Abstract
Acute lung injury (ALI) and acute kidney injury (AKI) are major causes of sepsis-induced mortality. The objective of the study is to evaluate the effect of tocilizumab (TCZ), an IL-6 receptor inhibitor, in sepsis-induced ALI and AKI using the cecal ligation and puncture (CLP) rat model of sepsis. Clinical and experimental studies have demonstrated the importance of IL-6 in sepsis; however, the role of TCZ has not been investigated. Rats subjected to CLP developed histological evidence of ALI and AKI at 24 h. We found that TCZ alleviated sepsis-induced ALI and AKI as evidenced by improvements in various pathological changes, a significant reduction in the lung wet/dry weight ratio and total protein content in bronchoalveolar lavage fluid (BALF), and a significant decrease in the elevated serum level of creatinine (CR) and blood urea nitrogen (BUN). TCZ induced an increase in the survival rate of treated rats. Additionally, TCZ markedly inhibited sepsis-induced pulmonary and renal inflammatory responses. Moreover, we found that treatment with TCZ inhibited oxidative stress and apoptosis in lung and kidney tissue. TCZ treatment significantly inhibited NF-κB activation, attenuating JNK signaling pathway and significantly up-regulated P-glycoprotein (P-gp) expression in pulmonary as well as in renal tissues. Our data provide novel evidence that TCZ has a protective effect against sepsis-induced ALI and AKI by blocking IL-6 receptor signaling. This could provide a molecular basis for a new medical treatment for sepsis-induced ALI and AKI.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sepsis, a common and a serious complication of postoperative critically ill patients, is a systemic inflammatory response syndrome caused by infection that leads to multiple organ dysfunction (Parke et al. 2003). It is a major cause of morbidity and mortality in ICU patients. The lung and the kidneys are the most vulnerable organs during sepsis and approximately 50% of patients with sepsis may experience acute lung injury (ALI) and acute kidney injury (AKI) (Cohen 2002; Uchino et al. 2005). Despite improvements in treatments and guidelines for sepsis management, the mortality rate for patients with sepsis-associated ALI and AKI is still as high as 70%. Thus, the identification of innovative, safe, and effective therapeutic and preventive approaches is a pressing need for successful sepsis treatment and avoidance of complications.
Although septic shock is the most common trigger of ALI and AKI, the underlying mechanisms are not completely understood. Previous studies have revealed that the pathophysiology of ALI and AKI in sepsis is complex and multi-factorial, including inflammatory pathways, endothelial dysfunction, hemodynamic changes, and microcirculatory disorders (Zarjou and Agarwal 2011). The inflammatory response as a result of sepsis has been considered a direct causative mechanism for ALI and AKI (Matejovic et al. 2011). Growing evidence suggests that ALI and AKI in sepsis have a prominent inflammatory component during both the initiation and extension phase of the lung and kidney injuries (Kinsey et al. 2008). Septic shock is characterized by an uncontrolled release of pro-inflammatory cytokines, especially interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α) as well as free oxygen radicals (De Backer et al. 2014; Iskander et al. 2013). This occurs as a consequence of bacterial invasion into the blood stream, which leads to activation of the complement system. Although bacterial invasion is a common trigger for complement activation, other pathways “aseptic” can lead to the same sepsis outcomes (Gao and Yan 2012).
IL-6 is a cytokine with broad-spectrum biological activities. It is important in controlling the induction of the acute phase response and also is critical for B cell and cytotoxic T-cell differentiation and maturation, immunoglobulin class switching and macrophage and monocyte function. These biological activities are pivotal in both in vivo and in vitro settings. IL-6 functions as an essential and sensitive indicator of inflammation within the body (Tanaka et al. 2016). Several studies among newborns and cancer patients have reported that elevated plasma levels of IL-6 may be used as a diagnostic marker of sepsis (de Bont et al. 1999; Martin et al. 2001). Moreover, elevated IL-6 was associated with a negative prognostic outcome for sepsis (Hack et al. 1989). Taken together, IL-6 is believed to play a crucial role in the pathogenesis of sepsis and associated mortality. Thus blocking the signaling pathway(s) activated by IL-6 may ameliorate the severity of this condition and provide potential therapeutic benefits.
Tocilizumab (TCZ) is a humanized monoclonal antibody against IL-6 receptor (IL-6R) that was approved in 2010 for the treatment of rheumatoid arthritis (RA) and juvenile idiopathic arthritis. TCZ has a wide range of beneficial anti-oxidant, anti-apoptotic, and anti-inflammatory effects (Ruiz-Limon et al. 2017; Wang et al. 2016). It has also been reported that TCZ improves the total lung injury score in a rat model of mechanical ventilation induced lung injury (Potito et al. 2017) and improved the histopathological, biochemical and physiological parameters in a rat model of severe acute pancreatitis and complicating lung injury (Chen et al. 2016). However, the effect of TCZ on sepsis-induced ALI and AKI and its related molecular mechanism(s) have not been studied in detail.
In the light of these previous findings, we investigated the possible beneficial effects of TCZ as a therapeutic strategy in a well-established experimental rat model of sepsis induced by cecal ligation and puncture (CLP). Furthermore, we investigated the possible mechanisms by which TCZ may ameliorate CLP-induced ALI and AKI.
Materials and methods
Animals and experimental induction of sepsis
Female albino Wistar rats (200 ± 20 g) were purchased from Nahda University at BeniSuef (NUB) Animal House (Beni-Suef, Egypt). The animals were kept at 23 °C under a 12 h light–dark cycle with free access to food and water until the day of the experiment.
Sepsis was induced via the CLP method as previously described by Deitch (1998). Briefly, rats were anesthetized by a mixture of ketamine (50 mg/kg) and xylazine (10 mg/kg) and an abdominal incision was made in the lower left quadrant of the body after shaving and disinfecting the abdominal wall using 10% povidone iodine solution. The cecum was then exteriorized and ligated with a 0.3-mm silk surgical suture thread, and then two punctures were made in the cecum by a through-and-through puncture in the ligated part using an 18-gauge syringe needle. To minimize variations in the severity of sepsis, the percentage of the length of ligated cecum from its tip up to the ileo-cecal junction was kept constant through all experiments (75%). The ligated cecum was squeezed gently and returned back into the abdominal cavity. Abdominal wall sutures were made in two layers and the animals received normal saline solution (subcutaneously) in a dose of 3 ml/100 gm for resuscitation. The sham-operated rats were subjected to the same surgical and resuscitation procedures without cecal ligation and puncture procedure. Twenty-four hours after CLP induction, rats were killed and blood was collected by exsanguination, left for 10 min then centrifuged to obtain serum samples. The trachea was intubated and lung lavage was performed using cold PBS (0.5 mL three times) to obtain the bronchoalveolar lavage fluid (BALF) (Wang et al. 2018). The lungs and kidneys were carefully dissected, washed and divided into two parts; the left lung and kidney were used for histopathological examination, while the right lung and kidney were homogenized in order to assess the different biochemical markers. All animal experiments were carried out according to the international ethical guidelines and the National Institutes of Health Guide concerning the Care and Use of Laboratory Animals. The experiments were approved by the Animal Care Committee of Minia University (Council No. 87-11/2018).
Survival study and experimental groups
TCZ was obtained from Actemra®; F. Hoffmann-La Roche, Basel, Switzerland. The survival study was done first in order to test the effect of two doses of TCZ on survival. Sixty rats (same weight and sex as previously described) were randomly divided into six experimental groups (n = 10 per group): sham operated, sham operated plus TCZ (4 mg/kg) group, sham operated plus TCZ (8 mg/kg), untreated septic group, septic rats treated with TCZ (4 mg/kg), and septic rats treated with TCZ (8 mg/kg). The mortality of rats was observed daily for 15 days. The dose that gave a better effect on survival (4 mg/kg) was utilized for the mechanistic study. Twenty-four rats were randomly divided into four experimental groups (n = 6 per group): sham-operated group; sham-operated plus TCZ group; sepsis group; sepsis plus TCZ group. TCZ was injected as a single intraperitoneal (i.p.) injection (4 mg/kg) 2 h after the induction of sepsis.
