Abstract
Background/Objectives
Sepsis-induced acute lung injury (ALI) is the typical complications of sepsis with a high global incidence and mortality. Inhibition of inflammatory response is a crucial and effective strategy for sepsis-induced ALI. Pedunculoside (PE) has been shown to have an anti-inflammatory effect on various diseases. However, the effect and mechanism of PE on sepsis-induced ALI remain unknown.
Materials/Methods
A mice model of sepsis-induced ALI was constructed by cecal ligation and puncture (CLP). The effect of PE on the CLP-induced mice were assessed using pathological staining, terminal deoxynucleotidyl transferase deoxyuridine triphosphate (dUTP) nick end labeling (TUNEL), reverse transcription quantitative polymerase chain reaction (RT-qPCR), enzyme-linked immunosorbent assay (ELISA) and western blot assays.
Results
PE reduced pathological symptoms and scores, apoptosis and the W/D ratio of lung tissues in CLP-induced mice. Besides, PE decreased the level of interleukin (IL)-1β, IL-6 and tumor necrosis factor (TNF)-α), pulmonary fibrosis and the expression of fibrosis markers. Mechanically, PE inhibited AKT/NF-κB signaling in CLP-induced mice. Activation of AKT/NF-κB pathway abolished the ameliorative effect of PE on the pathological symptoms, the release of inflammatory factors and pulmonary fibrosis of CLP-induced mice.
Conclusion
PE improved inflammation and pulmonary fibrosis by inhibiting AKT/NF-κB pathway in CLP-induced mice.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sepsis is a life-threatening disease in clinical practice that is related to an abnormal host response to infection (Singer et al. 2016). Globally, there are over 18 million cases of severe sepsis annually, with the incidence rising from 1.5 to 8.0% per year (Jawad et al. 2012). Moreover, about 14,000 deaths globally are attributed to complications from sepsis per day (Jawad et al. 2012). The lung is the most susceptible organ during sepsis (Sadowitz et al. 2011). The primary cause of sepsis-related mortality is acute lung injury (ALI) or acute respiratory distress syndrome (ARDS), which affects nearly half of patients with severe sepsis (Hwang et al. 2019; Shashaty et al. 2019). Inflammation is a key factor in sepsis-induced ALI, and the severity and duration of the inflammatory response eventually determine the prognosis of patients with sepsis-elicited ALI/ARDS (Xiong et al. 2020). Blocking the inflammatory response is therefore an essential and effective treatment for sepsis-induced ALI. It has been shown that paclitaxel, which has a major role in regulating inflammation, reduces the inflammatory response, improves lung function, and increases the survival rate of ALI (Wang et al. 2019). However, the application of corticosteroids can have various side effects in clinical practice, including immunosuppression, hypokalemia, dyslipidemia, hyperglycemia, myopathy and osteoporosis (Schäcke et al. 2002; Walker 2007). Therefore, it is still significant and imminent to develop safe and effective drugs to decrease the inflammatory response in the therapeutic treatment of sepsis-elicited ALI.
Pedunculoside (PE), a triterpene saponin, is a main bioactive component from the dried bark of Ilex rotunda Thunb (Wang et al. 2014), with a range of pharmacological properties, such as anti-inflammation (Ma et al. 2019), protection against liver injury (Wu et al. 2019), lowing hyperlipidemia (Liu et al. 2018) and improvement of intestinal flora (Yang et al. 2019). Notably, PE has been demonstrated to have an anti-inflammatory effect on various diseases. Kan et al. (Kan et al. 2021) show that PE prevents mastitis in mice by reducing inflammation and maintaining the integrity of blood-milk barrier. Liu et al. (Liu et al. 2020) report that PE protects against ulcerative colitis in mice via AKT/NF-κB and MAPK pathways. Ma et al. (Ma et al. 2019) reveal that PE inhibits collagen-induced arthritis in vitro and in vivo. However, whether PE improves sepsis-induced ALI by inhibiting inflammation remains to be determined.
Thus, mice were treated by cecal ligation and puncture (CLP) to construct a model of sepsis-induced ALI. Then, the effect of PE on the lung injury, inflammation and pulmonary fibrosis was investigated in CLP-induced mice. Moreover, the potential molecular mechanism was studies in vivo. We expect that our results can provide a solid foundation for the treatment of sepsis-induced ALI.
Materials and methods
Animal
C57BL/6 mice (6–8 weeks, 20–25 g) were purchased from Cyagen (Jiangsu, China) and fed in a specified pathogen free (SPF) animal house. Mice were given access to rodent chow and water freely, with 40-60% the relative humidity and a 12 h/12 hours light-dark cycle at (25 ± 2) ℃. All the experiments were complied with the National Institute of Health Guide for the Care and Use of Laboratory Animals, as well as approved by the Animal Research Ethics Committee of Beijing Tongren Hospital, Capital Medical University.
