Abstract
Innate immunity refers to defense mechanisms that are always present, ready to combat microbes and other offending agents. Innate immunity acts as a first-line defense and activates the conventional immune responses; however, it has been speculated that the importance of innate immunity in initiation and development of some disorders is more than just the “first line of defense”. Autoimmune diseases, caused by immune system overactivation, are among the most challenging scientific and clinical problems, and there is still much to be learned about their pathogenesis. We aimed to provide a comprehensive overview of available documents about the role of innate immunity in systemic autoimmune diseases including rheumatoid arthritis, systemic lupus erythematosus, Sjögren’s syndrome, polymyositis, and systemic sclerosis. This study highlights the innate immunity pathways or molecules that are under investigation for therapy of these diseases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Immunity is a complicated concept that can be divided into two primary arms: the innate and adaptive systems. Innate immunity as the first-line defense against pathogens consists of physical barriers, soluble factors, and cells. Adaptive immunity is made up of a vast array of special cells called B and T lymphocytes (Frizinsky et al. 2019; Watts et al. 2017). The breakdown of self-tolerance as the hallmark of autoimmunity is based on adaptive immunity, but innate immunity also has unique characteristics, which make it a central driver in some critical immune responses (Zouali and La Cava 2019). Autoimmunity is a consequence of the failure of self-tolerance and immune reaction against an autoantigen, which is classified as systemic or organ specific (Pozsgay et al. 2017). Systemic autoimmune diseases are a wide array of disorders including rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), Sjögren’s syndrome, polymyositis (PM), and systemic sclerosis (Pasoto et al. 2019). This heterogeneous group of disorders is characterized by the presence of ubiquitously expressed autoantigens and the involvement of multiple tissues and organs (Fridkis-Hareli 2008). To the best of our knowledge, although the association between adaptive immunity and autoimmune disease has been extensively studied, the importance of innate immunity in the development of autoimmune disorders is yet to be determined. Considering the lack of cure for this kind of disease, which leads to the need for long-lasting treatment, studies on the basic research give us new insights to help identify novel therapeutic targets. This study highlights how innate immunity might affect systemic autoimmune diseases and presents therapies targeting innate immunity components in systemic autoimmune diseases that are currently under investigation.
Rheumatoid arthritis
Rheumatoid arthritis is a systemic autoimmune disease that affects mainly joints, leading to chronic inflammation of joints, cartilage damage, bone erosion, and finally systemic complications (Croia et al. 2019). Although the etiology of RA has not been fully delineated, both innate and adaptive immune systems are indispensable in the pathogenesis of RA (Rana et al. 2018). In this review, we will focus on innate immune cells and their crucial roles in RA.
Macrophages (MQs) are significant immune cells and central players in the pathogenesis of RA. They are the main source of proinflammatory cytokines such as tumor necrosis factor (TNFα), interleukin-1 beta (IL-1β), and IL-6, which generate inflammatory responses and contribute to the destruction of cartilage and bone resorption in patients with RA (Udalova et al. 2016). Synovial MQ plays critical roles in the events driving inflammation, including immune cell recruitment, fibroblast cell expansion, and protease secretion, leading to synovium destruction (Kennedy et al. 2011). Researchers have indicated that the imbalance between M1 and M2 MQs has a critical role in the pathogenesis of RA (Wang et al. 2017). MQs in the synovial fluid of patients with RA produce large amounts of TNFα and IL-1β, important proinflammatory cytokines that are characteristically secreted by M1 MQ (Kennedy et al. 2011).
Neutrophils are the first immune cells that arrive at the inflammation site. Their function includes phagocytosis, production of reactive oxygen species (ROS), and generation of neutrophil extracellular traps (NETs) in host defense (Bach et al. 2020). NETs can be the key source of citrullinated autoantigens, which can trigger the progress of RA (Chen et al. 2018). Citrullinated autoantigens in NETs can be taken up by fibroblast-like synoviocytes (FLS), presented to T cells, and leading to expansion of T and B cell response in patients with RA (Carmona-Rivera et al. 2017). Proinflammatory cytokines such as TNFα, IL-6, IL-8, and IL-17A can increase NETs in RA neutrophils. Eventually, NETs stimulate more cytokine production and inflammation via activating FLS and MQs (Chen et al. 2018; Khandpur et al. 2013). Neutrophils in the synovial fluid of patients with RA produce B lymphocyte stimulator (BLyS) that contributes to the activation of autoreactive B lymphocytes (Assi et al. 2007); furthermore, synovial joint neutrophils produce receptor activator of nuclear factor kappa-B ligand (RANKL) that is implicated in the activation and differentiation of osteoclasts and bone erosion in patients with RA (O’Neil and Kaplan 2019). Immune complexes are the major activator of neutrophils in the RA joint, by interaction with the FCγ receptor on the neutrophils (Wright et al. 2014).
Dendritic cells (DCs), as dedicated professional antigen-presenting cells, are likely key players in the initiation of joint inflammation and implicated in RA development (Yu and Langridge 2017). There are an increased number of myeloid and plasmacytoid DCs (pDC) in the joints of patients with RA. Some studies have suggested that in the proinflammatory environment, such as synovial fluid of patients with RA, the function of DC is different. These cells have been proposed as an inflammatory DC subset (Lebre et al. 2008; Yu and Langridge 2017). Inflammatory DCs are the main inducer of IL-17-producing T helper (Th17) cells by the production of IL-23 in the RA synovium (Estrada-Capetillo et al. 2013; Yu and Langridge 2017). Smoking has some effects on DCs, such as modifying the antigens that DCs present and regulation of DC activity (Yu and Langridge 2017).
Natural killer (NK) cells play an important role in the pathogenesis of RA through the production of inflammatory cytokines and interaction with various immune cells in synovial tissue (Ahern and Brennan 2011). Some studies have indicated the increased NK cells in the synovium of patients with RA expressed elevated levels of activation markers and cytokines such as TNFα and interferon-γ (IFNγ) (Fogel et al. 2013). NK cells secrets IFNγ, which may involve in inflammation through induction of B cell activation, class switching, and DC maturation (Ahern and Brennan 2011).
FLS, non-immune cells in synovium, have a critical function in the pathogenesis of RA. In an inflammatory microenvironment, FLS produce chemokines such as CCL2, CCL5, CCL8, CXCL5, and CXCL10, which recruit monocytes and MQs, and subsequently contribute to the pathogenesis of RA and inflammation. Activated FLS produces high levels of RANKL, which is a significant factor for the differentiation of osteoclasts and bone resorption. FLS functions as an antigen-presenting cell and interacts with CD4+ T cells; furthermore, FLS has critical roles in the differentiation of T cells with the production of cytokines (Yoshitomi 2019). FLS produces large amounts of B cell-activating factor (BAFF) and IL-6 that contribute to the maturation and survival of B cells (Hunter and Jones 2015; Yoshitomi 2019).
The significant function of mucosal surfaces in the pathogenesis of RA has been proved. There is some evidence that the first hit in breaking self-tolerance for RA may originate at the epithelial surfaces. Many risk factors such as smoking and periodontal disease, at oral and lung levels, involve autoimmunity by driving the production of citrullinated autoantigens, and consequently anti-citrullinated protein antibodies (ACPA) (Lucchino et al. 2019; Pentony et al. 2017). A strong association between ACPAs and the progress of RA has been confirmed in various phases of the disease in patients with RA (Kurowska et al. 2017). Gut dysbiosis contributes to the inflammatory state through induced Th17 polarization and imbalance between Th17 and regulatory T cells (Lucchino et al. 2019).
Many studies show the pathologic role of mast cells (MC) in RA (Xu and Chen 2015). The number of MCs is increased in RA synovium. Activated MCs produce various mediators, cytokines, and chemokines that recruit inflammatory cells into synovium. The main source of IL-17A in RA synovium is MCs (Min et al. 2020). It is indicated that MCs enhance survival, activation, proliferation, and differentiation of naive B cells (Rivellese et al. 2018).
γδ T cells are mainly distributed in the mucosal and epithelial tissues. These cells have a significant role in autoimmune diseases such as RA. γδ T cells contribute to an escalated production of proinflammatory cytokines, pathogenic autoantibody, and finally lead to the initiation of this autoimmune disease (Sun 2013).
Various autoantibodies present in patients with RA, such as anti-collagen type II antibodies, ACPA, and rheumatoid factor that target antigens in cartilage and synovium, lead to the formation of immune complexes. These immune complexes can activate complement and consequently cause the chronic destruction of the joint (Dijkstra et al. 2019). Many studies have shown the presence of activated or cleaved complement components in the joint, and elements such as C1q–C4 complexes in the circulation of patients with RA (Dijkstra et al. 2019; Wouters et al. 2006). The level of C5a is elevated in the synovium of patients with RA, which is related to an increased number of infiltrating neutrophils. It seems that C3a and C5a are involved in the activation of the NLRP3 inflammasome pathway, which is important in RA inflammatory processes (Paoliello-Paschoalato et al. 2015) (Figs. 1, 2).
