Abstract
Benign prostatic hyperplasia (BPH) is a nonmalignant enlargement of the prostate common in older men. Diallyl sulfide (DAS), a major component of garlic, has been reported to possess antioxidant, anti-inflammatory, and antiproliferative effects. However, the underlying protective immunomodulatory mechanism of DAS on BPH remains vague. Herein, experimental BPH was induced in rats by daily subcutaneous injection of testosterone propionate (TP) (3 mg/kg, s.c.) for 4 weeks. In parallel, finasteride (Fin) (5 mg/kg, p.o) or DAS (50 mg/kg, p.o.) was administered orally during BPH induction. TP-induced histological alterations and the immune-inflammatory cascade. On the other hand, DAS or Fin administration alleviated all abnormalities induced testosterone. Fin and DAS administration markedly reduced prostate weight by 53% with Fin, and by 60% with DAS. Moreover, serum testosterone and DHT were reduced by 55% and 52%, respectively, with Fin and by 68% and 75%, respectively, with DAS, in concordance with decreased protein expression of androgen receptor (AR), and prostate-specific antigen (PSA). Furthermore, both regime lessen immune-inflammatory milieu, as evidenced by decrease CD4+ T-cells protein expression and associated inflammatory cytokines. Concomitantly, Fin and DAS exhibited marked mitigation in insulin-like growth factor-1 (IGF-1), transforming growth factor-beta1 (TGF-β1), and phosphorylated extracellular signal-regulated kinase (ERK1/2) signaling. Besides alleviating oxidative stress by 53% and 68% in prostatic MDA and by 27% and 7% in prostatic iNOS with Fin and DAS, respectively. In conclusion, this work highlighted a potential therapeutic approach of DAS as a dietary preventive agent against BPH via its anti-inflammatory and immunomodulatory effect along with suppression of the ERK pathway.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Benign prostatic hyperplasia (BPH) is one of the most common diseases in men. It is characterized by benign enlargement of the prostatic gland causing constriction of the urethra and subsequent lower urinary tract symptoms that include difficult urination, frequent voiding, bladder obstruction, and dysuria. Such symptoms have a negative impact on the quality of life and the productivity of BPH patients (Paolone 2010).
The androgenic pathway is the most dominant pathophysiology of BPH. Testosterone is activated by 5 alpha-reductase enzyme, which converts it to dihydrotestosterone (DHT). DHT is considered the most potent prostatic androgen due to its high affinity to the androgen receptor (Andriole et al. 2004). Others elaborate on the role of DHT in controlling differentiation and growth-promoting factors in BPH (Izumi et al. 2013). Moreover, hormonal regulation of BPH also coordinates with other peptide hormones, most importantly, insulin-like growth factors (IGFs) (Madersbacher et al. 2019). In the same context, metabolic and inflammatory pathways have been recognized to contribute to the development of BPH (Chughtai et al. 2011; Sebastianelli and Gacci 2018). This inflammatory cascade includes the activation of several chemokines, cytokines, and pro-differentiation factors such as transforming growth factor (TGF), IGF, and extracellular signal-regulated kinase (ERK) (Xu et al. 2017) with consequent overgrowth of stromal and epithelial prostatic cells. Additionally, the chronic prostatic inflammation is accompanied by histological damage that sensitizes immune responses which manifested as the accumulation of CD4+ T-lymphocytes (Krušlin et al. 2017).
Alpha-1 adrenergic receptor inhibitors represent the first-line therapy for BPH patients with prostates < 30 ml; they can offer rapid relief of symptoms but cannot prevent disease progression (Marberger 2013). Unfortunately, these drugs can also cause a loss of libido and erectile dysfunction. 5 alpha-reductase inhibitors such as finasteride (Fin) provide an additional therapeutic option to improve BPH symptoms and alleviate the risk of progression (Marberger 2013). However, 5 alpha-reductase inhibitors are not permitted for preventive strategies to men with no or mild urinary retention because of their unclear probable side effects (Madersbacher et al. 2019). Consequently, there is recent awareness concerning the use of natural products as effective and safe strategies to prevent the development of androgen-induced BPH (Ammar et al. 2015; Xu et al. 2018).
Garlic (Allium sativum) has been consumed worldwide as a traditional medicine for thousands of years. Garlic extracts have been reported to possess many biological activities including, antithrombotic, anti-diabetic, antiviral, fungicidal, bactericidal, antioxidant, and anticancer effects (Thomson and Ali 2003). Garlic is rich in organosulfur compounds, of which allicin is the most abundant, and it is naturally metabolized into several allyl sulfides (Zhang et al. 2006). Garlic organo-sulfur compounds enhance the immune system and suppress the abnormal tissue growth. Hence, the garlic can modify the immunity by switch the balance from a pro-inflammatory and immunosuppressive milieu to an enhanced anti-tumor response leading to tumor suppression (Schäfer and Kaschula 2014). The complex chemical composition of garlic elicits the need to study pure chemically active compounds of garlic at defined concentrations. Among Garlic organo-sulfur metabolites, diallyl sulfide (DAS) is the chief lipid-soluble and medicinally beneficial sulfur compound obtained from allicin (Rao et al. 2015). DAS possess immunotherapeutic effect via acting as an immune booster (Kuttan 2000). Other garlic-derived allyl sulfides, diallyl disulfide (DADS), and diallyl trisulfide (DATS) were also reported to have medicinal benefits (Wang et al. 2012; Yi and Su 2013); however, their consumption was associated with potential toxicity (Karmakar et al. 2007; Iciek et al. 2009). Though extensive work has been carried out to assess the protective and antitumor effects of DAS; no reports are available regarding the possible protective and immune-modulatory potential of DAS against BPH in vivo. This study aims to assess the possible protective effect of DAS against testosterone-induced BPH in rats and compare its activity to the standard used drug Fin.
Materials and methods
Animals
Adult male Wistar albino rats weighing 180–220 g, aged 8–10 weeks old, were purchased from the faculty of Pharmacy Animal Facility, Cairo University, Cairo, Egypt. Before setting up the experiment, the rats were adapted for 2 weeks at 24 ± 2 °C with an alternate 12 h day/dark cycle in the Faculty of Pharmacy Animal Facility. Standard chow diet and free water access were allowed throughout the experimental study.
The protocols used in this experiment were met with The Guide for Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85–23, revised 2011) and were approved by the Ethics Committee for Animal Experimentation at the Faculty of Pharmacy, Cairo University (Permission Number: 2504). All efforts were exerted to alleviate the suffering of animals and to reduce the number of animals used.
Drugs and chemicals
Testosterone propionate (TP) and diallyl sulfide (DAS) were purchased from Sigma-Aldrich Co. (St Louis, MO, USA). Finasteride (Fin) was purchased under the trade name Prostride (ADWIA Pharmaceuticals, Obour City, Egypt). All other chemicals were of the highest analytical grade and supplied by Sigma-Aldrich Co. (USA).