Animal model and treatment
Mice were accommodated for at least one week and then used for the experiments. A mouse model of sepsis-elicited ALI was constructed by cecal ligation and puncture (CLP) following the earlier description (Sang et al. 2022). Briefly, mice were intraperitoneally anesthetized with 1% pentobarbital sodium (1 mg/kg), and a 1–2 cm length incision was made in the middle of their abdomen. A 21-gauge needle was used to maul the cecum after it had been split and ligated with 5 − 0 B suture. Then, a droplet of feces was ejected after the needle was removed. The abdominal cavity was eventually sutured. Mice were randomly divided into eight groups (n = 6). (1) The control group: Healthy mice in the control group were orally given with the same amount of dimethylsulfoxide (DMSO, ST038, Beyotime, Shanghai, China); (2) The 20 mg/kg PE group: Healthy mice were orally given with PE with a dose of 20 mg/kg; (3) The sham groups: Mice in the sham group underwent the same surgery, excluding the ligation and puncture, and were then orally given with the same amount of DMSO; (4) The CLP group: Mice were treated with CLP and then orally given with the same amount of DMSO; (5–7) The CLP + PE groups (5 mg/kg, 10 mg/kg and 20 mg/kg): Mice were treated with CLP and then orally given with PE with a dose of 5 mg/kg, 10 mg/kg and 20 mg/kg, respectively; (8) The CLP + 20 mg/kg PE + SC79 group: Mice were treated with CLP, orally given with 20 mg/kg PE and intraperitoneally administrated with 10 mg/kg SC79 (an activator of AKT pathway) (123,871, Sigma, St. Louis, MO, USA). PE was bought from MedChemExpress (HY-N0458, Monmouth Junction, NJ, USA) with a purity of 99.17% and dissolved in DMSO. The dose of PE and SC79 used in the present study was based on the previous reports by Kan et al. (Kan et al. 2021) and Jing et al. (Jing et al. 2019), respectively. The sera, bronchoalveolar lavage fluid (BALF) and lung tissues from mice were collected for the following examinations.
Measurement of wet/dry (W/D) ratio of lung tissue
The lower lobe of the left lung was excised, and immediately weighed using an electronic balance. Tissues were then dried at 70 °C. After 48 h, the tissues were reweighed. The wet-to-dry ratio was calculated following the formula: wet weight/dry weight (Qu et al. 2022).
Pathological examination
Lung tissues were fixed into 4% paraformaldehyde (P0099, Beyotime) overnight. The tissue samples were embedded in paraffin and cut into slices with a 5 μm thickness. Then, the slices were incubated with Hematoxylin and Eosin Staining Kit (C0105S, Beyotime), and the imaged were captured under a light microscopy (Olympus, Tokyo, Japan). The pathological results were scored based on a previous study (Li et al. 2021). Four indexes, including bleeding, alveolar hyperemia, alveolar dilatation and neutrophil infiltration, were used for the pathological evaluation, with a 5-point damage scoring system. Therein, 0–4 indicated minimal, mild, moderate, serious and maximal, respectively. The total of all the points was used to determine the severity of lung injury. In addition, the slices were incubated with Masson’s Trichrome Stain Kit (G1340, Solarbio, Beijing, China) to assess the fibrosis of lung tissues.
Terminal deoxynucleotidyl transferase deoxyuridine triphosphate (dUTP) nick end labeling (TUNEL) assays
After the paraffin slices were dewaxed with xylene and dehydrated with graded ethanol, the slices were incubated with proteinase K (ST533, Beyotime) at room temperature for an hour. Then, the slices were washed with phosphate buffer saline (PBS, C0221A, Beyotime) for three times, and treated with 2% H2O2 for 20 min at room temperature. Next, the slices were rinsed with PBS thrice, and administrated with TUNEL reaction mixtures at 37 °C for 60 min. The slices were then incubated with 500 µL DAB (P0202, Beyotime) for 30 min at room temperature, and covered with 50 µL Streptavidin-HRP (A0305, Beyotime) for half an hour at room temperature. The slices were re-stained with hematoxylin (C0107, Beyotime) and pictured under a light microscope (Olympus). The percent of apoptosis cells was determined by TUNEL positive cells/the total cells number×100%.
Enzyme-linked immunosorbent assay (ELISA)
The concentrations of interleukin (IL)-1β, IL-6 and tumor necrosis factor (TNF)-α in BALF were examined using Mouse IL-1β ELISA Kit (PI301), Mouse IL-6 ELISA Kit (PI326) and Mouse TNF-α ELISA Kit (PT512) based on the working instructions. All three kits were bought from Beyotime. The absorbance was examined at 450 nm with a microplate reader (Thermo Fisher Scientific, Waltham, MA, USA).