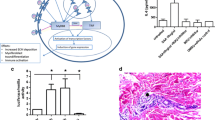
Mucosal surfaces considered as potential initiating sites of RA. Environmental risk factors (smoking and infections) and various susceptibility genes lead to localized innate immune responses in epithelium, especially airway epithelial cells. This localization of innate immune cells results in some changes in epithelium, including induction of oxidative stress and expression of protein-arginine deiminase (PADI), with consequent generation of citrullinated proteins and synovitis in the latter
Summary of innate immunity involved in the synovium. After the interaction between synovial CD4+ T cells and antigen-presenting cell (APC) presenting an unknown antigen, T cells differentiate to Th1 and Th17 and produce IFNγ and IL-17, respectively. These cytokines stimulate synovial MQ to secrete proinflammatory mediators, among which TNFα is paramount. TNFα regulates the balance between bone destruction and formation under normal conditions. In RA disease, TNFα stimulates osteoclastogenesis and inhibits the differentiation of activated osteoblast
Some of the innate immunity-related targets for the treatment of RA are listed in Table 1 (US Food and Drug Administration (FDA) approved and clinical trials).
Systemic lupus erythematosus
Systemic lupus erythematosus (SLE), also simply known as lupus, is a chronic multifaceted autoimmune disease with protean manifestation that affects multiple organs including kidneys, skin, heart, and lungs. It is characterized by the presence of specific autoantibodies for self-antigens, such as double-stranded DNA (dsDNA), ribonucleoproteins, histones, and certain cytoplasmic components (Herrada et al. 2019). Some studies have elucidated that various immune cells and proinflammatory cytokines play a significant role in SLE pathogenesis. The innate immune system, particularly MQs, have been indicated as a key player in the pathogenesis of SLE (Dema and Charles 2014). In this review, we focus on cellular and molecular components of innate immunity in SLE pathogenesis.
MQs are defective in the phagocytosis and clearance of apoptotic cells; thus, prolonged exposure of autoantigens to the adaptive immune cells provides survival signals for autoreactive B cells and consequently loss of tolerance to nuclear antigens released from apoptotic cells (Ma et al. 2019a; b). Plasticity is a major feature of MQs, which depends on cytokine milieu. MQs are classified as two main groups: classically activated MQs (M1) and alternatively activated MQs (M2). M1 MQs are induced by IFNγ and lipopolysaccharide (LPS) that are involved in inflammatory responses, whereas M2 MQs are induced by IL-4 and IL-13 that are involved in tissue remodeling (Chalmers et al. 2015; Labonte et al. 2014; Mantovani et al. 2004). Some data has suggested that M1 and M2 MQs have different roles in SLE development. M1 MQs increase the severity of the condition, while M2 MQs reduce it (Li et al. 2015). Therefore, MQ polarization modulates the development of SLE. It is reported that M2-polarizing cytokines such as IL-4 may have therapeutic effects to reduce SLE symptoms (Li et al. 2015).
Of circulating white blood cells, neutrophils are the largest in number. It is proposed that they play a pathogenic role in SLE. Patients with SLE have elevated numbers of apoptotic neutrophils in circulation; this scenario is related to the development of autoantibodies against DNA and disease activity (Herrada et al. 2019; Lande et al. 2011). Clearance of NETs is defective in patients with SLE and leads to the presentation of self-antigens, including immunogenic DNA, histones, and neutrophil proteins to the immune system and contributes to the development of autoantibodies and proinflammatory cytokines, driving the pathogenesis of SLE (Apel et al. 2018; Herrada et al. 2019). The neutrophils of patients with SLE have an abnormal function; their phagocytosis ability is decreased, while their production of ROS is increased (Alves et al. 2008). NETosis, a type of cell death, is elevated in the neutrophils of patients with SLE by the presence of antibody–ribonucleoprotein complexes, which activate various immune cells. Mouse models of SLE demonstrate elevated bone marrow NETosis and autoantibodies that distinguish NET components (Gupta and Kaplan 2016). It is reported that endonuclease DNase1 is required to degrade NETs. Some patients with SLE have DNase1 inhibitors, whereas other patients with SLE have high levels of antibodies that bind to NETs and protect them from DNase1 (Apel et al. 2018). A distinct subset of neutrophil-like cells termed low-density granulocytes (LDGs) are distinguished in patients with SLE that produce excess proinflammatory cytokines such as IL-6, IL-8, TNFα, and IFN-I. It is proposed that LDGs have an important role in SLE pathogenesis (Apel et al. 2018; Carmona-Rivera and Kaplan 2013).
Some studies documented dysregulated DCs play a critical role in the initiation and development of SLE (Mok 2015). DCs from patients with SLE show a considerable reduction of PD-L1 expression during active disease, whereas the expression of CD80/CD86 is elevated (Mackern‐Oberti et al. 2015).
Plasmacytoid DCs (pDCs) in patients with SLE produce high levels of IFNα that causes a positive-feedback loop in the activation of innate and adaptive immunity (Mok 2015). pDCs numbers are decreased in the blood of patients with SLE, but pDCs nonetheless accumulate in the damaged skin of patients with lupus (Herrada et al. 2019). Several reports have indicated that pDCs depletion reduces the activation and expansion of immune cells, limits autoantibody production, and restricts kidney inflammation in patients with SLE (Rowland et al. 2014).
Increased IFNα levels in in patients with SLE are correlated with both disease activity and severity. Sustained IFNα production, a signature of lupus, may lead to the development of autoreactive T and B cells (Huang et al. 2011; Ronnblom and Alm 2001).
NK cells may have a crucial role in the pathogenesis of SLE. It has been reported that the number of NK cells in patients with SLE is diminished and their cytotoxicity is impaired (Cho et al. 2011). Some studies have suggested a decrease in the number of natural killer T (NKT) cells in patients with SLE (Chen et al. 2015). IL-15 is a cytokine that plays a major role in NK differentiation and survival and was elevated in patients with SLE, a scenario which is related to disease activity (Lin et al. 2017). The activatory receptor CD69 is overexpressed in NK cells of patients with SLE with active disease (Lin et al. 2017). Some studies have indicated that NK cells produce high levels of IFNγ in patients with SLE with active disease and this has been associated with cytotoxicity and contributed to the dysregulation of the link between innate and adaptive immunity in SLE (Hervier et al. 2011).
Basophils are involved in skin lesions in patients with SLE and have a role in promoting tissue damage (Pan et al. 2017). Basophils are proposed as a biomarker of disease activity in SLE. These cells have an important role in inflammation and anti-nuclear antibody production by B cells (Charles et al. 2010). Prostaglandin D2 (PGD2) is increased in patients with SLE and interacts with PGD2 receptors on the surface of basophils, leading to migration of basophils to secondary lymphoid organs (Pellefigues et al. 2018).
The complement system plays controversial roles in the pathogenesis of SLE. This system has protective and pathologic functions. Complement exerts its protective role through clearance of immune complexes and apoptotic cells, as well as induction of tolerance (Pabón-Porras et al. 2019). Complement activation leading to the inflammatory response and tissue damage defines the pathologic role of complement (Markiewski and Lambris 2007). Complement deficiency is correlated to the pathogenesis of SLE (Pabón-Porras et al. 2019). C1q deficiencies, including genetic defects or anti-C1q autoantibodies, can cause SLE in 90% of patients. It is well established that C1q is involved in the regulation of immune cell differentiation and MQ polarization to a tolerogenic phenotype (Son et al. 2015). The low levels of C1q, C4, and C2 are associated with dysfunction in the clearance of apoptotic and debris cells (Pabón-Porras et al. 2019).
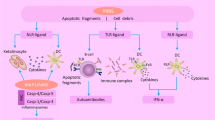
Keratinocytes may be a key player in the pathogenesis of SLE. These cells are activated by UV light and produce inflammatory cytokines that result in the recruitment of immune cells and the initiation of inflammatory responses (Pentony et al. 2017) (Fig. 3).
Role of innate immunity in SLE pathogenesis. Genetic and environmental factors contribute to a breakdown of self-tolerance in SLE. Exposure to sunlight induces apoptosis of keratinocytes, leading to the formation of blebs which contain both nuclear and cytoplasmic antigens on the surface of dying cells. These antigens are captured by APC cells such as B cells and pDCs. Activated pDC produces abnormally large amounts of IFN-γ leading to more production of autoantibodies by B cells. Autoantibodies induce NETosis and the release of antimicrobial peptides results in nuclear antigen complex formation. The net result is a cycle of antigen release and immune activation that leads to the production of high-affinity autoantibodies
Some of the innate immunity-related targets for the treatment of SLE are listed in Table 2 (clinical trials).
Sjögren’s syndrome
Sjögren’s syndrome (SS) is a systemic autoimmune disease that affects the exocrine glands, mainly involving the lachrymal and salivary ones, leading to dryness of the eyes and mouth. SS is classified into two forms: primary (pSS) and secondary (sSS). Primary SS is often associated with dysfunction in lacrimal and salivary flow and features a wide range of both organ-specific and systemic manifestations, whereas sSS occurs in association with another autoimmune disease, such as SLE or RA (Kiripolsky et al. 2017; Malladi et al. 2012). While the pathogenesis of pSS is currently not well understood like other autoimmune diseases, both innate and adaptive immune systems play a critical role in the disease pathogenesis. Many types of innate cells are implicated in SS.