Experimental design
Rats were randomly allocated into four groups (n = 10 rats/group). Group I received bidistilled water orally, and corn oil s.c daily and served as a control group. Group II received TP (3 mg/kg; s.c) dissolved in corn oil, daily for 4 weeks and served as a BPH control group (Ammar et al. 2015). Group III received Fin (5 mg/kg; p.o) suspended in bidistilled water (Sayed et al. 2016) while group IV received DAS (50 mg/kg; p.o) dissolved in corn oil (Kim et al. 2016). All treatments were administered daily for 4 weeks and parallel to TP injection (Fig. 1).
One day before animals’ sacrifice, animals were treated with their daily regimen, then rats were placed into metabolic cages to record urinary output over 3 h (Hardik et al. 2014; Kantah et al. 2017).
Toward the end of the experiment, under light anesthesia, blood samples were collected and sera were separated, then the animals were euthanized, and their prostate was rapidly removed and weighed. The isolated prostates were divided into 2 subsets; the first was used for histopathological and immunohistochemical examination where the prostates (n = 4 per group) were fixed in 10% (v/v) formalin for 24 h. In the second set of samples (n = 6 per group) the right and the left portions of the prostate lobes were stored in RIPA buffer and PBS, respectively, at − 80 °C to be used for further analyses. All experiments were performed in triplet for each rat in the group.
During sample analysis for the assessed parameters, the investigators were blinded to sample identity; where sample coding and decoding were carried out by an independent experimenter.
Biochemical parameters
Prostate weight and relative prostate weight
The isolated prostates were weighed individually then the relative prostate weight was calculated by dividing prostate weight over its corresponding animal weight (Vyas et al. 2013).
ELISA technique
Serum testosterone, and DHT were measured using ELISA kits. Serum testosterone was assessed using the Picokine Rat ELISA kit (BosterBio., CA, USA, Cat.# EK7014), while serum DHT was measured by rat specific ELISA kit (Cusabio, Wuhan, China, Cat.# CSB E07879r). The results are expressed as ng/ml for testosterone and as pg/ml for DHT. Additionally, prostatic tissue samples were homogenized and further used to measure prostatic IL-6, IL-8, and IL-17 using Rat ELISA kits provided by Biovision (CA, USA, Cat.# K4145-100), Abbexa LLC (Houston, USA, Cat.# abx576575) and MyBioSource (CA, USA, Cat.# MBS2503506), respectively. Moreover, prostatic inducible nitric oxide synthase (iNOS) and malondialdehyde (MDA) were assessed as well using ELISA kits provided by MyBioSource (CA, USA, Cat.# MBS263618 and MBS268427, respectively). All procedures were carried out in compliance with the manufacturer’s instructions. The results were presented as pg/mg tissue for IL-6, IL-8, and IL-17 as well as nmol/mg tissue for MDA and ng/mg tissue for iNOS.
Western blot analysis of total/p-ERK1/2, TGF-β1, and IGF protein levels
Protein levels of TGF-β1, IGF-1, and ERK1/2 were assessed using the Western Blot technique. For protein extraction, The ReadyPrep™ protein extraction kit (Bio-Rad, CA, USA, Cat.# 163–2086) was added to each sample of the homogenized tissues and Bradford protein assay kit (Bio basic, Markham Ontario, Canada, Cat.# SK3041) was applied to determine protein concentrations per sample. From each sample, protein concentration (20 μg) was loaded with an equal volume of 2 × Laemmli sample buffer at pH 6.8. Denaturation of protein was carried out by boiling the mixture mentioned above at 95 °C for 5 min before loading onto polyacrylamide gel electrophoresis. Samples were separated according to their molecular weights on a sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) using TGX Stain-Free™ FastCast™ Acrylamide kit (SDS-PAGE) (Bio-Rad, CA, USA, Cat.# 161–0181). The gel was assembled in a transfer sandwich, which was placed in the transfer tank with 1 × transfer buffer. Subsequently, the blot was run at 25 V for 7 min to allow protein bands to transfer from gel to membrane using Bio-Rad Trans-Blot Turbo. The membrane was blocked using 20 mM Tris pH 7.5, 150 mM NaCl, 0.1% Tween 20 and 3% bovine serum albumin at room temperature for 60 min.
Primary antibodies; anti-TGF-β (Abcam, MA,USA,Cat.#ab92486), anti-IGF-1 (Biovision, CA, USA, Cat.# 5121-30T), anti-p-ERK1/2 (R&D, MN, USA, Cat.# AF1018), and anti-total-ERK (Santa Cruz Biotechnology, Texas, USA, Cat.#sc-514302) were diluted in TBST. Moreover, incubation was done overnight in each primary antibody solution, against the blotted target protein, at 4 ͦ C, and then the blot was rinsed 3–5 times for 5 min with TBST. This was followed by incubation with HRP-conjugated to the anti-rabbit antibody (Dianova, Hamburg, Germany).
The chemiluminescent substrate, Clarity™ Western ECL substrate (Bio-Rad, CA, USA, Cat#170-5060), was applied to the blot according to the manufacturer’s recommendation. The chemiluminescent signals were captured using a CCD camera-based imager. The band intensity of the target proteins against the control sample of beta-actin (housekeeping protein) was read using image analysis software by protein normalization on the ChemiDoc MP imager (Bio-Rad, CA, USA).
Histopathological examination
The excised prostates were washed and fixed in formalin for 24 h followed by routine processing and cutting of the paraffin blocks by Leica RM 2155 microtome at 3–4 µm thickness. Then regular H&E staining procedures were applied. Histological evaluation of the different groups was completed, and photomicrographs were captured at the power of (× 100).
Immunohistochemistry
Sets of positively charged slides were prepared for the immunohistochemical examination. The four groups were immunostained by primary antibodies in a dilution of 1:50 for TGF-β1 (Abcam, MA, USA,Cat.#ab92486), androgenic receptor (AR;Cat.#M3562), prostate-specific antigen (PSA; Cat.#A0562), and CD4+ T (Cat.#M7310) which were purchased from Dako, Copenhagen, Denmark. The standard avidin–biotin-peroxidase system was utilized, and the primary and secondary kits, as well as DAB chromagen, were provided by Dako, Copenhagen, Denmark. The immune-stained sections were examined under light microscopy, and immunoreactive percentage areas in individual sections were traced and evaluated using the image analysis system (Leica Microsystems, QWin software 3000). Five fields (× 100) per slide are evaluated in the analysis.
Statistical analysis
Data are expressed as mean ± SD. For multiple comparisons one-way analysis of variance (ANOVA) was used, followed by Tukey post-hoc test. Moreover, Pearson's correlation analysis was used to evaluate the relationship between relative prostate weight, serum testosterone, urine output, and TGF-β1. The significance level was considered at p value < 0.05. Statistical analysis was carried out using GraphPad Prism 6.0 (San Diego, CA, USA).