Reverse transcription quantitative polymerase chain reaction (RT-qPCR)
The lung tissues were lysed with TRIzol reagent (15,596,026, Thermo Fisher Scientific) to extract the total RNA. The total RNA was inversely transcribed into cDNA using the Bio-Rad ScripTM cDNA Synthesis Kit (1,708,890, Bio-Rad Laboratories, Inc., Hercules, CA, USA) according to the instruction for use. 2× SYBR Master mix (RR820A, Takara, Dalian, China) was used for the RT-qPCR assay on the Bio-Rad CFX Manager software (Bio-Rad Laboratories, Inc.), with the following procedures: 5 min at 95 °C, followed by 40 cycles between 95 °C for 15 s and 59 °C for 45 s, and 72 °C for 60 s. The gene expressions were calculated by the 2−ΔΔCT method with the GAPDH as the internal reference gene. The primer sequences were as follows: 5′-GGAACGCACGTACCAGGAG-3′ (alpha smooth muscle actin (α-SMA) forward), 5′-TGGCTATTCAGGCTGTGCTGTC-3′ (α-SMA reverse); 5′-ATCCAGTCCACAGCCATTCC-3′ (fibronectin (FN) forward), 5′-GGAAGGGTAACCAGTTGGGG-3′ (FN reverse); 5′-CCTGGCAAAGACGGACTCAAC-3′ (collagen I forward), 5′- -3′ (collagen I reverse); 5′-GCTGAAGTCATAACCGCCACTG-3′ (GAPDH forward), and 5′-AGCCTTCTCCATGGTGGTGAAGAC-3′ (GAPDH reverse).
Western blot
Lung tissues were treated with RIPA lysis buffer (P0013B, Beyotime). The protein concentrations of lung tissues and sera were measured using BCA Protein Assay Kit (P0012S, Beyotime). The protein samples (20 µg) were dissolved with 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and then transferred onto PVDF membranes (IPVH00010, EMD Millipore, Billerica, MA, USA). The membranes were blocked with 5% skimmed milk powder (P0216, Beyotime) for 60 min at room temperature, and incubated with primary antibodies at 4˚C overnight. Subsequently, the membranes were treated with the secondary antibodies Goat Anti-Rabbit IgG H&L (HRP) (1:20000, ab6721) for 60 min at room temperature. The membranes were visualized by a BeyoECL Plus kit (P0018S, Beyotime), and the gray value was quantified by Image-ProPlus software (Media Cybernetics, Inc., Rockville, MD, USA). GAPDH served as the internal reference. The primary antibodies included anti-IL-1β (1:1000, ab254360), anti-IL-6 (1:1000, ab259341), anti-TNF-α (1:1000, ab66579), anti-α-SMA (1:1000, ab5694), anti-FN (1:1000, ab2413), anti-collagen I (1:2000, ab21286), anti-AKT (1:500, ab8805), anti- phosphorylated AKT (p-AKT) (1:500, ab38449), anti-NF-κB (1:1000, ab16502), anti-p-NF-κB (1:1000, ab76302), and anti-GAPDH (1:10000, ab181602). All the primary and secondary antibodies were bought from Abcam (Cambridge, UK).
Statistical analysis
Statistical analysis was performed by SPSS 20.0 software (IBM Corp., Armonk, NY, USA). All results were presented as mean ± standard deviation (SD). The data were analyzed by the one-way analysis of variance (ANOVA) followed by Post Hoc Bonferroni test. The differences were defined as statistically significant difference when P < 0.05.
Results
PE improved lung injury in CLP-induced mice
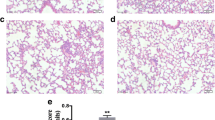
To address the effect of PE (Fig. 1A) on sepsis-induced lung injury, mice were treated with CLP and then administrated with PE. No pathological changes were found in the control mice (Fig. 1B). Treatment of 20 mg/kg PE also had no pathological impact on mice compared to the control mice (Fig. 1B), indicating that 20 mg/kg PE was safe for mice. Then, mice were administrated with CLP to construct a model of sepsis-induced lung injury. No pathological changes were found in the sham mice (Fig. 2A). A series of pathological changes, including severe inflammatory infiltration, the narrowed alveolar cavity, the thickened septum and the congested alveolar wall, were discovered in lung tissues of CLP mice (Fig. 2A). However, these pathological changes in CLP mice were increasingly improved with the treatment of 5, 10 and 20 mg/kg of PE (Fig. 2A). Also, PE with 5, 10 and 20 mg/kg increasingly lowered the histological injury scores (Fig. 2A). Besides, the percent of apoptotic cells in lung tissues was significantly elevated in CLP-induced mice, which was markedly declined by the administration of 10 and 20 mg/kg PE gradually (Fig. 2B). Moreover, the treatment of 10 and 20 mg/kg PE significantly decreased the W/D ratio of lung tissues in CLP-induced mice (Fig. 2C). Therefore, PE attenuated pathological damage, apoptosis and edema in CLP-induced mice.
