Abstract
Parkinson’s disease (PD) pathogenesis inevitably involves neuroinflammatory responses attained through contribution of both neuron and glial cells. Investigation done in both experimental models of PD and in samples of PD patients suggested the involvement of both central and peripheral inflammatory responses during PD pathogenesis. Such neuroinflammatory responses could be regulated by neuron-glia interaction which is one of the recently focused areas in the field of disease diagnosis, pathogenesis and therapeutics. Such aggravated neuroinflammatory responses during PD are very well associated with augmented levels of cyclooxygenase (COX). An increased expression of cyclooxygenase (COX) with a concomitant increase in the prostaglandin E2 (PGE2) levels has been observed during PD pathology. Ibuprofen is one of the non-steroidal anti-inflammatory drugs (NSAID) and clinically being used for PD patients. This review focuses on the neuroinflammatory responses during PD pathology as well as the effect of ibuprofen on various disease related signaling factors and mechanisms involving nitrosative stress, neurotransmission, neuronal communication and peroxisome proliferator-activated receptor-γ. Such mechanistic effect of ibuprofen has been mostly reported in experimental models of PD and clinical investigations are still required. Since oxidative neuronal death is one of the major neurodegenerative mechanisms in PD, the antioxidant capacity of ibuprofen along with its antidepressant effects have also been discussed. This review will direct the readers towards fulfilling the existing gaps in the mechanistic aspect of ibuprofen and enhance its clinical relevance in PD therapeutics and probably in other age-related neurodegenerative diseases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
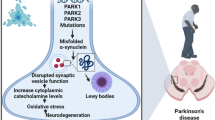
Parkinson’s disease (PD) is a chronic neurological disorder, phenotypically, biochemically and anatomically identified through motor dysfunction, dopamine depletion and progressive loss of dopaminergic terminals and neurons in the striatum and substantia nigra, respectively (Srivastava et al. 2012; Yadav et al. 2012; Singh et al. 2012). The cardinal symptoms of PD are resting tremor, bradykinesia, rigidity and postural instability (Singh et al. 2012; Dixit et al. 2013). In PD patients of advanced disease stage the aggregates of fibrillar α-synuclein are found to be deposited at multiple brain regions (Stefanis 2012; Giráldez-Pérez et al. 2014; Recasens and Dehay 2014; Longhena et al. 2017). Studies have suggested the various etiologies of disease but whereby it starts and progresses are yet difficult to understand (Srivastava et al. 2012; Carvalho et al. 2015). Factors such as aging, genetic factors, environmental toxins, neuronal metabolic disturbances and inflammation are considered to be major contributors in PD pathogenesis (Dias et al. 2013). However, the studies indicated that the persistent inflammatory responses along with activated microglia are the plebeian features of PD pathology as observed in both experimental models and PD patients (Esposito et al. 2007; Glass et al. 2010). Such aggravated inflammatory response significantly bestowed in propagation of disease related adverse signaling mechanisms which could be regulated by utilization of anti-inflammatory agent. Ibuprofen is a non-steroidal anti-inflammatory drug (NSAID) which has a significant anti-inflammatory effect exerted through the inhibition of cyclooxygenase (COX), a precursor for the prostaglandin synthesis (Esposito et al. 2007; Ajmone-Cat et al. 2010). Elevated levels of COX and prostaglandins have been found to be involved in neurodegenerative disease pathogenesis including PD (Bartels and Leenders 2010; Corwin et al. 2018). COX level in the brain is very well associated with the pro-inflammatory activities which could be instrumental in the neurodegenerative signaling and drugs affecting such consociation of signaling mechanism may be relevant in therapeutics. Ibuprofen has antiinflammtory effects and is considered as the safest conventional NSAID that could be safe for chronic utilization during PD pathogenesis (Bushra and Aslam 2010). In this review we will discuss about the neuroinflammatory responses and microglial activation during PD pathogenesis, preclinical findings in support of utilization of ibuprofen in PD therapeutics and reported mechanistic interference of ibuprofen in PD-related neurodegenerative signaling mechanisms.
Neuroinflammation and Parkinsonism
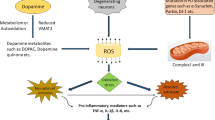
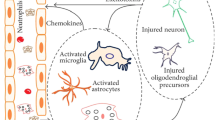
Inflammation is the first line of defense in the body against any foreign invaders or injuries however, the excessive inflammatory responses could be harmful and initiate deleterious signaling (Kim and Joh 2006). Since neurons are post-mitotic these could not have the ability to recover from any injury and are susceptible to adverse signaling or auto destructive immune responses (Colafrancesco and Villoslada 2011). Abundant information has been reported regarding the etiology of PD but it is not clear how the disease initiates and how it progresses (Ardestani 2010). Research of the last decades has grabbed attention towards evaluation of role of glial cells in disease pathology as both experimental models and studies in patient’s have suggested the activation of glial cells which is closely associated with inflammatory responses and neuron-glia crosstalk may play significant role in this mechanism (McGeer and McGeer 2004; Esposito et al. 2007; Tansey and Goldberg 2010; Perry 2012; More et al. 2013; Yan et al. 2014; Bruck et al. 2016; Wang et al. 2015; Booth et al. 2017). PD could not solely characterized by the neuroinflammation as it shares various other pathological mechanisms which are also detected in other neurodegenerative diseases. Neuroinflammatory responses during disease could play primary role in exacerbating the disease mechanisms and further investigations are required to understand their colligated mechanisms (Minghetti 2004; Chen et al. 2016). Studies in postmortem brain of PD patients revealed that substantia nigra region is enriched in microglia therefore more prone for the microglia mediated inflammatory reactions (McGeeret al. 2001; Hartmann 2004; Tufekci et al. 2011). Disease related microglia activation follow the release of interleukin (IL)-1β, IL-6, tumor necrosis factor-α (TNF-α) and interferon- γ (IFN-γ) that may ultimately result in the death of neurons through activation of inducible nitric oxide (iNOS) or the apoptotic receptor and signaling (Saha and Pahan 2006; Förstermann and Sessa 2012; Peferoen et al. 2014). Interestingly some other factors like high content of iron and low content of glutathione in the dopaminergic neurons makes the substantia nigra more prone for inflammation related injury (Dias et al. 2013). These inflammatory responses involve the increased selective activity of COX-2 thus increased level of PGE2 and this augmented level could be further aggravated during neuronal apoptosis and microglial activation and worsen the conditions (Minghetti 2004; Bartels and Leenders 2010).
