Abstract
In the present study, the effect of inosine was evaluated on learning and memory of 18 months old aged female rats. Inosine (50, 100 and 200 mg/kg; i.p.) was administered to separate groups of rats for 15 successive days. Donepezil (1 mg/kg; i.p.), an acetylcholinesterase inhibitor, was used as a standard drug. Behavioral models such as Morris water maze and elevated plus maze were used to evaluate the effect of drugs on learning and memory of rats. After behavioral studies, animals were killed and their brain was isolated and further processed for estimation of various biochemical parameters such as acetylcholinesterase activity, oxidative stress markers, proinflammatory marker and histological examinations. Inosine (100 and 200 mg/kg) significantly improved learning and memory of aged rats. Further, inosine significantly reduced lipid peroxidation and nitrite, and increased the levels of reduced glutathione and superoxide dismutase. However, no significant difference in AChEs activity was observed in inosine-treated rats as compared to aged control rats. TNF-α level was found to be ameliorated in aged rats by inosine. Histopathological evaluation showed that inosine-treated aged rats have less number of pyknotic neurons in hippocampal CA1 region as compared to aged control rats. In conclusion, inosine significantly improved learning and memory of aged female rats possibly through its antioxidant as well as anti-inflammatory effect and improvement of neuronal survival in the hippocampal CA1 region. However, additional studies are required to further explore the downstream signaling pathways involved in the neuroprotective effect of inosine in aged animals.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aging is a natural phenomenon that can be explained as a progressive decline in efficacy of physiological functions of the body organs (Lopez-Otin et al. 2013). The life span of human beings has been significantly increased in the 21st century due to advances in therapeutics and medicines. As the population is aging, the incidence of age-related neurodegenerative diseases, such as Alzheimer’s disease, is growing. According to WHO, around 50 million people have dementia worldwide, and there are nearly 10 million new cases every year. The main cause of dementia is Alzheimer’s disease which contributes to about 60–70% of total cases of dementia (WHO 2017). In the aged population, there is a decrease in levels of certain hormones (estrogen in females and testosterone in males) which may lead to cognitive dysfunction in aged women and men (Harburger et al. 2007; Sandstrom et al. 2006). Recently, it has been reported that women have more prevalence rate of Alzheimer’s disease and other dementia than men. Almost two-thirds of Americans with Alzheimer’s are women. Of the 5.3 million people aged 65 and older with Alzheimer’s in the United States, 3.3 million are women and 2.0 million are men (Alzheimer’s Association, 2017). Rats display similar age-related disturbances in spatial memory and, therefore, aged rats may be used as a model for the cognitive deficits associated with aging in humans (van Groen et al. 2002).
Aging of brain adversely affects cognitive functions (van Groen et al. 2002), leading to maladaptation of synaptic processes resulting in aberrant perception, cognitive dysfunction and neurodegeneration. The reason behind maladaptation of synaptic function is imbalance between the generation of reactive oxygen species and antioxidant enzyme activity in neurons (Fukui et al., 2001). Further, it is well known that the brain is highly susceptible to reactive oxygen species because of the following reasons, (1) it is rich in fatty acids, which are sensible to peroxidation; (2) it does not have a powerful antioxidant defence system; (3) it has large number of mitochondria; (4) it consumes a lot of oxygen; therefore, it is exposed to oxy-radicals accumulation (Freeman and Keller 2012; Uttara et al. 2009).
In addition to the free radical theory of aging, the neuroinflammatory theory has also gained the interest of neuroscientists (Barrientos et al. 2015; Chung et al. 2006), whereby the activation of redox-sensitive transcriptional factors by age-related oxidative stress causes the upregulation of proinflammatory gene expression. The mobility of reactive microglia towards the degenerative areas is the crux of neuroinflammation (David et al. 1997). In general, migration of microglia towards the site of injury is a part of an innate immune response that usually helps to resolve potential pathogenic conditions (Lull and Block 2010). However, continuous noxious stimuli result in the transformation of quiescent microglia to reactive phenotype which further leads to the production of intracerebral neurotoxic molecules like superoxide free radicals and pro-inflammatory cytokines such as IL-1β, IL-6, TNF-α (Lombardi et al. 1999), thereby causing chronic neuroinflammation that may consequence into development of neurodegenerative disorders such as AD in old age (Blasko et al. 2004).
With this background, the current hypothesis was aimed to identify a compound with an approach of multiple targets that will help to protect the brain from the devastating cellular cascades that operate in aging brain. Further, safety profile of the compound has also been taken into consideration, as most of the drugs show toxicity at chronic administration. Inosine, a breakdown product of adenosine, is normally present in humans and has good safety and tolerability profile even at high doses (Kaster et al. 2013; Starling et al. 1996). It has been reported to possess antidepressant (Kaster et al. 2013), immunomodulatory and neuroprotective action (Hasko et al. 2004). Inosine has also been shown to have an axonal regeneration effect either by mimicking or augmenting the effects of nerve growth factor (Benowitz et al. 2002). In addition, it has been reported to have neuroprotective effect in various neurological diseases including multiple sclerosis (Markowitz et al. 2009), stroke (Chen et al. 2002), Parkinson’s disease (Cipriani et al. 2014) and focal brain injuries (Smith et al. 2007). Further, inosine has been reported to have some enhancing effect on learning and memory of aged rats and mice. In middle aged and young animals, inosine inhibited learning (Gordon 1974). In another study, inosine suppressed spatial memory deficits of irradiated mice (Hou et al. 2007). But the effect of inosine on aging-induced cognitive dysfunction in female rats has not been reported yet. Moreover, mechanisms for effect of inosine on learning and memory of aged female rats have also not been reported. Therefore, in the present study, the effect of inosine was studied in a spontaneous model of dementia using aged female rats.