Results
Effect of Fin and DAS on the body weight, prostate weight, relative prostate weight, and urine output in TP-induced BPH in rats.
Concerning body weight, there wasn't any difference among groups. Meanwhile, testosterone injected animals positively increased prostate weight and its relative weight (p value < 0.0001) (Tables 1 and 2) and negatively affected urine output compared with the control group (p value < 0.0001). Conversely, Fin and DAS administration markedly reduced prostate weight to about 53% (p value < 0.0001) and 60% (p value < 0.001), respectively, and the relative prostate weight to 59.6% (p value < 0.0001) and 71% (p value < 0.01), respectively, compared with the TP-induced BPH group. However, neither Fin nor DAS managed to return the relative prostate weight to the normal value (Table 1).
Effect of Fin and DAS on serum testosterone and DHT levels in TP-induced BPH in rats.
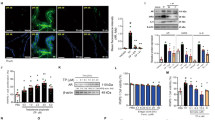
TP-induced BPH group showed intensely elevated serum testosterone and DHT to about 550% and 516%, respectively, compared with the control rats (p value < 0.0001) (Fig. 2a, b). However, Fin and DAS treated groups ameliorated the elevation in testosterone to 55% and 68%, respectively (p value < 0.0001) (Fig. 2a). Moreover, DAS administration decreased serum DHT levels to approximately 75% of the TP group (p value < 0.0001); however, it was still greater than the standard Fin treated rats that only reached 52% of the TP group (Fig. 2b).
Effect of Fin (5 mg/kg; p.o) and DAS (50 mg/kg; p.o) on serum levels of a testosterone, b DHT as well as the protein expression of c AR and d PSA and their corresponding expression area % (e, f), respectively, in TP (3 mg/kg, s.c)-induced BPH. Protein expression detected by high staining intensity. Data are expressed as the mean ± SD (n = 6) of each experiment performed in triplet for ELISA technique and 3 for immunohistochemistry analysis. Statistical analysis was performed using one-way analysis of variance (ANOVA) followed by Tukey’s multiple comparison test, *p < 0.05, ***p < 0.001, and ****p < 0.0001: significantly different from control. ##p < 0.01, ###p < 0.001, and ####p < 0.0001: significantly different from the TP-induced BPH group. @p < 0.05, and @@@p < 0.001: significantly different from the Fin + TP group. Fin and DAS were administered 1 h before TP. AR androgenic receptor, BPH benign prostatic hyperplasia, DAS diallyl sulfide, DHT dihydrotestosterone, Fin finasteride, p.o peroral, PSA prostate specific antigen, s.c. subcutaneously, TP: testosterone propionate
Effect of Fin and DAS on AR, and PSA protein expressions in TP-induced BPH in rats
The results showed that AR and PSA protein expressions were below the detection level in the normal prostate as demonstrated by the negative immunohistochemical reaction. On the other hand, AR and PSA showed high protein expressions detected by high staining intensity in TP-induced BPH group. Interestingly, upon treatment with Fin or DAS, there was a significant weak expression of both proteins as compared with the BPH group (Fig. 2c, d). Interestingly, these effects were represented by Fin and DAS ability to induce a reduction in the calculated area percentage of AR by 65% and 55%, respectively (Fig. 2e) and of PSA by 57% and 70%, respectively (Fig. 2f), with the predominant effect of Fin.
Effect of Fin and DAS on prostate pro-inflammatory cytokines, and CD4+ T-cells immunohistochemical reactivity in TP-induced BPH in rats
There was a significant rise in prostatic IL-6, IL-8, and IL-17 protein levels in TP rats by 318%, 429%, and 486%, respectively, compared with the control group (p value < 0.0001) (Fig. 3a–c). While co-treatment with Fin or DAS significantly reduced the level of the aforementioned parameters (p value < 0.0001). It worth to mention that DAS caused normalization in the IL-6 level, which did not occur even with Fin treatment (p value = 0.1133).
Effect of Fin (5 mg/kg; p.o) and DAS (50 mg/kg; p.o) on protein levels of prostatic a IL-6, b IL-8, and c IL-17, as well as the protein expression of d CD4+ T and its expression area % e in TP (3 mg/kg, s.c)-induced BPH. Thin arrow in TP-induced BPH group showing minimal staining of stromal T-lymphocytes. Protein expression detected by high staining intensity. Data are expressed as the mean ± SD (n = 6) of each experiment performed in triplet for ELISA technique and 3 for immunohistochemistry analysis. Statistical analysis was performed using a one-way analysis of variance (ANOVA) followed by Tukey’s multiple comparison test. **p < 0.01, and ****p < 0.0001: significantly different from control. ##p < 0.01, and ####p < 0.0001: significantly different from the TP-induced BPH group. @p < 0.05, @@p < 0.01, and @@@@p < 0.0001: significantly different from the Fin + TP group. Fin and DAS were administered 1 h before TP. BPH benign prostatic hyperplasia, DAS diallyl sulfide, DHT dihydrotestosterone, Fin finasteride, p.o per oral, s.c. subcutaneously, TP testosterone propionate
The immunohistochemical examination demonstrated an increment in CD4+ T immunostaining in the BPH group, evidenced by up-leveling in its corresponding area percentage when compared with the control group (Fig. 3d, e). However, co-treatment with either Fin or DAS revealed minimal staining of CD4+ T (Fig. 3d, e).
Effect of Fin and DAS on ERK phosphorylation, TGF-β1, and IGF-1 protein levels in TP-induced BPH in rats
It is well recognized that the mitogen-activated protein kinases (MAPK) pathway is involved in cell proliferation and apoptosis. Therefore, we examined whether the role of MAPKs in the mechanism of DAS-mediated positive effects in BPH is significant. The expression of phosphorylated ERK /total ERK was markedly increased in the BPH group by 385.5% when compared with the normal control group (p value < 0.0001). DAS markedly downregulated this protein level by 8.4%, compared to testosterone received animals (p value = 0.0136). However, Fin significantly decreased the expression by 21.3% compared with the TP group (p value < 0.0001) (Fig. 4a). Intriguingly, Fin and DAS alleviated the dramatic increment in the expression of TGF-β1 as well as IGF-1 (Fig. 4b, c) when compared with the TP-induced BPH group (p value < 0.0001). These observations imply the valuable effect of DAS against growth factors-mediated BPH in rats. Moreover, the relative prostate weight was strongly correlated to the TGF-β1 (p value < 0.0001) (Table 2).