Histopathological examination
Lung and kidney tissues were processed by standard histological methods: fixation in 10% buffered formalin (24 h), embedding in paraffin, and sectioning through 5 µm thick paraffin sections. Sections were prepared and then routinely stained with hematoxylin and eosin (H&E) dyes. Stained slides were microscopically analyzed using light microscopy (Olympus CX 23L).
A blinded pathological assessment was done to lung and kidney tissues for each group. Levels of lung injury was graded on a scale of 0–4 (0, absent; 1, light; 2, moderate; 3, strong; 4, intense) for congestion, edema, infiltration of inflammatory cells, and hemorrhage. Lung injury score was calculated as the mean of the scores for the separate parameters. Kidney injury was assessed for the percentages of tubules that showed cellular necrosis that were scored as follows: 0 = none, 1 = 0–20%, 2 = 20%–50%, 3 = 50%–70%, 4 = more than 70%. For each animal, at least 10 fields were examined.
Lung wet/dry (W/D) weight ratio
Lung W/D weight ratio was assessed to evaluate the lung edema (Wang et al. 2018). By using a sensitive electric scale, a fresh portion of the lung was weighed and then it was dried in an oven at 70 °C for 72 h until the weight remained constant. Dry weights were measured, and the W/D weight ratios were calculated.
Total leukocytic cell counts in bronchoalveolar lavage fluid (BALF)
BALF was concentrated by centrifugation (1000 rpm, 10 min at 4 °C), and the cell pellet was resuspended in 0.5 ml PBS and the total cells were counted using a hemocytometer.
Assessment of total protein in BALF
Total protein level was determined in the supernatant of the BALF using a commercial kit (BioMed, Egypt). Total protein was determined based on its reaction with copper ion that produces a blue violet color proportional to the concentration of the protein in the sample.
Assessment of serum creatinine and urea
Serum urea (BUN) and creatinine (CR) levels were determined using commercial kits obtained from Biodiagnostic, Egypt. BUN and CR were determined according to the method of Fawcett and Scott and the method of Bartles et al., respectively (Bartels et al. 1972; Fawcett and Scott 1960).
Determination of lung and kidney oxidative stress parameters
Lung and kidney tissues were homogenized in ice-cold phosphate buffer saline as 10% (0.1 g/ml) using a Polytron homogenizer. The crude homogenate has been centrifuged for 20 min at 4 °C. The supernatant was then aspirated and used for the determination of the following parameters:
-
Malondialdehyde (MDA), a measure of lipid peroxidation, was determined according to the method of Uchiyama and Mihara (Mihara and Uchiyama 1978).
-
Nitric oxide (NO) was assayed colorimetrically by measuring the accumulation of its stable degradation products total nitrite in lung and kidney tissues using Griess reagent (Green et al. 1982).
-
Superoxide dismutase activity in kidney and lung homogenates was determined spectrophotometrically by the method of Mc-Cords and Fridovich (McCord and Fridovich 1969).
-
Lung and kidney tissue catalase activity was determined using commercial kits (Biodiagnostic, Egypt), according to the method of Fossati et al. (1980).
Determination of total anti-oxidant capacity
Serum total anti-oxidant capacity (TAC) was determined using commercial kits (Biodiagnostic, Egypt). TAC was determined colorimetrically at 505 nm (Koracevic et al. 2001).
Determination of TNF-α, IL-6, and IL-1β
Commercially available ELISA kits were used to measure inflammatory cytokines according to the manufacturer’s instructions (Elabscience Biotechnology, Houston, Texas, USA). Concentrations were calculated by generating a standard curve using standard proteins and were measured spectrophotometrically at 450 nm.
Western blotting analyses
For estimation of caspase-3 and IL-6 expression, western blotting was performed as previously described (Ewees et al. 2018). Briefly, tissue was homogenized, total protein was estimated and then equal amounts of protein from each tissue homogenate was loaded per lane and separated on a 10% sodium dodecyl sulfate (SDS)–Tris glycine polyacrylamide gel electrophoresis (PAGE) gel. Protein bands were transferred to a nitrocellulose membrane using a semi-dry blotter (Bio-Rad). The blots were subsequently blocked with TBS-T buffer containing 5% skim milk powder for 1 h at room temperature. Then, the blots were probed overnight at 4 °C with rabbit polyclonal anti-caspase-3 (Neo Markers Co., CA), mouse monoclonal anti-IL-6 (Santa-Cruz Co., Germany), rabbit monoclonal anti-p-JNK (ThermoFisher scientific Co., USA), rabbit monoclonal total JNK (ThermoFisher scientific Co., USA) or mouse monoclonal anti-β-actin antibodies (Sigma-Aldrich Co., USA). Subsequently, incubation with alkaline phosphatase-coupled secondary antibody (Sigma-Aldrich Co., USA) was performed for 1 h at room temperature. Blots were finally analyzed using BCIP/NBT colorimetric detection method. Protein bands on the blots were analyzed using Image-J program (Image J 1.48 V, Wayne Rasband National Institutes of Health, USA).
Immunohistochemical assessment of lung and kidney NF-κB p65 and Bcl-2
For immunohistochemical staining, streptavidin–biotin immunoperoxidase complex procedure was applied. 5-μm-thick sections were transferred to adhesive slides from representative formalin-fixed, paraffin-embedded blocks. The sections were deparaffinized in xylene and dehydrated through a series of graded alcohols. Endogenous peroxidase activity was blocked by incubation with 0.3% hydrogen peroxide in methanol for 30 min. Antigen retrieval was done by microwave treatment in sodium citrate buffer, pH 6, for both NF-κB p65 (nuclear factor kappa-light-chain-enhancer of activated B cells) and Bcl-2 (B cell lymphoma-2) for 15 min.
Tissue sections were then incubated with rabbit anti-NF-κB p65 (Thermo Fisher Scientific, 1:50 dilution) for 1 h at room temperature and rabbit anti-Bcl-2 (Thermo Fisher Scientific, 1:100 dilution) for 30 min at room temperature. This was followed by incubation with a biotinylated anti-rabbit secondary antibody for 30 min at room temperature. The reaction was then visualized with an avidin–biotin complex immunoperoxidase system using 3,3′ diaminobenzidine (DAB) as a chromogen. Sections were then counterstained with hematoxylin, dehydrated, cleared, and mounted with distyrene, plasticizer, and xylene (DPX). For NF-κB p65, nuclear ± cytoplasmic staining was considered positive. Immunostaining score was performed by counting the number of positively stained cells in 1000 counted cells selected randomly at magnification X 400. For Bcl-2, cytoplasmic staining is considered positive. A semiquantitative immune-scoring for Bcl-2 expression was done using H-score, H-score is determined by adding the results of multiplication of the percentage of positive cells with staining intensity ordinal value, scored from 0 (negative staining), 1 + (mild staining), 2 + (moderate staining) to 3 + (intense staining) with final score ranging from 0 to 300.
Determination of P-glycoprotein
P-glycoprotein was measured by a commercially available ELISA kit according to the manufacturer’s instructions (USB Biological Life Science; Massachusetts, USA). Concentrations were calculated by generating a standard curve using standard proteins and were measured spectrophotometrically at 450 nm.
Statistical analysis
Data were expressed as mean ± SEM. All statistical analyses were performed using GraphPad Prism (version 6.0; San Diego, CA, USA). Analysis of variance (ANOVA) test was used for multiple comparisons followed by Tukey–Kramer as a post-ANOVA test. Survival analysis was performed using the log-rank Mantel–Cox test. The results were considered statistically significant if the p values were < 0.05.