Materials and methods
Animals
Wistar female young rats (3 months old) and female aged rats (18 months old) were procured from Disease Free Small Animal House, Lala Lajpat Rai University of Veterinary and Animal Sciences, Hisar (Haryana, India). The animals were maintained under standard laboratory conditions with alternating light and dark cycles of 12 h each and had free access to food and water. Food given to rats consisted of freshly cooked dalia (broken wheat). The animals were acclimatized for at least 5 days before behavioral experiments. Experiments were carried out between 09:00 and 17:00 h. The experimental protocol was approved by the Institutional Animals Ethics Committee (IAEC/2016/10-17; 5-09-2016) and care of laboratory animals was taken as per guidelines of Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Ministry of Environment, Forests and Climate Change, Govt. of India, New Delhi.
Drugs
Inosine was purchased from Sigma-Aldrich (St. Louis, MO, USA). Donepezil was obtained as a gift sample from Ranbaxy Laboratories Pvt. Ltd., Gurgaon (Haryana), India. Inosine and donepezil were dissolved in normal saline and administered intraperitoneally.
Experimental protocol
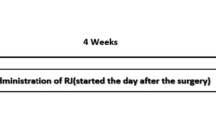
In this study, there were 12 groups of rats and each group comprised a minimum of eight animals. The time schedule for entire experimental protocol has been depicted in Fig. 1. The details of experimental groups are as follows:
Time schedule for administration of vehicle, donepezil, and inosine in aged rats. The young and aged control groups received vehicle only. In drug-treated groups, donepezil and inosine were administered daily for a period of 15 days. The behavioral observations were started from day 11 onward, and the animals were killed after the last behavioral test on day 15. The hippocampus and frontal cortex of rat brain were isolated to carry out biochemical, molecular and histopathological examinations. EPM elevated plus maze, H&E hematoxylin and eosin, MWM Morris water maze
Groups for elevated plus maze
Group I (Control group for young rats): Vehicle (Normal Saline) was administered i.p. to young rats for 15 consecutive days. Transfer latency (TL) was measured 30 min after the injection of normal saline on 14th day and 15th day.
Group II: (Control group for aged rats): Vehicle (Normal Saline) was administered i.p. to aged rats for 15 consecutive days. TL was measured 30 min after the injection of saline on 14th day and 15th day.
Group III: Donepezil (1 mg/kg; i.p.) was administered to aged rats for 15 consecutive days. TL was measured 30 min after the injection of donepezil on 14th day and 15th day.
Groups IV, V, VI: Inosine (50, 100 and 200 mg/kg; i.p., respectively) was administered to aged rats for 15 consecutive days. TL was measured 30 min after the injection of inosine on 14th day and 15th day.
After behavioral testing on elevated plus maze, animals were killed by cervical dislocation and their brain was isolated for biochemical (malondialdehyde, nitrite, GSH, SOD and acetylcholinesterase activity) and histopathological studies. Doses and route of administration of inosine and donepezil were selected on the basis of the previous literature (Dachir et al. 2014; Sonkusare et al. 2005).
Groups for Morris water maze
Group VII (Control group for young rats): Normal saline was administered i.p. to young rats for 15 consecutive days. Escape latency (EL) was measured on 11th to 14th day and time spent in target quadrant (TSTQ) was measured on 15th day.
Group VIII (Control group for aged rats): Normal saline was administered i.p. to aged rats for 15 consecutive days. EL was measured on 11th to 14th day and TSTQ was measured on 15th day.
Group IX: Donepezil (1 mg/kg; i.p.) was administered to aged rats for 15 consecutive days. EL was measured on 11th to 14th day and TSTQ was measured on 15th day.
Groups X, XI and XII: Inosine (50, 100 and 200 mg/kg; i.p., respectively) was administered to aged rats for 15 consecutive days. EL was measured on 11th to 14th day and TSTQ was measured on 15th day.
After measuring TSTQ in Morris water maze on 15th day, animals were tested for locomotor activity using actophotometer. After testing of locomotor activity, animals were killed and their brain was isolated for estimation of proinflammatory cytokines, i.e., TNF-α using ELISA.
Behavioral assessments
Elevated plus maze and Morris water maze were used as behavioral models to evaluate effect of drugs on learning and memory of rats. The details of these models are as follows:
Elevated plus-maze
Elevated plus-maze consisted of two opposite open arms (50 × 10 cm), crossed with two closed arms of the same dimensions with 40 cm high walls (Sharma and Kulkarni 1992). The arms were connected with central square (10 × 10 cm) and the entire maze was elevated to a height of 50 cm from the floor. Each rat was placed at the end of one of the open arms, facing outwards. The time taken by the animal with all its four paws in the closed arm on 14th day (acquisition trial) was noted and was called as initial transfer latency (ITL). Cutoff time was fixed at 90 s, and in case a rat could not find the closed arm within this period, it was gently pushed into one of the closed arms and allowed to explore the maze for another 30 s. The second trial (retention trial) was performed 24 h after the acquisition trial (day 15) and retention transfer latency (RTL) was noted.
Morris water maze performance task
The acquisition and retention of a spatial navigation task were examined using a Morris water maze (Kumar et al. 2012; Morris 1984). Water maze consists of a cylindrical pool (180 cm in diameter and 50 cm in height) filled with water maintained at approximately 28 ± 1 °C and measuring 30 cm deep. Water was made opaque using non-toxic and non-irritant dye (titanium dioxide). The tank was divided into four equal quadrants (Q1–4), and a submerged platform (10 × 10 cm2) was placed 2 cm below the surface of the water in the middle of the target quadrant (north Q1). The position of the platform was kept unaltered throughout the training session. The water maze was also kept in the same position throughout the study. During testing, the investigator wearing a white lab coat stood at the west edge of the pool.
Acquisition test (learning)
All the rats underwent training over four consecutive days, starting from day 11 of drug treatment, and consisting of 4 swimming trials per day, each at an interval of 30 min approximately. Each animal was subjected to training trials for 4 consecutive days, the starting position was changed with each exposure as mentioned below and target quadrant (Q1) remained constant throughout the training period:
Day 1 Q1 Q2 Q3 Q4.
Day 2 Q2 Q3 Q4 Q1.
Day 3 Q3 Q4 Q1 Q2.
Day 4 Q4 Q1 Q2 Q3.