20 mg/kg PE was safe for mice. Mice were randomly divided into the control group and 20 mg/kg PE group. Mice in the 20 mg/kg PE group were orally given with 20 mg/kg PE, while mice in the control group were orally given with the same amount of DMSO. (A) The two-dimension (2D) and three-dimension (3D) structure of PE. (B) The pathological changes of lung tissues were assessed by HE staining. Scale bar = 50 μm
PE alleviated lung injury in CLP-induced mice. Mice were randomly divided into sham group, CLP group, CLP + 5 mg/kg PE group, CLP + 10 mg/kg PE group and CLP + 20 mg/kg PE group. Mice in the sham group were treated with the same surgery except for ligation and puncture, and then orally given with the same amount of DMSO; Mice in the CLP group were administrated with CLP and then orally given with the same amount of DMSO; Mice in the CLP + 5 mg/kg PE group, CLP + 10 mg/kg PE and CLP + 20 mg/kg PE group were treated with CLP and then orally given with PE with a dose of 5 mg/kg, 10 mg/kg and 20 mg/kg, respectively. (A) The pathological changes were examined by HE staining, and the histological injury scores were calculated. Scale bar = 50 μm. (B) The apoptosis of lung tissues was determined by TUNEL assays, and the percent of apoptosis cells was calculated by TUNEL positive cells/the total cells number×100%. Black arrow indicated the apoptotic cells. Scale bar = 50 μm. (C) The W/D ratio of lung tissue was measured following the formula: wet weight/dry weight. **P < 0.01 and ***P < 0.001 vs. sham; #P < 0.05 and ##P < 0.01 vs. CLP. N = 6
PE attenuated the inflammatory response in CLP-induced mice
To investigate the effect of PE on the inflammatory response in CLP-induced mice, the release of inflammatory factors was determined. The concentrations of IL-1β, IL-6 and TNF-α were observably increased in BALF from CLP-treated mice, which were significantly neutralized with the treatment of 10 and 20 mg/kg PE (Fig. 3A-C). Also, the injection of PE (10 and 20 mg/kg) markedly decreased the relative protein expressions of IL-1β, IL-6 and TNF-α in CLP-induced mice (Fig. 3D and E). Thus, PE reduced the release of inflammatory cytokines in CLP-treated mice.
PE attenuated the inflammatory response in CLP-induced mice. (A-C) The concentrations of IL-1β, IL-6 and TNF-α in BALF were measured by ELISA. (D and E) The relative protein expressions of IL-1β, IL-6 and TNF-α in lung tissues were examined by western blot. Data were normalized with GAPDH. **P < 0.01 and ***P < 0.001 vs. sham; #P < 0.05 and ##P < 0.01 vs. CLP. N = 6
PE reduced pulmonary fibrosis in CLP-induced mice
To evaluate the effect of PE on pulmonary fibrosis in CLP-treated mice, the lung tissues were subjected to the MASSON staining, as well as the quantification of the level of fibrosis markers. PE with 5, 10 and 20 mg/kg increasingly mitigated the pulmonary fibrosis in CLP-induced mice (Fig. 4A). Meanwhile, both the transcriptional and translational expressions of α-SMA, FN and collagen I were markedly upregulated in lung tissues from CLP-induced mice, which were markedly counteracted with the treatment of 10 and 20 mg/kg PE (Fig. 4B-D). Hence, PE diminished CLP-induced pulmonary fibrosis.
PE reduced pulmonary fibrosis in CLP-induced mice. (A) The lung tissues were subjected to MASSON staining. Scale bar = 50 μm. (B) The relative mRNA expressions of α-SMA, FN and collagen I were examined by RT-qPCR. Data were normalized with GAPDH gene. (C and D) The relative protein expressions of α-SMA, FN and collagen I were detected by western blot. Data were normalized with GAPDH. **P < 0.01 and ***P < 0.001 vs. sham; #P < 0.05 and ##P < 0.01 vs. CLP. N = 6
PE inhibited AKT/NF-κB pathway in CLP-induced mice
To explore the mechanism of PE in the CLP-induced mice, the expression of AKT/NF-κB signaling pathway was examined by western blot. A prominent upregulation in the level of p-AKT/AKT and p-NF-κB/NF-κB was observed in CLP-induced mice, which was markedly declined with the treatment of 10 and 20 mg/kg PE (Fig. 5A). Consistently, the treatment of 10 and 20 mg/kg PE significantly decreased the level of p-AKT/AKT and p-NF-κB/NF-κB in sera from CLP-induced mice (Fig. 5B). Altogether, PE suppressed the expression of AKT/NF-κB pathway in CLP-induced mice.
PE suppressed the expression of AKT/NF-κB signaling pathway in CLP-induced mice. (A) The relative protein expressions of p-AKT, AKT, p-NF-κB and NF-κB in lung tissues were measured by western blot. Data were normalized with GAPDH. (B) The relative protein expressions of p-AKT, AKT, p-NF-κB and NF-κB in sera were determined by western blot. Data were normalized with GAPDH. **P < 0.01 vs. sham; #P < 0.05 and ##P < 0.01 vs. CLP. N = 6
PE alleviated CLP-induced lung injury by inhibiting AKT/NF-κB pathway in mice
To further confirm the direct role of AKT/NF-κB pathway, SC79, the activator of AKT pathway, was introduced into mice. The pathological symptoms in CLP-induced mice were improved by the administration of PE, which were notably abolished by the injection of SC79 (Fig. 6A). Also, the treatment of SC79 markedly restored the PE-reduced the concentrations of IL-1β, IL-6 and TNF-α in CLP-treated mice (Fig. 6B). Besides, the application of SC79 counteracted the alleviative effect of PE on pulmonary fibrosis in CLP-treated mice (Fig. 7A). The PE-reduced the levels of p-AKT/AKT and p-NF-κB/NF-κB in CLP-treated mice were markedly recovered by the treatment of SC79 (Fig. 7B). Taken together, PE improved pathological symptoms, the release of inflammatory cytokines and pulmonary fibrosis by inhibiting AKT/NF-κB pathway in CLP-treated mice (Fig. 8).