Some studies have also shown that MQs are critical mediators of SS pathogenesis (Ma et al. 2019a; b). The presence of M1 and M2 MQs has been indicated in the salivary glands in the SS mouse model (Baban et al. 2013; Ma et al. 2019a, b). Proinflammatory M1 polarization is the major phenotype of SS MQ and systemic and local concentrations of IL-6 are importantly increased in patients with SS (Tishler et al. 1999). Also, patients with SS with more active disease display higher levels of IL-12, while patients with less active disease display higher levels of IL-35 (Fogel et al. 2018). Levels of monocyte chemoattractant protein-1 (MCP-1/CCL2), IFNγ, and proinflammatory cytokines or chemokines that are secreted by monocyte and MQ, such as, IL-6, IL-18, IFN-I, and BAFF, are importantly elevated in patients with SS (Hernández-Molina et al. 2011; Willeke et al. 2009; Brkic et al. 2013; Yoshimoto et al. 2011). It has also been reported that the level of IκBα in SS monocytes is decreased, which leads to NFκB signaling pathway dysregulation and production of proinflammatory cytokines (Lisi et al. 2012).
DCs play a significant role in SS, as they function as antigen-presenting cells in ectopic germinal centers in the salivary gland (Bombardieri and Pitzalis 2012). Some studies suggest that DCs are increased in salivary tissue of patients with pSS as compared to controls (Ozaki et al. 2010). Plasmacytoid DCs, a specific DC subset, are activated by Toll-like receptors (TLRs) and produce several proinflammatory cytokines, including IFNα (Vogelsang et al. 2006).
Primary DCs are the main source of IFN-I in response to foreign nucleic acids. IFN-I might be involved in the pathogenesis of SS (Hillen et al. 2019). Self-nucleic acids in the form of autoantibody complexes and apoptotic cell fragments are present in patients with pSS and considerably activate production of IFN-I by pDCs (Ainola et al. 2018). It is indicated that pDCs play a critical role in pSS pathogenesis (Hillen et al. 2019). The activated phenotype and enhanced production of proinflammatory cytokines by pSS-pDCs can affect salivary gland inflammation dramatically (Hillen et al. 2019).
Some studies have shown that NK cells are involved in SS pathogenesis, although the precise role of these cells is unknown. NK cells have been related to salivary gland inflammation in patients with pSS. NK cells express NCR3/NKp30 that regulates IFN-II secretion and correlation with DCs. In patients with pSS, NKp30 expression is elevated in comparison to controls. Salivary gland epithelial cells express NKp30 ligand, and interaction of this ligand with NKp30 leads to the production of Th1 cytokines (Rusakiewicz et al. 2013). In contrast to the salivary gland, in patients with pSS, the number and activity of peripheral blood NK cells are significantly reduced as compared to healthy controls (Izumi et al. 2006). It is possible that NK cells are protective in early stages of the disease and later play a pathogenic role in advanced disease (Kiripolsky et al. 2017). It was proven that invariant natural killer T (iNKT) cells were significantly decreased in pSS, and a possible correlation between the low number of iNKT and autoreactive tissue injury was suggested (Rizzo et al. 2019).
It is known that in patients with SS, neutrophil functions such as phagocytosis, chemotaxis, and chemokinesis were normal, while their adherence ability was impaired (Gudbjörnsson et al. 1991).
Some studies have reported a significant role of salivary gland epithelial cells in SS pathogenesis (Kiripolsky et al. 2017; Manoussakis and Kapsogeorgou 2007). Furthermore, it has been shown that salivary gland epithelial cells express high levels of TLR2, TLR3, and TLR4; thus, they contribute to the induction of innate immune responses upon recognition of foreign pathogens (Deshmukh et al. 2009; Low and Witte 2011; Manoussakis and Kapsogeorgou 2007).
Some of the innate immunity-related targets for the treatment of Sjögren’s syndrome are listed in Table 3 (clinical trials).
Polymyositis
Polymyositis (PM) is an unusual chronic inflammatory connective tissue disease that involves muscles that undergo atrophy over time, so patients with PM cannot climb stairs or even walk. Muscle involvement is different in different parts of the body, and muscle weakness can lead to problems for patients such as dysphagia and breathing difficulties. PM is an autoimmune disease with no clear etiology. As with the other autoimmune conditions, PM is more common in women (Dalakas 1991; Dalakas and Pongratz 2003; Hilton-Jones 2011). Genetics is likely to be associated with the disease, and the presence of specific human leukocyte antigen (HLA) genes such as DRB1*0301 alleles increases the likelihood of PM (Shamim et al. 2000). PM is strongly associated with other inflammatory, viral, and cancerous diseases (Dalakas and Hohlfeld 2003; Hill et al. 2001). Although muscles do not usually express MHC I, they express MHC I and even MHC II widely in PM. Cytotoxic T cells (CTLs) attack healthy fibers of muscles which express MHC I, which seems to be the main reason for tissue injury. Upregulation of co-stimulatory molecules helps the activation of CTLs, but not classic co-stimulatory molecules. Muscles have their own co-stimulatory molecules called BB-1 (Behrens et al. 1998). Muscle biopsies show CTLs migrating into the basal lamina where they accumulate to attack muscle fibers (Arahata and Engel 1986). The mechanism by which fibers are destroyed is via perforin and granzyme, yet muscle fibers express Fas (Behrens et al. 1997). There is little evidence of innate immunity involvement in the immunopathology of PM.
Many inflammatory cytokines are increased in serum of patients with PM, such as IL-1, IL-2, IL-6, and IL-10 as well as TNFα, IFN-γ, and TGFβ (Tews and Goebel 1996). IL-1 and TNFα have a devastating effect on muscles; TNFα induces a proteolytic effect via glucocorticoids, whereas IL-1β exerts its effect through an independent glucocorticoid pathway (Zamir et al. 1992). Overexpression of chemokines has also been observed, including CXCL8, CCL9, CCL2, CXCL9, and CXCL10. They help in directing T cells to the inflammatory sites such as endomysial in inflammatory cells (De Bleecker et al. 2002).
Increased TLRs were observed in biopsies from myopathies, especially TLR3 and TLR7, which are activated by the nucleic acids. Necrotic muscle cells can activate TLR3 which triggers the production of a large amount of IL-6 from myoblasts, leading to the maintenance of inflammatory response in the muscles (Tournadre et al. 2012).
High mobility group box 1 protein (HMGB1) is a nuclear protein that can be secreted by immune cells in inflammatory conditions (Harris et al. 2012). It is secreted by MQs and DCs in connective tissue and enhances MHC I expression by binding to TLR4 in muscle fibers (Ulfgren et al. 2004).
The role of eosinophils in myopathies has also been established; patients who have eosinophilia in muscle biopsy, especially endomysium, have more necrotic fibers (Kumamoto et al. 1996).
DCs accumulate in muscle tissue to present antigens, and plasmacytoid DCs secrete IFN-I (Eloranta et al. 2007). IFN-I plays an essential role in PM. One of the mechanisms that lead to IFN secretion is via cathelicidins, which include LL-37, which is the only cathelicidin expressed in humans. Along with all its antimicrobial, anti-inflammatory, and even proinflammatory functions, LL-37 increases IFN-I (Hilchie et al. 2013). LL-37 footprints have been seen in inflammatory and autoimmune diseases (Kahlenberg and Kaplan 2013). Continuous immune system contact with IFN-I can cause immune tolerance failure and autoimmune diseases, such as myositis (Lu et al. 2017).
Systemic sclerosis
Systemic sclerosis (SSc, scleroderma) is a chronic, heterogeneous autoimmune disease. Thickening of the skin, fibrosis of connective tissue, and vessel dysfunction are results of excessive collagen secretion from the fibroblasts and its deposition which can sometimes involve internal organs (Dowson et al. 2017). Myofibroblasts are the active form of fibroblasts which secrete collagen persistently and is found in fibrotic lesions (Artlett et al. 2011). The main cause of SS is still unclear but the immune system has an important role and autoantibodies are found in most patients. T cells, especially Th2, interact with fibroblasts through profibrotic cytokines such as IL-4, IL-6, and IL-13, which results in aggravation of fibrosis (O'Reilly et al. 2012). The innate immune system plays a critical role in both the onset and progression of the disease (Pattanaik et al. 2015).
The role of many pattern recognition receptors (PRRs) has been proven in autoimmune diseases (Duffy and O'Reilly 2016), such as TLRs in SSc. TLRs identify damage-associated molecular patterns (DAMPs) that have been released from endogenous cells, e.g., they may be secreted in response to stress or damage; also the response of TLRs to PAMPs that leads to the activation of an intracellular signaling pathway, and the improper and over-response of the TLRs to their ligands can be involved in the onset and exacerbation of the disease (Ciechomska et al. 2013a, b). CD14+ monocytes and DCs are activated by TLR4, resulting in a large amount of CCL8 and IL-10 secretion in patients with SSc. CCL8 and IL-10 are chemoattractants of T cells and fibrotic factors which increase in the serum of patients with scleroderma (van Lieshout et al. 2009). Serum amyloid A (SAA), which is a ligand for TLR2 and a type of DAMPs family, can be elevated in patients with scleroderma. This pathway leads to the production of IL-6 through NF-кB (O'Reilly et al. 2014a, b), and IL-6 directly increases collagen secretion from fibroblasts (O'Reilly et al. 2014a, b). Differentiation of fibroblasts into myofibroblasts is common in patients with scleroderma, which results in increased TLR9 because 60% of myofibroblasts express TLR9 (Fang et al. 2016). When TLR9 is activated by its ligand, CpG, secretion of TGFβ would increase. TGFβ has a major role in the differentiation of myofibroblasts and the production of collagen (Fang et al. 2016). MyD88 has a key role in TLR signaling and SSc pathogenesis, and researchers could decrease fibrogenesis by inhibiting MyD88 (Singh et al. 2012).