Microglial activation and Parkinson’s Disease
Microglia cells are the phagocytic cells of the brain, act as immune sentinel and capable in instrumentation of various neuroinflammatory reactions during disease pathology (Farooqui et al. 2007; Graeber et al. 2011; Perry and Teeling 2013). Activated microglia are considered as hallmark of disease as well as neuroinflammation which act as scavenger and engulf the cellular debris of the brain (Fu et al. 2014; Sochocka et al. 2017). It has been reported that microglia are the major source of prostaglandins (PGs) which synthesized through membrane phospholipids mediated release of arachidonic acid (AA) (Ajmone-Cat et al. 2010; Ricciotti and FitzGerald 2011). Studies in postmortem brain of PD patients and in experimental models of PD have suggested the inescapable role of microglia during disease and suggested that these activated microglia play pathological role in disease pathogenesis however this still unexplored whether these activated microglia are the cause or consequence of the degenerating dopaminergic neurons (Su et al. 2008; Theodore et al. 2008; Griffiths et al. 2010; Chao et al. 2014). It has also been reported that with age the microglia numbers increased in substantia nigra which may be contributory in disease pathogenesis but require further investigations (Croisier et al. 2005; Bartels and Leenders 2010). It has also been reported that microglial cells induced chronic inflammatory responses play significantly in the death of dopaminergic neurons (Lull and Block 2010; Singh et al 2011). Under physiological conditions the microglial response remains tightly regulated however PD pathology involves their unregulated responses and consequent neurodegeneration involving chronic neuroinflammation as one of the culprit responsible for the progressive loss of dopaminergic neurons (Tansey and Goldberg 2010). The experimental data from PD patients also showed the increased level of pro-inflammatory cytokines, eicosanoids and oxidative stress in the brain and peripheral system which evidently support the pathological role of activated microglia in PD patients (Quian et al. 2010; Wang et al. 2015). Positron emission tomography in drug-naive PD patients utilizing radiotracer [(11)C](R)-PK11195, for activated microglia showed its significantly higher level in comparison to control subjects reflecting close association of activated microglia during disease onset (Ouchi et al. 2005). Since early diagnosis of PD in patients is not very well established and human brain tissue cannot be utilized for estimations, therefore the investigations have been done in brain tissue of experimental rodent models. It has been observed that various neurotoxin like lipopolysaccharide (LPS), aggregated or nitrated form of α-synuclein could directly induce the microgliosis (Glass et al. 2010). Several other neurotoxins like 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine (MPTP), 6-hydroxydopamine (6-OHDA) and rotenone may directly induce the neurotoxicity with the assistance of microglial activation. In comparison to glial cells the neurons have weaker antioxidant capacity, as these are dependent on glial cells for GSH replenishment, therefore more susceptible for injury and degeneration during microglial induced inflammatory responses (Dringen et al. 1999; Bove et al. 2005; Bozyczko-Coyne 2007; Hernandez-Baltazar et al. 2017).
Ibuprofen: mechanistic aspects
Ibuprofen, a first derivative of propionic acid, is one of the most popular commonly used safest conventional NSAID (Bushra and Aslam 2010). Chemically, ibuprofen is a diastereoisomeric chiral drug with equal ratio of R ( +) ibuprofen and S ( +) ibuprofen. Pharmacokinetic studies have shown the presence of enantiomers of ibuprofen in plasma as determined by direct stereo selective high performance liquid chromatography (Menzel-Soglowek et al. 1990). Majority of pharmacologically active form of drug is contributed by its S-enantiomer as it prohibits the prostaglandin synthesis (Jamali and Brocks 2015). In the early to mid‐1970s (R) ‐ibuprofen was first reported for unidirectional enzymatic conversion of inactive R enantiomer to the active S enantiomer. This is so because approximately 40–60% metabolism of R (−) enantiomer takes place into the S ( +) form in liver (Rainsford 2012). Its anti-inflammatory effect is due to its inhibitory activity on cyclooxygenases (COX) that are responsible for the prostaglandins synthesis and play an important role in exacerbation of inflammatory responses mediated neuronal death (Simmons et al. 2004; Bartels and Leenders 2010; Teismann 2012). Ibuprofen also inhibits COX, reduces the nitric oxide (NO) production and activates the peroxisome proliferator-activated receptor-γ (PPAR-γ) (Esplugues 2002; Asanuma and Miyazaki 2007; Chaturvedi and Beal, 2008; Tsuji et al. 2009). As we and others have reviewed earlier that NO critically involve in the neuronal damage during PD pathology (Tuteja et al. 2004; Calabrese et al. 2007; Knott and Bossy-Wetzel 2009) thus the ibuprofen mediated inhibition of disease related augmented level of NO could offer neuroprotective responses. PPAR is a signaling receptor that takes part in the inhibition of the apoptotic pathway and thus plays an important role in providing protection against PD (Wu 2010; Wnuk and Kajta 2017). Several experimental and epidemiological studies have shown the neuroprotective effects of ibuprofen (Asanuma and Miyazaki 2007; Gao et al. 2011). Inflammation is an inescapable event in the PD pathogenesis therefore it is worth to review the mechanistic aspect of ibuprofen and its clinical utilization. Various pre-clinical studies have been performed in experimental animal models which have shown the protective effects of ibuprofen on dopaminergic cell loss (Casper et al. 2000; Hsieh et al. 2011; Singh et al. 2016; Tripathi et al. 2018). In the following sections the therapeutic application of ibuprofen has been discussed.