Results
Effect of tocilizumab on sepsis-induced mortality
Sham, septic and TCZ-treated rats were monitored every 12 h for 15 days after induction of sepsis. The survival rate was recorded (Fig. 1). All rats in the CLP group died within 72 h after the CLP surgery. In contrast, TCZ (4 mg/kg) treatment dramatically reduced mortality by 80% (p < 0.001). In addition, TCZ (8 mg/kg) treatment significantly increased the survival rate by 30% (p < 0.05). No death of rats was observed in the sham or in the sham + TCZ groups. The sham + TCZ rats showed no changes in behavior and were generally healthy and looked normal. This confirms the absence of potential adverse effects of our drug. These results confirmed the protective effect of the lower dose of TCZ in rats with sepsis. Based on these results we moved forward with TCZ (4 mg/kg) in the subsequent experiments.
Effect of TCZ on CLP-induced mortality. Tocilizumab treatment markedly improved the survival of septic rats. TCZ (4 and 8 mg/kg) was administered to rats 2 h after CLP. Mortality was monitored daily for 15 days. Data are presented as the survival percentage of animals (n = 10 rats per group). *Significant difference at p < 0.05 compared to CLP group, **significant difference at p < 0.01 vs. CLP group, ***p < 0.001 vs. CLP group
Evaluation of serum IL-6 at different time points following sepsis
Next, we evaluated serum IL-6 level, as a direct prognostic marker for morbidity and mortality in sepsis in CLP operated rats receiving 4 mg/kg TCZ. The results showed a significant (p < 0.001) increase in serum level of IL-6 in septic rats (65.5 ± 2.7 pg/ml) compared to their sham control (41.9 ± 1.9 pg/ml). Administration of 4 mg/kg of TCZ normalized this value (Supplementary Table 1).
To tackle time changes in serum IL-6, we performed a time course experiment in which both sham and CLP operated rats with or without 4 mg/kg TCZ administration, have been scarified 3, 6 and 24 h following surgical manipulation. Results show persistent high serum levels of IL-6 in septic rats, the highest value has been reported early after sepsis induction. A dose of 4 mg/kg of TCZ, at all recorded time points, significantly abolished this elevation to normal control levels (Supplementary Table 1).
Protective effect of TCZ against sepsis-induced ALI
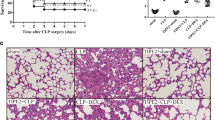
Histopathological examination of H&E stained lung sections revealed that neither sham nor sham + TCZ groups showed pulmonary histological alterations (Fig. 2a). In the CLP group, lung tissue showed interstitial infiltration by neutrophils and increased alveolar septal thickness. TCZ treatment in CLP + TCZ (4 mg/kg) group significantly reduced septal thickness and inflammatory cell infiltration in comparison to the CLP group. Lung injury scores were significantly higher in the CLP group than in the sham and sham + TCZ groups, meanwhile, it was markedly reduced in the CLP + TCZ group than in the CLP group (Fig. 2a).
Protective effect of TCZ against sepsis-induced ALI. a TCZ treatment reduces sepsis-induced histopathological lesions in rat lungs (H&E stain, 200 ×). The sham group (i) showed normal lung alveoli lined with normal alveolar epithelium (arrow indicates terminal bronchiole lined with columnar epithelium). Sham + TCZ group (ii) showed normal alveoli lined with normal alveolar epithelium (arrowhead). CLP group (iii) showed increased alveolar septal thickness (arrow) associated with perivascular mononuclear cells infiltration (arrowhead). CLP + TCZ group (iv) showed increase of alveolar spaces and decrease the thickness of the interstitial tissue (arrowheads) with a significant reduction in the histopathological lesions. Analysis of lung injury score (v). b The W/D weight ratio of lung tissue from different groups. c The total protein content of BALF from different groups. d The total number of cells in BALF from different groups. The results shown are representative of at least three independent experiments. Each bar represents the mean ± SEM (n = 6). *, ** and *** significant difference at p < 0.05, p < 0.01 and p < 0.001 compared to CLP. Scale, 100 μm
Tissue inflammation in the form of tissue edema as wet/dry weight of lung tissue increased significantly (p < 0.001) in the CLP group (4.11 ± 0.33 to 6.28 ± 0.44, for sham and CLP, respectively). In CLP + TCZ group, TCZ treatment significantly reduced lung wet/dry weight ratio compared to the CLP group (Fig. 2b) (p < 0.001 in each group).
To further confirm the effect of TCZ on ALI, we measured the total protein content in BALF. As shown in Fig. 2c, the total protein content was significantly higher in CLP rats compared to sham and sham + TCZ rats. Treatment of CLP animals with TCZ resulted in a lower total protein content, measuring almost normal values. Moreover, we detected the total number of cells in BALF as shown in Fig. 2d. TCZ-treated CLP rats showed significantly lower numbers of these cells; similar to the values measured in the sham-operated animals. In addition, we performed differential leukocyte counts in BALF (supplementary Table 2), and the results indicated a significant increase in neutrophil infiltration in CLP group when compared to the sham and TCZ-treated ones. These results indicated that TCZ might have a protective effect against sepsis-induced ALI.
Protective effect of TCZ against sepsis-induced AKI
Next, we explored the effect of TCZ on renal integrity and function of rats. The H&E-stained renal tissues showed normal kidney tubules in the sham and sham + TCZ groups. In contrast, CLP group showed degenerative and necrotic changes (Fig. 3a). These findings were confirmed by significantly higher kidney injury scores in the CLP group, which were significantly reduced by TCZ treatment (Fig. 3a).
Protective effect of TCZ against sepsis-induced AKI. a TCZ treatment reduces sepsis-induced histopathological lesions in rat kidneys (H&E stain, 200 ×). The sham group (i) showed normal renal glomeruli and tubules (arrowhead and arrow, respectively). Sham + TCZ group (ii) showed normal looking renal glomeruli (arrowhead) and tubules (arrow), CLP group (iii) showed degenerative changes within the lining epithelium of the medullary renal tubules (arrow), CLP + TCZ (iv) group showed normal looking renal glomeruli (arrowhead) with marked diminished CLP-induced epithelial degeneration (arrow). Analysis of kidney injury score (v). Effect of TCZ on serum BUN (b) and on serum CR (c). The results shown are representative of at least three independent experiments. Each value represents the mean ± SEM (n = 6). *, ** and *** significant difference at p < 0.05, p < 0.01 and p < 0.001 compared to CLP. Scale, 100 μm
The kidney function parameters; BUN and CR were markedly increased in septic rats in comparison to sham animals (50.52 ± 3.43 and 1.73 ± 0.17 for BUN and CR, respectively). On the other hand, administration of TCZ markedly inhibited this increase (22.93 ± 6.57 and 1.04 ± 0.77 for BUN and CR, respectively) (Fig. 3b, c).
Tocilizumab improves pulmonary and renal oxidative and anti-oxidant profile in septic rats
Next, we evaluated the effect of TCZ on oxidative stress and anti-oxidant defense in lung and renal tissues: lipid peroxidation and total nitrite levels as well as SOD and catalase activities were evaluated in lung tissues. In addition, serum total anti-oxidant capacity was measured. The results, presented in Table 1, show that in the CLP group, lung MDA and total nitrite levels were elevated compared to sham indicative of increased oxidative stress during sepsis. On the other hand, the activities of SOD and catalase in lung tissues as well as serum total anti-oxidant capacity were lower than those of the sham-operated rat group (p < 0.001). TCZ administration had protective effects against these alterations. Treatment with TCZ was found to significantly reduce the levels of MDA and total nitrite to 50%, when compared to the CLP group (p < 0.001). Moreover, TCZ significantly increased the SOD and catalase activities as well as the total anti-oxidant capacity when compared to the CLP group (p < 0.001). Similar data were obtained in kidney tissues as shown in Table 2.
Tocilizumab modulates IL-6 and NF-κB as well as JNK expression in pulmonary and renal tissues in septic rats
As shown in Fig. 4, the results of western blotting revealed that the overexpression of IL-6 caused by CLP in rats was totally normalized after TCZ treatment in both lung and renal tissues (Fig. 4a, b). In addition, we detected important pro-inflammatory cytokines TNF-α and IL-1β in BALF (Supplementary Table 2), and the results showed a significant increase in both TNF-α and IL-1β in CLP operated group as well as a significant decrease in lung tissues after TCZ treatment.