Effect of Fin (5 mg/kg; p.o) and DAS (50 mg/kg; p.o) on protein levels of a p-ERK1/2/total ERK1/2, b TGF-β1, c IGF-1 as well as the protein expression of d TGF-β1 and its expression area % e in TP (3 mg/kg, s.c)-induced BPH. Protein expression detected by high staining intensity. Data are expressed as the mean ± SD (n = 6) of each experiment performed in triplet for Western Blot technique and 3 for immunohistochemistry analysis. Statistical analysis was performed using a one-way analysis of variance (ANOVA) followed by Tukey’s multiple comparison test, ***p < 0.001, and ****p < 0.0001: significantly different from control. #p < 0.05, and ####p < 0.0001: significantly different from the TP-induced BPH group. @@@p < 0.001, and @@@@p < 0.0001: significantly different from the Fin + TP group. Fin and DAS were administered 1 h before TP. BPH benign prostatic hyperplasia, DAS diallyl sulfide, ERK 1/2 extracellular signal-regulated kinase 1/2, DHT dihydrotestosterone, Fin finasteride, IGF-1 insulin-like growth factor-1, p.o peroral, PSA prostate specific antigen, s.c. subcutaneously, TGF-β1 transforming growth factor-beta1, TP testosterone propionate
Effect of Fin and DAS on lipid peroxidation, and iNOS levels in TP-induced BPH in rats
The BPH group displayed an upsurge in oxidative stress biomarkers reflected by elevated lipid peroxidation as manifested by increased MDA level as well as increased iNOS by sevenfold and threefold, respectively, compared with the control group (p value < 0.0001) (Fig. 5a, b). Conversely, Fin and DAS treatment showed powerful antioxidant effects by dampening MDA level by 53% and 68%, respectively (p value < 0.0001) (Fig. 5a) and reducing iNOS by 27% and 7%, respectively (Fig. 5b) when compared with the TP-induced BPH group (p value < 0.0001).
Effect of Fin (5 mg/kg; p.o) and DAS (50 mg/kg; p.o) on levels of a MDA, and b iNOS in the prostate tissue in TP (3 mg/kg, s.c)-induced BPH. Protein expression detected by high staining intensity. Data are expressed as the mean ± SD (n = 6) of each experiment performed in triplet for ELISA technique. Statistical analysis was performed using one-way analysis of variance (ANOVA) followed by Tukey’s Multiple Comparison test, ****p < 0.0001: significantly different from control. ####p < 0.0001: significantly different from the TP-induced BPH group. @@@@p < 0.0001: significantly different from the Fin + TP group. Fin and DAS were administered 1 h before TP. BPH benign prostatic hyperplasia, DAS diallyl sulfide, Fin finasteride, iNOS inducible nitric oxide synthase, MDA malondialdehyde, p.o peroral, s.c. subcutaneously, TP testosterone propionate
Histopathological examination
The histological analysis of H&E staining showed no remarkable hyperplasia or hypertrophy in the prostate of the normal control group (Fig. 6a). However, TP-induced BPH group showing marked hyperplasia and hypertrophy. Crowded acini and minimal mucosal infoldings were noted, together with inspissated secretions (thick arrow, Fig. 6b). While, co-treatment with either Fin or DAS showed mild hyperplasia or hypertrophy, mildly crowded acini, and focal mucosal infoldings (thin arrow, Fig. 6c, d, respectively).
Histological examination of the prostatic tissues of different groups, H&E X100. a Control: showed no remarkable hyperplasia or hypertrophy in the prostate. b TP: thick arrow showed marked hyperplasia and hypertrophy, crowded acini and minimal mucosal infoldings, together with inspissated secretions. c Fin + TP and d DAS + TP: thin arrow showed mild hyperplasia or hypertrophy, mildly crowded acini, and focal mucosal infoldings
Discussion
DAS, one of the garlic organosulfur active compound, has been reported to possess anti-inflammatory (Li et al. 2015), antioxidant (Chen et al. 2014), antimicrobial (Davis 2005), and anticancer (Zou et al. 2016) activities. In the current study, the administration of DAS concurrently for 28 days showed promising efficacy in relieving BPH in a dependent and independent manner from the androgenic cue.
The DHT forms a complex with AR (Gao et al. 2005) and undergoes nuclear translocation, DNA activation with stimulation of growth-promoting genes, and PSA production. The elevated PSA and AR contribute to prostatic hyperplasia (Culig et al. 1996; Izumi et al. 2013); these events show up that the androgen/AR signaling pathway is the masterpiece in BPH development; hence its targeting becomes one of the most effective strategies in BPH therapy (Izumi et al. 2013). In the same context, studies have demonstrated that estrogens and their receptors α and β (ERα, ERβ) also have an essential role in normal prostate growth. Activation of ERα is associated with the proliferation and anti-apoptotic response; however, ERβ activation has antiproliferative and proapoptotic impacts (Fano et al. 2017), these are also strongly related to the inflammatory reaction (Vásquez-Velásquez et al. 2020) and the ERK pathway (Zhang et al. 2008).
Herein, the BPH rat model showed significant histopathological abnormalities, with an increase in serum testosterone and DHT levels tied with overexpression of AR protein expression. These findings are in line with previous studies (Atawia et al. 2013; Wang et al. 2016). In the present work, DAS attenuated the androgenic axis (testosterone/DHT/AR protein expression), starting with suppressed testosterone level partly via subsequent reduction of IL-6. It was reported in previous studies that IL-6 could enhance intracrine androgen synthesis with the activation of AR to promote prostate growth (Chun et al. 2009; Culig and Puhr 2012). DAS diminished AR expression, the basal core for the androgenic cascade; this may be attributed to the antioxidant property of DAS recorded here and was confirmed by other studies which showed the ability of DAS to upregulate NrF2 signaling in in vitro models (Ho et al. 2012; Rao et al. 2015). Khurana and Sikka (2018) documented the potential of NrF2 to suppress AR. Stan and Singh (2009) studied the chemopreventive ability of organosulfur compounds and allyl compounds against prostate cancer using 3 prostate cell lines (LNCap, C4-2 and Tramp-c1) and in the transgenic adenocarcinoma mouse prostate. They concluded that DAS directly suppress AR promoter activity, AR translocation and sequentially affected PSA level in LNCaP cell line utilizing immunoblotting technique and confirmed this mechanism by structure–activity relationships between allyl compounds and AR promoter activity. This study proves direct evidence of DAS interaction with the androgenic axis namely; AR and PSA. This finding confirms the results of the current manuscript for DAS on AR and PSA in in vivo BPH rat model. To yield insights into the prementioned AR signaling, the present study examined the protein expression of PSA, which represents a prostatic cellular differentiation and proliferation marker (Schalken 2004). Interestingly, DAS reduced the protein expression of testosterone-triggered PSA. Similarly, the consumption of garlic extract in prostate cancer patients showed a significant reduction in PSA levels (Durak et al. 2003).
