran J Biol Sci 5(2):27–40. http://zisti.iauvaramin.ac.ir/article_539967.html
Mendoza-Palomar N, Balasch-Carulla M, González-Di Lauro S, Céspedes MC, Andreu A, Frick MA, Linde MÁ, Soler-Palacin P (2017) Escherichia coli early-onset sepsis: trends over two decades. Eur J Pediatr 176(9):1227–1234
Mirjalili MH, Amiri F, Goldansaz SM (2020) Chemical variation of Ziziphora clinopodioides Lam. Subsp. rigida (Boiss) Rech. f. from the Central of Iran. J Med Plants By-product
Mobarakian M, Nikbakht Dastjerdi M, Shakarami J, Abtahi SM (2021) Mechanisms of cytotoxicity-inducing effect of 1, 8-cineole, a plant terpenoid, on lepidopteran (Spodoptera frugiperda) cell line. J Appl Biotechnol Rep 8(1):32–36
Mohammadhosseini M (2017) The ethnobotanical, phytochemical and pharmacological properties and medicinal applications of essential oils and extracts of different Ziziphora species. Ind Crops Prod 105:164–192
Moosavy M-H, Basti AA, Misaghi A, Salehi TZ, Abbasifar R, Mousavi HAE, Alipour M, Razavi NE, Gandomi H, Noori N (2008) Effect of Zataria multiflora Boiss. essential oil and nisin on Salmonella typhimurium and Staphylococcus aureus in a food model system and on the bacterial cell membranes. Food Res Int 41(10):1050–1057
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65(1–2):55–63
Mozaffarian V (1996) A dictionary of Iranian plant names. Farhang Moaser, Tehran, p 396
Napoli E, Di Vito M (2021) Toward a new future for essential oils. Antibiotics 10:207
Nasiri Tarzejani E, Nasri S (2020) Cytotoxicity of methanol extracts of Ziziphora tenuior L. on Hela cell line by MTT assay. New Cell Mol Biotechnol J 10(38):69–82
Nazer MR, Darvishi M (2017) Prescribe and use of antibiotics and its role in microbial resistance and its effects on resistance economy. Yafteh 19(3):49–62
Norouzi R, Norouzi M (2018) The study of quantitative and qualitative variations in essential oil of Salvia reuteriana Boiss. in wild and field conditions. J Plant Process Funct 7(23):347–360
Omara T, Kiprop AK, Kosgei VJ (2021) Intraspecific variation of phytochemicals, antioxidant, and antibacterial activities of different solvent extracts of Albizia coriaria leaves from some agroecological zones of Uganda. Evid Based Complement Altern Med 2021:2335454
Oueslati MH, Abutaha N, Al-Ghamdi F, Nehdi IA, Nasr FA, Mansour L, Mohammed A-Z, Harrath AH (2020) Analysis of the chemical composition and in vitro cytotoxic activities of the essential oil of the aerial parts of Lavandula atriplicifolia Benth. J King Saud Univ Sci 32(2):1476–1481
Panahi Y, Ghanei M, Hadjiakhoondi A, Ahmadi-Koulaei S, Delnavazi M-R (2020) Free radical scavenging principles of Salvia reuteriana Boiss. aerial parts. Iran J Pharm Res IJPR 19(2):283
Popović-Djordjević J, Cengiz M, Ozer MS, Sarikurkcu C (2019) Calamintha incana: essential oil composition and biological activity. Ind Crops Prod 128:162–166
Potente G, Bonvicini F, Gentilomi GA, Antognoni F (2020) Anti-candida activity of essential oils from Lamiaceae plants from the Mediterranean Area and the Middle East. Antibiotics 9(7):395
Pourhosseini S, Mirjalili M, Nejad Ebrahimi S, Sonboli A (2018) Essential oil quantity and quality of different plant organs from Perovskia abrotanoides Karel in natural habitat of North Khorasan province. J Plant Prod (agronomy, Breeding and Horticulture) 40(4):53–62
Rajabi Z, Ebrahimi M, Farajpour M, Mirza M, Ramshini H (2014) Compositions and yield variation of essential oils among and within nine Salvia species from various areas of Iran. Ind Crops Prod 61:233–239
Razavi N, Molavi Choobini Z, Salehian Dehkordi M, Saleh Riyahi S, Salehian Dehkordi M, Molavi CS (2016) Overview of the antibacterial properties of essential oils and extracts of medicinal plants in Iran. J Shahrekord Univ Med Sci 17(6):41–52
Richter SS, Heilmann KP, Beekmann SE, Miller NJ, Miller AL, Rice CL, Doern CD, Reid SD, Doern GV (2005) Macrolide-resistant Streptococcus pyogenes in the United States, 2002–2003. Clin Infect Dis 41(5):599–608
Roshani M, Heidary M, Goudarzi H, Hashemi A, Eslami G, Yousefi N (2016) Investigating the antibacterial effect of methanoland acetone extracts of urtica dioica and zataria multifloraagainst metallo beta-lactamase producing Pseudomonas aeru December 5, 2015 ginosa. Sci J Ilam Univ Med Sci 24(3):70–78