For each trial, the rat was placed at the edge of the pool in the centre of the appropriate quadrant, facing the wall of water maze, and latency to find the platform was recorded. Cutoff time for finding the platform was kept 90 s. If the rat could not find the submerged platform in 90 s, then the animal was gently placed on it and allowed to stay there for the next 20 s. Escape latency time (ELT) to locate the hidden platform in water maze was noted as an index of acquisition or learning.
Retention test (memory)
Following training for 4 days, a retention test was performed on day 5 (day 15 of drug treatment). The platform was removed from water maze. Each rat was placed in the quadrant (Q3) opposite to the target quadrant (Q1) and allowed to explore the target quadrant for 90 s. Time spent in target quadrant (TSTQ) in search of the missing platform was noted which indicated index of retrieval or retention.
Assessment of locomotor activity
Immediately after recording TSTQ on 15th day, the locomotor activity (horizontal activity) of animals was assessed using actophotometer (INCO, Ambala, India). This instrument operates on photoelectric cells which are connected in circuit with a counter. When the beam of light falling on the photocell is cut off by the animal, a count is recorded. Each animal was placed in the actophotometer for a period of 5 min and locomotor counts were recorded (Kulkarni and Dhir 2008). The apparatus was placed in a sound attenuated and ventilated room.
Biochemical assessments
Following behavioral assessments, the animals were killed by cervical dislocation and their brain was isolated. Frontal cortex and hippocampus were separated and then weighed. Tissue homogenates 10% (w/v) of both frontal cortex and hippocampus were prepared in 0.1 M phosphate buffer (pH 7.4). The homogenates were centrifuged at 10,000×g at 4 °C for 15 min. Aliquots of supernatants were separated and used for biochemical estimations. UV–Vis spectrophotometer (SPECTROstar® Nano, Ortenberg, Germany) was used as an instrument for various biochemical estimations. ELISA plate reader (Spectramax Plus, Molecular Device, USA) was used for proinflammatory TNF-α measurement.
Estimation of acetylcholinesterase activity
Acetylcholinesterase (AChE) activity was assessed in the hippocampal and cortical regions by the method of Ellman et al. (1961). The assay mixture contained 50 μl of tissue homogenate, 3 ml of sodium phosphate buffer (pH 8.0), 100 μl of acetylthiocholine iodide, and 100 μl of 0.01 M 5,5′ dithio-bis-2-nitro benzoic acid (DTNB, Ellman reagent). The change in absorbance was measured for 2 min at 30 s interval at 412 nm using a UV–Vis spectrophotometer. Results were expressed as μM of acetylthiocholine iodide hydrolyzed per min per mg of protein.
Measurement of lipid peroxidation
The quantitative measurement of lipid peroxidation (LPO) was carried out according to the method as described by Wills (1966) with slight modifications. In brief, 0.5 ml of supernatant from tissue homogenate was incubated with 0.5 ml of Tris HCl for 2 h at 37 °C. After the incubation, it was treated with 1 ml of ice-cold trichloroacetic acid (10% w/v) reagent followed by addition of 1 ml thiobarbituric acid (0.67% w/v) and placed in boiling water bath for 15 min, cooled, centrifuged and then clear supernatant was removed. The absorbance of supernatant was measured at 535 nm against blank using UV–vis spectrophotometer. The values were calculated using molar extinction coefficient of chromophore (1.56 × 105 M−1 cm−1).
Estimation of reduced glutathione
Reduced glutathione (GSH) was estimated according to the method described by Ellman (1959). A 1.0 ml of homogenate was precipitated with 1.0 ml of 4% w/v sulfosalicylic acid by keeping the mixture at 4 °C for 1 h, and the samples were immediately centrifuged at 1200×g for 15 min at 4 °C. The assay mixture contained 1.0 ml of supernatant, 2.0 ml of phosphate buffer (0.1 M, pH 8.0), and 0.2 ml of 0.01 M DTNB. The yellow color developed was read immediately at 412 nm using a UV–Vis spectrophotometer. Results were calculated using the molar extinction coefficient of chromophore (1.36 × 104 M−1cm−1) and expressed as nM of GSH per mg of protein.
Estimation of superoxide dismutase activity
Superoxide dismutase (SOD) activity was assayed according to the method of Kono (1978), wherein the reduction of nitro blue tetrazolium was inhibited by SOD and measured at 560 nm using a UV–Vis spectrophotometer. In brief, the reaction was initiated by the addition of the 500 μl of hydroxylamine hydrochloride to the assay mixture containing 2 ml nitroblue tetrazolium and 100 μl tissue homogenate sample. The results were expressed as units/mg protein where one unit of enzyme is defined as the amount of enzyme inhibiting the rate of reaction by 100 percent.
Estimation of nitrite
Accumulation of nitrite, an indicator of the production of nitric oxide (NO), was determined with a colorimetric assay with Greiss reagent as described by Green et al. (1982). In brief, 500 μl of supernatant and 500 μl of Greiss reagent (250 µl of 1.0% w/v sulfanilamide and 250 µl of 0.1% w/v N-naphthylethylenediamine) were mixed, and the mixture was incubated in the dark for 10 min at room temperature. Absorbance was recorded at 546 nm with a UV–Vis spectrophotometer. The concentration of nitrite in the supernatant was determined from a sodium nitrite standard curve.
Protein estimation
Protein estimation was done by biuret method using bovine serum albumin as standard (Gornall et al. 1949).
Estimation of pro-inflammatory marker
TNF-α measurement was done using rat TNF-α immunoassay kits (Quantikine® Elisa; R&D Systems, Minneapolis, USA) in hippocampus and frontal cortex regions of brain. The assays employ the sandwich enzyme immunoassay technique. The intensity of the color developed was measured with the help of an ELISA reader (Spectramax Plus, Molecular Device, USA) and the sample values were then read from the standard curve.
Assessment of histological changes
After behavioral testing on elevated plus maze, animals were killed by cervical dislocation and their brain was isolated for histopathological studies. The brains were rapidly removed and fixed by immersion in formalin (10%v/v). The brain tissues were cut into 3 mm thickness and its blocks were embedded in paraffin. The brain sections (4 μm thick) were prepared and stained with hemotoxylin and eosin stain. Furthermore, hippocampal CA1 region of the brain was examined under bright field illumination using AHBT-51 microscope (Olympus Vanox Research Microscope, Japan) and photographed (Kim et al. 2014).