SC79 abrogated the mitigatory effect of PE on pathological symptoms and inflammatory response in CLP-induced mice. Mice were randomly divided into sham group, CLP group, CLP + 20 mg/kg PE group and CLP + 20 mg/kg PE + SC79 group. Mice in the sham group were treated with the same surgery except for ligation and puncture, and then orally given with the same amount of DMSO; Mice in the CLP group were administrated with CLP and then orally given with the same amount of DMSO; Mice in the CLP + 20 mg/kg PE group were treated with CLP and then orally given with 20 mg/kg PE; Mice in the CLP + 20 mg/kg PE + SC79 group were treated with CLP, orally given with 20 mg/kg PE and intraperitoneally administrated with 10 mg/kg SC79. (A) The pathological changes were determined by HE staining, and the histological injury scores were exhibited. Scale bar = 50 μm. (B) The concentrations of IL-1β, IL-6 and TNF-α in BALF were measured by ELISA. **P < 0.01 and ***P < 0.001 vs. sham; ##P < 0.01 vs. CLP; &P < 0.01 vs. CLP + 20 mg/kg PE. N = 6
SC79 reversed the effect of PE on pulmonary fibrosis and the expression of AKT/NF-κB signaling pathway in CLP-induced mice. (A) The lung tissues were subjected to MASSON staining. Scale bar = 50 μm. (B) The relative protein expressions of p-AKT, AKT, p-NF-κB and NF-κB in lung tissues were measured by western blot. Data were normalized with GAPDH. **P < 0.01 vs. sham; ##P < 0.01 vs. CLP; &P < 0.01 vs. CLP + 20 mg/kg PE. N = 6
The summary of the present study. PE reduced the CLP-induced lung injury, and the release of inflammatory factors and pulmonary fibrosis at the molecular level. Mechanically, PE inhibited AKT/NF-κB signaling pathway in CLP-induced mice. Activation of AKT/NF-κB signaling pathway abolished the ameliorative effect of PE on CLP-induced mice. Collectively, PE improved inflammation and pulmonary fibrosis by suppressing AKT/NF-κB signaling pathway in CLP-induced mice
Discussion
A mouse model of sepsis-induced lung injury was constructed by CLP, and then the effect and mechanism of PE were addressed on the CLP-induced mice. PE reduced the pathological symptoms and scores, apoptosis and the W/D ratio of lung tissues from CLP-induced mice. At the molecular level, PE attenuated the release of inflammatory factors and pulmonary fibrosis in CLP-induced mice. Mechanically, PE inhibited AKT/NF-κB signaling in CLP-evoked mice. Activation of AKT/NF-κB pathway abolished the ameliorative effect of PE on lung injury, inflammation and pulmonary fibrosis in CLP-treated mice. Collectively, PE improved inflammation and pulmonary fibrosis via suppressing AKT/NF-κB signaling in CLP-induced mice.
Plant extracts are a type of fascinating resource for the development of drug. Sun et al. (Sun et al. 2015) report that total ginsenosides combined with ulinastatin provide protection against sepsis-induced ALI/ARDS in clinical practice. Ali et al. (Ali et al. 2021) reveal that aescin isolated from the horse chestnut (Aesculus hippocastanum) along with coenzyme Q10 prevent sepsis-induced ALI. In the present study, PE reduced the CLP-induced pathological symptoms and scores, and the W/D ratio (an index of edema) in mice. It is clarified that the development of sepsis-induced ALI is associated with an upregulation of inflammatory and apoptotic pathways, which can cause disruption of alveolar epithelial cells, an increase in epithelial permeability and an influx of edema fluid into the alveolar space (Johnson and Matthay 2010). This study found that PE decreased the percent of apoptotic cells in CLP-induced mice. Lung epithelial cell apoptosis has been shown to be essential to the pathogenesis of ALI. Kawasaki et al. (Kawasaki et al. 2000) demonstrate that inhibiting apoptosis in lipopolysaccharide (LPS)-induced ALI mice prominently lowers the degree of ALI and the mortality of mice. Altogether, PE improved lung injury in sepsis-induced ALI.