Russo A, Formisano C, Rigano D, Senatore F, Delfine S, Cardile V, Rosselli S, Bruno M (2013) Chemical composition and anticancer activity of essential oils of Mediterranean sage (Salvia officinalis L.) grown in different environmental conditions. Food Chem Toxicol 55:42–47
Russo A, Cardile V, Graziano AC, Formisano C, Rigano D, Canzoneri M, Bruno M, Senatore F (2015) Comparison of essential oil components and in vitro anticancer activity in wild and cultivated Salvia verbenaca. Nat Prod Res 29(17):1630–1640
Russo A, Formisano C, Rigano D, Cardile V, Arnold NA, Senatore F (2016) Comparative phytochemical profile and antiproliferative activity on human melanoma cells of essential oils of three lebanese Salvia species. Ind Crops Prod 83:492–499
Rustaiyan A, Masoudi S, Ameri N, Samiee K, Monfared A (2006) Volatile constituents of Ballota aucheri Boiss., Stachys benthamiana Boiss. and Perovskia abrotanoides Karel. growing wild in Iran. J Essent Oil Res 18(2):218–221
Ryall B, Davies JC, Wilson R, Shoemark A, Williams HD (2008) Pseudomonas aeruginosa, cyanide accumulation and lung function in CF and non-CF bronchiectasis patients. Eur Respir J 32(3):740–747
Sain S, Naoghare P, Saravana Devi S, Daiwile A, Krishnamurthi K, Arrigo P, Chakrabarti T (2014) Beta caryophyllene and caryophyllene oxide, isolated from Aegle marmelos, as the potent anti-inflammatory agents against lymphoma and neuroblastoma cells. Anti-Inflamm Anti-Allergy Agents Med Chem 13(1):45–55
Sajjadi SE, Mehregan I, Khatamsaz M, Asgari G (2005) Chemical composition of the essential oil of Perovskia abrotanoides Karel. growing wild in Iran. Flavour Fragr J 20(4):445–446
Salehi P, Sonboli A, Eftekhar F, Nejad-Ebrahimi S, Yousefzadi M (2005) Essential oil composition, antibacterial and antioxidant activity of the oil and various extracts of Ziziphora clinopodioides subsp. rigida (B OISS.) R ECH f from Iran. Biol Pharm Bull 28(10):1892–1896
Salimpour F, Mazooji A, Darzikolaei SA (2011) Chemotaxonomy of six Salvia species using essential oil composition markers. J Med Plants Res 5(9):1795–1805
Salimpour F, Mazooji A, Mazhar F, Barzin G (2014) Comparative study of antibacterial properties of four species of Salvia L. as a medicinal plant. 37(4):205–210
Salvador MJ, Carvalho JE, Wisniewski-Jr A, Kassuya CA, Santos ÉP, Riva D, Stefanello MÉA (2011) Chemical composition and cytotoxic activity of the essential oil from the leaves of Casearia lasiophylla. Rev Bras Farmacogn 21:864–868
Sertel S, Eichhorn T, Plinkert P, Efferth T (2011) Anticancer activity of Salvia officinalis essential oil against HNSCC cell line (UMSCC1). HNO 59(12):1203–1208
Sfeir J, Lefrançois C, Baudoux D, Derbré S, Licznar P (2013) In vitro antibacterial activity of essential oils against Streptococcus pyogenes. Evid Based Complement Altern Med 2013:269161
Shahbazi Y (2015) Chemical composition and in vitro antibacterial effect of Ziziphora clinopodioides essential oil. Pharm Sci 21(2):51–56
Shahbazi Y, Shavisi N (2016) Interactions of Ziziphora clinopodioides and Mentha spicata essential oils with chitosan and ciprofloxacin against common food-related pathogens. LWT Food Sci Technol 71:364–369
Shahbazi Y (2020) Antibacterial effects of ziziphora clinopodioides and mentha spicata essential oils against common food-borne pathogen biofilms on stainless steel surface. Bulg J Vet Med 23(1):29–43
Shaikh N, Leonard E, Martin JM (2010) Prevalence of streptococcal pharyngitis and streptococcal carriage in children: a meta-analysis. Pediatrics 126(3):e557–e564
Shakhseniaie M, Nikoonahad Lotfabadi N, Haghirossadat F (2020) Effect of rosemary essential oil on the expression of BCL-XL, an anti-apoptotic gene, in MCF-7 cell line breast cancer. Daneshvar Med Basic Clin Res J 27(4):45–52
Sharifzadeh A, Javan AJ, Shokri H, Abbaszadeh S, Keykhosravy K (2016) Evaluation of antioxidant and antifungal properties of the traditional plants against foodborne fungal pathogens. J Mycol Med 26(1):e11–e17
Shokri H, Sharifzadeh A, Tamai IA (2012) Anti-Candida zeylanoides activity of some Iranian plants used in traditional medicine. J Mycol Med 22(3):211–216
Silva A, da Silva H, Monteiro A, Lemos T, de Souza S, Militão G, Santos H, Alves P, Romero N, Santiago G (2020) Chemical composition, larvicidal and cytotoxic activities of the leaf essential oil of Bauhinia cheilantha (Bong.) Steud. S Afr J Bot 131:369–373