Statistical analysis
Graph Pad Prism (Graph Pad Software, San Diego, CA, USA) was used for all statistical analysis. The results are expressed as mean ± SEM. Based on the number of variables in each parameter, one-way ANOVA followed by Tukey’s multiple comparison test was used to analyze the mean of the data of locomotor activity, time spent in target quadrant (retention) of Morris water maze, transfer latency in elevated plus maze and biochemical parameters. Two-way ANOVA followed by Bonferroni’s post hoc test was used to analyze the mean of the data of escape latency (acquisition) in Morris water maze, since in these data, we have simultaneously analyzed the effect of time (days 11–14) and treatment on escape latency of rats. In all tests, p < 0.05 was considered as statistically significant.
Results
Effect of inosine on transfer latencies of rats using elevated plus maze
In the present study, there was no significant change in TL on day 14 for all the groups irrespective of the treatment given. On 15th day (that is, 24 h after first exposure to elevated plus maze), there was significant increase in TL by aged rats as compared to young rats, indicating significant impairment of memory. Inosine (200 mg/kg) significantly decreased TL on day 15 (retention trial) in aged rats as compared to aged control animals, indicating significant improvement of memory. However, the lowest dose (50 mg/kg) and middle dose (100 mg/kg) of inosine did not significantly decrease TL during retention trial on 15th day in aged rats, indicating non-significant effect of these doses of inosine on memory of aged rats. Memory improving effect of inosine (200 mg/kg) was comparable to donepezil (Fig. 2).
Effect of inosine on transfer latency of rats in elevated plus maze. N = 8 each group. Data are in mean ± SEM. Data were analyzed by one-way ANOVA followed by Tukey’s test for multiple comparisons **p < 0.01 versus young control, #p < 0.05 versus aged control, INO inosine. For ITL (14th day): F(5, 42) = 0.6286; p = 0.6788. For RTL (15th day): F(5,42) = 4.634; p = 0.0019
Effect of inosine on escape latency and time spent in target quadrant by rats using Morris water maze
Effect on acquisition (day 11–14): the acquisition data showed that EL of the aged control animals increased significantly on 13th and 14th day of drug treatment (3rd and 4th day of training session) as compared to young control rats, indicating aging-induced significant impairment of learning. Inosine (200 mg/kg) and donepezil (1 mg/kg) per se significantly decreased EL in aged rats on 12th, 13th and 14th days (2nd, 3rd and 4th days of training session) as compared to young rats, indicating improvement of learning in aged rats. The lowest dose (50 mg/kg) and middle dose (100 mg/kg) of inosine did not significantly affect EL by aged rats during training trials (from 11th day to 14th day), indicating non-significant effect of these doses of inosine on learning of aged rats (Fig. 3a).
Effect of inosine on escape latency (a) and time spent in target quadrant (b) by rats in Morris water maze test. N = 8 each group. Data are in mean ± SEM. Acquisition data (escape latency in a), as plotted in line diagram, were analyzed by two-way ANOVA followed by Bonferroni’s post hoc test for multiple comparisons. Retention data (time spent in target quadrant in b) in probe trial was analyzed by one-way ANOVA followed by Tukey’s test for multiple comparisons. **p < 0.01 versus young control, ***p < 0.001 versus young control, #p < 0.05 versus aged control, ##p < 0.01 versus aged control, ###p < 0.001 versus aged control, INO: inosine. Interaction (time × treatment) for escape latency. Time; F(3,108) = 106.6; p < 0.0001. Treatment; F(5,108) = 12.21; p < 0.0001. F(5, 41) = 7.521; p < 0.0001 for TSTQ
Effect on retention (day 15): In the retention trial on day 15, when the platform was removed, aged control group failed to recall the precise location of the platform as evident from significantly less time spent in target quadrant as compared to young control rats, indicating cognitive impairment in aged rats. Inosine (200 mg/kg) and donepezil (1 mg/kg) per se treatment in aged animals significantly increased time spent in target quadrant as compared to vehicle-treated aged rats, indicating improvement of memory. The lowest dose (50 mg/kg) and middle dose (100 mg/kg) of inosine did not significantly affect TSTQ by aged rats on 15th day, indicating non-significant effect of these doses of inosine on memory of aged rats. (Figure 3b).
Effect of inosine on locomotor activity of rats
Spontaneous locomotor activity did not differ significantly among all the treatment groups as assessed on the last day of treatment, i.e., day 15 (Fig. 4), suggesting that inosine did not affect locomotor activity of rats.
Effect of inosine on brain acetylcholinesterase activity
The AChE activity in hippocampus and frontal cortex of aged control animals was found to be reduced significantly (p < 0.001 and 0.001 respectively) as compared to young control animals. Inosine (50, 100 and 200 mg/kg) did not show any significant effect on AChE activity in aged rats as compared to vehicle-treated aged rats. However, donepezil significantly decreased brain acetylcholinesterase activity in aged rats as compared to vehicle-treated aged rats (Fig. 5).
Effect of inosine on brain acetylcholinesterase activity in aged rats. N = 8 each group. Data are in mean ± SEM. Data were analyzed by one-way ANOVA followed by Tukey’s test for multiple comparisons. ***p < 0.001 versus young control, #p < 0.05 versus aged control, ##p < 0.01 versus aged control, INO: inosine. F(5,36) = 21.17; p < 0.0001 (Hippocampus). F(5,36) = 17.40; p < 0.0001 (Cortex)
Effect of inosine on brain malondialdehyde, GSH and SOD
Malondialdehyde (MDA) levels increased significantly in both hippocampus and frontal cortex of vehicle-treated aged rats as compared to vehicle-treated young rats (Fig. 6a). Treatment with donepezil (p < 0.001) significantly reduced elevated MDA level in both hippocampus and frontal cortex of aged rats as compared to vehicle-treated aged rats. Inosine (100 mg/kg) significantly reduced MDA levels in both frontal cortex (p < 0.01) and hippocampus (p < 0.05) as compared to aged control rats. Highest dose (200 mg/kg) of inosine significantly decreased MDA levels in frontal cortex only (Fig. 6a).