TCZ attenuated the inflammation in sepsis-induced ALI and AKI. Effect of TCZ on lung expression levels a and on kidney expression levels b of IL-6 relative to β-actin expression by western blotting analysis. c Effect of TCZ on the expression of NF-ĸB p65 in lung tissues of CLP-treated rat. Sham group (i) showing mild expression of NF-ĸB mostly within the cytoplasm of the alveolar lining epithelium, NF-ĸB-p65 IHC, X400, Sham + TCZ group (ii) showing mild cytoplasmic expression of NF-ĸB within alveolar lining epithelium, CLP group (iii) showing marked increase of NF-ĸB expression (nuclear and cytoplasmic) within both alveolar lining epithelium and the interstitial inflammatory cells, NF-ĸB-p65 IHC, X400, CLP + TCZ group (iv) showing marked decrease of NF-ĸB expression within the alveolar lining epithelium, NF-ĸB-p65 IHC, X400, Analysis of NF-ĸB immunoexpression among different groups (v). d Effect of TCZ on the expression of NF-ĸB p65 in kidney tissues of CLP-treated rat. Sham group (i) showing mild cytoplasmic expression of NF-ĸB mostly within the renal tubular lining epithelium, NF-ĸB-p65 IHC, X200, Sham + TCZ group (ii) showing mild cytoplasmic expression of NF-ĸB within the renal tubular lining epithelium, NF-ĸB-p65 IHC, X200, CLP group (iii) showing marked increase of NF-ĸB expression within nucleus and cytoplasm of the renal tubular epithelial lining, NF-ĸB-p65 IHC, X200, CLP + TCZ group (iv)showing marked decrease of both cytoplasmic and nuclear expression of NF-ĸB within the renal tubular epithelial lining, NF-ĸB-p65 IHC, X200, Analysis of NF-ĸB immunoexpression among different groups (v). The results shown are representative of at least three independent experiments. Each value represents the mean ± SEM (n = 6). *, ** and *** significant difference at p < 0.05, p < 0.01 and p < 0.001 compared to CLP
Moreover, we detected the protein expression of NF-κB in lung and kidney tissues. NF-κB is a key mediator of inflammation in lungs and kidney. An important mutual relationship between IL-6 and NF-κB has been previously detected in regulating the protein expression of each other. The expression of NF-κB p65 in sham group showed mainly light brown immunostaining in cytoplasm and low staining in the nuclei, while significant positive expression of NF-κB p65 was identified as strong brown staining in both cytoplasm and nuclei. In lung tissues, the sham group showed minimal NF-κB staining, whereas CLP group showed a significantly higher staining (67.50 ± 14.00 versus 301.66 ± 30.60, respectively, p < 0.001) (Fig. 4c). On the other hand, the CLP + TCZ group showed a significantly lower NF-κB immunoexpression compared to the CLP group (p < 0.001) (Fig. 4c).
In kidney tissues, as shown in Fig. 4d, CLP group showed a significantly higher (p < 0.001) NF-κB immunostaining compared to the sham group, whereas a significant reduction in positive NF-κB immunoexpression was noticed in CLP + TCZ group as compared to CLP group (p < 0.001).
Jun N-terminal kinase (JNK) is a major signaling pathway in mediating inflammation (Waetzig et al. 2005) and apoptosis (Dhanasekaran and Reddy 2017). We explored the protein expression of p-JNK, the active mediator of JNK signaling, normalized to total JNK in CLP groups, and the effect of TCZ administration on its signaling activation. We demonstrated a significant increase in p-JNK protein expression in CLP-treated rats, both in lung and kidney tissues. Administration of TCZ attenuated this activation, and decreased the JNK activity almost to basal control levels (Supplementary Fig. 1).
Tocilizumab reduces sepsis-induced apoptosis in pulmonary and renal tissues of septic rats
Cytoplasmic expression of Bcl-2 was investigated as a marker of cellular anti-apoptotic signals in lung and kidney tissue. In lung tissues, as shown in Fig. 5, compared with the sham group, Bcl-2 cytoplasmic immunoexpression in alveolar epithelial and pulmonary vascular endothelial cell was significantly reduced in the sepsis group. TCZ treatment in CLP + TCZ group caused a significant elevation in Bcl-2 expression compared to CLP group (p < 0.01). Also in kidney tissues, a significantly reduced Bcl-2 immunostaining was identified in CLP group compared to the sham group (p < 0.001), whereas significantly increased Bcl-2 expression was identified in CLP + TCZ group as compared to sepsis group (p < 0.001) as shown in Fig. 5B.
TCZ inhibited ALI and AKI-induced apoptosis in lung and kidney tissue. a TCZ treatment increased Bcl2 in lung tissues of CLP-treated rat. Sham group (i) showing high cytoplasmic expression of Bcl2 within the alveolar lining epithelium, Bcl2 IHC, X400, Sham + TCZ group (ii) showing high Bcl2 immunoexpression within alveolar lining epithelium, Bcl2 IHC, X400, CLP group (iii) showing marked decrease of Bcl2 expression within the alveolar lining epithelium, Bcl2 IHC, X400, CLP + TCZ group (iv) showing increase of Bcl2 expression within the alveolar lining epithelium, Bcl2 IHC, X400, Analysis of Bcl2 immunoexpression among different groups (v). b TCZ treatment increased Bcl2 in the kidney tissues of CLP-treated rat. Sham group (i) showing marked cytoplasmic expression of Bcl2 within renal tubular epithelial lining, Bcl2 IHC, X200, Sham + TCZ group (ii) showing high Bcl2 immunoexpression within renal tubular epithelial lining, Bcl2 IHC, X200, CLP group (iii) showing marked decrease of Bcl2 expression within renal tubular epithelial lining, Bcl2 IHC, X200, CLP + TCZ group (iv) showing increased Bcl2 cytoplasmic expression within renal tubular epithelial lining, Bcl2 IHC, X200, Analysis of Bcl2 immunoexpression among different groups (v). The apoptosis-related protein, caspase-3, was detected by western blotting in lung c and in kidney d tissues. β-actin was used as a loading control. The results shown are representative of at least three independent experiments. Each value represents the mean ± SEM (n = 6). *, ** and *** significant difference at p < 0.05, p < 0.01 and p < 0.001 compared to CLP
In addition, we assessed the expression of caspase-3 as an essential apoptosis-related protein using western blotting. As shown in Fig. 5c, d, the expression of caspase-3 protein was statistically higher in the sepsis group, while administration of TCZ completely normalized its expression in CLP + TCZ rats, both in lung as well as kidney tissues, compared to CLP rats (p < 0.01 and p < 0.001, respectively).
Tocilizumab attenuation of ALI and AKI in septic rats might be mediated by the up-regulation of P-glycoprotein in lung and kidney tissues
P-glycoprotein (P-gp) is a phosphoglycoprotein, encoded by mdr-1a and mdr-1b genes in rodents. It protects against xenobiotics and endogenous compounds and prevents drug accumulation and toxicity at the site of P-gp expression. In order to investigate the possible role of P-gp in sepsis and in TCZ ameliorative effect, we have explored its expression in rat lung and kidney tissues. P-gp expression has been significantly decreased by almost 50% in rat showed a significant group, in comparison to the sham group (Fig. 6). Interestingly, TCZ administration in CLP + TCZ group significantly up-regulated P-gp expression to levels close to the sham group. These data suggest a possible protective role for P-glycoprotein in lungs and kidneys, a mechanism that is disturbed under septic conditions and effectively restored by using TCZ.