Inflammatory response is dominant for the development of sepsis-induced ALI. Sepsis causes dysregulation of the alveolar-capillary barrier and alveolar walls, which invites the inflammatory cells to lung tissues, thereby generating superfluous inflammatory mediators, such as IL-1β, IL-6 and TNF-α (Li et al. 2022). Moreover, the cytokines in turn function on leukocytes to activate positive feedback of pro-inflammatory signals (Li et al. 2022). Elevated levels of IL-1 and TNF-α in the serum and BALF are associated with a poor prognosis in patients with ALI (Agouridakis et al. 2002). Affluent studies have demonstrated that suppression the release of inflammatory cytokines benefits to the treatment of sepsis-induced ALI. For instance, 6-Gingerol has demonstrated promise as a treatment for sepsis-induced ALI by lowering lung levels of IL-1β, IL-6 and TNF-α (Pan et al. 2023). Cornus iridoid glycoside reduces the level of IL-1β, IL-6 and TNF-α, which helps to ameliorate sepsis-induced ALI (Tang and Tang 2022). Resveratrol has also exhibited a suppressive effect on the levels of these inflammatory factors in CLP-induced mice (Wang et al. 2021). Similar to these findings, our results revealed that PE suppressed the concentrations of IL-1β, IL-6 and TNF-α in BALF and the protein expressions of IL-1β, IL-6 and TNF-α in CLP-indued mice. Consistently, PE has been elucidated to reduce the concentrations of IL-1β, IL-6 and TNF-α in rheumatoid arthritis (Ma et al. 2019), ulcerative colitis (Liu et al. 2020) and mastitis (Kan et al. 2021). Altogether, PE attenuated the inflammatory response in sepsis-induced ALI.
The overabundance release of inflammatory mediators can further lead to fibrosis during sepsis-induced ALI (Zou et al. 2018). Xue et al. (Xue and Li 2020) reveal that pterostilbene protect rats from sepsis-induced ALI via the JAK2/STAT3 pathway, in which it prominently improves sepsis-induced pulmonary fibrosis. Zou et al. (Zou et al. 2018) report that blocking fibroblast growth factor-inducible 14 (Fn14) significantly reduces the degree of lung fibrosis, which eventually ameliorates the outcomes of sepsis-induced ALI. In this study, PE decreased the pulmonary fibrosis, as well as the protein expressions of fibrosis markers, including α-SMA, FN and collagen I in CLP-induced mice. α-SMA is a recognized protein marker associated with myofibroblasts (Nho et al. 2022), and FN and collagen I are essential extracellular matrix (ECM) proteins that are mostly produced by fibroblasts (Nho et al. 2022). The activation of fibroblast is typically indicated by the dysregulation of α-SMA, FN as well as collagen I (Nho et al. 2022). Altogether, these results demonstrated that PE reduced pulmonary fibrosis in sepsis-induced ALI.
AKT is strongly implicated in the multiple pathogenesis, including apoptosis and inflammation (Manning and Toker 2017). AKT activation can transmit inflammatory signals downward, increase the release of inflammatory factors and upregulate the inflammatory response (Yahfoufi et al. 2018). NF-κB, a significant inflammatory signal pathway, locates the downstream of the AKT signal. The P65 protein translocates into the nucleus and directly contributes to the transcription and translation of inflammatory mediators, including IL-6, IL-1β, and TNF-α, thereby intensifying the inflammatory response (Lawrence 2009). Unremitting NF-κB activation is closely associated with lung injury, where the intensity and duration of NF-κB activity affect the severity of endotoxin-induced ALI (Everhart et al. 2006). It has been shown that AKT regulates the generation of proinflammatory factors by inducing the nuclear translocation of NF-κB in endotoxin-induced ALI (Yum et al. 2001). Research has demonstrated that deactivating the AKT/NF-κB signal can effectively prevent sepsis-induced ALI (Li et al. 2021; Xin et al. 2021). In the present study, the injection of PE significantly counteracted the elevation of the level of p-AKT/AKT and p-NF-κB/NF-κB in CLP-treated mice, suggesting that PE inhibited AKT/NF-κB signaling in CLP-induced mice. It has been discovered that PE suppresses the phosphorylation of the AKT/NF-κB signaling in mastitis (Kan et al. 2021). In this study, the activation of AKT/NF-κB signaling abolished the ameliorative effect of PE on lung injury, inflammation and pulmonary fibrosis in CLP-treated mice. Collectively, PE alleviated inflammation and pulmonary fibrosis by inhibiting AKT/NF-κB pathway in sepsis-induced ALI.
In conclusion, PE improved the pathological symptoms in CLP-induced mice. Molecularly, PE attenuated apoptosis, inflammation and pulmonary fibrosis in CLP-induced mice. Mechanically, PE inhibited the activation of AKT/NF-κB signaling. Therefore, PE ameliorated inflammation and pulmonary fibrosis by suppressing AKT/NF-κB pathway in sepsis-induced ALI. Although SC79 was used for the rescue experiments, the results showed the changes of NF-κB or AKT expression by PE treatment separately, in-depth studies of the relationship between NF-κB and AKT by PE treatment are lacking. Thus, more experiments will be carried out later to prove the relationship between NF-κB and AKT under PE treatment. Briefly, the results elucidate that PE may be a potential agent for the treatment of sepsis-induced ALI.