Monocytes are important cells in SSc. These cells have an important role in extracellular matrix (ECM) remodeling in patients with SSc through TLR8 (ssRNA) and tissue inhibitor of metalloproteinase-1 (TIMP-1) secretion (Duffy and O'Reilly 2016). Fibroblasts break down ECM by secreting matrix metalloproteinase-1 (MMP-1) and maintaining homeostasis; TIMP-1 inhibits MMP-1 and leads to the accumulation of ECM in patients with SSc (Ciechomska et al. 2013a, b). The number of monocytes and MQs elevates in the peripheral blood and the inflammatory sites in patients with scleroderma; this makes the situation worse because these cells are resistant to apoptosis (López-Cacho et al. 2014), and releasing fibrotic factors (Higashi-Kuwata et al. 2010). It has also been reported that IL-4, IL-13, and IL-10 are higher than normal in the serum of patients with scleroderma (Scala et al. 2004), and this causes the formation of M2 MQs, which means more TGFβ and more fibrosis (Higashi-Kuwata et al. 2010).
For years, researchers have been trying to find a link between SSc and the increase or decrease in complement components. It seems that the presence of autoantibodies helps to activate the complement and activation factors increase in all three pathways (Okrój et al. 2016). Vascular endothelium damage is a common cause of scleroderma, which seems to be an important item in the induction of the disease. Factor H is a regulator of complement and its principal function is to protect host cells against complement. Host cells may be damaged by dysfunction of factor H and release cellular contents, react with existing autoantibodies, and form immune complexes (Scambi et al. 2010).
The inflammasome is a multi-factor assembly which is mainly activated by TLRs that lead to activated caspase-1, resulting in IL-1β and IL-18 activation by caspase-1. NLRP3 is the best-known inflammasome and has the main role in many autoinflammatory and autoimmune diseases (Martinon et al. 2002). The role of the IL-1β and IL-1α has been confirmed in fibrosis by an autocrine effect on fibroblasts (Zhang et al. 2014), and serum levels of IL-1β are higher in patients with SSc than in healthy controls (Hussein et al. 2005). Contrary to all efforts, the role of IL-18 has not yet been elucidated in the pathogenesis of SSc (Pan et al. 2011). IL-18 seems to have an antifibrotic effect unlike IL-1β (Kim et al. 2010) but it is increased in patients with SSc (Lin et al. 2019).
The mast cell accumulation has been seen in the affected skin of patients with SSc and has an important role in diseases associated with fibrosis (Hügle 2014) because mast cells have dense granules that contain proinflammatory cytokines, TGFβ, and histamine which help to form myofibroblasts. Mast cells and myofibroblasts interact through gap junctions or send vesicles and help each other to increase inflammation (Hugle et al. 2012).
Some of the innate immunity-related targets for the treatment of SSc are listed in Table 4 (clinical trials).
Conclusion
There is a great unmet need to identify how autoimmunity can be initiated, progressed, and propagated. In this context, innate immunity acts as both a provider of inflammatory conditions for adaptive immunity function and as an independent part or co-player to the adaptive immune system. The dysregulation of innate immunity has been shown in many disorders, so manipulation of related pathways has so far drawn attention, as far as the targeting of TNFα has revolutionized management and outcomes of RA disease. Also, a large number of other therapies are currently being tested in clinical trials; however, there is still much to be learned about this issue and related misconceptions.
References
Ahern DJ, Brennan FM (2011) The role of natural killer cells in the pathogenesis of rheumatoid arthritis: major contributors or essential homeostatic modulators? Immunol Lett 136(2):115–121
Ainola M, Porola P, Takakubo Y, Przybyla B, Kouri V, Tolvanen T et al (2018) Activation of plasmacytoid dendritic cells by apoptotic particles–mechanism for the loss of immunological tolerance in Sjögren's syndrome. Clin Exp Immunol 191(3):301–310
Alves CM, Marzocchi-Machado CM, Louzada-Junior P, Azzolini AEC, Polizello ACM, De Carvalho IF, Lucisano-Valim YM (2008) Superoxide anion production by neutrophils is associated with prevalent clinical manifestations in systemic lupus erythematosus. Clin Rheumatol 27(6):701–708
Apel F, Zychlinsky A, Kenny EF (2018) The role of neutrophil extracellular traps in rheumatic diseases. Nat Rev Rheumatol 14(8):467–475
Arahata K, Engel AG (1986) Monoclonal antibody analysis of mononuclear cells in myopathies. III: Immunoelectron microscopy aspects of cell-mediated muscle fiber injury. Ann Neurol 19(2):112–125
Artlett CM, Sassi-Gaha S, Rieger JL, Boesteanu AC, Feghali-Bostwick CA, Katsikis PD (2011) The inflammasome activating caspase 1 mediates fibrosis and myofibroblast differentiation in systemic sclerosis. Arthritis Rheum 63(11):3563–3574. https://doi.org/10.1002/art.30568
Assi LK, Wong SH, Ludwig A, Raza K, Gordon C, Salmon M et al (2007) Tumor necrosis factor α activates release of B lymphocyte stimulator by neutrophils infiltrating the rheumatoid joint. Arthritis Rheum 56(6):1776–1786
Baban B, Liu JY, Abdelsayed R, Mozaffari MS (2013) Reciprocal relation between GADD153 and Del-1 in regulation of salivary gland inflammation in Sjögren syndrome. Exp Mol Pathol 95(3):288–297
Bach M, Moon J, Moore R, Pan T, Nelson JL, Lood C (2020) A neutrophil activation biomarker panel in prognosis and monitoring of patients with rheumatoid arthritis. Arthritis Rheumatol 72(1):47–56
Behrens L, Bender A, Johnson MA, Hohlfeld R (1997) Cytotoxic mechanisms in inflammatory myopathies. Co-expression of Fas and protective Bcl-2 in muscle fibres and inflammatory cells. Brain J Neurol 120(6):929–938
Behrens L, Kerschensteiner M, Misgeld T, Goebels N, Wekerle H, Hohlfeld R (1998) Human muscle cells express a functional costimulatory molecule distinct from B7.1 (CD80) and B7.2 (CD86) in vitro and in inflammatory lesions. J Immunol 161(11):5943–5951
Boisen AF, Rasmussen EB, Kragstrup TW (2019) AB0069 the downstream effect of adalimumab involves inhibition of synovial cxcl subfamily chemokine expression. Ann Rheum Dis 78:1498–1499
Bombardieri M, Pitzalis C (2012) Ectopic lymphoid neogenesis and lymphoid chemokines in Sjogren's syndrome: at the interplay between chronic inflammation, autoimmunity and lymphomagenesis. Curr Pharm Biotechnol 13(10):1989–1996
Brkic Z, Maria NI, van Helden-Meeuwsen CG, van de Merwe JP, van Daele PL, Dalm VA et al (2013) Prevalence of interferon type I signature in CD14 monocytes of patients with Sjögren's syndrome and association with disease activity and BAFF gene expression. Ann Rheum Dis 72(5):728–735
Carmona-Rivera C, Kaplan MJ (2013) Low-density granulocytes: a distinct class of neutrophils in systemic autoimmunity. Semin Immunopathol 35(4):455–463
Carmona-Rivera C, Carlucci PM, Moore E, Lingampalli N, Uchtenhagen H, James E et al (2017) Synovial fibroblast-neutrophil interactions promote pathogenic adaptive immunity in rheumatoid arthritis. Sci Immunol 2(10):eaag3358
Catrina AI, Lampa J, Ernestam S, Af Klint E, Bratt J, Klareskog L, Ulfgren AK (2002) Anti-tumour necrosis factor (TNF)-α therapy (etanercept) down-regulates serum matrix metalloproteinase (MMP)-3 and MMP-1 in rheumatoid arthritis. Rheumatology 41(5):484–489. https://doi.org/10.1093/rheumatology/41.5.484%JRheumatology
Chaichian Y, Wallace DJ, Weisman MH (2019) A promising approach to targeting type 1 IFN in systemic lupus erythematosus. J Clin Invest 129(3):958–961
Chalmers SA, Chitu V, Ramanujam M, Putterman C (2015) Therapeutic targeting of macrophages in lupus nephritis. Discov Med 20(108):43–49
Charles N, Hardwick D, Daugas E, Illei GG, Rivera J (2010) Basophils and the T helper 2 environment can promote the development of lupus nephritis. Nat Med 16(6):701
Chen J, Wu M, Wang J, Li X (2015) Immunoregulation of NKT cells in systemic lupus erythematosus. J Immunol Res 2015:206731