Effect of Ibuprofen on various PD models
Age related physiological and morphological alterations are contributory factors in most of the neurodegenerative diseases involving PD. Such alterations include the neuroinflammatory responses among which most are glial cell mediated as reviewed previously (Singh et al. 2011; Asanuma and Miyazaki 2007; Radtke et al. 2017; Heneka 2019) (Table 1). To evaluate the dopaminergic neuronal death related neuroinflammatory responses the LPS induced experimental models is being frequently utilized by researchers and readers may refer the article by Jeong et al. (2010). Studies have also been conducted with other neurotoxins like, MPTP, 1-methyl-4-phenylpyridinium (MPP+) and 6-hydroxydopamine (6-OHDA) (Zawada et al. 2011). The data obtained from the studies on neurotoxins induced experimental models showed the increased density of microglial activation demonstrating the critical role of microgliosis during disease pathogenesis (Bove et al. 2005; Bozyczko-Coyne 2007; Glass et al. 2010; Hernandez-Baltazar et al. 2017). Along with microgliosis the experimental models also showed the significant depletion of PD related pathological markers like decreased TH immunoreactivity along with augmented level of COX-2 (Singh et al. 2016; Tripathi et al. 2018). Since the reports have suggested that microglia remain higher in population in substantia nigra thus dopaminergic neurons of this region are more susceptible for neuroinflammatory responses related neuronal death (Esposito et al. 2007; Bartels and Leenders 2010). With such observations the utilization of NSAID has been suggested in PD therapeutics specifically on ibuprofen (Gao et al. 2011; Casper et al. 2000). The neuroprotective efficacy of NSAIDs depends on their dose regime and duration and also on the stage of disease (Chen et al. 2003, 2005; Esposito et al. 2007). Epidemiological studies have also supported the role of ibuprofen as an anti-PD as it reduces the disease related augmented activity of COX (Chen et al. 2003, 2005; Gao et al. 2011). Studies in experimental in vitro and rodent models have suggested the prophylactic effect of ibuprofen in PD pathology (Casper et al. 2000; Tsuji et al. 2009; Hsieh et al. 2011; Ramazani et al. 2019). In vitro studies have shown MPTP induced excitotoxicity in dopaminergic neurons could be prevented with ibuprofen (Hsieh et al. 2011). Data obtained from a meta-analysis carried on the regular ibuprofen users showed that these individuals have forty percent reduced risk of PD onset (Moore et al. 2010). Meta-analysis in patients also showed the therapeutic effect of ibuprofen and must be explored further in clinics (Gagne and Power 2010) .
Effect of ibuprofen on synapses
Activated microglial cells are responsible for the release of various pro-inflammatory cytokines, chemokines, reactive oxygen species (ROS), reactive nitrogen species (RNS) and excitatory amino acids at synapses and cell bodies during disease pathogenesis and contribute to the neurodegeneration (Mosley et al. 2006; Wilkinson and Landreth 2006; Uttara et al. 2009). The dopaminergic terminals are responsible for the unloading of dopamine in the striatum. It has been reported that MPTP induced parkinsonian model exhibit the COX dependent microglial activation (Członkowska et al. 2002). Microglial cells could also cause neuritic beading or synaptic stripping along dendrites which lead to synaptic disconnection and consequent loss of neurotrophic factors support and affect the neuronal communication (Vijitruth et al. 2006; Takeuchi et al. 2005; Schiefer et al. 1999; Volpe et al. 1998; Isacson et al. 1985). In LPS exposed microglia it has been observed that COX-2 inhibition reduces the level of NO suggesting that microglia mediate the secondary injury through various inflammatory factors and also suggested the positive modulatory effect of exogenous COX-2 inhibitor on activated microglial related neuronal toxicity (Minghetti et al. 1997). Due to high iron in dopaminergic neurons of substantia nigra these are highly susceptible for oxidative and inflammatory injury and COX-2 mediated enzymatic reactions contributes to dopaminergic neuronal death by oxidizing dopamine to a reactive quinine or through direct oxidation of DNA (Nikolic and van Breemen. 2001; Hastings 1995). The COX-2 also responsible for the prostaglandin synthesis which remain actively present in dendritic spines, responsible for the amplification of synaptic signaling (Kaufmann et al. 1996). Since ibuprofen is a proved COX-2 inhibitor it could prevent the discussed COX-2 mediated effects and might prevent the neuronal degeneration though detailed investigations are required. It has also been reported that anti-inflammatory agent like ibuprofen could prevent the microglia proliferation through modulation of cell cycle progression and apoptosis (Elsisi et al. 2005). Ibuprofen also offer the protection against terminal degeneration of dopaminergic neurons by inhibition of COX-2 activity (Sanchez-Pernaute et al. 2004). Though various studies are available indicating the neuroprotective role of ibuprofen in neuroprotection in context to functional factors in synapses however the detailed investigation is still lacking and need further investigations.
Effect of ibuprofen against NO production
PD pathology is very well correlated with increased level of iNOS as reported by us and others previously. Increased level of NO is reported to enhance the production of proinflammatory prostaglandins by increasing the COX activity without the involvement of cGMP (Salvemini et al. 1993). Since ibuprofen is able to inhibit the iNOS expression thus regulate the level of NO during pathological conditions and offer the neuroprotective effects (Aeberhard et al. 1995; Stratman et al. 1997; Asanuma and Miyazaki 2007). Such increased activity of iNOS and COX-2 involve the activation of nuclear factor-kB (NF-kB) which also reported to be involve during PD pathogenesis and contribute in production of inflammatory cytokines (Lee et al. 2009). Rock et al. 2004; Lawrence 2009). It has been reported that LPS exposed microglial cells exhibit the increased NF-kB activity which in turn activates the production of the inducible form of COX and facilitate the neuronal death (Choi et al. 2009). Both iNOS and neuronal NOS are expressed in the microglial cells and neurons and are responsible for the production of NO (Kraft and Harry 2011; Contestabile et al. 2012). Inflammatory stimuli or oxidative environment to microglial cells and neurons showed elevated levels of NF-kB which facilitate the production of inflammatory cytokines (IL-1b, IL-6, IFN-γ), COX-2, iNOS, TNF-α and apoptosis-promoting factors (p53, Bax) (Kim and Joh 2006). These activated inflammatory mediators further enhance the production of NO and worsen the diseased condition (Ajmone-Cat et al. 2010). In an in vitro NO- generating system the NO quenching activity of ibuprofen was estimated and it had been observed that it could prevent the dopaminergic neuronal death directly in dose dependent manner through scavenging of NO (Asanuma and Miyazaki 2007). The free radical scavenging capacity of ibuprofen has been reported in various models (Van Antwerpen and Nève 2004).