Aside from the androgenic pathway, chronic inflammation plays a crucial role in the pathogenesis of BPH (Pace et al. 2011; Gandaglia et al. 2013). Several studies have confirmed a relevance relationship between inflammation and prostatic insults such as BPH; aberrant arachidonic acid metabolism, which accompanied with an elevation in cyclooxygenase-2 and 5-lipoxygenase expressions as well as prostaglandin-2 and leukotriene A4 levels, has been linked with cellular proliferation in BPH (Altavilla et al. 2012). In the same context, overflow of infiltrated T-cells increased pro-inflammatory cytokine secretion. Co-culture of human BPH cells with active CD4+ T-cells enhanced Th17 production of IL-17, which stimulates the stromal cells to produce inflammatory molecules viz; IL-6, IL-8, and iNOS (Steiner et al. 2003; Kramer et al. 2007). As well, Kuwabara et al. (2017) presented IL-17 as a bridge between T-cell activation and the inflammatory cascade in chronic inflammatory diseases; this is concomitant with IL-17 presence in BPH provoked by TP as previously observed (Yang et al. 2014). Herein, for the first time, the increments of CD4+ T-cells in the TP-induced rat model, were recorded. Together, no previous data noted the immunoregulatory properties of Fin and DAS afforded by suppressed CD4+ T-cells, as well as IL-17 in the TP-induced BPH model. The inhibitory effect of DAS on CD4+ T-cells and their downstream may be partially attributed to the depression of PSA, which has been reported to enhance CD4+ T-cells (Klyushnenkova et al. 2004). Consequentially, DAS reduced IL-17; this result is similar to that of Allium sativum, which inhibits IL-17 gene expression in vitro (Moutia et al. 2016).
The light shed on the main executors of prostate cell development in BPH; IL-6 and IL-8 where their levels upsurge in the TP-induced model and these elevations were noted by Yang et al. (2014). Herein, the elevation of IL-6 is critical since it shifts the TGFβ-1-enhanced differentiation of naive T-cells toward pathogenic Th17 cells (Veldhoen et al. 2006; Bettelli et al. 2006). In the present study, the ameliorative effect of DAS on local growth proteins could be linked to the noted reduction of CD4+ T-cells and the break-up of the IL-17 link. Thus IL-17, IL-6, and TGFβ-1 can afford a sustained positive loop and augmentation of the immune-inflammatory process in BPH (Steiner et al. 2003; Bettelli et al. 2006). Beside the previous data, IL-8 appears to be the most reliable and predictive surrogate marker to diagnose BPH (Penna et al. 2007). In the present data, DAS suppressed pro-inflammatory IL-8 production and this similar to the in vitro study in virally infected cell lines (Hall et al. 2017). This chemokine acts as an autocrine/paracrine growth factor for BPH cells (Penna et al. 2009). The anti-IL-8 afforded by DAS may be partially referred to the DAS-suppression of IL-6 that subsequently affects IL-8 expression (Khurana and Sikka 2018). Additionally, DAS reduced prostatic weight with improved urinary bladder voiding could be mediated through amelioration of IL-8 and subsequent reduction of growth factors, namely TGF-β (Ficarra et al. 2014) and IGF; this outlined the anti-growth, immunomodulatory, and anti-inflammatory impacts of DAS that mitigated the cycle of chronic immuno-inflammation.
Prostatic cells release TGFβ-1 and IGF-1 in response to cytokine-induced damage that drives local growth; this milieu was observed and highly expressed histologically in human prostate hyperplasia tissue (Scott Lucia and Lambert 2008; Afdal et al. 2019). In the current study, TGFβ-1 and IGF-1 protein levels, were elevated in BPH rats, which is concurrent with the study by Kim et al. (2015). Moreover, DAS inhibited TGFβ1-induced myofibroblast formation by activating Nrf2-related antioxidant enzyme in lung medical research council cell strain 5 (MRC-5) cells (Ho et al. 2017). Regarding IGF-1, DADS lowered its protein expression dose-dependently in the prostate cancer cell line (Arunkumar et al. 2012). This modulation may feature the antiproliferative and anti-mitogenic effects of DAS reported herein.
Many growth factors like IGF are known as ERK cascade stimulants and are found to be over-expressed in BPH (Habib and Chisholm 1991). ERK activation is essential for prostate growth and development (Papatsoris and Papavassiliou 2001), and it was enhanced in BPH (Youn et al. 2017). Peterziel et al. (1999) reported that androgens could contribute either directly to or by the production of mitogens in activating the ERK cascade. On the other hand, Gao et al. (2006) demonstrated that the function of ERK as a prostate growth stimulator was independent of androgen signaling. In our findings, ERK was dramatically triggered in the BPH group and then was decreased markedly by DAS treatment. In agreement with our results, Ko et al. (2018) demonstrated that garlic oil and DADS considerably inhibited ERK activation in airway inflammation elicited by cigarette smoke and lipopolysaccharides in mice. Once again, a low level of growth factors in DAS treated group would reduce ERK1/2 activation. This finding strongly supports the idea that DAS protective action in BPH is partially mediated by ERK dephosphorylation.
Persistent inflammation activates the production of ROS and RNS with nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kB) that encodes the expression of iNOS (Paulis 2018). iNOS is the main factor in all prostatic inflammatory cells, which releases reactive nitrogen that damage cells (Baltaci et al. 2001). Previously, DAS was shown to raise immunocompetence through inhibition of inflammatory cytokines in lipopolysaccharide (LPS)-stimulated RAW264.7 macrophages, thereafter suppression of NO and PGE2 release (Chang et al. 2005). Macrophages are the typical source for both immune oxidative responses as well as iNOS. The expression of iNOS can be transcriptionally upregulated by pro-inflammatory cytokines (Kleinert et al. 2003). The current study showed a marked elevation in the iNOS protein level in BPH animals. Elevated iNOS in TP-treated rats is further clarified by Gradini et al. (1999) who demonstrated the incorporation of testosterone in iNOS induction. The reduced iNOS in DAS-treated rats in the present study was also observed in LPS-treated cell lines and viral infected cell lines (Chang and Chen 2005; Hall et al. 2017).
Oxidative stress plays an essential role in the pathology of BPH (Ammar et al. 2015). The current study showed an elevated end-product of lipid peroxidation in BPH rats in concomitant with Sun et al. (2020). Indeed, the antioxidant activity of DAS is manifested by decreased MDA level. This complies with the inhibitory effect of DAS on oxidative stress triggered by testosterone in BPH model in mice (Prasad et al. 2006). Moreover, it was reported that DAS blocked the increase in lipid peroxidation and myeloperoxidase activity caused by bleomycin (Kalayarasan et al. 2008). Notably, DAS pretreatment also induced Nrf2 expression upregulation, this action was confirmed to be the crucial molecular shift responsible for the antioxidant effects of DAS in a rat thoracic aorta smooth muscle cell line (A7r5) and lung MRC-5 cells (Ho et al. 2012; Rao et al. 2015).
Conclusion
Taken together, this work provides insights to a potential therapeutic approach of DAS in BPH via its anti-inflammatory, immunomodulatory and ERK pathway repressor actions.