Chen W, Wang Q, Ke Y, Lin J (2018) Neutrophil function in an inflammatory milieu of rheumatoid arthritis. J Immunol Res 2018:8549329
Cho Y-N, Kee S-J, Lee S-J, Seo S-R, Kim T-J, Lee S-S et al (2011) Numerical and functional deficiencies of natural killer T cells in systemic lupus erythematosus: their deficiency related to disease activity. Rheumatology 50(6):1054–1063
Ciechomska M, Cant R, Finnigan J, van Laar JM, O'Reilly S (2013) Role of toll-like receptors in systemic sclerosis. Expert Rev Mol Med 15:e9
Ciechomska M, Huigens CA, Hügle T, Stanly T, Gessner A, Griffiths B et al (2013b) Toll-like receptor-mediated, enhanced production of profibrotic TIMP-1 in monocytes from patients with systemic sclerosis: role of serum factors. Ann Rheum Dis 72(8):1382–1389. https://doi.org/10.1136/annrheumdis-2012-201958
Croia C, Bursi R, Sutera D, Petrelli F, Alunno A, Puxeddu I (2019) One year in review 2019: pathogenesis of rheumatoid arthritis. Clin Exp Rheumatol 37:347–357
Cunnane G, Madigan A, Murphy E, FitzGerald O, Bresnihan BJR (2001) The effects of treatment with interleukin-1 receptor antagonist on the inflamed synovial membrane in rheumatoid arthritis. Rheumatology 40(1):62–69
Dalakas MC (1991) Polymyositis, dermatomyositis, and inclusion-body myositis. N Engl J Med 325(21):1487–1498
Dalakas MC, Hohlfeld R (2003) Polymyositis and dermatomyositis. Lancet 362(9388):971–982. https://doi.org/10.1016/S0140-6736(03)14368-1
Pongratz D (2006) Therapeutic options in autoimmune inflammatory myopathies (dermatomyositis, polymyositis, inclusion body myositis). J Neurol 253:v64–v65
De Bleecker JL, De Paepe B, Vanwalleghem IE, Schröder JM (2002) Differential expression of chemokines in inflammatory myopathies. Neurology 58(12):1779–1785
Dema B, Charles N (2014) Advances in mechanisms of systemic lupus erythematosus. Discov Med 17(95):247–255
Deshmukh US, Nandula SR, Thimmalapura PR, Scindia YM, Bagavant H (2009) Activation of innate immune responses through Toll-like receptor 3 causes a rapid loss of salivary gland function. J Oral Pathol Med 38(1):42–47
Dijkstra DJ, Joeloemsingh JV, Bajema IM, Trouw LA (2019) Complement activation and regulation in rheumatic disease. Semin Immunol 45:101339
Dörner T, Weinblatt M, Van Beneden K, Dombrecht E, De Beuf K, Schoen P, Zeldin RK (2017) FRI0239 Results of a phase 2b study of vobarilizumab, an anti-interleukin-6 receptor nanobody, as monotherapy in patients with moderate to severe rheumatoid arthritis. Ann Rheum Dis 76:575
Dowson C, Simpson N, Duffy L, O'Reilly S (2017) Innate immunity in systemic sclerosis. Curr Rheumatol Rep 19(1):2. https://doi.org/10.1007/s11926-017-0630-3
Duffy L, O'Reilly SC (2016) Toll-like receptors in the pathogenesis of autoimmune diseases: recent and emerging translational developments. ImmunoTargets Therapy 5:69–80. https://doi.org/10.2147/ITT.S89795
Eloranta M-L, Barbasso Helmers S, Ulfgren A-K, Rönnblom L, Alm GV, Lundberg IE (2007) A possible mechanism for endogenous activation of the type I interferon system in myositis patients with anti-Jo-1 or anti-Ro 52/anti-Ro 60 autoantibodies. Arthritis Rheum 56(9):3112–3124. https://doi.org/10.1002/art.22860
Eng GP, Bouchelouche P, Bartels EM, Bliddal H, Bendtzen K, Stoltenberg MJPO (2016) Anti-drug antibodies, drug levels, interleukin-6 and soluble TNF receptors in rheumatoid arthritis patients during the first 6 months of treatment with adalimumab or infliximab: a descriptive cohort study. PLoS One 11(9):e0162316
Estrada-Capetillo L, Hernández-Castro B, Monsiváis-Urenda A, Alvarez-Quiroga C, Layseca-Espinosa E, Abud-Mendoza C et al (2013) Induction of Th17 lymphocytes and Treg cells by monocyte-derived dendritic cells in patients with rheumatoid arthritis and systemic lupus erythematosus. Clin Dev Immunol 2013:584303
Fang F, Marangoni RG, Zhou X, Yang Y, Ye B, Shangguang A et al (2016) Toll-like receptor 9 signaling is augmented in systemic sclerosis and elicits transforming growth factor beta-dependent fibroblast activation. Arthritis Rheumatol 68(8):1989–2002. https://doi.org/10.1002/art.39655
FDA (2008) Certolizumab pegol label information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2008/125160s000lbl.pdf. Accessed 18 Apr 2008
Felten R, Dervovic E, Chasset F, Gottenberg J-E, Sibilia J, Scher F, Arnaud LJAR (2018) The 2018 pipeline of targeted therapies under clinical development for systemic lupus erythematosus: a systematic review of trials. Autoimmunity Rev 17(8):781–790
Felten R, Scher F, Sibilia J, Chasset F, Arnaud LJJBS (2019) Advances in the treatment of systemic lupus erythematosus: from back to the future, to the future and beyond. Joint Bone Spine 86(4):429–436
Fogel LA, Yokoyama WM, French AR (2013) Natural killer cells in human autoimmune disorders. Arthritis Res Ther 15(4):216
Fogel O, Rivière E, Seror R, Nocturne G, Boudaoud S, Ly B et al (2018) Role of the IL-12/IL-35 balance in patients with Sjögren syndrome. J Allergy Clin Immunol 142(1):258–268
Food and Drug Administration (2009) Golimumab pegol label information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2009/125289s000lbl.pdf. Accessed 20 Mar 2009
Fridkis-Hareli M (2008) Immunogenetic mechanisms for the coexistence of organ-specific and systemic autoimmune diseases. J Autoimmune Dis 5(1):1
Frizinsky S, Haj-Yahia S, Maayan DM, Lifshitz Y, Maoz-Segal R, Offengenden I et al (2019) The innate immune perspective of autoimmune and autoinflammatory conditions. Rheumatology 58(6):1–8
Gandolfo S, De Vita SJE (2019) Emerging drugs for primary Sjögren’s syndrome. Expert Opin Emerg Drugs 24(2):121–132
Gillooly K, Zhang Y, Yang X, Zupa-Fernandez A, Cheng L, Strnad J et al (2016) BMS-986165 is a highly potent and selective allosteric inhibitor of Tyk2, blocks IL-12, IL-23 and type I interferon signaling and provides for robust efficacy in preclinical models of systemic lupus erythematosus and inflammatory bowel disease [abstract]. Arthritis Rheumatol 68 (suppl 10)
Gudbjörnsson B, Feltelius N, Hällgren R, Venge P (1991) Neutrophil function in patients with primary Sjögren's syndrome: relation to infection propensity. Ann Rheum Dis 50(10):685–690
Gupta S, Kaplan MJ (2016) The role of neutrophils and NETosis in autoimmune and renal diseases. Nat Rev Nephrol 12(7):402
Harris HE, Andersson U, Pisetsky DS (2012) HMGB1: a multifunctional alarmin driving autoimmune and inflammatory disease. Nat Rev Rheumatol 8(4):195–202. https://doi.org/10.1038/nrrheum.2011.222
Hernández-Molina G, Michel-Peregrina M, Hernández-Ramírez DF, Sánchez-Guerrero J, Llorente L (2011) Chemokine saliva levels in patients with primary Sjögren’s syndrome, associated Sjögren’s syndrome, pre-clinical Sjögren’s syndrome and systemic autoimmune diseases. Rheumatology 50(7):1288–1292
Herrada AA, Escobedo N, Iruretagoyena M, Valenzuela RA, Burgos PI, Cuitino L, Llanos C (2019) Innate immune cells' contribution to systemic lupus erythematosus. Front Immunol 10:772
Hervier B, Beziat V, Haroche J, Mathian A, Lebon P, Ghillani-Dalbin P et al (2011) Phenotype and function of natural killer cells in systemic lupus erythematosus: excess interferon-γ production in patients with active disease. Arthritis Rheum 63(6):1698–1706
Higashi-Kuwata N, Jinnin M, Makino T, Fukushima S, Inoue Y, Muchemwa FC et al (2010) Characterization of monocyte/macrophage subsets in the skin and peripheral blood derived from patients with systemic sclerosis. Arthritis Res Ther 12(4):R128–R128. https://doi.org/10.1186/ar3066
Hilchie AL, Wuerth K, Hancock RE (2013) Immune modulation by multifaceted cationic host defense (antimicrobial) peptides. Nat Chem Biol 9(12):761
Hill CL, Zhang Y, Sigurgeirsson B, Pukkala E, Mellemkjaer L, Airio A et al (2001) Frequency of specific cancer types in dermatomyositis and polymyositis: a population-based study. Lancet 357(9250):96–100
Hillen MR, Pandit A, Blokland SL, Hartgring SA, Bekker CP, van der Heijden EH et al (2019) Plasmacytoid DCs from patients with Sjögren's syndrome are transcriptionally primed for enhanced pro-inflammatory cytokine production. Front Immunol 10:2096