Effects of ibuprofen on peroxisome proliferator-activated receptor-γ
Peroxisome proliferator-activated receptors (PPAR) are also known as the nuclear hormone receptors present in the fat tissue, spleen and brain (Chaturvedi and Beal 2008). These are the ligand inducible transcription factors regulate essentially required genes of various metabolic processes and cell differentiation along with their anti-inflammatory effects during neurodegeneration (Desvergne and Wahli 1999; Straus and Glass 2001; Kapadia et al. 2008; Yonutas and Sullivan 2013). PPAR are also related to the development of tissue differentiation, inflammation, mitochondrial function, wound healing, lipid metabolism and glucose metabolism regulation (Chaturvedi and Beal 2008). It has been reported that PPAR agonists exhibited the neuroprotective effects by inhibiting oxidative stress, inflammation and apoptosis (Bordet et al. 2006; Heneka and Landreth 2007). A study on various NSAIDs on various cell systems has demonstrated that the pharmacological effects of NSAIDs may be related to both their profile of inhibition of prostaglandin endoperoxide H synthase enzymes and the activation of PPAR α and / or γ isoforms (Jaradat et al. 2001). It has also been reported that like pioglitazone (PPAR γ agonist), ibuprofen is also capable to reduce the neurodegeneration related glial inflammation and gliosis thus offer protective effects against neuroinflammatory responses (Heneka et al. 2005). It has also been reported that ibuprofen has PPAR-γ agonistic property which offered neuroprotection during methamphitamine induced neurotoxicity (Tsuji et al. 2009). PPAR γ mediated inhibitory effect of ibuprofen has also been suggested on migration and proliferation of cardiac smooth muscle cells (Dannoura et al. 2014). The ibuprofen activated PPAR-γ regulates the transcriptional activity of transcription factors including signal transducer and activator of transcription (STAT), NF-κB, activating transcription factor-1 (ATF-1/4), reduces the iNOS and COX-2 levels and may contribute in neuroprotective activities (Chaturvedi and Beal. 2008). Ibuprofen activates PPAR-γ receptor through its receptor specific binding ability and could offer neuroprotective activity. Reports have suggested that ibuprofen significantly rescued the dopaminergic neurons from the toxic effects of glutamate, 6-OHDA and MPP+ in mesencephalic cultures involving role of transcription factors and inflammatory responses (Casper et al. 2000; Tsuji et al. 2009; Hsieh et al. 2011; Chaturvedi and Beal 2008). In view of above evidences the studies are required to evaluate how ibuprofen interact with PPAR γ and establish the therapeutic dose regime of it in clinics.
Ibuprofen as an antidepressant and antioxidant
The possible mechanisms involved in PD pathogenesis are mitochondrial dysfunction, oxidative stress and neuroinflammation. Mitochondrial dysfunction is responsible for the excessive ROS generation that in turn causes oxidative stress and energy crisis and direct the vulnerable neurons towards apoptosis. The free radicals that are generated could cause lipid peroxidation in the cell. A study in rotenone exposed rats showed that ibuprofen exhibited the antioxidant properties in addition to COX inhibition (Zaminelli et al. 2014). Some other in vivo and in vitro studies conducted in various neurotoxin induced experimental models have shown similar results. Use of ibuprofen has shown potential neuroprotective effects in such studies by means of restoring the antioxidant potential (Hsieh et al. 2011; Zaminelli et al. 2014; Naeem et al. 2017; Mandal et al. 2018). Few medical reports have suggested that ibuprofen showed antidepressant effect however, further detailed investigations are required to utilize this in clinics with the said aspect.
Conclusion
It is apparent from the studies that PD pathogenesis is closely associated with the inflammatory processes mostly mediated through activated glial cells (Singh et al. 2016; Joshi and Singh 2018). Use of NSAIDs is recommended for Parkinson’s pathology however the reported studies with meta-analysis implicate the diverse and controversial findings (Ren et al. 2018) suggesting further explorations. This review has focused on effects of ibuprofen on various disease related factors and mechanisms and suggested that use of ibuprofen may have entailment in diverse aspects of disease pathogenesis (Fig. 1). Since PD is multifactorial disease and involve various degenerative pathways along with neuroinflammation, the combination therapy may execute better therapeutic effects. In addition, the treatment regimen will also be for longer duration so optimal dose, timing, its beneficial/adverse effects must also be explored.
Code availability
Not applicable.
References
Aeberhard EE, Henderson SA, Arabolos NS, Griscavage JM, Castro FE, Barrett CT, Ignarro LJ (1995) Nonsteroidal anti-inflammatory drugs inhibit expression of the inducible nitric oxide synthase gene. Biochem Biophys Res Commun 208(3):1053–1059. https://doi.org/10.1006/bbrc.1995.1441
Ajmone-Cat MA, Bernardo A, Greco A, Minghetti L (2010) Non-steroidal anti-inflammatory drugs and brain inflammation: effects on microglial functions. Pharmaceuticals (Basel) 3(6):1949–1965
Ardestani MS (2010) Parkinson's disease, the inflammatory pathway and anti-inflammatory drug: an overview. J Med Sci 10(3):49–58
Asanuma M, Miyazaki I (2007) Common anti-inflammatory drugs are potentially therapeutic for Parkinson’s disease? Exp Neurol 206(2):172–178