TCZ reversed sepsis-induced down-regulation of P-glycoprotein expression in lung and kidney of ALI and AKI. The level of P-glycoprotein expression was detected by ELISA kit in lung a and in kidney b tissues from different groups. The results shown are representative of at least three independent experiments. Each value represents the mean ± SEM (n = 6). *, ** and *** significant difference at p < 0.05, p < 0.01 and p < 0.001 compared to CLP
Discussion
The purpose of the present study was to evaluate the effect of TCZ, an IL-6 receptor blocker, on survival as well as ALI and AKI associated with sepsis. We found that TCZ in two different doses (4 and 8 mg/kg) resulted in reduced mortality, however, the lower dose showed a more robust effect on survival. We continued our study using the 4 mg/kg dose to investigate the pathways involved in the observed protective effect of TCZ focusing on lung and kidney status in septic animals. Septic animals treated with TCZ showed an overall improvement in kidney and lung functions. TCZ was found to attenuate elevated oxidative stress associated with sepsis. It also improved anti-oxidant defense in the form of increased activity of pulmonary and renal catalase and SOD enzymes as well as increased serum total anti-oxidant capacity. Moreover, TCZ treatment resulted in a diminished inflammatory response evident in reduction of tissue IL-6 and NFκB expression. Reduction of sepsis-induced apoptosis was also observed in the form of increased expression of the anti-apoptotic signal Bcl2 and reduced Caspase-3 level. A role of P-glycoprotein in sepsis was for the first time demonstrated in our study, with a reduced level in the kidneys and lungs of septic animal that was reversed by TCZ treatment. We employed the CLP model, which is currently considered as the gold standard for sepsis-related studies and closely resembles the pathophysiology of human sepsis (Hubbard et al. 2005).
In our study, better survival was achieved using the low dose of TCZ (4 mg/kg) versus its high dose (8 mg/kg). One explanation of this finding is that a minimal level of IL-6 signaling is probably required to maintain some balance in the immune system especially in the acute phase of the early septic events. Indeed, the risk of infection from TCZ administration has been reported to increase with the high dose (8 mg/kg compared to 4 mg/kg), while it remains stable with prolonged treatment (Delaloye and Ribi 2017). In 2003, a study examining the effect of a different IL-6 neutralizing antibody reported that a higher dose of the antibody resulted in lower survival compared to a lower dose (Riedemann et al. 2003). Similar results were shown in a previous study examining the effect of TCZ on a model of pancreatitis and lung injury where lower doses were associated with better outcomes (Chen et al. 2016). In the same study, the authors examined the safety of relatively high doses of TCZ and found no organs pathology or adverse effects.
The present study reports that TCZ reduced the lung W/D weight ratio induced by the CLP protocol. Histological analysis of lung tissue in rats with CLP-induced ALI supported this finding by showing increased interstitial infiltration by neutrophils and alveolar septal thickness, which was attenuated by TCZ administration. These findings indicated that TCZ could prevent the development of pulmonary edema and inflammatory cells infiltration into the lungs, a critical characteristic in the pathogenesis of ALI (Abraham 2003).
Similarly, histopathological examination of the kidneys showed renal tubular epithelial cell degeneration with obvious necrotic changes in CLP group. Treatment with TCZ, however, decreased the severity of the lesions, alleviated the extent of renal injury, and reduced the infiltration of inflammatory cells. These findings were supported by an ameliorative effect of TCZ on CLP induced elevation in BUN and CR levels. Taken together, TCZ treatment results in improved kidney function in sepsis.
To further evaluate the therapeutic effect of TCZ on CLP-induced ALI and AKI, we evaluated oxidative stress status in the lung and kidney tissues. TCZ treatment showed a reduction of oxidative stress and improvement of anti-oxidant mechanisms. These results support our finding of a protective effect of TCZ on sepsis as endothelial damage, neutrophil enhancement, cytokine release, and mitochondrial injury are among the proinflammatory effects of ROS (Andrades et al. 2009; Hotchkiss and Karl 2003; Barichello et al. 2006), which lead to microvascular dysfunction and organ dysfunction (Peralta et al. 1993). Decreased SOD and CAT activities were also reported in sepsis (Maurya et al. 2014). Ritter et al. demonstrated that high MDA and SOD levels are taken as markers of early mortality in rats with sepsis (Ritter et al. 2003). Collectively, the reduced mortality associated with TCZ use can be explained by its ability to improve the oxidative stress status induced by sepsis. Moreover, the ability of TCZ to abort the IL-6 signaling may be a direct contributing mechanism for its impressing restoration for the anti-oxidant/oxidant balance in septic rats. Our data are in accordance with the literature reporting the alleviation of the oxidative stress and tissue damage in patients with RA upon the treatment with TCZ (Ruiz-Limon et al. 2017).
Cell apoptosis in pulmonary and renal tissues is involved in the development and progression of lung and kidney injuries during sepsis (Chang et al. 2012; Kockara and Kayatas 2013). Research shows that the activation of caspase-3 plays a critical role in the apoptosis of lung and kidney tissue cells. Bcl-2, among the Bcl family members, is an important regulatory factor of apoptosis (Low et al. 2011). Bcl-2 can prevent activation of the pro-apoptotic protein Bax (Postolow et al. 2011; Rigoulet et al. 2011). Our data demonstrated that TCZ inhibits lung and kidney apoptosis via normalization of the increased level of caspase-3 protein expression in septic rat lung and kidney tissues. Our results showed also that TCZ increased the survival of pulmonary alveolar epithelial and the renal tubular cells through up-regulation of the survival mediator Bcl2 evident in immunostaining of lung tissue.
Another important finding of the present study was that TCZ attenuated the up-regulation of the proinflammatory cytokine IL-6. Because IL-6 is extensively produced in the lung, the level of IL-6 is taken as a good index to assess the early injury of the lung (Spooner et al. 1992). Elevated lung IL-6 can be attributed to neutrophil infiltration evident in H&E stained lung sections. Neutrophils have been shown in previous studies to be an important source of IL-6 (Rincon and Irvin 2012). Interestingly, IL-6 can be also produced from primary lung epithelial cells where constitutive increase in IL-6 mRNA is observed (Neveu et al. 2011). When these cells are subjected to different stimuli including elevated oxidative stress, these cells produce a large amount of IL-6, which contributes to pulmonary dysfunction and hence development of ALI (Rincon and Irvin 2012).
In the kidneys, for every tenfold increase in IL-6, the risk for AKI increased by 16% in sepsis patients (Chawla et al. 2007). Similar to the lungs, neutrophil infiltration is not the only source for elevated kidney IL-6. Other kidney cells including endothelial cells, podocytes, mesangial cells, and tubular epithelial cells are capable of secreting IL-6 under certain conditions (Su et al. 2017).
IL-6 levels were previously shown to increase in the lungs and kidneys, and contribute to the progression of sepsis in a murine CLP model (Yeh et al. 2005). Our data showed a remarkable decrease in IL-6 protein expression in both lung and kidney of septic rats with administration of TCZ. This finding is in accordance with previous studies in patients with RA, which demonstrated a reduction in IL-6 serum levels with TCZ administration (Nishimoto et al. 2014). Previous studies have reported that TCZ rebalances the production of different immune cells in RA patients, favoring a protective response in the context of inflammatory disease (Samson et al. 2012; Pesce et al. 2013). TCZ suppresses Th17 cells, which are involved in IL-6 production (Mills 2008). Thus in our results, the success of TCZ treatment to reduce the intrinsic production of IL-6 during sepsis might be attributed to Th17 cells suppression and can be considered as an important biologic finding with major beneficial consequences in the septic cascade. We report the maximal increase in serum IL-6 to occur early in sepsis, thus success to decrease its level and to neutralize its deleterious effects may be of the highest benefit if it is in early stages when there is an exaggerated inflammatory response with excessive secretion of proinflammatory cytokines. This data is in accordance with previous observations by Riedemann et al. (2003).