References
Afdal A, Darwin E, Yanwirasti Y, Hamid R (2019) The expression of transforming growth factor beta-1 and interleukin-6 on human prostate: prostate hyperplasia and prostate cancer. Open Access Maced J Med Sci 7:1905–1910. https://doi.org/10.3889/oamjms.2019.548
Altavilla D, Minutoli L, Polito F et al (2012) Effects of flavocoxid, a dual inhibitor of COX and 5-lipoxygenase enzymes, on benign prostatic hyperplasia. Br J Pharmacol 167:95–108. https://doi.org/10.1111/j.1476-5381.2012.01969.x
Ammar AE, Esmat A, Hassona MDH et al (2015) The effect of pomegranate fruit extract on testosterone-induced BPH in rats. Prostate 75:679–692. https://doi.org/10.1002/pros.22951
Andriole G, Bruchovsky N, Chung LWK et al (2004) Dihydrotestosterone and the prostate: the scientific rationale for 5α-reductase inhibitors in the treatment of benign prostatic hyperplasia. J Urol 172:1399–1403. https://doi.org/10.1097/01.JU.0000139539.94828.29
Arunkumar R, Sharmila G, Elumalai P et al (2012) Effect of diallyl disulfide on insulin-like growth factor signaling molecules involved in cell survival and proliferation of human prostate cancer cells in vitro and in silico approach through docking analysis. Phytomedicine 19:912–923. https://doi.org/10.1016/j.phymed.2012.04.009
Atawia RT, Tadros MG, Khalifa AE et al (2013) Role of the phytoestrogenic, pro-apoptotic and anti-oxidative properties of silymarin in inhibiting experimental benign prostatic hyperplasia in rats. Toxicol Lett 219:160–169. https://doi.org/10.1016/j.toxlet.2013.03.002
Baltaci S, Orhan D, Gögüs Ç et al (2001) Inducible nitric oxide synthase expression in benign prostatic hyperplasia, low- and high-grade prostatic intraepithelial neoplasia and prostatic carcinoma. BJU Int 88:100–103. https://doi.org/10.1046/j.1464-410x.2001.02231.x
Bettelli E, Carrier Y, Gao W et al (2006) Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 441:235–238. https://doi.org/10.1038/nature04753
Chang H-P, Chen Y-H (2005) Differential effects of organosulfur compounds from garlic oil on nitric oxide and prostaglandin E2 in stimulated macrophages. Nutrition 21:530–536. https://doi.org/10.1016/j.nut.2004.07.018
Chang H-P, Huang S-Y, Chen Y-H (2005) Modulation of cytokine secretion by garlic oil derivatives is associated with suppressed nitric oxide production in stimulated macrophages. J Agric Food Chem 53:2530–2534. https://doi.org/10.1021/jf048601n
Chen W, Qi J, Feng F et al (2014) Neuroprotective effect of allicin against traumatic brain injury via Akt/endothelial nitric oxide synthase pathway-mediated anti-inflammatory and anti-oxidative activities. Neurochem Int 68:28–37. https://doi.org/10.1016/j.neuint.2014.01.015
Chughtai B, Lee R, Te A, Kaplan S (2011) Role of inflammation in benign prostatic hyperplasia. Rev Urol 13:147–150. https://doi.org/10.3909/riu0535
Chun JY, Nadiminty N, Dutt S et al (2009) Interleukin-6 regulates androgen synthesis in prostate cancer cells. Clin Cancer Res 15:4815–4822. https://doi.org/10.1158/1078-0432.CCR-09-0640
Culig Z, Hobisch A, Cronauer MV et al (1996) Regulation of prostatic growth and function by peptide growth factors. Prostate 28:392–405. https://doi.org/10.1002/(SICI)1097-0045(199606)28:6<392:AID-PROS9>3.0.CO;2-C
Culig Z, Puhr M (2012) Interleukin-6: a multifunctional targetable cytokine in human prostate cancer. Mol Cell Endocrinol 360:52–58. https://doi.org/10.1016/j.mce.2011.05.033
Davis SR (2005) An overview of the antifungal properties of allicin and its breakdown products—the possibility of a safe and effective antifungal prophylactic. Mycoses 48:95–100. https://doi.org/10.1111/j.1439-0507.2004.01076.x
Durak I, Yilmaz E, Devrim E et al (2003) Consumption of aqueous garlic extract leads to significant improvement in patients with benign prostate hyperplasia and prostate cancer. Nutr Res 23:199–204. https://doi.org/10.1016/S0271-5317(02)00495-5
Fano D, Vásquez-Velásquez C, Gonzales-Castañeda C et al (2017) N-Butanol and aqueous fractions of red maca methanolic extract exerts opposite effects on androgen and oestrogens receptors (alpha and beta) in rats with testosterone-induced benign prostatic hyperplasia. Evid Based Complement Altern Med. https://doi.org/10.1155/2017/9124240
Ficarra V, Rossanese M, Zazzara M et al (2014) The role of inflammation in lower urinary tract symptoms (LUTS) due to benign prostatic hyperplasia (BPH) and its potential impact on medical therapy. Curr Urol Rep 15:463. https://doi.org/10.1007/s11934-014-0463-9
Gandaglia G, Briganti A, Gontero P et al (2013) The role of chronic prostatic inflammation in the pathogenesis and progression of benign prostatic hyperplasia (BPH). BJU Int 112:432–441. https://doi.org/10.1111/bju.12118
Gao H, Ouyang X, Banach-Petrosky WA et al (2006) Combinatorial activities of Akt and B-Raf/Erk signaling in a mouse model of androgen-independent prostate cancer. Proc Natl Acad Sci USA 103:14477–14482. https://doi.org/10.1073/pnas.0606836103
Gao W, Bohl CE, Dalton JT (2005) Chemistry and structural biology of androgen receptor. Chem Rev 105:3352–3370. https://doi.org/10.1021/cr020456u
Gradini R, Realacci M, Ginepri A et al (1999) Nitric oxide synthases in normal and benign hyperplastic human prostate: immunohistochemistry and molecular biology. J Pathol 189:224–229. https://doi.org/10.1002/(SICI)1096-9896(199910)189:2<224:AID-PATH422>3.0.CO;2-K
Habib FK, Chisholm GD (1991) The role of growth factors in the human prostate. Scand J Urol Nephrol Suppl 138:53–58
Hall A, Troupin A, Londono-Renteria B, Colpitts T (2017) Garlic organosulfur compounds reduce inflammation and oxidative stress during dengue virus infection. Viruses 9:159. https://doi.org/10.3390/v9070159
Hardik S, Hardik M, Deepti J, Ghanashyam P (2014) Pharmacological investigation of an ayurvedic formulation on testosterone propionate-induced benign prostatic hyperplasia rats. J Exp Integr Med 4:131. https://doi.org/10.5455/jeim.280314.or.099