Bartels AL, Leenders KL (2010) Cyclooxygenase and neuroinflammation in Parkinson’s disease neurodegeneration. Curr Neuropharmacol 8:62–68
Booth HDE, Hirst WD, Wade-Martins R (2017) The role of astrocyte dysfunction in Parkinson's disease pathogenesis. Trends Neurosci 40(6):358–370
Bordet R, Ouk T, Petrault O, Gelé P, Gautier S, Laprais M, Deplanque D, Duriez P, Staels B, Fruchart JC, Bastide M (2006) PPAR: a new pharmacological target for neuroprotection in stroke and neurodegenerative diseases. BiochemSoc Trans 34(Pt 6):1341–1346
Bové J, Prou D, Perier C, Przedborski S (2005) Toxin-induced models of Parkinson's disease. NeuroRx 2(3):484–494
Bozyczko-Coyne D, Williams M (2007) Therapeutic areas I: central nervous system, pain, metabolic syndrome, urology, gastrointestinal and cardiovascular. Comprehen Med Chem 6:193–228
Brück D, Wenning GK, Stefanova N, Fellner L (2016) Glia and alpha-synuclein in neurodegeneration: a complex interaction. Neurobiol Dis 85:262–274
Bushra R, Aslam N (2010) An overview of clinical pharmacology of Ibuprofen. Oman Med J 25(3):155–1661
Calabrese V, Mancuso C, Calvani M, Rizzarelli E, Butterfield DA, Stella AM (2007) Nitric oxide in the central nervous system: neuroprotection versus neurotoxicity. Nat Rev Neurosci 8(10):766–775
Carvalho KM, Winter E, Antunes AMDS (2015) Evaluation of development of R&D into Parkinson’s disease through technology monitoring using patent documents and scientific articles. Intern J Res Pharm Biosci 2(3):17–24
Casper D, Yaparpalvi U, Rempel N, Werner P (2000) Ibuprofen protects dopaminergic neurons against glutamate toxicity in vitro. NeurosciLett 289:201–204
Chao Y, Wong SC, Tan EK (2014) Evidence of inflammatory system involvement in Parkinson’s disease. Biomed Res Int 14:308654
Chaturvedi RK, Beal MF (2008) PPAR: a therapeutic target in Parkinson’s disease. J Neurochem 106(2):506–518
Chen H, Zhang SM (2003) Nonsteroidal anti-inflammatory drugs and the risk of Parkinson disease. Arch Neurol 60:1059–1064
Chen H, Jacobs E, Schwarzschild MA, McCullough ML, Calle EE, Thun MJ, Ascherio A (2005) Nonsteroidal anti-inflammatory drug use and the risk for Parkinson's disease. Ann Neurol 58:963–967
Chen WW, Zhang X, Huang WJ (2016) Role of neuroinflammation in neurodegenerative diseases (Review). Mol Med Rep 13(4):3391–3396
Choi Y, Lee MK, Lim SY, Sung SH, Kim YC (2009) Inhibition of inducible NO synthase, cyclooxygenase-2 and interleukin-1beta by torilin is mediated by mitogen-activated protein kinases in microglial BV2 cells. Br J Pharmacol 156(6):933–940
Colafrancesco V, Villoslada P (2011) Targeting NGF-pathway for developing neuroprotective therapies for multiple sclerosis and other neurological diseases. Arch Biol 149:183–192
Contestabile A, Monti B, Polazzi E (2012) Neuronal-glial interactions define the role of nitric oxide in neural functional processes. Curr Neuropharmacol 10(4):303–310
Corwin C, Nikolopoulou A, Pan AL, Nunez-Santos M, Vallabhajosula S, Serrano P, Figueiredo-Pereira ME (2018) Prostaglandin D2/J2 signaling pathway in a rat model of neuroinflammation displaying progressive parkinsonian-like pathology: potential novel therapeutic targets. J Neuroinflam 15(1):272. https://doi.org/10.1186/s12974-018-1305-3
Croisier E, Moran LB, Dexter DT, Pearce RK, Graeber MB (2005) Microglial inflammation in the parkinsoniansubstantianigra: relationship to alpha-synuclein deposition. J Neuroinflam 3(2):14
Członkowska A, Kurkowska-Jastrzebska I, Członkowski A, Peter D, Stefano GB (2002) Immune processes in the pathogenesis of Parkinson's disease - a potential role for microglia and nitric oxide. Med Sci Monitor Internat Med J Experiment Clin Res 8(8):165–177
Dannoura A, Giraldo A, Pereira I, Gibbins JM, Dash PR, Bicknell KA, Brooks G (2014) Ibuprofen inhibits migration and proliferation of human coronary artery smooth muscle cells by inducing a differentiated phenotype: role of peroxisome proliferator-activated receptor γ. J Pharm Pharmacol 66(6):779–792. https://doi.org/10.1111/jphp.12203
Desvergne B, Wahli W (1999) Peroxisome proliferator-activated receptors: nuclear control of metabolism. Endocr Rev 20(5):649–688
Dias V, Junn E, Mouradian MM (2013) The role of oxidative stress in Parkinson’s disease. J Parkinsons Dis 3(4):461–491
Dixit A, Srivastava G, Verma D, Mishra M, Singh PK, Prakash O, Singh MP (2013) Minocycline, levodopa and MnTMPyP induced changes in the mitochondrial proteome profile of MPTP and maneb and paraquat mice models of Parkinson's disease. Biochim Biophys Acta 1832(8):1227–1240
Dringen R, Pfeiffer B, Hamprecht B (1999) Synthesis of the antioxidant glutathione in neurons: supply by astrocytes of CysGly as precursor for neuronal glutathione. J Neurosci Off Soc Neurosci 19(2):562–569. https://doi.org/10.1523/JNEUROSCI.19-02-00562.1999
Elsisi NS, Darling-Reed S, Lee EY, Oriaku ET, Soliman KF (2005) Ibuprofen and apigenin induce apoptosis and cell cycle arrest in activated microglia. Neurosci Lett 375(2):91–96. https://doi.org/10.1016/j.neulet.2004.10.087
Esplugues JV (2002) NO as a signalling molecule in the nervous system. Br J Pharmacol 135(5):1079–1095
Esposito E, Di Matteo V, Benigno A, Pierucci M, Crescimanno G, Di Giovanni G (2007) Non-steroidal anti-inflammatory drugs in Parkinson's disease. Exp Neurol 205(2):295–312
Farooqui AA, Horrocks LA, Farooqui T (2007) Modulation of inflammation in brain: a matter of fat. J Neurochem 101(3):577–599
Förstermann U, Sessa WC (2012) Nitric oxide synthases: regulation and function. Eur Heart J 33(7):829–837