Data availability
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
References
Agouridakis P, Kyriakou D, Alexandrakis MG, Prekates A, Perisinakis K, Karkavitsas N, Bouros D (2002) The predictive role of serum and bronchoalveolar lavage cytokines and adhesion molecules for acute respiratory distress syndrome development and outcome. Respir Res 3:25
Ali FEM, Ahmed SF, Eltrawy AH, Yousef RS, Ali HS, Mahmoud AR, Abd-Elhamid TH (2021) Pretreatment with Coenzyme Q10 Combined with Aescin protects against Sepsis-Induced Acute Lung Injury. Cells Tissues Organs 210:195–217
Everhart MB, Han W, Sherrill TP, Arutiunov M, Polosukhin VV, Burke JR, Sadikot RT, Christman JW, Yull FE, Blackwell TS (2006) Duration and intensity of NF-kappaB activity determine the severity of endotoxin-induced acute lung injury. J Immunol 176:4995–5005
Hwang JS, Kim KH, Park J, Kim SM, Cho H, Lee Y, Han IO (2019) Glucosamine improves survival in a mouse model of sepsis and attenuates sepsis-induced lung injury and inflammation. J Biol Chem 294:608–622
Jawad I, Lukšić I, Rafnsson SB (2012) Assessing available information on the burden of sepsis: global estimates of incidence, prevalence and mortality. J Glob Health 2:010404
Jing ZT, Liu W, Xue CR, Wu SX, Chen WN, Lin XJ, Lin X (2019) AKT activator SC79 protects hepatocytes from TNF-α-mediated apoptosis and alleviates d-Gal/LPS-induced liver injury. Am J Physiol Gastrointest Liver Physiol 316:G387–g396
Johnson ER, Matthay MA (2010) Acute lung injury: epidemiology, pathogenesis, and treatment. J Aerosol Med Pulm Drug Deliv 23:243–252
Kan X, Hu G, Huang B, Guo W, Huang Y, Chen Y, Xu P, Cai X, Fu S, Liu J (2021) Pedunculoside protects against LPS-induced mastitis in mice by inhibiting inflammation and maintaining the integrity of blood-milk barrier. Aging 13:19460–19474
Kawasaki M, Kuwano K, Hagimoto N, Matsuba T, Kunitake R, Tanaka T, Maeyama T, Hara N (2000) Protection from lethal apoptosis in lipopolysaccharide-induced acute lung injury in mice by a caspase inhibitor. Am J Pathol 157:597–603
Lawrence T (2009) The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb Perspect Biol 1:a001651
Li J, Ma J, Li M, Tao J, Chen J, Yao C, Yao S (2021) GYY4137 alleviates sepsis-induced acute lung injury in mice by inhibiting the PDGFRβ/Akt/NF-κB/NLRP3 pathway. Life Sci 271:119192
Li W, Li D, Chen Y, Abudou H, Wang H, Cai J, Wang Y, Liu Z, Liu Y, Fan H (2022) Classic Signaling Pathways in Alveolar Injury and Repair Involved in Sepsis-Induced ALI/ARDS: New Research Progress and Prospect. Dis Markers 2022: 6362344
Liu C, Shen YJ, Tu QB, Zhao YR, Guo H, Wang J, Zhang L, Shi HW, Sun Y (2018) Pedunculoside, a novel triterpene saponin extracted from Ilex rotunda, ameliorates high-fat diet induced hyperlipidemia in rats. Biomed Pharmacother 101:608–616
Liu K, Li G, Guo W, Zhang J (2020) The protective effect and mechanism of pedunculoside on DSS (dextran sulfate sodium) induced ulcerative colitis in mice. Int Immunopharmacol 88:107017
Ma X, Chen G, Wang J, Xu J, Zhao F, Hu M, Xu Z, Yang B, Guo J, Sun S, Liu M (2019) Pedunculoside attenuates pathological phenotypes of fibroblast-like synoviocytes and protects against collagen-induced arthritis. Scand J Rheumatol 48:383–392
Manning BD, Toker A (2017) AKT/PKB signaling: navigating the network. Cell 169:381–405
Nho RS, Ballinger MN, Rojas MM, Ghadiali SN, Horowitz JC (2022) Biomechanical Force and Cellular Stiffness in Lung Fibrosis. Am J Pathol 192:750–761
Pan Q, Liu P, Wan M (2023) 6-Gingerol attenuates sepsis-induced acute lung injury by suppressing NLRP3 inflammasome through Nrf2 activation. Folia Histochem Cytobiol
Qu M, Chen Z, Qiu Z, Nan K, Wang Y, Shi Y, Shao Y, Zhong Z, Zhu S, Guo K, Chen W, Lu X, Wang Z, Zhang H, Miao C (2022) Neutrophil extracellular traps-triggered impaired autophagic flux via METTL3 underlies sepsis-associated acute lung injury. Cell Death Discov 8:375
Sadowitz B, Roy S, Gatto LA, Habashi N, Nieman G (2011) Lung injury induced by sepsis: lessons learned from large animal models and future directions for treatment. Expert Rev Anti Infect Ther 9:1169–1178
Sang A, Wang Y, Wang S, Wang Q, Wang X, Li X, Song X (2022) Quercetin attenuates sepsis-induced acute lung injury via suppressing oxidative stress-mediated ER stress through activation of SIRT1/AMPK pathways. Cell Signal 96:110363