Hilton-Jones D (2011) Observations on the classification of the inflammatory myopathies. Presse Med 40(4):e199–e208
Huang Z, Fu B, Zheng SG, Li X, Sun R, Tian Z, Wei H (2011) Involvement of CD226+ NK cells in immunopathogenesis of systemic lupus erythematosus. J Immunol 186(6):3421–3431
Hügle T (2014) Beyond allergy: the role of mast cells in fibrosis. Swiss Med Weekly 144:w13999
Hugle T, White K, van Laar JM (2012) Cell-to-cell contact of activated mast cells with fibroblasts and lymphocytes in systemic sclerosis. Ann Rheum Dis 71(9):1582. https://doi.org/10.1136/annrheumdis-2011-200809
Huizinga TW, Fleischmann RM, Jasson M, Radin AR, van Adelsberg J, Fiore S et al (2014) Sarilumab, a fully human monoclonal antibody against IL-6Rα in patients with rheumatoid arthritis and an inadequate response to methotrexate: efficacy and safety results from the randomised SARIL-RA-MOBILITY Part A trial. Ann Rheumatic Diseases 73(9):1626–1634
Hunter CA, Jones SA (2015) IL-6 as a keystone cytokine in health and disease. Nat Immunol 16(5):448–457
Hussein MR, Hassan HI, Hofny ER, Elkholy M, Fatehy NA, Abd Elmoniem AE et al (2005) Alterations of mononuclear inflammatory cells, CD4/CD8+ T cells, interleukin 1beta, and tumour necrosis factor alpha in the bronchoalveolar lavage fluid, peripheral blood, and skin of patients with systemic sclerosis. J Clin Pathol 58(2):178–184. https://doi.org/10.1136/jcp.2004.019224
Ikari Y, Isozaki T, Tsubokura Y, Kasama TJC (2019) Peficitinib inhibits the chemotactic activity of monocytes via proinflammatory cytokine production in rheumatoid arthritis fibroblast-like synoviocytes. Cells 8(6):561
Iwamoto N, Sato S, Sumiyoshi R, Chiba K, Miyamoto N, Arinaga K et al (2019) Comparative study of the inhibitory effect on bone erosion progression with denosumab treatment and conventional treatment in rheumatoid arthritis patients: study protocol for an open-label randomized controlled trial by HR-pQCT. Trials 20(1):1–8
Izumi Y, Ida H, Huang M, Iwanaga N, Tanaka F, Aratake K et al (2006) Characterization of peripheral natural killer cells in primary Sjögren’s syndrome: impaired NK cell activity and low NK cell number. J Lab Clin Med 147(5):242–249
Jiang H, Gao H, Wang Q, Wang M, Wu BJB (2020) Molecular mechanisms and clinical application of Iguratimod: a review. Biomed Pharmacother 122:109704
Kahlenberg JM, Kaplan MJ (2013) Little peptide, big effects: the role of LL-37 in inflammation and autoimmune disease. J Immunol 191(10):4895–4901
Karonitsch T, Beckmann D, Dalwigk K, Niederreiter B, Studenic P, Byrne RA et al (2018) Targeted inhibition of Janus kinases abates interfon gamma-induced invasive behaviour of fibroblast-like synoviocytes. Rheumatology 57(3):572–577
Kennedy A, Fearon U, Veale DJ, Godson C (2011) Macrophages in synovial inflammation. Front Immunol 2:52
Khandpur R, Carmona-Rivera C, Vivekanandan-Giri A, Gizinski A, Yalavarthi S, Knight JS et al (2013) NETs are a source of citrullinated autoantigens and stimulate inflammatory responses in rheumatoid arthritis. Sci Transl Med 5(178):178ra140
Kim HJ, Song SB, Choi JM, Kim KM, Cho BK, Cho DH, Park HJ (2010) IL-18 downregulates collagen production in human dermal fibroblasts via the ERK pathway. J Invest Dermatol 130(3):706–715. https://doi.org/10.1038/jid.2009.302
Kiripolsky J, McCabe LG, Kramer JM (2017) Innate immunity in Sjögren's syndrome. Clin Immunol 182:4–13
Klavdianou K, Lazarini A, Fanouriakis AJB (2020) Targeted biologic therapy for systemic lupus erythematosus: emerging pathways and drug pipeline. BioDrugs 34(2):133–147
Kumamoto T, Ueyama H, Fujimoto S, Nagao S, Tsuda T (1996) Clinicopathologic characteristics of polymyositis patients with numerous tissue eosinophils. Acta Neurol Scand 94(2):110–114. https://doi.org/10.1111/j.1600-0404.1996.tb07039.x
Kurowska W, Kuca-Warnawin EH, Radzikowska A, Maśliński W (2017) The role of anti-citrullinated protein antibodies (ACPA) in the pathogenesis of rheumatoid arthritis. Central-Eur J Immunol 42(4):390
Labonte AC, Tosello-Trampont A-C, Hahn YS (2014) The role of macrophage polarization in infectious and inflammatory diseases. Mol Cells 37(4):275
Lande R, Ganguly D, Facchinetti V, Frasca L, Conrad C, Gregorio J et al (2011) Neutrophils activate plasmacytoid dendritic cells by releasing self-DNA–peptide complexes in systemic lupus erythematosus. Sci Transl Med 3(73):73ra19
Lebre MC, Jongbloed SL, Tas SW, Smeets TJ, McInnes IB, Tak PP (2008) Rheumatoid arthritis synovium contains two subsets of CD83-DC-LAMP-dendritic cells with distinct cytokine profiles. Am J Pathol 172(4):940–950
Li F, Yang Y, Zhu X, Huang L, Xu J (2015) Macrophage polarization modulates development of systemic lupus erythematosus. Cell Physiol Biochem 37(4):1279–1288
Lin S-J, Kuo M-L, Hsiao H-S, Lee P-T, Chen J-Y, Huang J-L (2017) Activating and inhibitory receptors on natural killer cells in patients with systemic lupus erythematosis-regulation with interleukin-15. PLoS One 12(10):e0186223
Lin E, Vincent FB, Sahhar J, Ngian G-S, Kandane-Rathnayake R, Mende R et al (2019) Analysis of serum interleukin(IL)-1α, IL-1β and IL-18 in patients with systemic sclerosis. Clin Transl Immunol 8(4):e1045. https://doi.org/10.1002/cti2.1045
Lisi S, Sisto M, Lofrumento DD, D’Amore M (2012) Altered IkBα expression promotes NF-kB activation in monocytes from primary Sjögren’s syndrome patients. Pathology 44(6):557–561
López-Cacho JM, Gallardo S, Posada M, Aguerri M, Calzada D, Mayayo T et al (2014) Association of immunological cell profiles with specific clinical phenotypes of scleroderma disease. Biomed Res Int 2014:148293. https://doi.org/10.1155/2014/148293
Low HZ, Witte T (2011) Aspects of innate immunity in Sjögren's syndrome. Arthritis Res Ther 13(3):218
Lu X, Tang Q, Lindh M, Dastmalchi M, Alexanderson H, Popovic Silwerfeldt K et al (2017) The host defense peptide LL-37 a possible inducer of the type I interferon system in patients with polymyositis and dermatomyositis. J Autoimmun 78:46–56. https://doi.org/10.1016/j.jaut.2016.12.003
Lucchino B, Spinelli FR, Iannuccelli C, Guzzo MP, Conti F, Franco MD (2019) Mucosa-environment interactions in the pathogenesis of rheumatoid arthritis. Cells 8(7):700
Ma C, Xia Y, Yang Q, Zhao Y (2019) The contribution of macrophages to systemic lupus erythematosus. Clin Immunol 207:1–9
Ma W-T, Gao F, Gu K, Chen D-K (2019) The role of monocytes and macrophages in autoimmune diseases: a comprehensive review. Front Immunol 10:1140
Mackern-Oberti JP, Llanos C, Riedel CA, Bueno SM, Kalergis AM (2015) Contribution of dendritic cells to the autoimmune pathology of systemic lupus erythematosus. Immunology 146(4):497–507
Malladi AS, Sack KE, Shiboski SC, Shiboski CH, Baer AN, Banushree R et al (2012) Primary Sjögren's syndrome as a systemic disease: a study of participants enrolled in an international Sjögren's syndrome registry. Arthritis Care Res 64(6):911–918
Manoussakis MN, Kapsogeorgou EK (2007) The role of epithelial cells in the pathogenesis of Sjögren’s syndrome. Clin Rev Allergy Immunol 32(3):225–230
Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M (2004) The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol 25(12):677–686
Markiewski MM, Lambris JD (2007) The role of complement in inflammatory diseases from behind the scenes into the spotlight. Am J Pathol 171(3):715–727
Martinon F, Burns K, Tschopp J (2002) The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-β. Mol Cell 10(2):417–426
Marzaioli V, Canavan M, Floudas A, Wade S, Low C, Veale D, Fearon U (2019) P067 Tofacitinib impairs monocyte-derived dendritic cell differentiation in rheumatoid arthritisand psoriatic arthritis. Ann Rheum Dis 78:A28
Mavragani CP, Moutsopoulos HMJJA (2019) Sjögren's syndrome: old and new therapeutic targets. J Autoimmun 110:102364
Min HK, Kim K-W, Lee S-H, Kim H-R (2020) Roles of mast cells in rheumatoid arthritis. Korean J Internal Med 35(1):12
Mitchell TS, Moots RJ, Wright HL (2017) Janus kinase inhibitors prevent migration of rheumatoid arthritis neutrophils towards interleukin-8, but do not inhibit priming of the respiratory burst or reactive oxygen species production. Clin Exp Immunol 189(2):250–258