Effect of inosine on brain a lipid peroxidation, b reduced glutathione and c superoxide dismutase levels in aged rats. N = 8 each group. Data are in mean ± SEM. Data were analyzed by one-way ANOVA followed by Tukey’s test for multiple comparisons. ***p < 0.001 versus young control, #p < 0.05 versus aged control, ##p < 0.01 versus aged control, ###p < 0.001 versus aged control, INO: inosine. For lipid peroxidation: F(5,36) = 8.271; p < 0.0001 (Hippocampus). F(5,36) = 11.42; p < 0.0001 (Cortex). For reduced glutathione: F(5,36) = 11.01; p < 0.0001 (Hippocampus). F(5,36) = 8.739; p < 0.0001 (Cortex). For superoxide dismutase: F(5,36) = 14.42; p < 0.0001 (Hippocampus). F(5,36) = 20.64; p < 0.0001 (Cortex)
Reduced glutathione (GSH) levels and SOD activity decreased significantly (p < 0.001) in both hippocampus and frontal cortex of aged rats as compared to vehicle-treated young rats. Donepezil significantly (p < 0.001) increased GSH levels as compared to vehicle-treated aged rats. Inosine (100 mg/kg) significantly (p < 0.05) reversed aging-induced decrease in GSH levels in hippocampus only. Inosine (100 mg/kg) significantly restored the decreased SOD activity in both hippocampus (p < 0.05) and frontal cortex (p < 0.05) of aged rats as compared to aged control rats. Increase in SOD activity by the highest dose (200 mg/kg) of inosine was better in both hippocampus (p < 0.01) and in frontal cortex (p < 0.001) as compared to lower dose (100 mg/kg) of inosine (Fig. 6b, c).
Effect of inosine on brain nitrite levels
Nitrite levels were found to be significantly increased in both hippocampus (p < 0.001) and frontal cortex (p < 0.01) of aged rats as compared to vehicle-treated young rats. Donepezil significantly (p < 0.001) decreased nitrite levels in both hippocampus and frontal cortex as compared to aged control rats. Inosine (100 mg/kg) significantly (p < 0.01) decreased nitrite level in hippocampus only. But the highest dose (200 mg/kg) of inosine significantly reduced nitrite levels in both hippocampus (p < 0.01) and frontal cortex (p < 0.05). The lowest dose (50 mg/kg) of inosine failed to show any significant effect on nitrite levels in aged rats (Fig. 7).
Effect of inosine on brain nitrite levels in aged rats. N = 8 each group. Data are in mean ± SEM. Data were analyzed by one-way ANOVA followed by Tukey’s test for multiple comparisons. **p < 0.01 versus young control, ***p < 0.001 versus young control, #p < 0.05 versus aged control, ##p < 0.01 versus aged control, ###p < 0.001 versus aged control, INO: inosine. F(5,36) = 12.35; p < 0.0001 (Hippocampus). F(5,36) = 7.376; p < 0.0001 (Cortex)
Effect of inosine on expression of proinflammatory cytokines in aged rats
The expression of TNF-α was found to be significantly high in tissue homogenate of hippocampus and frontal cortex of aged control rats as compared to vehicle-treated young rats (p < 00.001). Further, the expression of TNF-α was significantly (p < 00.001) decreased by inosine (200 mg/kg) in aged rats as compared to aged control rats (Fig. 8).
Effect of inosine on brain TNF-α levels in aged rats. N = 6 each group. Data are in mean ± SEM. Data were analyzed by one-way ANOVA followed by Tukey’s test for multiple comparisons. ***p < 0.001 versus young control, ###p < 0.001 versus aged control, INO: inosine. F(5,30) = 51.25; p < 0.0001 (Hippocampus). F(5,30) = 14.42; p < 0.0001 (Cortex)
Effect of inosine on neurons in hippocampal CA1 region in aged rats
Microscopic histopathological analysis showed that hippocampal CA1 neurons in young control (Fig. 9a) were healthy with robust and oval shape and arranged linearly. On the other hand, photomicrographs from hippocampus CA1 region of aged control rat brain showed a significant loss of neurons as compared to young control group (Fig. 9b). Further, microscopic examination revealed that neurons in the aged control were large and sparsely arranged. Few of the degenerated cells were sickle shaped or with an altered morphology. Donepezil (1 mg/kg; Fig. 9c) significantly decreased the neuronal loss in the hippocampus of aged rats. Inosine (50 mg/kg) failed to increase the number of healthy neurons in aged rats (Fig. 9d). However, higher doses (100 and 200 mg/kg) of inosine showed more number of healthy neurons with oval shape and clear cytoplasm as compared to aged control rats indicating their protective action (Fig. 9e, f).
Effect of inosine on histopathological changes in the CA-1 region of hippocampus of aged rats. a–f Photomicrographs (10X) of H&E stained brain hippocampus CA1 sections. a Vehicle-treated young control group showing healthy neurons; b aged control group showing dark stained degenerated neurons; c donepezil (1 mg/kg, i.p.) showing neuroprotection against aging-induced neurodegeneration; d INO (50 mg/kg; i.p.) did not show any protective effect in aging-induced neurodegeneration; e INO (100 mg/kg, i.p.) showing protection against aging-induced neurodegeneration; f INO (200 mg/kg, i.p.) showing more protection against aging-induced neurodegeneration; INO inosine. Calibration bar = 100 μm
Discussion
In the present study, the effect of inosine on various behavioral, biochemical, neuroinflammatory, and histological changes was evaluated in aged female rats. The aged rats showed significant impairment of learning and memory, increased oxidative stress and pro-inflammatory TNF-α level, and also induced histopathological changes in both hippocampus and frontal cortex of rat brain. Further, these abnormalities were significantly attenuated by treatment with inosine.