Schäcke H, Döcke WD, Asadullah K (2002) Mechanisms involved in the side effects of glucocorticoids. Pharmacol Ther 96:23–43
Shashaty MGS, Reilly JP, Faust HE, Forker CM, Ittner CAG, Zhang PX, Hotz MJ, Fitzgerald D, Yang W, Anderson BJ, Holena DN, Lanken PN, Christie JD, Meyer NJ, Mangalmurti NS (2019) Plasma receptor interacting protein kinase-3 levels are associated with acute respiratory distress syndrome in sepsis and trauma: a cohort study. Crit Care 23:235
Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, Hotchkiss RS, Levy MM, Marshall JC, Martin GS, Opal SM, Rubenfeld GD, van der Poll T, Vincent JL, Angus DC (2016) The Third International Consensus definitions for Sepsis and septic shock (Sepsis-3). JAMA 315:801–810
Sun R, Li Y, Chen W, Zhang F, Li T (2015) Total ginsenosides synergize with ulinastatin against septic acute lung injury and acute respiratory distress syndrome. Int J Clin Exp Pathol 8:7385–7390
Tang X, Tang H (2022) Cornus iridoid glycoside alleviates sepsis-induced acute lung injury by regulating NF-κB and Nrf2/HO-1 pathways. Allergol Immunopathol (Madr) 50:121–128
Walker BR (2007) Glucocorticoids and cardiovascular disease. Eur J Endocrinol 157:545–559
Wang C, Chao Z, Sun W, Wu X, Ito Y (2014) Enrichment and purification of pedunculoside and syringin from the barks of Ilex rotunda with macroporous resins. J Liq Chromatogr Relat Technol 37:572–587
Wang YM, Ji R, Chen WW, Huang SW, Zheng YJ, Yang ZT, Qu HP, Chen H, Mao EQ, Chen Y, Chen EZ (2019) Paclitaxel alleviated sepsis-induced acute lung injury by activating MUC1 and suppressing TLR-4/NF-κB pathway. Drug Des Devel Ther 13:3391–3404
Wang C, Yuan J, Du J (2021) Resveratrol alleviates acute lung injury through regulating PLSCR-3-mediated mitochondrial dysfunction and mitophagy in a cecal ligation and puncture model. Eur J Pharmacol 913:174643
Wu L, Kang A, Shan C, Chai C, Zhou Z, Lin Y, Bian Y (2019) LC-Q-TOF/MS-oriented systemic metabolism study of pedunculoside with in vitro and in vivo biotransformation. J Pharm Biomed Anal 175:112762
Xin Y, Zou L, Lang S (2021) 4-Octyl itaconate (4-OI) attenuates lipopolysaccharide-induced acute lung injury by suppressing PI3K/Akt/NF-κB signaling pathways in mice. Exp Ther Med 21:141
Xiong S, Hong Z, Huang LS, Tsukasaki Y, Nepal S, Di A, Zhong M, Wu W, Ye Z, Gao X, Rao GN, Mehta D, Rehman J, Malik AB (2020) IL-1β suppression of VE-cadherin transcription underlies sepsis-induced inflammatory lung injury. J Clin Invest 130:3684–3698
Xue H, Li M (2020) Protective effect of pterostilbene on sepsis-induced acute lung injury in a rat model via the JAK2/STAT3 pathway. Ann Transl Med 8:1452
Yahfoufi N, Alsadi N, Jambi M, Matar C (2018) The Immunomodulatory and Anti-Inflammatory Role of Polyphenols. Nutrients 10
Yang B, Li H, Ruan Q, Xuan S, Chen X, Cui H, Liu Z, Jin J, Zhao Z (2019) Effects of Gut Microbiota and Ingredient-Ingredient Interaction on the Pharmacokinetic properties of Rotundic Acid and Pedunculoside. Planta Med 85:729–737
Yum HK, Arcaroli J, Kupfner J, Shenkar R, Penninger JM, Sasaki T, Yang KY, Park JS, Abraham E (2001) Involvement of phosphoinositide 3-kinases in neutrophil activation and the development of acute lung injury. J Immunol 167:6601–6608
Zou Y, Bao S, Wang F, Guo L, Zhu J, Wang J, Deng X, Li J (2018) FN14 blockade on pulmonary microvascular endothelial cells improves the outcome of Sepsis-Induced Acute Lung Injury. Shock 49:213–220
Acknowledgements
Not applicable.
Author information
Authors and Affiliations
Contributions
Conceptualization: Xiangbo Li and Qiumei Cao; Methodology: Xiangbo Li and Ruiming Xu; Formal analysis: Xiangbo Li and Kaiguo Zhou; Investigation: Xiangbo Li, Ruiming Xu and Kaiguo Zhou; Writing - original draft: Xiangbo Li; Writing - review & editing: Qiumei Cao; Funding Acquistion: Qiumei Cao; Supervision: Qiumei Cao.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare there are no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, X., Xu, R., Zhou, K. et al. Ameliorative effect of pedunculoside on sepsis-induced acute lung injury, inflammation and pulmonary fibrosis in mice model via suppressing AKT/NF-κB pathway. J Mol Histol (2024). https://doi.org/10.1007/s10735-024-10222-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10735-024-10222-4