Fu R, Shen Q, Xu P, Luo JJ, Tang Y (2014) Phagocytosis of microglia in the central nervous system diseases. Mol Neurobiol 49(3):1422–1434
Gagne JJ, Power MC (2010) Anti-inflammatory drugs and risk of Parkinson disease: a meta-analysis. Neurology 74(12):995–1002. https://doi.org/10.1212/WNL.0b013e3181d5a4a3
Gao X, Chen H, Schwarzschild MA, Ascherio A (2011) Use of ibuprofen and risk of Parkinson disease. Neurology 76(10):863–869
Giráldez-Pérez R, Antolín-Vallespín M, Muñoz M, Sánchez-Capelo A (2014) Models of α-synuclein aggregation in Parkinson’s disease. Acta Neuropathol Commun 13(2):176
Glass CK, Saijo K, Winner B, Marchetto MC, Gage FH (2010) Mechanisms underlying inflammation in neurodegeneration. Cell 140(6):918–934
Graeber MB, Li W, Rodriguez ML (2011) Role of microglia in CNS inflammation. FEBSLett 585(23):3798–3805
Griffiths MR, Gasque P, Neal JW (2010) The regulation of the CNS innate immune response is vital for the restoration of tissue homeostasis (repair) after acute brain injury: a brief review. Int J Inflam 9:151097
Hartmann A (2004) Postmortem studies in Parkinson’s disease. Dialogues ClinNeurosci 6(3):281–293
Hastings TG (1995) Enzymatic oxidation of dopamine: the role of prostaglandin H synthase. J Neurochem 64(2):919–924. https://doi.org/10.1046/j.1471-4159.1995.64020919.x
Heneka MT (2019) Microglia take centre stage in neurodegenerative disease. Nat Rev Immunol 19(2):79–80. https://doi.org/10.1038/s41577-018-0112-5
Heneka MT, Landreth GE (2007) PPARs in the brain. Biochim Biophys Acta 1771(8):1031–1045
Heneka MT, Sastre M, Dumitrescu-Ozimek L, Hanke A, Dewachter I, Kuiperi C, O'Banion K, Klockgether T, Van Leuven F, Landreth GE (2005) Acute treatment with the PPARgamma agonist pioglitazone and ibuprofen reduces glial inflammation and Abeta1–42 levels in APPV717I transgenic mice. Brain j neurol 128(6):1442–1453. https://doi.org/10.1093/brain/awh452
Hernandez-Baltazar D, Zavala-Flores LM, Villanueva-Olivo A (2017) The 6-hydroxydopamine model and parkinsonian pathophysiology: novel findings in an older model. Neurología 32(8):533–539
Hsieh YC, Mounsey RB, Teismann P (2011) MPP(+)-induced toxicity in the presence of dopamine is mediated by COX-2 through oxidative stress. NaunynSchmiedebergs Arch Pharmacol 384(2):157–167
Isacson O, Brundin P, Gage FH, Björklund A (1985) Neural grafting in a rat model of Huntington’s disease: progressive neurochemical changes after neostriatal ibotenate lesions and striatal tissue grafting. Neuroscience 16(4):799–817. https://doi.org/10.1016/0306-4522(85)90095-8
Jamali F, Brocks DR (2015) The Pharmacokinetics of Ibuprofen in Humans and Animals. In: Rainsford KD (ed) Ibuprofen: Discovery. Development and Therapeutics, First Edition, pp 81–130
Jaradat MS, Wongsud B, Phornchirasilp S, Rangwala SM, Shams G, Sutton M, Romstedt KJ, Noonan DJ, Feller DR (2001) Activation of peroxisome proliferator-activated receptor isoforms and inhibition of prostaglandin H(2) synthases by ibuprofen, naproxen, and indomethacin. Biochem Pharmacol 62(12):1587–1595. https://doi.org/10.1016/s0006-2952(01)00822-x
Jeong HK, Jou I, Joe EH (2010) Systemic LPS administration induces brain inflammation but not dopaminergic neuronal death in the substantianigra. ExpMol Med 42(12):823–832
Joshi N, Singh S (2018) Updates on immunity and inflammation in Parkinson disease pathology. J Neurosci Res 96(3):379–390. https://doi.org/10.1002/jnr.24185
Kapadia R, Yi JH, Vemuganti R (2008) Mechanisms of anti-inflammatory and neuroprotective actions of PPAR-gamma agonists. Front Biosci J Virt Lib 13:1813–1826. https://doi.org/10.2741/2802
Kaufmann WE, Worley PF, Pegg J, Bremer M, Isakson P (1996) COX-2, a synaptically induced enzyme, is expressed by excitatory neurons at postsynaptic sites in rat cerebral cortex. Proc Natl Acad Sci U S A 93:2317–2321
Kim YS, Joh TH (2006) Microglia, major player in the brain inflammation: their roles in the pathogenesis of Parkinson’s disease. ExpMol Med 38(4):333–347
Knott AB, Bossy-Wetzel E (2009) Nitric oxide in health and disease of the nervous system. Antioxid Redox Signal 11(3):541–554
Kraft AD, Harry GJ (2011) Features of microglia and neuroinflammation relevant to environmental exposure and neurotoxicity. Int J Environ Res Public Health 8(7):2980–3018
Lawrence T (2009) The nuclear factor NF-kappaB pathway in inflammation. Cold Spring HarbPerspect Biol 1(6):a001651
Lee JA, Song HY, Ju SM, Lee SJ, Kwon HJ, Eum WS, Jang SH, Choi SY, Park JS (2009) Differential regulation of inducible nitric oxide synthase and cyclooxygenase-2 expression by superoxide dismutase in lipopolysaccharide stimulated RAW 264.7 cells. Exp Mol Med 41(9):629–637
Longhena F, Faustini G, Missale C, Pizzi M, Spano P, Bellucci A (2017) The contribution of α-Synuclein spreading to Parkinson's DiseaseSynaptopathy. Neural Plast 2017:5012129
Lull ME, Block ML (2010) Microglial activation and chronic neurodegeneration. Neurotherapeutics 7(4):354–365
Mandal S, Mandal SD, Chuttani K, Sawant KK, Subudhi BB (2018) Preclinical study of ibuprofen loaded transnasal mucoadhesive microemulsion for neuroprotective effect in MPTP mice model. Iran J Pharma Res IJPR 17(1):23–38
McGeer PL, McGeer EG (2004) Inflammation and neurodegeneration in Parkinson's disease. Parkinsonism Relat Disord 10(Suppl 1):S3–7
McGeer E, Yasojima K, McGeer LP (2001) Inflammation in the pathogenesis of Parkinson’s disease. BCMJ 3:138–141