Elevated plus maze and Morris water maze were used as behavioral models to assess the effect of inosine on learning and memory of rats. These two behavioral models are widely used to evaluate the effect of drugs on learning and memory of rats (Dhull et al. 2012; Kumar et al. 2016). Inosine administered for 15 successive days significantly improved learning and memory of aged female rats as compared to vehicle-treated aged rats. There was no significant effect of inosine and donepezil on locomotor activity of aged rats, indicating that memory improving effect of inosine is independent of locomotor activity of rats.
Impairment of learning and memory in aged female rats might be due to decrease in estrogen level (Gibbs 2000). Aging process increases the free radicals load that further leads to lipid peroxidation, protein carbonylation, DNA acetylation followed by degeneration of neuronal cells (Sultana et al. 2006). Due to short lifespan, the direct detection of reactive oxygen species is difficult and, hence, the amount is often judged from the alteration of antioxidant status and the accumulation of relatively stable products of lipid peroxidation, i.e., malondialdehyde (Radak et al. 2011). The current study showed that MDA levels increased in the hippocampus and frontal cortex of aged control rats as compared to young control rats, thus, confirming the previous findings that peroxidation of lipid molecules increases with the aging process (Barja 2002; Singh et al. 2009). Further, endogenous antioxidant defence system was found to be compromised in aged rats as indicated by decreased GSH and SOD levels in both hippocampus as well as frontal cortex of aged control rats as compared to young control rats. Superoxide dismutase is known to be a part of endogenous antioxidant defence system. However, its activity gets decreased with aging, leading to accumulation of reactive free radicals inside the cells, thereby causing oxidative stress (Gülinnaz Alper et al. 1998; Warner 1994). In addition, brain nitrite level was also found to be significantly high in aged control rats as compared to young control rats. The latter results support the hypothesis that necrosis increases during aging due to the formation of highly toxic peroxynitrite, a product of nitric oxide and superoxide anion (Ljubuncic et al. 2010; Maruyama et al. 2001). Inosine administration significantly reduced the oxidative and nitrosative stress in aged rats as indicated by decrease in MDA, nitrite levels and increase in GSH levels and SOD activity. This is also supported by an earlier study where inosine treatment prevented oxidative damage to DNA, decreased the generation of reactive oxygen species, and protected mice against gamma-radiation-induced death (Gudkov et al. 2006).
Primed activation of glial cells, especially astrocytes and microglia, in the central nervous system results in dysregulation of cytokines expression and their release which further leads to neuroinflammation followed by neurodegeneration, ultimately resulting in cognitive dysfunction (Lourbopoulos et al. 2015; Morel et al. 2015). As reported earlier, the expression of proinflammatory cytokines increases with aging in brain as well as in peripheral system (Sierra et al. 2007; Ye and Johnson 1999), which is consequently related to memory impairment and greater risk of age-related dementia, including vascular dementia and Alzheimer’s disease (Spangenberg et al. 2016; Udeochu et al. 2016). A microarray study showed that about 35% of total mRNA increase in the aged mouse brain was inflammatory-related proteins (Lee et al. 2000). Consistent with these studies, we also observed an increased level of TNF-α in both hippocampal (~ 2.7 fold) and frontal cortex (~ 2.6 fold) of aged rats as compared to young control rats. Previous literature also supports the current findings describing the enhanced TNF-α level in the hippocampus and cortex of aging brain (Campuzano et al. 2009; Casolini et al. 2002). In the present study, inosine treatment significantly reduced the elevated TNF-α level both in hippocampus and frontal cortex of aged rats.
The cholinergic system in the brain is known to play a significant role in learning and memory. However, any alteration to cholinergic neurotransmission results in cognitive dysfunction. AChE is responsible for the metabolism of ACh neurotransmitter into acetyl-CoA and choline at the synapse. Thus, decreased AChEs activity can be correlated with improved cognitive function due to increase in ACh level. In the present study, we observed significant reduction in AChEs activity in hippocampus and frontal cortex of aged control rats as compared to young control rats. These results are in consistent with the previous reports describing a decline in AChEs activity with aging in both preclinical as well as clinical studies (Das et al. 2001; Perry et al. 1992). Donepezil treatment significantly decreased AChE activity in aged rats as compared to aged control rats. Inosine treatment failed to show any effect on AChE activity in the aged rats but inosine significantly improved learning and memory of aged rats as compared to vehicle-treated aged rats. These results suggest that improvement of learning and memory of aged rats by inosine might not be through inhibition of acetylcholinesterase. Thus, antioxidant and anti-inflammatory actions of inosine might be responsible for its improving effect on learning and memory of aged rats.
Histopathological examination in the present study showed more number of damaged neurons with shrunk or sickle-shaped and irregular morphology in hippocampal CA1 region of aged rats as compared to young control rats which is consistent with the previous finding showing sparsely arranged, unclear cellular structures and irregularly shaped CA1 hippocampal neuronal cells in aged rats (Wu et al. 2017). Inosine (100 and 200 mg/kg) resulted in significant reduction of pyknotic neurons in aged rats as compared to aged control rats. This is the first histopathological study showing improvement of neuronal survival in hippocampal CA1 region of aged rats by inosine.
Conclusion
Inosine significantly improved learning and memory of aged female rats possibly through its antioxidant as well as anti-inflammatory effects; and improvement of neuronal survival in hippocampal CA1 region of aged rats. Moreover, the current findings suggest the use of inosine as a neutraceutical to preserve brain cognitive activities during aging process. However, additional study is required to further explore the downstream signaling pathways involved in the neuroprotective effect of inosine in aged animals.