Ho C-Y, Cheng Y-T, Chau C-F, Yen G-C (2012) Effect of diallyl sulfide on in vitro and in vivo Nrf2-mediated pulmonic antioxidant enzyme expression via activation ERK/p38 signaling pathway. J Agric Food Chem 60:100–107. https://doi.org/10.1021/jf203800d
Ho CY, Lu CC, Jhang JJ, Yen GC (2017) Diallyl sulfide attenuates transforming growth factor-β-stimulated pulmonary fibrosis through Nrf2 activation in lung MRC-5 fibroblast. J Funct Foods 28:314–320. https://doi.org/10.1016/j.jff.2016.10.025
Iciek M, Kwiecień I, Włodek L (2009) Biological properties of garlic and garlic-derived organosulfur compounds. Environ Mol Mutagen 50:247–265. https://doi.org/10.1002/em.20474
Izumi K, Mizokami A, Lin W-J et al (2013) Androgen receptor roles in the development of benign prostate hyperplasia. Am J Pathol 182:1942–1949. https://doi.org/10.1016/j.ajpath.2013.02.028
Kalayarasan S, Sriram N, Sudhandiran G (2008) Diallyl sulfide attenuates bleomycin-induced pulmonary fibrosis: critical role of iNOS, NF-κB, TNF-α and IL-1β. Life Sci 82:1142–1153. https://doi.org/10.1016/j.lfs.2008.03.018
Kantah M, Singh B, Sweed H et al (2017) Beneficial effect of a multifunctional polyphytocompound in experimental prostatic hyperplasia in rats. Clin Pharmacol Biopharm 6:1–7. https://doi.org/10.4172/2167-065X.1000169
Karmakar S, Banik NL, Patel SJ, Ray SK (2007) Garlic compounds induced calpain and intrinsic caspase cascade for apoptosis in human malignant neuroblastoma SH-SY5Y cells. Apoptosis 12:671–684. https://doi.org/10.1007/s10495-006-0024-x
Khurana N, Sikka S (2018) Targeting crosstalk between Nrf-2, NF-κB and androgen receptor signaling in prostate cancer. Cancers (Basel) 10:352. https://doi.org/10.3390/cancers10100352
Kim S-H, Lee I-C, Ko J-W et al (2016) Mechanism of protection by diallyl disulfide against cyclophosphamide-induced spermatotoxicity and oxidative stress in rats. Mol Cell Toxicol 12:301–312. https://doi.org/10.1007/s13273-016-0035-9
Kim SK, Seok H, Park HJ et al (2015) Inhibitory effect of curcumin on testosterone induced benign prostatic hyperplasia rat model. BMC Complement Altern Med 15:380. https://doi.org/10.1186/s12906-015-0825-y
Kleinert H, Schwarz PM, Förstermann U (2003) Regulation of the expression of inducible nitric oxide synthase. Biol Chem 384:1343–1364. https://doi.org/10.1515/BC.2003.152
Klyushnenkova EN, Ponniah S, Rodriguez A et al (2004) CD4 and CD8 T-lymphocyte recognition of prostate specific antigen in granulomatous prostatitis. J Immunother 27:136–146. https://doi.org/10.1097/00002371-200403000-00007
Ko JW, Jeong SH, Kwon HJ et al (2018) Preventive effect of garlic oil and its organosulfur component diallyl-disulfide on cigarette smoke-induced airway inflammation in mice. Nutrients 10:1–12. https://doi.org/10.3390/nu10111659
Kramer G, Mitteregger D, Marberger M (2007) Is benign prostatic hyperplasia (BPH) an immune inflammatory disease? Eur Urol 51:1202–1216. https://doi.org/10.1016/j.eururo.2006.12.011
Krušlin B, Tomas D, Džombeta T et al (2017) Inflammation in prostatic hyperplasia and carcinoma-basic scientific approach. Front Oncol 7:77. https://doi.org/10.3389/fonc.2017.00077
Kuttan G (2000) Immunomodulatory effect of some naturally occuring sulphur-containing compounds. J Ethnopharmacol 72:93–99. https://doi.org/10.1016/S0378-8741(00)00211-7
Kuwabara T, Ishikawa F, Kondo M, Kakiuchi T (2017) The role of IL-17 and related cytokines in inflammatory autoimmune diseases. Mediat Inflamm. https://doi.org/10.1155/2017/3908061
Li C, Lun W, Zhao X et al (2015) Allicin alleviates inflammation of trinitrobenzenesulfonic acid-induced rats and suppresses P38 and JNK pathways in caco-2 cells. Mediat Inflamm 2015:1–11. https://doi.org/10.1155/2015/434692
Madersbacher S, Culig Z, Sampson N, Culig Z (2019) Pathophysiology of benign prostatic hyperplasia and benign prostatic enlargement: a mini-review. Gerontology 65:458–464. https://doi.org/10.1159/000496289
Marberger M (2013) Medical management of lower urinary tract symptoms in men with benign prostatic enlargement. Adv Ther 30:309–319. https://doi.org/10.1007/s12325-013-0022-7
Moutia M, Seghrouchni F, Abouelazz O et al (2016) Allium sativum L. regulates in vitro IL-17 gene expression in human peripheral blood mononuclear cells. BMC Complement Altern Med 16:377. https://doi.org/10.1186/s12906-016-1365-9
Pace G, Di Massimo C, De Amicis D et al (2011) Inflammation and endothelial activation in benign prostatic hyperplasia and prostate cancer. Int Braz J Urol 37:617–622. https://doi.org/10.1590/S1677-55382011000500008
Paolone DR (2010) Benign prostatic hyperplasia. Clin Geriatr Med 26:223–239. https://doi.org/10.1016/j.cger.2010.02.010
Papatsoris AG, Papavassiliou AG (2001) Molecular “palpation” of BPH: a tale of MAPK signalling? Trends Mol Med 7:288–292. https://doi.org/10.1016/S1471-4914(01)02015-9
Paulis G (2018) Inflammatory mechanisms and oxidative stress in prostatitis: the possible role of antioxidant therapy. Res Rep Urol 10:75–87. https://doi.org/10.2147/RRU.S170400
Penna G, Fibbi B, Amuchastegui S et al (2009) Human benign prostatic hyperplasia stromal cells as inducers and targets of chronic immuno-mediated inflammation. J Immunol 182:4056–4064. https://doi.org/10.4049/jimmunol.0801875
Penna G, Mondaini N, Amuchastegui S et al (2007) Seminal plasma cytokines and chemokines in prostate inflammation: interleukin 8 as a predictive biomarker in chronic prostatitis/chronic pelvic pain syndrome and benign prostatic hyperplasia. Eur Urol 51:524–533. https://doi.org/10.1016/j.eururo.2006.07.016
Peterziel H, Mink S, Schonert A et al (1999) Rapid signalling by androgen receptor in prostate cancer cells. Oncogene 18:6322–6329. https://doi.org/10.1038/sj.onc.1203032