Sookto T, Srithavaj T, Thaweboon S, Thaweboon B, Shrestha B (2013) In vitro effects of Salvia officinalis L. essential oil on Candida albicans. Asian Pac J Trop Biomed 3(5):376–380. https://doi.org/10.1016/S2221-1691(13)60080-5
Surmaghi MS, Amin YAG, Mahmoodi Z (1992) Survey of Iranian plants for saponins alkaloids flavonoids and tannins. IV. DARU J Pharm Sci 2(2–3):1–11
Sylvester PW (2011) Optimization of the tetrazolium dye (MTT) colorimetric assay for cellular growth and viability. In: Drug design and discovery. Springer, pp 157–168
Tabatabaei YF, Alizadeh BB, Heidari SM, Mortazavi SA (2014) The in vitro study of antimicrobial effect of Teucrium polium extract on infectious microorganisms
Triantaphyllou GB, Boskou DK (2001) Antioxidative properties of water extracts obtained from herbs of the species Lamiaceae. Int J Food Sci Nutr 52(4):313–317
Villalta G, Salinas M, Calva J, Bec N, Larroque C, Vidari G, Armijos C (2021) Selective BuChE inhibitory activity, chemical composition, and enantiomeric content of the essential oil from Salvia leucantha Cav. collected in Ecuador. Plants 10(6):1169
Wang G, Li X, Huang F, Zhao J, Ding H, Cunningham C, Coad J, Flynn D, Reed E, Li Q (2005) Antitumor effect of β-elemene in non-small-cell lung cancer cells is mediated via induction of cell cycle arrest and apoptotic cell death. Cell Mol Life Sci CMLS 62(7):881–893
Wang W, Li N, Luo M, Zu Y, Efferth T (2012) Antibacterial activity and anticancer activity of Rosmarinus officinalis L. essential oil compared to that of its main components. Molecules 17(3):2704–2713
Wijesundara NM, Rupasinghe HV (2018) Essential oils from Origanum vulgare and Salvia officinalis exhibit antibacterial and anti-biofilm activities against Streptococcus pyogenes. Microb Pathog 117:118–127
Wijesundara NM, Rupasinghe H (2019) Herbal tea for the management of pharyngitis: inhibition of streptococcus pyogenes growth and biofilm formation by herbal infusions. Biomedicines 7(3):63
World Health Organization (WHO) (2002) Prevention of hospital-acquired infections: a practical guide, 2nd edn. Geneva. WHO/CDS/CSR/EPH/2002.12
Yi F, Sun J, Bao X, Ma B, Sun M (2019) Influence of molecular distillation on antioxidant and antimicrobial activities of rose essential oils. LWT Food Sci Technol 102:310–316
Younessi M, Farmani B, Alirezalu K, Fathizadeh O, Sabzi Nojadeh M (2019) Study of phytochemical composition and antibacterial effects of Artemisia fragrans Willd. essential oil in different seasons. Food Sci Technol 16(91):357–367
Yousefbeyk F, Gohari AR, Hashemighahderijani Z, Ostad SN, Sourmaghi MHS, Amini M, Golfakhrabadi F, Jamalifar H, Amin G (2014) Bioactive terpenoids and flavonoids from Daucus littoralis Smith subsp. hyrcanicus Rech. f, an endemic species of Iran. DARU J Pharm Sciences 22(1):1–6
Yousefbeyk F, Tabaside J, Ostad S, Salehi Sourmaghi M, Amin G (2016) Investigation of chemical composition and cytotoxic activity of aerial parts of Ziziphora clinopodioides Lam. Res J Pharmacogn 3(2):47–51
Zaker A, Asili J, Abrishamchi P, Tayarani-Najaran Z, Mousavi SH (2017) Cytotoxic and apoptotic effects of root extract and tanshinones isolated from Perovskiaabrotanoides Kar. Iran J Basic Med Sci 20(12):1377
Zarezadeh A, Sefidkon F, Aghdaei ST, Mirhosseini A, Arabzadeh M, Mirjalili M (2017) Investigation on quality and quantity of essential oil cultivated different Satureja species in Yazd province. Iran J Med Aromat Plants 33(3)
Zargari A (1995) Iranian medicinal plants, vol 4.Tehran University Press, Tehran
Zhao J, Li QQ, Zou B, Wang G, Li X, Kim JE, Cuff CF, Huang L, Reed E, Gardner K (2007) In vitro combination characterization of the new anticancer plant drug β-elemene with taxanes against human lung carcinoma. Int J Oncol 31(2):241–252
Funding
The authors have not disclosed any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have not disclosed any competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ghavam, M. In vitro biological potential of the essential oil of some aromatic species used in Iranian traditional medicine. Inflammopharmacol 30, 855–874 (2022). https://doi.org/10.1007/s10787-022-00934-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10787-022-00934-y