Abbreviations
- AChE:
-
Acetylcholinesterase
- AD:
-
Alzheimer’s disease
- ANOVA:
-
Analysis of variance
- CPCSEA:
-
Committee for the Purpose of Control and Supervision of Experiments on Animals
- DTNB:
-
5,5′ dithio-bis-2-nitro benzoic acid
- EL:
-
Escape latency
- GSH:
-
Reduced glutathione
- ITL:
-
Initial transfer latency
- LPO:
-
Lipid peroxidation
- MDA:
-
Malondialdehyde
- NO:
-
Nitric oxide
- RTL:
-
Retention transfer latency
- SOD:
-
Superoxide dismutase
- TL:
-
Transfer latency
- TSTQ:
-
Time spent in target quadrant
- WHO:
-
World Health Organization
References
Alzheimer’s Association (2017) Alzheimer’s disease facts and figures. Alzheimer’s Dementia 13:325–373
Barja G (2002) Rate of generation of oxidative stress-related damage and animal longevity. Free Radic Biol Med 33:1167–1172
Barrientos RM, Kitt MM, Watkins LR, Maier SF (2015) Neuroinflammation in the normal aging hippocampus. Neurosci 309:84–99
Benowitz LI, Goldberg DE, Irwin N (2002) Inosine stimulates axon growth in vitro and in the adult CNS. Prog Brain Res 137:389–399
Blasko I, Stampfer-Kountchev M, Robatscher P, Veerhuis R, Eikelenboom P, Grubeck-Loebenstein B (2004) How chronic inflammation can affect the brain and support the development of Alzheimer’s disease in old age: the role of microglia and astrocytes. Aging Cell 3:169–176
Campuzano O, Castillo-Ruiz MM, Acarin L, Castellano B, Gonzalez B (2009) Increased levels of proinflammatory cytokines in the aged rat brain attenuate injury-induced cytokine response after excitotoxic damage. J Neurosci Res 87:2484–2497
Casolini P, Catalani A, Zuena AR, Angelucci L (2002) Inhibition of COX-2 reduces the age-dependent increase of hippocampal inflammatory markers, corticosterone secretion, and behavioral impairments in the rat. J Neurosci Res 68:337–343
Chen P, Goldberg DE, Kolb B, Lanser M, Benowitz LI (2002) Inosine induces axonal rewiring and improves behavioral outcome after stroke. Proc Natl Acad Sci USA 99:9031–9036
Chung HY, Sung B, Jung KJ, Zou Y, Yu BP (2006) The molecular inflammatory process in aging. Antioxid Redox Signal 8:572–581
Cipriani S, Bakshi R, Schwarzschild MA (2014) Protection by inosine in a cellular model of Parkinson’s disease. Neurosci 274:242–249
Dachir S, Shabashov D, Trembovler V, Alexandrovich AG, Benowitz LI, Shohami E (2014) Inosine improves functional recovery after experimental traumatic brain injury. Brain Res 25:78–88
Das A, Dikshit M, Nath C (2001) Profile of acetylcholinesterase in brain areas of male and female rats of adult and old age. Life Sci 68:1545–1555
David JP et al (1997) Glial reaction in the hippocampal formation is highly correlated with aging in human brain. Neurosci Lett 235:53–56
Dhull DK, Jindal A, Dhull RK, Aggarwal S, Bhateja D, Padi SS (2012) Neuroprotective effect of cyclooxygenase inhibitors in ICV-STZ induced sporadic Alzheimer’s disease in rats. J Mol Neurosci 46:223–235
Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82:70–77
Ellman GL, Courtney KD, Andres V Jr, Feather-Stone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–95
Freeman LR, Keller JN (2012) Oxidative stress and cerebral endothelial cells: regulation of the blood-brain-barrier and antioxidant based interventions. Biochim Biophys Acta 5:822–829
Fukui K, Onodera K, Shinkai T, Suzuki S, Urano S (2001) Impairment of learning and memory in rats caused by oxidative stress and aging, and changes in antioxidative defense system. Ann N Y Acad Sci 928:168–175
Gibbs RB (2000) Long-term treatment with estrogen and progesterone enhances acquisition of a spatial memory task by ovariectomized aged rats. Neurobiol Aging 21:107–116
Gordon P (1974) US Patent, Publication No. US3857940 A
Gornall AG, Bardawill CJ, David MM (1949) Determination of serum proteins by means of the biuret reaction. J Biol Chem 177:751–766
Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR (1982) Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem 126:131–138
Gudkov SV, Shtarkman IN, Smirnova VS, Chernikov AV, Bruskov VI (2006) Guanosine and inosine display antioxidant activity, protect DNA in vitro from oxidative damage induced by reactive oxygen species, and serve as radioprotectors in mice. Radiat Res 165:538–545
Gülinnaz Alper EYS, Kanit Lütfiye, Mentes Gülriz, Ersoz Biltan, Kutay Fatma Z (1998) Age-related alterations in superoxide dismutase and catalase activities in rat brain. Trop J Medical Sci 29:491–494
Harburger LL, Bennett JC, Frick KM (2007) Effects of estrogen and progesterone on spatial memory consolidation in aged females. Neurobiol Aging 28:602–610
Hasko G, Sitkovsky MV, Szabo C (2004) Immunomodulatory and neuroprotective effects of inosine. Trends Pharmacol Sci 25:152–157
Hou B, Xu ZW, Yang CW, Gao Y, Zhao SF, Zhang CG (2007) Protective effects of inosine on mice subjected to lethal total-body ionizing irradiation. J Radiat Res 48:57–62
Kaster MP, Budni J, Gazal M, Cunha MP, Santos AR, Rodrigues AL (2013) The antidepressant-like effect of inosine in the FST is associated with both adenosine A1 and A 2A receptors. Purinergic Signal 9:481–486
Kim HG, Lee JS, Choi MK, Han JM, Son CG (2014) Ethanolic extract of Astragali radix and Salviae radix prohibits oxidative brain injury by psycho-emotional stress in whisker removal rat model. PLoS ONE 9:e98329
Kono Y (1978) Generation of superoxide radical during autoxidation of hydroxylamine and an assay for superoxide dismutase. Arch Biochem Biophys 186:189–195
Kulkarni SK, Dhir A (2008) On the mechanism of antidepressant-like action of berberine chloride. European J Pharmacol 589:163–172
Kumar A, Prakash A, Pahwa D, Mishra J (2012) Montelukast potentiates the protective effect of rofecoxib against kainic acid-induced cognitive dysfunction in rats. Pharmacol Biochem Behav 103:43–52