Menzel-Soglowek S, Geisslinger G, Brune K (1990) Stereoselective high-performance liquid chromatographic determination of ketoprofen, ibuprofen and fenoprofen in plasma using a chiral alpha 1-acid glycoprotein column. J Chromatogr 532(2):295–303. https://doi.org/10.1016/s0378-4347(00)83780-9
Minghetti L (2004) Cyclooxygenase-2 (COX-2) in inflammatory and degenerative brain diseases. J Neuropathol Exp Neurol 63(9):901–910
Minghetti L, Nicolini A, Polazzi E, Créminon C, Maclouf J, Levi G (1997) Inducible nitric oxide synthase expression in activated rat microglial cultures is downregulated by exogenous prostaglandin E2 and by cyclooxygenase inhibitors. Glia 19(2):152–160
Moore AH, Bigbee MJ, Boynton GE, Wakeham CM, Rosenheim HM, Staral CJ, Morrissey JL, Hund AK (2010) Non-steroidal anti-inflammatory drugs in Alzheimer’s Disease and Parkinson's Disease: reconsidering the role of neuroinflammation. Pharmaceuticals (Basel) 3(6):1812–1841
More SV, Kumar H, Kim IS, Song SY, Choi DK (2013) Cellular and molecular mediators of neuroinflammation in the pathogenesis of Parkinson's disease. Med Inflamm 2013:952375
Mosley RL, Benner EJ, Kadiu I, Thomas M, Boska MD, Hasan K, Laurie C, Gendelman HE (2006) Neuroinflammation, oxidative stress and the pathogenesis of Parkinson’s disease. Clin Neuro sci Res 6(5):261–281
Naeem S, Ikram R, Khan SS, Rao SS (2017) NSAIDs ameliorate cognitive and motor impairment in a model of parkinsonism induced by chlorpromazine. Pakistan J Pharma Sci 30(3):801–808
Nikolic D, van Breemen RB (2001) DNA oxidation induced by cyclooxygenase-2. Chem Res Toxicol 14(4):351–354. https://doi.org/10.1021/tx010004x
Ouchi Y, Yoshikawa E, Sekine Y, Futatsubashi M, Kanno T, Ogusu T, Torizuka T (2005) Microglial activation and dopamine terminal loss in early Parkinson’s disease. Ann Neurol 57(2):168–175. https://doi.org/10.1002/ana.20338
Peferoen L, Kipp M, van der Valk P, van Noort JM, Amor S (2014) Oligodendrocyte-microglia cross-talk in the central nervous system. Immunology 141(3):302–313
Perry VH (2012) Innate inflammation in Parkinson’s disease. Cold Spring HarbPerspect Med 2(9):a009373
Perry VH, Teeling J (2013) Microglia and macrophages of the central nervous system: the contribution of microglia priming and systemic inflammation to chronic neurodegeneration. Semin Immunopathol 35(5):601–612
Radtke FA, Chapman G, Hall J, Syed YA (2017) Modulating neuroinflammation to treat neuropsychiatric disorders. Biomed Res Int 2017:5071786. https://doi.org/10.1155/2017/5071786
Rainsford KD (2012) Ibuprofen: Pharmacology. Therapeutics and Side Effects, Springer, Heidelberg New York Dordrecht London
Ramazani E, Tayarani-Najaran Z, Fereidoni M (2019) Celecoxib, indomethacin, and ibuprofen prevent 6-hydroxydopamine-induced PC12 cell death through the inhibition of NFκB and SAPK/JNK pathways. Iran J Basic Med Sci 22(5):477–484. https://doi.org/10.22038/IJBMS.2019.34011.8091
Recasens A, Dehay B (2014) Alpha-synuclein spreading in Parkinson's disease. Front Neuroanat 18(8):159
Ren L, Yi J, Yang J, Li P, Cheng X, Mao P (2018) Nonsteroidal anti-inflammatory drugs use and risk of Parkinson disease: A dose-response meta-analysis. Medicine 97(37):e12172. https://doi.org/10.1097/MD.0000000000012172
Ricciotti E, FitzGerald GA (2011) Prostaglandins and inflammation. Arterioscler Throm Vasc Biol 31(5):986–1000
Rock RB, Gekker G, Hu S, Sheng WS, Cheeran M, Lokensgard JR, Peterson PK (2004) Role of microglia in central nervous system infections. ClinMicrobiol Rev 17(4):942–964
Saha RN, Pahan K (2006) Regulation of inducible nitric oxide synthase gene in glial cells. Antioxid Redox Signal 8(5–6):929–947
Salvemini D, Misko TP, Masferrer JL, Seibert K, Currie MG, Needleman P (1993) Nitric oxide activates cyclooxygenase enzymes. Proc Natl Acad Sci USA 90(15):7240–7244
Sánchez-Pernaute R, Ferree A, Cooper O, Yu M, Brownell AL, Isacson O (2004) Selective COX-2 inhibition prevents progressive dopamine neuron degeneration in a rat model of Parkinson's disease. J Neuroinflam 1(1):6. https://doi.org/10.1186/1742-2094-1-6
Schiefer J, Kampe K, Dodt HU, Zieglgänsberger W, Kreutzberg GW (1999) Microglial motility in the rat facial nucleus following peripheral axotomy. J Neurocytol 28(6):439–453. https://doi.org/10.1023/a:1007048903862
Simmons DL, Botting RM, Hla T (2004) Cyclooxygenase isozymes: the biology of prostaglandin synthesis and inhibition. Pharmacol Rev 56(3):387–437
Singh S, Swarnkar S, Goswami P, Nath C (2011) Astrocytes and microglia: responses to neuropathological conditions. Internat J Neurosci 121(11):589–597. https://doi.org/10.3109/00207454.2011.598981
Singh AK, Tiwari MN, Upadhyay G, Patel DK, Singh D, Prakash O, Singh MP (2012) Long term exposure to cypermethrin induces nigrostriatal dopaminergic neurodegeneration in adult rats: postnatal exposure enhances the susceptibility during adulthood. Neurobiol Aging 33:404–415
Singh A, Tripathi P, Prakash O, Singh MP (2016) Ibuprofen abates cypermethrin-induced expression of pro-inflammatory mediators and mitogen-activated protein kinases and averts the nigrostriatal dopaminergic neurodegeneration. MolNeurobiol 53:6849–6858
Sochocka M, Diniz BS, Leszek J (2017) Inflammatory response in the CNS: friend or Foe? MolNeurobiol 54(10):8071–8089
Srivastava G, Dixit A, Yadav S, Patel DK, Prakash O, Singh MP (2012) Resveratrol potentiates cytochrome P450 2 d22-mediated neuroprotection in maneb- and paraquat-induced parkinsonism in the mouse. Free RadicBiol Med 52(8):1294–1306
Stefanis L (2012) α-Synuclein in Parkinson's disease. Cold Spring HarbPerspect Med 2(2):a009399