NF-κB is one of the most important transcription factors controlling genes that encode for many pro-inflammatory cytokines, such as IL-6, IL-1β and MCP-1, and is involved in the gene expression of sepsis-induced inflammation response and the pathophysiology in ALI and AKI (Liu and Malik 2006; Sanz et al. 2010; Souza et al. 2012). IL-6 has been shown to induce NF-κB activation and nuclear translocation in the intestinal epithelium (Wang et al. 2003). In order to explore whether TCZ affects NF-κB activity under septic conditions, we determined its relative protein expression through immunostaining of lung and kidney tissues of septic rats, with and without the treatment of TCZ. Our study has demonstrated that NF-κB expression and nuclear translocation was increased during sepsis, while treatment with TCZ significantly decreased its expression and dampened its activity as evidenced by its minimal cytoplasmic expression. These data provide a strong anti-inflammatory effect for TCZ, possibly through inhibiting a crucial molecule that can promote the expression of other inflammatory cytokines and augments the inflammatory response in sepsis both in ALI and in AKI. Attenuated NF-κB signaling has been shown previously to mediate protection against sepsis (Zou et al. 2018).
JNK is a critical signaling pathway in mediating inflammation and apoptosis. Activation of JNK signaling was reported upon LPS (Lipopolysaccharide) injection (Waetzig et al. 2005) and in CLP model in rodents (Pizzino et al. 2015). Using a JNK signaling inhibitor ameliorated the early and late steps of the inflammatory and apoptotic cascade in a murine model of CLP-induced sepsis (Pizzino et al. 2015). Moreover, an interesting relation has been documented between IL-6 action and JNK signaling pathway. IL-6 action in regulating iron-related proteins in BV2 microglial cell lines was promoted through JNK kinase activation (Zhou et al. 2017). Also, IL-6 activity in promoting cell migration in oral squamous cell carcinoma was mediated by JNK activation, and using IL-6R monoclonal antibody significantly abolished such activation (Chuang et al. 2014). In the present study, there was a marked stimulation of the JNK cascade in CLP group, while TCZ administration in CLP + TCZ group significantly reduced the JNK activation to almost its basal activity. This may imply that TCZ mediated its anti-inflammatory and its anti-apoptotic beneficial effects, through limiting IL-6 signaling and attenuating JNK signaling pathway in CLP operated rats.
A new role of P-glycoprotein (P-gp) was introduced in our study. P-glycoprotein is an ATP-dependent efflux transporter. It is overexpressed at the origin of multiple drug resistance (MDR) phenomenon occurring during tumor chemotherapy (Juliano and Ling 1976). Its protein expression is also influenced by a number of xenobiotic (Thiebaut et al. 1987), and endogenous compounds (Sukhai and Piquette-Miller 2000), and it is reported to prevent drug accumulation and toxicity at the site of P-gp expression (Thiebaut et al. 1987). We investigated whether severe inflammation under septic conditions can influence P-gp protein expression in lung and kidneys barriers, and whether P-gp can be regulated by the treatment of TCZ. Our data has shown a reduction in both lung and kidney P-gp expression in septic rats. TCZ-treated rats had levels comparable to non-septic rats, suggesting that TCZ can stimulate the expression of P-gp or prevent the inhibition of P-gp, which is consistent with its protective role in extruding toxins and harmful elements during sepsis. Our data are in accordance with the literature; P-gp expression was reported to be strongly reduced in the gut mucosa of individuals with active inflammation compared with controls and was negatively correlated with the levels of IL-6 mRNA (Englund et al. 2007). A direct link between IL-6 signaling and P-gp expression was shown in previous studies performed in vitro on cultured hepatocytes in which incubation with IL-6 resulted in a higher expression of P-gp (Sukhai et al. 2001). Several research groups also demonstrated a decrease in P-gp protein expression in various disease states, as in kidneys of rats with chronic renal failure, in the mucosa of patients with active ulcerative colitis, as well as in human striatum in Parkinson’s disease when compared to the normal control group (Gutmann et al. 2008; Naud et al. 2011; Westerlund et al. 2008). These data provides a novel finding for the role of P-gp in the pathogenesis of sepsis and adds an important insight to the protective efficacy of TCZ in sepsis-induced ALI and AKI.
While a previous study investigated the possible role for IL-6 in the biology of sepsis by neutralizing circulating IL-6 using an anti-IL-6 AB (Riedemann et al. 2003), it is worth mentioning that our study is the first one which demonstrates the effect of IL-6 signaling in a model of sepsis by using the monoclonal antibody approved drug, TCZ. It is important to state that being a humanized antibody, involvement of other off-targets for TCZ in addition to IL-6 is possible and warrants further research to be evaluated.
The results of this study, as well as previous studies on the effects of anti-inflammatory antibodies during experimental sepsis, introduce a glimpse of hope for sepsis patients. Unfortunately, clinical trials examining the effect of anti-cytokine therapy (for example anti-TNF-alpha) found either no significant benefit or a very small increase in survival in septic patients (Reinhart et al. 2001; Gallagher et al. 2001; Panacek et al. 2004). Interestingly, most of these studies used plasma IL-6 as a marker for initiation of this therapy suggesting an important role of IL-6 in this regard. Identification of the exact time point(s) at which anti-cytokine intervention should be initiated is a critically important factor that should be the focus of future studies.
Conclusion
In conclusion, we have demonstrated that treatment with TCZ attenuates sepsis-induced acute lung and acute kidney injuries, so that it has a significant protective effect on the development and progression of septic ALI and AKI. The underlying mechanism of TCZ on sepsis-induced lung and kidney injuries may be closely related to its blocking effects to the IL-6 receptor with its anti-oxidant, anti-apoptotic, and anti-inflammatory roles. TCZ could inhibit the production of the pro-inflammatory IL-6 and attenuate cellular infiltration by decreasing the activation of NF-κB, attenuating the JNK signaling pathway and restoring the normal expression of P-gp in order to prevent tissue damage secondary to the septic cascade. Timing and dosing of TCZ is necessary to keep a balanced level of IL-6 serum content during early sepsis, which may thereby be a key for successful therapy of these patients.