Kumar A, Ekavali Mishra J, Chopra K, Dhull DK (2016) Possible role of P-glycoprotein in the neuroprotective mechanism of berberine in intracerebroventricular streptozotocin-induced cognitive dysfunction. Psychopharmacol 233:137–152
Lee CK, Weindruch R, Prolla TA (2000) Gene-expression profile of the ageing brain in mice. Nat Genet 25:294–297
Ljubuncic P, Gochman E, Reznick AZ (2010) Nitrosative stress in aging—its importance and Biological implications in NF-κB signaling. In: Bondy S, Maiese K (eds) Aging and age-related disorders. Humana Press, Totowa, pp 27–54
Lombardi VR, Garcia M, Rey L, Cacabelos R (1999) Characterization of cytokine production, screening of lymphocyte subset patterns and in vitro apoptosis in healthy and Alzheimer’s Disease individuals. J Neuroimmunol 97:163–171
Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G (2013) The hallmarks of aging. Cell 153:1194–1217
Lourbopoulos A, Erturk A, Hellal F (2015) Microglia in action: how aging and injury can change the brain’s guardians. Front Cell Neurosci 9
Lull ME, Block ML (2010) Microglial activation and chronic neurodegeneration. Neurotherapeutics 7:354–365
Markowitz CE, Spitsin S, Zimmerman V, Jacobs D, Udupa JK, Hooper DC, Koprowski H (2009) The treatment of multiple sclerosis with inosine. J Altern Complement Med 15:619–625
Maruyama W, Kato Y, Yamamoto T, Oh-Hashi K, Hashizume Y, Naoi M (2001) Peroxynitrite induces neuronal cell death in aging and age-associated disorders: a review. J Am Aging Assoc 24:11–18
Morel GR, Andersen T, Pardo J, Zuccolilli GO, Cambiaggi VL, Herenu CB, Goya RG (2015) Cognitive impairment and morphological changes in the dorsal hippocampus of very old female rats. Neurosci 303:189–199
Morris R (1984) Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods 11:47–60
Perry EK et al (1992) Convergent cholinergic activities in aging and Alzheimer’s disease. Neurobiol Aging 13:393–400
Radak Z, Zhao Z, Goto S, Koltai E (2011) Age-associated neurodegeneration and oxidative damage to lipids, proteins and DNA. Mol Aspects Med 32:305–315
Sandstrom NJ, Kim JH, Wasserman MA (2006) Testosterone modulates performance on a spatial working memory task in male rats. Horm Behav 50(1):18–26
Sharma AC, Kulkarni SK (1992) Evaluation of learning and memory mechanisms employing elevated plus-maze in rats and mice. Prog Neuropsychopharmacol Biol Psychiatry 16:117–125
Sierra A, Gottfried-Blackmore AC, McEwen BS, Bulloch K (2007) Microglia derived from aging mice exhibit an altered inflammatory profile. Glia 55:412–424
Singh KKS, Kumari K, Singh G, Kaur A (2009) Alterations in lipid peroxidation and certain antioxidant enzymes in different age groups under physiological conditions. J Hum Ecol 27:143–147
Smith JM et al (2007) Inosine promotes recovery of skilled motor function in a model of focal brain injury. Brain 130:915–925
Sonkusare S, Srinivasan K, Kaul C, Ramarao P (2005) Effect of donepezil and lercanidipine on memory impairment induced by intracerebroventricular streptozotocin in rats. Life Sci 77:1–14
Spangenberg EE et al (2016) Eliminating microglia in Alzheimer’s mice prevents neuronal loss without modulating amyloid-beta pathology. Brain 139:1265–1281
Starling RD, Trappe TA, Short KR, Sheffield-Moore M, Jozsi AC, Fink WJ, Costill DL (1996) Effect of inosine supplementation on aerobic and anaerobic cycling performance. Med Sci Sports Exerc 28:1193–1198
Sultana R, Perluigi M, Butterfield DA (2006) Protein oxidation and lipid peroxidation in brain of subjects with Alzheimer’s disease: insights into mechanism of neurodegeneration from redox proteomics. Antioxid Redox Signal 8:2021–2037
Udeochu JC, Shea JM, Villeda SA (2016) Microglia communication: parallels between aging and Alzheimer’s disease. Clin Exp Neuroimmunol 7:114–125
Uttara B, Singh AV, Zamboni P, Mahajan RT (2009) Oxidative stress and neurodegenerative diseases: a review of upstream and downstream antioxidant therapeutic options. Curr Neuropharmacol 7:65–74
van Groen T, Kadish I, Wyss JM (2002) Old rats remember old tricks; memories of the water maze persist for 12 months. Behav Brain Res 136:247–255
Warner HR (1994) Superoxide dismutase, aging, and degenerative disease. Free Radic Biol Med 17:249–258
WHO, Dementia (2017) http://www.who.int/mediacentre/factsheets/fs362/en/. Accessed 13 Dec 2017
Wills ED (1966) Mechanisms of lipid peroxide formation in animal tissues. Biochem J 99:667–676
Wu H, Zhao J, Chen M, Wang H, Yao Q, Fan J, Zhang M (2017) The anti-aging effect of erythropoietin via the ERK/Nrf2-ARE pathway in aging rats. J Mol Neurosci 61:449–458
Ye SM, Johnson RW (1999) Increased interleukin-6 expression by microglia from brain of aged mice. J Neuroimmunol 93:139–148
Acknowledgements
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. The authors would like to thank the Honorable Vice-Chancellor, Guru Jambheshwar University of Science and Technology for providing financial support and infrastructural facilities to carry out this work.
Author information
Authors and Affiliations
Contributions
DD conceptualized the project; PR planned and performed the experiments (animal behavioral study, biochemical estimations, ELISAs, histology); DD and PR analyzed all the data and wrote the manuscript. Authors critically reviewed the manuscript and approved for final submission.
Corresponding author
Ethics declarations
Conflict of interest
There is no conflict of interest among authors.
Rights and permissions
About this article
Cite this article
Ruhal, P., Dhingra, D. Inosine improves cognitive function and decreases aging-induced oxidative stress and neuroinflammation in aged female rats. Inflammopharmacol 26, 1317–1329 (2018). https://doi.org/10.1007/s10787-018-0476-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10787-018-0476-y