Mitoma H, Horiuchi T, Tsukamoto H, Tamimoto Y, Kimoto Y, Uchino A (2008) Mechanisms for cytotoxic effects of anti-tumor necrosis factor agents on transmembrane tumor necrosis factor α-expressing cells: comparison among infliximab, etanercept, and adalimumab. Arthritis Rheum 58(5):1248–1257
Mohamed MEF, Beck D, Camp HS, Othman AAJ (2020) Preferential inhibition of JAK1 relative to JAK3 by upadacitinib: exposure-response analyses of ex vivo data from 2 phase 1 clinical trials and comparison to tofacitinib. J Clin Pharmacol 60(2):188–197
Mok MY (2015) Tolerogenic dendritic cells: role and therapeutic implications in systemic lupus erythematosus. Int J Rheumatic Dis 18(2):250–259
Nesbitt A, Fossati G, Bergin M, Stephens P, Stephens S, Foulkes R et al (2007) Mechanism of action of certolizumab pegol (CDP870): in vitro comparison with other anti-tumor necrosis factor α agents. Inflamm Bowel Dis 13(11):1323–1332
Nesbitt A, Lamour S, Bracher MJ (2009) PEG component of certolizumab pegol inhibits stimulated mast cell degranulation. Am J Gastroenterol 104:S444
NIH (2020) Efficacy and safety study of p144 to treat skin fibrosis in systemic sclerosis. https://ClinicalTrials.gov/show/NCT00574613. Accessed 11 Feb 2013
NIH (2020) Fresolimumab in systemic sclerosis. https://ClinicalTrials.gov/show/NCT01284322. Accessed 16 July 2014
NIH (2020) Imatinib mesylate (Gleevec) in the treatment of systemic sclerosis. https://ClinicalTrials.gov/show/NCT00555581. Accessed 6 Feb 2018
NIH (2020) Nilotinib in the treatment of systemic sclerosis. https://ClinicalTrials.gov/show/NCT01166139. Accessed 4 Oct 2017
NIH (2020) A phase 1 study of MEDI7734 in type I interferon-mediated autoimmune diseases. https://ClinicalTrials.gov/show/NCT02780674. Accessed 21 Dec 2018
NIH (2020) Proof of biological activity of SAR100842 in systemic sclerosis. https://ClinicalTrials.gov/show/NCT01651143. Accessed 25 Mar 2016
NIH (2020) Safety evaluation of dasatinib in subjects with scleroderma pulmonary fibrosis. https://ClinicalTrials.gov/show/NCT00764309. Accessed 29 Feb 2012
NIH (2020) Safety, tolerability, and pharmacokinetics of CAT-192 (human anti-TGF-beta1 monoclonal antibody) in patients with early stage diffuse systemic sclerosis. https://ClinicalTrials.gov/show/NCT00043706. Accessed 5 Mar 2015
NIH (2020) Study of iguratimod in Sjögren's syndrome. https://ClinicalTrials.gov/show/NCT03023592. Accessed 18 Jan 2017
NIH (2020) A Study of RoActemra/Actemra (Tocilizumab) versus placebo in patients with systemic sclerosis. https://ClinicalTrials.gov/show/NCT01532869. Accessed 23 Sept 2016
NIH (2020) A study to evaluate safety and tolerability of multiple doses of MEDI-546 in adult subjects with scleroderma. https://ClinicalTrials.gov/show/NCT00930683. Accessed 8 May 2012
NIH (2020) A trial to compare nintedanib with placebo for patients with scleroderma related lung fibrosis. https://ClinicalTrials.gov/show/NCT02597933. Accessed 13 Dec 2019
O’Neil LJ, Kaplan MJ (2019) Neutrophils in rheumatoid arthritis: breaking immune tolerance and fueling disease. Trends Mol Med 25(3):215–227
Ohta S, Tsuru T, Terao K, Mogi S, Suzaki M, Shono E et al (2014) Mechanism-based approach using a biomarker response to evaluate tocilizumab subcutaneous injection in patients with rheumatoid arthritis with an inadequate response to synthetic DMARDs (MATSURI study). J Clin Pharmacol 54(1):109–119
Okrój M, Johansson M, Saxne T, Blom AM, Hesselstrand R (2016) Analysis of complement biomarkers in systemic sclerosis indicates a distinct pattern in scleroderma renal crisis. Arthritis Res Ther 18(1):267–267. https://doi.org/10.1186/s13075-016-1168-x
Oon S, Wilson NJ, Wicks IJ (2016) Targeted therapeutics in SLE: emerging strategies to modulate the interferon pathway. Clin Transl Immunol 5(5):e79
O'Reilly S, Hugle T, van Laar JM (2012) T cells in systemic sclerosis: a reappraisal. Rheumatology (Oxford) 51(9):1540–1549. https://doi.org/10.1093/rheumatology/kes090
O'Reilly S, Cant R, Ciechomska M, Finnigan J, Oakley F, Hambleton S, van Laar JM (2014a) Serum amyloid A induces interleukin-6 in dermal fibroblasts via Toll-like receptor 2, interleukin-1 receptor-associated kinase 4 and nuclear factor-κB. Immunology 143(3):331–340. https://doi.org/10.1111/imm.12260
O'Reilly S, Ciechomska M, Cant R, van Laar JM (2014b) Interleukin-6 (IL-6) trans signaling drives a STAT3-dependent pathway that leads to hyperactive transforming growth factor-β (TGF-β) signaling promoting SMAD3 activation and fibrosis via Gremlin protein. J Biol Chem 289(14):9952–9960. https://doi.org/10.1074/jbc.M113.545822
Ozaki Y, Ito T, Son Y, Amuro H, Shimamoto K, Sugimoto H et al (2010) Decrease of blood dendritic cells and increase of tissue-infiltrating dendritic cells are involved in the induction of Sjögren's syndrome but not in the maintenance. Clin Exp Immunol 159(3):315–326
Pabón-Porras MA, Molina-Ríos S, Flórez-Suárez JB, Coral-Alvarado PX, Méndez-Patarroyo P, Quintana-López G (2019) Rheumatoid arthritis and systemic lupus erythematosus: pathophysiological mechanisms related to innate immune system. SAGE Open Med 7:2050312119876146
Pan HF, Wang J, Leng RX, Li XP, Ye DQ (2011) Interleukin-18: friend or foe for systemic sclerosis? J Invest Dermatol 131(12):2495. https://doi.org/10.1038/jid.2011.224 (author reply 2496-2497)
Pan Q, Feng Y, Peng Y, Zhou H, Deng Z, Li L et al (2017) Basophil recruitment to skin lesions of patients with systemic lupus erythematosus mediated by CCR1 and CCR2. Cell Physiol Biochem 43(2):832–839
Paoliello-Paschoalato AB, Marchi LF, Andrade MF, Kabeya LM, Donadi EA, Lucisano-Valim YM (2015) Fcγ and complement receptors and complement proteins in neutrophil activation in rheumatoid arthritis: contribution to pathogenesis and progression and modulation by natural products. Evidence-Based Complem Alternat Med eCAM 2015:429878
Pasoto SG, de Oliveira Martins VA, Bonfa E (2019) Sjögren’s syndrome and systemic lupus erythematosus: links and risks. Open Access Rheumatol 11:33
Pattanaik D, Brown M, Postlethwaite BC, Postlethwaite AE (2015) Pathogenesis of systemic sclerosis. Front Immunol. https://doi.org/10.3389/fimmu.2015.00272
Pellefigues C, Dema B, Lamri Y, Saidoune F, Chavarot N, Lohéac C et al (2018) Prostaglandin D 2 amplifies lupus disease through basophil accumulation in lymphoid organs. Nat Commun 9(1):725
Pentony P, Duquenne L, Dutton K, Mankia K, Gul H, Vital E, Emery P (2017) The initiation of autoimmunity at epithelial surfaces: a focus on rheumatoid arthritis and systemic lupus erythematosus. Discov Med 24(133):191–200
Pohlmeyer C, Cui Z-H, Han P, Clarke A, Jones R, Mollova N et al (2018) AB0484 Monotherapy with filgotinib, a jak1-selective inhibitor, reduces disease severity and alters immune cell subsets in the nzb/w f1 murine model of lupus. Ann Rheum Dis 77:1403
Pozsgay J, Szekanecz Z, Sármay G (2017) Antigen-specific immunotherapies in rheumatic diseases. Nat Rev Rheumatol 13(9):525
Rana AK, Li Y, Dang Q, Yang F (2018) Monocytes in rheumatoid arthritis: circulating precursors of macrophages and osteoclasts and their heterogeneity and plasticity role in RA pathogenesis. Int Immunopharmacol 65:348–359
Rivellese F, Mauro D, Nerviani A, Pagani S, Fossati-Jimack L, Messemaker T et al (2018) Mast cells in early rheumatoid arthritis associate with disease severity and support B cell autoantibody production. Ann Rheum Dis 77(12):1773–1781
Rizzo C, La Barbera L, Lo Pizzo M, Ciccia F, Sireci G, Guggino G (2019) Invariant NKT cells and rheumatic disease: focus on primary Sjogren syndrome. Int J Mol Sci 20(21):5435
Ronnblom L, Alm GV (2001) An etiopathogenic role for the type I IFN system in SLE. Trends Immunol 22(8):427–431
Rowland SL, Riggs JM, Gilfillan S, Bugatti M, Vermi W, Kolbeck R et al (2014) Early, transient depletion of plasmacytoid dendritic cells ameliorates autoimmunity in a lupus model. J Exp Med 211(10):1977–1991