References
Abraham E (2003) Neutrophils and acute lung injury. Crit Care Med 31:S195–S199
Andrades ME, Ritter C, Dal-Pizzol F (2009) The role of free radicals in sepsis development. Front Biosci (Elite Ed) 1:277–287
Barichello T, Fortunato JJ, Vitali AM, Feier G, Reinke A, Moreira JC, Quevedo J, Dal-Pizzol F (2006) Oxidative variables in the rat brain after sepsis induced by cecal ligation and perforation. Crit Care Med 34:886–889
Bartels H, Bohmer M, Heierli C (1972) Serum creatinine determination without protein precipitation. Clin Chim Acta 37:193–197
Chang CL, Leu S, Sung HC, Zhen YY, Cho CL, Chen A, Tsai TH, Chung SY, Chai HT, Sun CK, Yen CH, Yip HK (2012) Impact of apoptotic adipose-derived mesenchymal stem cells on attenuating organ damage and reducing mortality in rat sepsis syndrome induced by cecal puncture and ligation. J Transl Med 10:244
Chawla LS, Seneff MG, Nelson DR, Williams M, Levy H, Kimmel PL, Macias WL (2007) Elevated plasma concentrations of IL-6 and elevated APACHE II score predict acute kidney injury in patients with severe sepsis. Clin J Am Soc Nephrol 2:22–30
Chen KL, Lv ZY, Yang HW, Liu Y, Long FW, Zhou B, Sun XF, Peng ZH, Zhou ZG, Li Y (2016) Effects of tocilizumab on experimental severe acute pancreatitis and associated acute lung injury. Crit Care Med 44:e664–e677
Chuang JY, Huang YL, Yen WL, Chiang IP, Tsai MH, Tang CH (2014) Syk/JNK/AP-1 signaling pathway mediates interleukin-6-promoted cell migration in oral squamous cell carcinoma. Int J Mol Sci 15:545–559
Cohen J (2002) The immunopathogenesis of sepsis. Nature 420:885–891
De Backer D, Orbegozo Cortes D, Donadello K, Vincent JL (2014) Pathophysiology of microcirculatory dysfunction and the pathogenesis of septic shock. Virulence 5:73–79
De Bont ES, Vellenga E, Swaanenburg JC, Fidler V, Visser-Van Brummen PJ, Kamps WA (1999) Plasma IL-8 and IL-6 levels can be used to define a group with low risk of septicaemia among cancer patients with fever and neutropenia. Br J Haematol 107:375–380
Deitch EA (1998) Animal models of sepsis and shock: a review and lessons learned. Shock 9:1–11
Delaloye J, Ribi C (2017) Infections associated with immunobiologics. In: Cohen J, Powderly WG, Opal SM (eds) Infectious diseases, 4th edn. Elsevier, Amsterdam
Dhanasekaran DN, Reddy EP (2017) JNK-signaling: a multiplexing hub in programmed cell death. Genes Cancer 8:682–694
Englund G, Jacobson A, Rorsman F, Artursson P, Kindmark A, Ronnblom A (2007) Efflux transporters in ulcerative colitis: decreased expression of BCRP (ABCG2) and Pgp (ABCB1). Inflamm Bowel Dis 13:291–297
Ewees MG, Messiha BAS, Abdel-Bakky MS, Bayoumi AMA, Abo-Saif AA (2018) Tempol, a superoxide dismutase mimetic agent, reduces cisplatin-induced nephrotoxicity in rats. Drug Chem Toxicol 1:1–8
Fawcett JK, Scott JE (1960) A rapid and precise method for the determination of urea. J Clin Pathol 13:156–159
Fossati P, Prencipe L, Berti G (1980) Use of 3,5-dichloro-2-hydroxybenzenesulfonic acid/4-aminophenazone chromogenic system in direct enzymic assay of uric acid in serum and urine. Clin Chem 26:227–231
Gallagher J, Fisher C, Sherman B, Munger M, Meyers B, Ellison T, Fischkoff S, Barchuk WT, Teoh L, Velagapudi R (2001) A multicenter, open-label, prospective, randomized, dose-ranging pharmacokinetic study of the anti-TNF-alpha antibody afelimomab in patients with sepsis syndrome. Intensive Care Med 27:1169–1178
Gao H, Yan C (2012) New insights for C5a and C5a receptors in sepsis. Front Immunol 3:368
Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR (1982) Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem 126:131–138
Gutmann H, Hruz P, Zimmermann C, Straumann A, Terracciano L, Hammann F, Lehmann F, Beglinger C, Drewe J (2008) Breast cancer resistance protein and P-glycoprotein expression in patients with newly diagnosed and therapy-refractory ulcerative colitis compared with healthy controls. Digestion 78:154–162
Hack CE, De Groot ER, Felt-Bersma RJ, Nuijens JH, Strack Van Schijndel RJ, Eerenberg-Belmer AJ, Thijs LG, Aarden LA (1989) Increased plasma levels of interleukin-6 in sepsis. Blood 74:1704–1710
Hotchkiss RS, Karl IE (2003) The pathophysiology and treatment of sepsis. N Engl J Med 348:138–150
Hubbard WJ, Choudhry M, Schwacha MG, Kerby JD, Rue LW 3rd, Bland KI, Chaudry IH (2005) Cecal ligation and puncture. Shock 24(Suppl 1):52–57
Iskander KN, Osuchowski MF, Stearns-Kurosawa DJ, Kurosawa S, Stepien D, Valentine C, Remick DG (2013) Sepsis: multiple abnormalities, heterogeneous responses, and evolving understanding. Physiol Rev 93:1247–1288
Juliano RL, Ling V (1976) A surface glycoprotein modulating drug permeability in Chinese hamster ovary cell mutants. Biochim Biophys Acta 455:152–162
Kinsey GR, Li L, Okusa MD (2008) Inflammation in acute kidney injury. Nephron Exp Nephrol 109:e102–e107
Kockara A, Kayatas M (2013) Renal cell apoptosis and new treatment options in sepsis-induced acute kidney injury. Ren Fail 35:291–294
Koracevic D, Koracevic G, Djordjevic V, Andrejevic S, Cosic V (2001) Method for the measurement of antioxidant activity in human fluids. J Clin Pathol 54:356–361
Liu SF, Malik AB (2006) NF-kappa B activation as a pathological mechanism of septic shock and inflammation. Am J Physiol Lung Cell Mol Physiol 290:L622–L645
Low IC, Kang J, Pervaiz S (2011) Bcl-2: a prime regulator of mitochondrial redox metabolism in cancer cells. Antioxid Redox Signal 15:2975–2987
Martin H, Olander B, Norman M (2001) Reactive hyperemia and interleukin 6, interleukin 8, and tumor necrosis factor-alpha in the diagnosis of early-onset neonatal sepsis. Pediatrics 108:E61
Matejovic M, Chvojka J, Radej J, Ledvinova L, Karvunidis T, Krouzecky A, Novak I (2011) Sepsis and acute kidney injury are bidirectional. Contrib Nephrol 174:78–88
Maurya H, Mangal V, Gandhi S, Prabhu K, Ponnudurai K (2014) Prophylactic antioxidant potential of gallic acid in murine model of sepsis. Int J Inflam 2014:580320
Mccord JM, Fridovich I (1969) Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem 244:6049–6055
Mihara M, Uchiyama M (1978) Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal Biochem 86:271–278
Mills KH (2008) Induction, function and regulation of IL-17-producing T cells. Eur J Immunol 38:2636–2649
Naud J, Michaud J, Beauchemin S, Hebert MJ, Roger M, Lefrancois S, Leblond FA, Pichette V (2011) Effects of chronic renal failure on kidney drug transporters and cytochrome P450 in rats. Drug Metab Dispos 39:1363–1369
Neveu WA, Bernardo E, Allard JL, Nagaleekar V, Wargo MJ, Davis RJ, Iwakura Y, Whittaker LA, Rincon M (2011) Fungal allergen beta-glucans trigger p38 mitogen-activated protein kinase-mediated IL-6 translation in lung epithelial cells. Am J Respir Cell Mol Biol 45:1133–1141
Nishimoto N, Amano K, Hirabayashi Y, Horiuchi T, Ishii T, Iwahashi M, Iwamoto M, Kohsaka H, Kondo M, Matsubara T, Mimura T, Miyahara H, Ohta S, Saeki Y, Saito K, Sano H, Takasugi K, Takeuchi T, Tohma S, Tsuru T, Ueki Y, Yamana J, Hashimoto J, Matsutani T, Murakami M, Takagi N (2014) Drug free REmission/low disease activity after cessation of tocilizumab (Actemra) Monotherapy (DREAM) study. Mod Rheumatol 24:17–25
Panacek EA, Marshall JC, Albertson TE, Johnson DH, Johnson S, Macarthur RD, Miller M, Barchuk WT, Fischkoff S, Kaul M, Teoh L, Van Meter L, Daum L, Lemeshow S, Hicklin G, Doig C (2004) Efficacy and safety of the monoclonal anti-tumor necrosis factor antibody F(ab’)2 fragment afelimomab in patients with severe sepsis and elevated interleukin-6 levels. Crit Care Med 32:2173–2182