Prasad S, Kalra N, Shukla Y (2006) Modulatory effects of diallyl sulfide against testosterone-induced oxidative stress in Swiss albino mice. Asian J Androl 8:719–723. https://doi.org/10.1111/j.1745-7262.2006.00201.x
Rao PSS, Midde NM, Miller DD et al (2015) Diallyl sulfide: potential use in novel therapeutic interventions in alcohol, drugs, and disease mediated cellular toxicity by targeting cytochrome P450 2E1. Curr Drug Metab 16:486–503. https://doi.org/10.2174/1389200216666150812123554
Sayed RH, Saad MA, El-Sahar AE (2016) Dapoxetine attenuates testosterone-induced prostatic hyperplasia in rats by the regulation of inflammatory and apoptotic proteins. Toxicol Appl Pharmacol 311:52–60. https://doi.org/10.1016/j.taap.2016.09.024
Schäfer G, Kaschula C (2014) The Immunomodulation and anti-inflammatory effects of garlic organosulfur compounds in cancer chemoprevention. Anticancer Agents Med Chem 14:233–240. https://doi.org/10.2174/18715206113136660370
Schalken JA (2004) Molecular and cellular prostate biology: origin of prostate-specific antigen expression and implications for benign prostatic hyperplasia. BJU Int 93:5–9
Scott Lucia M, Lambert JR (2008) Growth factors in benign prostatic hyperplasia: basic science implications. Curr Urol Rep 9:272–278. https://doi.org/10.1007/s11934-008-0048-6
Sebastianelli A, Gacci M (2018) Current status of the relationship between metabolic syndrome and lower urinary tract symptoms. Eur Urol Focus 4:25–27. https://doi.org/10.1016/j.euf.2018.03.007
Stan SD, Singh SV (2009) Transcriptional repression and inhibition of nuclear translocation of androgen receptor by diallyl trisulfide in human prostate cancer cells. Clin Cancer Res 15:4895–4903. https://doi.org/10.1158/1078-0432.CCR-09-0512
Steiner GE, Newman ME, Paikl D et al (2003) Expression and function of pro-inflammatory interleukin IL-17 and IL-17 receptor in normal, benign hyperplastic, and malignant prostate. Prostate 56:171–182. https://doi.org/10.1002/pros.10238
Sun C, Peng Y, Wu Y et al (2020) The effect of Metapanax delavayi leaf extract on testosterone-induced benign prostatic hyperplasia in rats. J Funct Foods. https://doi.org/10.1016/j.jff.2020.103797
Thomson M, Ali M (2003) Garlic [Allium sativum]: a review of its potential use as an anti-cancer agent. Curr Cancer Drug Targets 3:67–81. https://doi.org/10.2174/1568009033333736
Vásquez-Velásquez C, Gasco M, Fano-Sizgorich D, Gonzales GF (2020) Inflammatory pathway employed by Red Maca to treat induced benign prostatic hyperplasia in rats. Andrologia 52:1–8. https://doi.org/10.1111/and.13516
Veldhoen M, Hocking RJ, Atkins CJ et al (2006) TGFβ in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity 24:179–189. https://doi.org/10.1016/j.immuni.2006.01.001
Vyas BA, Desai NY, Patel PK et al (2013) Effect of Boerhaavia diffusa in experimental prostatic hyperplasia in rats. Indian J Pharmacol 45:264–269. https://doi.org/10.4103/0253-7613.111946
Wang C, Luo F, Zhou Y et al (2016) The therapeutic effects of docosahexaenoic acid on oestrogen/androgen-induced benign prostatic hyperplasia in rats. Exp Cell Res 345:125–133. https://doi.org/10.1016/j.yexcr.2015.03.026
Wang H-C, Pao J, Lin S-Y, Sheen L-Y (2012) Molecular mechanisms of garlic-derived allyl sulfides in the inhibition of skin cancer progression. Ann N Y Acad Sci 1271:44–52. https://doi.org/10.1111/j.1749-6632.2012.06743.x
Xu C, Mathews AE, Rodrigues C et al (2018) Aged garlic extract supplementation modifies inflammation and immunity of adults with obesity: a randomized, double-blind, placebo-controlled clinical trial. Clin Nutr ESPEN 24:148–155. https://doi.org/10.1016/j.clnesp.2017.11.010
Xu H, Fu S, Chen Y et al (2017) Oxytocin: its role in benign prostatic hyperplasia via the ERK pathway. Clin Sci 131:595–607. https://doi.org/10.1042/CS20170030
Yang X, Yuan L, Xiong C et al (2014) Abacopteris penangiana exerts testosterone-induced benign prostatic hyperplasia protective effect through regulating inflammatory responses, reducing oxidative stress and anti-proliferative. J Ethnopharmacol 157:105–113. https://doi.org/10.1016/j.jep.2014.09.025
Yi L, Su Q (2013) Molecular mechanisms for the anti-cancer effects of diallyl disulfide. Food Chem Toxicol 57:362–370. https://doi.org/10.1016/j.fct.2013.04.001
Youn DH, Park J, Kim HL et al (2017) Chrysophanic acid reduces testosterone-induced benign prostatic hyperplasia in rats by suppressing 5α-reductase and extracellular signal-regulated kinase. Oncotarget 8:9500–9512. https://doi.org/10.18632/oncotarget.13430
Zhang P, Noordine M-L, Cherbuy C et al (2006) Different activation patterns of rat xenobiotic metabolism genes by two constituents of garlic. Carcinogenesis 27:2090–2095. https://doi.org/10.1093/carcin/bgl064
Zhang Z, Duan L, Du X et al (2008) The proliferative effect of estradiol on human prostate stromal cells is mediated through activation of ERK. Prostate 68:508–516. https://doi.org/10.1002/pros.20722
Zou X, Liang J, Sun J et al (2016) Allicin sensitizes hepatocellular cancer cells to anti-tumor activity of 5-fluorouracil through ROS-mediated mitochondrial pathway. J Pharmacol Sci 131:233–240. https://doi.org/10.1016/j.jphs.2016.04.017
Funding
This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
EME, HSH, ASK, SMI conceived and designed the experiments. EME, HSH, HAAA, ASK, SMI: carried out the experiments. EME, SMI: analysed the data. EME, HSH, HAAA, ASK, SMI: wrote the manuscript. EME, HSH, ASK, SMI: contributed reagents and tools of analysis.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Elbaz, E.M., Amin, H.A.A., Kamel, A.S. et al. Immunomodulatory effect of diallyl sulfide on experimentally-induced benign prostate hyperplasia via the suppression of CD4+T/IL-17 and TGF-β1/ERK pathways. Inflammopharmacol 28, 1407–1420 (2020). https://doi.org/10.1007/s10787-020-00743-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10787-020-00743-1