Rusakiewicz S, Nocturne G, Lazure T, Semeraro M, Flament C, Caillat-Zucman S et al (2013) NCR3/NKp30 contributes to pathogenesis in primary Sjögren’s syndrome. Sci Transl Med 5(195):195ra196
Sambataro D, Sambataro G, Dal Bosco Y, Polosa RJ (2017) Present and future of biologic drugs in primary Sjögren’s syndrome. Expert Opin Biol Therapy 17(1):63–75
Scala E, Pallotta S, Frezzolini A, Abeni D, Barbieri C, Sampogna F et al (2004) Cytokine and chemokine levels in systemic sclerosis: relationship with cutaneous and internal organ involvement. Clin Exp Immunol 138(3):540–546. https://doi.org/10.1111/j.1365-2249.2004.02642.x
Scambi C, La Verde V, De Franceschi L, Barausse G, Poli F, Benedetti F et al (2010) Comparative proteomic analysis of serum from patients with systemic sclerosis and sclerodermatous GVHD. Evidence of defective function of factor H. PLoS One 5(8):e12162. https://doi.org/10.1371/journal.pone.0012162
Shamim EA, Rider LG, Miller FW (2000) Update on the genetics of the idiopathic inflammatory myopathies. Curr Opin Rheumatol 12(6):482–491
Shealy DJ et al (2010) Characterization of golimumab, a human monoclonal antibody specific for human tumor necrosis factor α. mAbs 2:428–439
Sierra-Sepúlveda A, Esquinca-González A, Benavides-Suárez SA, Sordo-Lima DE, Caballero-Islas AE, Cabral-Castañeda AR, Rodríguez-Reyna TS (2019) Systemic sclerosis pathogenesis and emerging therapies, beyond the fibroblast. Biomed Res Int 2019:4569826
Singh MV, Swaminathan PD, Luczak ED, Kutschke W, Weiss RM, Anderson ME (2012) MyD88 mediated inflammatory signaling leads to CaMKII oxidation, cardiac hypertrophy and death after myocardial infarction. J Mol Cell Cardiol 52(5):1135–1144. https://doi.org/10.1016/j.yjmcc.2012.01.021
Son M, Diamond B, Santiago-Schwarz F (2015) Fundamental role of C1q in autoimmunity and inflammation. Immunol Res 63(1–3):101–106
Sun L (2013) Roles of γ δ T Cells in the Pathogenesis of Autoimmune Diseases. Clin Dev Immunol 2013:985753
Taylor P, Westhovens R, Aa AV, Jamoul C, Li W, Goyal L et al (2017) THU0206 The jak1-selective inhibitor filgotinib reduces multiple markers of inflammation linked to various pathologic cell types and processes in rheumatoid arthritis patients. Ann Rheum Dis 76(Suppl 2):281–282. https://doi.org/10.1136/annrheumdis-2017-eular.5799
Tcherepanova I, Curtis M, Sale M, Miesowicz F, Nicolette CJ (2013) SAT0193 Results of a randomized placebo controlled phase ia study of AGS-009, a humanized anti-interferon-α monoclonal antibody in subjects with systemic lupus erythematosus. Ann Rheum Dis 71(Suppl 3):536–537
Ternant D, Ducourau E, Fuzibet P, Vignault C, Watier H, Lequerré T et al (2015) Pharmacokinetics and concentration–effect relationship of adalimumab in rheumatoid arthritis. Br J Clin Pharmacol 79(2):286–297. https://doi.org/10.1111/bcp.12509
Tews DS, Goebel HH (1996) Cytokine expression profile in idiopathic inflammatory myopathies. J Neuropathol Exp Neurol 55(3):342–347
Tishler M, Yaron I, Shirazi I, Yossipov Y, Yaron M (1999) Increased salivary interleukin-6 levels in patients with primary Sjögren's syndrome. Rheumatol Int 18(4):125–127
Tournadre A, Lenief V, Eljaafari A, Miossec P (2012) Immature muscle precursors are a source of interferon-β in myositis: role of Toll-like receptor 3 activation and contribution to HLA class I up-regulation. Arthritis Rheum 64(2):533–541. https://doi.org/10.1002/art.33350
Udalova IA, Mantovani A, Feldmann M (2016) Macrophage heterogeneity in the context of rheumatoid arthritis. Nat Rev Rheumatol 12(8):472
Ulfgren A-K, Grundtman C, Borg K, Alexanderson H, Andersson U, Harris HE, Lundberg IE (2004) Down-regulation of the aberrant expression of the inflammation mediator high mobility group box chromosomal protein 1 in muscle tissue of patients with polymyositis and dermatomyositis treated with corticosteroids. Arthritis Rheum 50(5):1586–1594. https://doi.org/10.1002/art.20220
van den Hoogen LL, van Laar JM (2020) Targeted therapies in systemic sclerosis, myositis, antiphospholipid syndrome, and Sjögren's syndrome. Best Pract Res Clin Rheumatol 34(1):101485
van Lieshout AWT, Vonk MC, Bredie SJH, Joosten LBA, Netea MG, van Riel PLCM et al (2009) Enhanced interleukin-10 production by dendritic cells upon stimulation with Toll-like receptor 4 agonists in systemic sclerosis that is possibly implicated in CCL18 secretion. Scand J Rheumatol 38(4):282–290. https://doi.org/10.1080/03009740802572467
Van Vollenhoven RF, Hahn BH, Tsokos GC, Wagner CL, Lipsky P, Touma Z et al (2018) Efficacy and safety of ustekinumab, an IL-12 and IL-23 inhibitor, in patients with active systemic lupus erythematosus: results of a multicentre, double-blind, phase 2, randomised, controlled study. Lancet 392(10155):1330–1339
Vogelsang P, Jonsson M, Dalvin S, Appel S (2006) Role of dendritic cells in Sjögren's syndrome. Scand J Immunol 64(3):219–226
Wang Y, Han C-C, Cui D, Li Y, Ma Y, Wei W (2017) Is macrophage polarization important in rheumatoid arthritis? Int Immunopharmacol 50:345–352
Watts ER, Ryan E, Walmsley SR, Whyte MKB (2018) Microenvironmental regulation of innate immune cell function. In: Cavaillon JM, Singer M (eds) Molecular and cellular mechanisms to the clinic, 1st edn. Wiley-VCHVerlagGmbH&Co.KGaA, France, pp 947–970
Wijbrandts CA, Remans PH, Klarenbeek PL, Wouters D, van den Bergh Weerman MA, Smeets TJ et al (2008) Analysis of apoptosis in peripheral blood and synovial tissue very early after initiation of infliximab treatment in rheumatoid arthritis patients. Arthritis Rheum 58(11):3330–3339
Willeke P, Schlüter B, Schotte H, Domschke W, Gaubitz M, Becker H (2009) Interferon-γ is increased in patients with primary Sjogren's syndrome and Raynaud's phenomenon. Semin Arthritis Rheum 39(3):197–202
Wouters D, Voskuyl AE, Molenaar ET, Dijkmans BA, Hack CE (2006) Evaluation of classical complement pathway activation in rheumatoid arthritis: measurement of C1q–C4 complexes as novel activation products. Arthritis Rheum 54(4):1143–1150
Wright HL, Moots RJ, Edwards SW (2014) The multifactorial role of neutrophils in rheumatoid arthritis. Nat Rev Rheumatol 10(10):593
Xu Y, Chen G (2015) Mast cell and autoimmune diseases. Mediat Inflamm 2015:246126
Yoshimoto K, Tanaka M, Kojima M, Setoyama Y, Kameda H, Suzuki K et al (2011) Regulatory mechanisms for the production of BAFF and IL-6 are impaired in monocytes of patients of primary Sjögren's syndrome. Arthritis Res Ther 13(5):170
Yoshitomi H (2019) Regulation of immune responses and chronic inflammation by fibroblast-like synoviocytes. Front Immunol 10:1395
Yu MB, Langridge WH (2017) The function of myeloid dendritic cells in rheumatoid arthritis. Rheumatol Int 37(7):1043–1051
Zamir O, Hasselgren PO, Higashiguchi T, Frederick JA, Fischer JE (1992) Tumour necrosis factor (TNF) and interleukin-1 (IL-1) induce muscle proteolysis through different mechanisms. Mediators Inflamm 1(4):247–250. https://doi.org/10.1155/S0962935192000371
Zhang L, Yan JW, Wang YJ, Wan YN, Wang BX, Tao JH et al (2014) Association of interleukin 1 family with systemic sclerosis. Inflammation 37(4):1213–1220. https://doi.org/10.1007/s10753-014-9848-7
Zouali M, La Cava A (2019) Editorial: innate immunity pathways in autoimmune diseases. Front Immunol 10:1245. https://doi.org/10.3389/fimmu
Funding
The authors gratefully acknowledge the student research committee of Mazandaran University of Medical Science, Sari, Iran for financially supporting this research.
Author information
Authors and Affiliations
Contributions
PZ and AH contributed to the idea design and literature search. AR and MT helped in data interpretation. MS, SH, and MD wrote the manuscript. ST contributed to designing the figures. FKH and DB contributed in language editing. All authors discussed the results and contributed to the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hejrati, A., Rafiei, A., Soltanshahi, M. et al. Innate immune response in systemic autoimmune diseases: a potential target of therapy. Inflammopharmacol 28, 1421–1438 (2020). https://doi.org/10.1007/s10787-020-00762-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10787-020-00762-y