Parke AL, Liu PT, Parke DV (2003) Multiple organ dysfunction syndrome. Inflammopharmacology 11:87–95
Peralta JG, Llesuy S, Evelson P, Carreras MC, Flecha BG, Poderoso JJ (1993) Oxidative stress in skeletal muscle during sepsis in rats. Circ Shock 39:153–159
Pesce B, Soto L, Sabugo F, Wurmann P, Cuchacovich M, Lopez MN, Sotelo PH, Molina MC, Aguillon JC, Catalan D (2013) Effect of interleukin-6 receptor blockade on the balance between regulatory T cells and T helper type 17 cells in rheumatoid arthritis patients. Clin Exp Immunol 171:237–242
Pizzino G, Bitto A, Pallio G, Irrera N, Galfo F, Interdonato M, Mecchio A, De Luca F, Minutoli L, Squadrito F, Altavilla D (2015) Blockade of the JNK signalling as a rational therapeutic approach to modulate the early and late steps of the inflammatory cascade in polymicrobial sepsis. Mediators Inflamm 2015:591572
Postolow F, Fediuk J, Nolette N, Hinton M, Dakshinamurti S (2011) Hypoxia and nitric oxide exposure promote apoptotic signaling in contractile pulmonary arterial smooth muscle but not in pulmonary epithelium. Pediatr Pulmonol 46:1194–1208
Potito J, Marcos J, Sotelo D, Stringa P, Laguens G, Beldarrain M, Correger ER (2017) Blocking Il-6 in rats attenuates mechanical ventilation lung injury. D109. Understanding the Mechanisms of Acute Lung Injury. American Thoracic Society
Reinhart K, Menges T, Gardlund B, Harm Zwaveling J, Smithes M, Vincent JL, Tellado JM, Salgado-Remigio A, Zimlichman R, Withington S, Tschaikowsky K, Brase R, Damas P, Kupper H, Kempeni J, Eiselstein J, Kaul M (2001) Randomized, placebo-controlled trial of the anti-tumor necrosis factor antibody fragment afelimomab in hyperinflammatory response during severe sepsis: the RAMSES Study. Crit Care Med 29:765–769
Riedemann NC, Neff TA, Guo RF, Bernacki KD, Laudes IJ, Sarma JV, Lambris JD, Ward PA (2003) Protective effects of IL-6 blockade in sepsis are linked to reduced C5a receptor expression. J Immunol 170:503–507
Rigoulet M, Yoboue ED, Devin A (2011) Mitochondrial ROS generation and its regulation: mechanisms involved in H(2)O(2) signaling. Antioxid Redox Signal 14:459–468
Rincon M, Irvin CG (2012) Role of IL-6 in asthma and other inflammatory pulmonary diseases. Int J Biol Sci 8:1281–1290
Ritter C, Andrades M, Frota Junior ML, Bonatto F, Pinho RA, Polydoro M, Klamt F, Pinheiro CT, Menna-Barreto SS, Moreira JC, Dal-Pizzol F (2003) Oxidative parameters and mortality in sepsis induced by cecal ligation and perforation. Intensive Care Med 29:1782–1789
Ruiz-Limon P, Ortega R, Arias de la Rosa I, Abalos-Aguilera MDC, Perez-Sanchez C, Jimenez-Gomez Y, Peralbo-Santaella E, Font P, Ruiz-Vilches D, Ferrin G, Collantes-Estevez E, Escudero-Contreras A, Lopez-Pedrera C, Barbarroja N (2017) Tocilizumab improves the proatherothrombotic profile of rheumatoid arthritis patients modulating endothelial dysfunction, NETosis, and inflammation. Transl Res 183:87–103
Samson M, Audia S, Janikashvili N, Ciudad M, Trad M, Fraszczak J, Ornetti P, Maillefert JF, Miossec P, Bonnotte B (2012) Brief report: inhibition of interleukin-6 function corrects Th17/Treg cell imbalance in patients with rheumatoid arthritis. Arthritis Rheum 64:2499–2503
Sanz AB, Sanchez-Nino MD, Ramos AM, Moreno JA, Santamaria B, Ruiz-Ortega M, Egido J, Ortiz A (2010) NF-kappaB in renal inflammation. J Am Soc Nephrol 21:1254–1262
Souza AC, Volpini RA, Shimizu MH, Sanches TR, Camara NO, Semedo P, Rodrigues CE, Seguro AC, Andrade L (2012) Erythropoietin prevents sepsis-related acute kidney injury in rats by inhibiting NF-kappaB and upregulating endothelial nitric oxide synthase. Am J Physiol Renal Physiol 302:F1045–F1054
Spooner CE, Markowitz NP, Saravolatz LD (1992) The role of tumor necrosis factor in sepsis. Clin Immunol Immunopathol 62:S11–S17
Su H, Lei C-T, Zhang C (2017) Interleukin-6 signaling pathway and its role in kidney disease: an update. Front Immunol 8:405
Sukhai M, Piquette-Miller M (2000) Regulation of the multidrug resistance genes by stress signals. J Pharm Pharm Sci 3:268–280
Sukhai M, Yong A, Pak A, Piquette-Miller M (2001) Decreased expression of P-glycoprotein in interleukin-1beta and interleukin-6 treated rat hepatocytes. Inflamm Res 50:362–370
Tanaka T, Narazaki M, Masuda K, Kishimoto T (2016) Regulation of IL-6 in immunity and diseases. Adv Exp Med Biol 941:79–88
Thiebaut F, Tsuruo T, Hamada H, Gottesman MM, Pastan I, Willingham MC (1987) Cellular localization of the multidrug-resistance gene product P-glycoprotein in normal human tissues. Proc Natl Acad Sci USA 84:7735–7738
Uchino S, Kellum JA, Bellomo R, Doig GS, Morimatsu H, Morgera S, Schetz M, Tan I, Bouman C, Macedo E, Gibney N, Tolwani A, Ronco C, Beginning and Ending Supportive Therapy for the Kidney, I. (2005) Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA 294:813–818
Waetzig V, Czeloth K, Hidding U, Mielke K, Kanzow M, Brecht S, Goetz M, Lucius R, Herdegen T, Hanisch UK (2005) c-Jun N-terminal kinases (JNKs) mediate pro-inflammatory actions of microglia. Glia 50:235–246
Wang L, Walia B, Evans J, Gewirtz AT, Merlin D, Sitaraman SV (2003) IL-6 induces NF-kappa B activation in the intestinal epithelia. J Immunol 171:3194–3201
Wang S, Zhou J, Kang W, Dong Z, Wang H (2016) Tocilizumab inhibits neuronal cell apoptosis and activates STAT3 in cerebral infarction rat model. Bosn J Basic Med Sci 16:145–150
Wang Y, Yang W, Zhao X, Zhang R (2018) Experimental study of the protective effect of simvastatin on lung injury in rats with sepsis. Inflammation 41:104–113
Westerlund M, Belin AC, Olson L, Galter D (2008) Expression of multi-drug resistance 1 mRNA in human and rodent tissues: reduced levels in Parkinson patients. Cell Tissue Res 334:179–185
Yeh CL, Hsu CS, Yeh SL, Chen WJ (2005) Dietary glutamine supplementation modulates Th1/Th2 cytokine and interleukin-6 expressions in septic mice. Cytokine 31:329–334
Zarjou A, Agarwal A (2011) Sepsis and acute kidney injury. J Am Soc Nephrol 22:999–1006
Zhou S, Du X, Xie J, Wang J (2017) Interleukin-6 regulates iron-related proteins through c-Jun N-terminal kinase activation in BV2 microglial cell lines. PLoS One 12:e0180464
Zou P, Ji HM, Zhao JW, Ding XM, Zhen ZG, Zhang X, Nie XQ, Xue LX (2018) Protective effect of isoliquiritigenin against cerebral injury in septic mice via attenuation of NF-kappaB. Inflammopharmacology 27:809
Acknowledgements
The authors greatly appreciate the technical assistance of Ms. Shaymaa Ramzy Senousy Khalil, B.Pharm (Minia University), and Dr. Sarah MacEachern, MD PhD (University of Calgary) for reading the manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
YFI was responsible for the experimental design. YFI and AFA contributed to the execution of experiments, collection of the samples, data statistics, manuscript writing and composition. RAM was responsible for the histopathology and the immunohistochemistry studies and AMAB was responsible for the western blot experiments and analysis. YFI was responsible for the supervision and management of the project. All authors have contributed to and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no potential conflict of interests with respect to the research, authorship, and/or publication of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ibrahim, Y.F., Moussa, R.A., Bayoumi, A.M.A. et al. Tocilizumab attenuates acute lung and kidney injuries and improves survival in a rat model of sepsis via down-regulation of NF-κB/JNK: a possible role of P-glycoprotein. Inflammopharmacol 28, 215–230 (2020). https://doi.org/10.1007/s10787-019-00628-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10787-019-00628-y