Stratman NC, Carter DB, Sethy VH (1997) Ibuprofen: effect on inducible nitric oxide synthase. Brain Res Mol Brain Res 50(1–2):107–112. https://doi.org/10.1016/s0169-328x(97)00168-x
Straus DS, Glass CK (2001) Cyclopentenone prostaglandins: new insights on biological activities and cellular targets. Med Res Rev 21:185–210
Su X, Maguire-Zeiss KA, Giuliano R, Prifti L, Venkatesh K, Federoff HJ (2008) Synuclein activates microglia in a model of Parkinson's disease. Neurobiol Aging 29(11):1690–1701
Takeuchi H, Mizuno T, Zhang G, Wang J, Kawanokuchi J, Kuno R, Suzumura A (2005) Neuritic beading induced by activated microglia is an early feature of neuronal dysfunction toward neuronal death by inhibition of mitochondrial respiration and axonal transport. J Biol Chem 280(11):10444–10454. https://doi.org/10.1074/jbc.M413863200
Tansey MG, Goldberg MS (2010) Neuroinflammation in Parkinson’s disease: its role in neuronal death and implications for therapeutic intervention. Neurobiol Dis 37(3):510–518
Teismann P (2012) COX-2 in the neurodegenerative process of Parkinson’s disease. BioFactors (Oxford, England) 38(6):395–397. https://doi.org/10.1002/biof.1035
Theodore S, Cao S, McLean PJ, Standaert DG (2008) Targeted overexpression of human alpha-synuclein triggers microglial activation and an adaptive immune response in a mouse model of Parkinson disease. J Neuropathol Exp Neurol 67(12):1149–1158
Tripathi P, Singh A, Bala L, Patel DK, Singh MP (2018) Ibuprofen protects from cypermethrin-induced changes in the striatal dendritic length and Spine Density. MolNeurobiol MolNeurobiol 55(3):2333–2339
Tsuji T, Asanuma M, Miyazaki I, Miyoshi K, Ogawa N (2009) Reduction of nuclear peroxisome proliferator-activated receptor gamma expression in methamphetamine-induced neurotoxicity and neuroprotective effects of ibuprofen. Neurochem Res 34:764–774
Tufekci KU, Genc S, Genc K (2011) The endotoxin-induced neuroinflammation model of Parkinson’s disease. Parkinsons Dis 18:487–450
Tuteja N, Chandra M, Tuteja R, Misra MK (2004) Nitric oxide as a unique bioactive signaling messenger in physiology and pathophysiology. J Biomed Biotechnol 4:227–237
Uttara B, Singh AV, Zamboni P, Mahajan RT (2009) Oxidative stress and neurodegenerative diseases: a review of upstream and downstream antioxidant therapeutic options. Curr Neuropharmacol 7(1):65–74
Van Antwerpen P, Nève J (2004) In vitro comparative assessment of the scavenging activity against three reactive oxygen species of non-steroidal anti-inflammatory drugs from the oxicam and sulfoanilide families. Eur J Pharmacol 496(1–3):55–61. https://doi.org/10.1016/j.ejphar.2004.06.017
Vijitruth R, Liu M, Choi DY, Nguyen XV, Hunter RL, Bing G (2006) Cyclooxygenase-2 mediates microglial activation and secondary dopaminergic cell death in the mouse MPTP model of Parkinson’s disease. J Neuroinflam 3:6. https://doi.org/10.1186/1742-2094-3-6
Volpe BT, Wildmann J, Altar CA (1998) Brain-derived neurotrophic factor prevents the loss of nigral neurons induced by excitotoxic striatal-pallidal lesions. Neuroscience 83(3):741–748. https://doi.org/10.1016/s0306-4522(97)00424-7
Wang Q, Liu Y, Zhou J (2015) Neuroinflammation in Parkinson’s disease and its potential as therapeutic target. Transl Neurodegen 12(4):19
Wilkinson BL, Landreth GE (2006) The microglial NADPH oxidase complex as a source of oxidative stress in Alzheimer's disease. J Neuroinflam 9(3):30
Wnuk A, Kajta M (2017) Steroid and xenobiotic receptor signalling in apoptosis and autophagy of the nervous system. Int J Mol Sci 18(11):E2394
Wu KK (2010) peroxisome proliferator-activated receptors protect against apoptosis via 14-3-3. PPAR Res 2:417646
Yadav S, Dixit A, Agrawal S, Singh A, Srivastava G, Singh AK, Srivastava PK, Prakash O, Singh MP (2012) Rodent models and contemporary molecular techniques: notable feats yet incomplete explanations of Parkinson's disease pathogenesis. MolNeurobiol 46(2):495–512
Yan J, Fu Q, Cheng L, Zhai M, Wu W, Huang L, Du G (2014) Inflammatory response in Parkinson’s disease (Review). Mol Med Rep 10(5):2223–2233
Yonutas HM, Sullivan PG (2013) Targeting PPAR isoforms following CNS injury. Curr Drug Targets 14(7):733–742. https://doi.org/10.2174/1389450111314070003
Zaminelli T, Gradowski RW, Bassani TB, Barbiero JK, Santiago RM, Maria-Ferreira D, Baggio CH, Vital MA (2014) Antidepressant and antioxidative effect of Ibuprofen in the rotenone model of Parkinson’s disease. Neurotox Res 26(4):351–362
Zawada WM, Banninger GP, Thornton J, Marriott B, Cantu D, Rachubinski AL, Das M, Griffin WS, Jones SM (2011) Generation of reactive oxygen species in 1-methyl-4-phenylpyridinium (MPP+) treated dopaminergic neurons occurs as an NADPH oxidase-dependent two-wave. Cascade J Neuroinflam 8:129
Funding
Authors acknowledge Science and Engineering Research Board for sanctioning the fellowship grant (PDF/2017/000984) to support financially. We would like to acknowledge Indian Council of Medical Research for financial support (2016-0264/CMB/ADHOC-BMS).
Author information
Authors and Affiliations
Contributions
AS wrote and designed the manuscript, PT wrote the manuscript. SS designed and critically reviewed the manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
Authors declare that they have no conflict of interest.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Singh, A., Tripathi, P. & Singh, S. Neuroinflammatory responses in Parkinson’s disease: relevance of Ibuprofen in therapeutics. Inflammopharmacol 29, 5–14 (2021). https://doi.org/10.1007/s10787-020-00764-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10787-020-00764-w