Abstract
The thermal properties of graphene oxide (GO) nanoparticles’ colloidal suspensions prepared using the microwave-assisted hydrothermal method were determined. The mode-mismatched dual-beam thermal lens technique was employed to measure the thermal diffusivity of GO nanoparticles for different concentrations in the base fluid. By fitting the experimental data to the theoretical expression, the characteristic time constant was determined. The thermal diffusivity of the fluids seemed to be strongly dependent on the presence of the nanoparticles, increasing from 15.02 ± 0.16 × 10−4 cm2·s−1 to 27.59 ± 0.51 × 10−4 cm2·s−1 for concentrations ranging from 0.82 %V to 4.00 %V of GO/H2O. As nanofluids concentration increased, a higher value of thermal diffusivity was obtained. The optical properties, morphology and chemical structure and functional groups of the nanoparticles were characterized by UV–Vis spectroscopy, transmission electron microscopy (TEM) and Fourier Transform infrared spectroscopy (FTIR). Two main absorption peaks at 230 nm and at 303 nm in the UV–Vis spectra were observed. TEM images revealed a uniform size distribution and spherical in shape NPs with mean diameter of 7.4 nm. This novel type of nanofluids have potential applications for heat transfer fluids like solar collectors and heat exchange systems.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Photothermal techniques are of special relevance to evaluate the thermophysical properties of fluids. The thermal diffusivity is defined as the rate at which heat transfer into a material having units of m2·s−1 and is expressed as D = k/ρcp, where k is the thermal conductivity, ρ is the mass density and cp is the specific heat capacity. Several traditional techniques exist to measure these thermal properties, such as photoacoustic [1], pyroelectric [2], photothermal deflection [3], resonant cavity [4], and thermal lens, among others. In this work it is of special importance the thermal lens (TL) technique for which a probe and an excitation laser are used. Their beams are guided collinearly in the opposite direction in the mismatched-mode to measure the thermal diffusivity of several substances such as nanofluids [5,6,7], paraffin [8], edible oils [9], nanocomposites graphene-based polymers [10], modified TiO2 nanoparticles and nanofluids that show high energy efficiency in cooling computer systems [11], hybrid nanofluids [12], nanofluids of conductive materials and phase change materials [7, 13], among others. The use of the thermal lens is a very important tool in the thermal characterization of liquids and semitransparent films due to it is a very sensitive, and non-invasive technique. Edible oil, biodiesel and water are for example some of the conventional liquids for heat transfer. However, their efficiency as heat transfer in liquids can be improved. Then, it is necessary to improve their thermal properties. To improve heat transport, metallic nanoparticles are added to fluids such as oil and water. The concentration and dispersion of these particles would improve the thermal properties of the fluid compared to the base liquid, and consequently, the range of applications would be expanded. For example, graphene has high rigidity, outstanding electrical properties, and high thermal conductivity (3000 W·mK−1 to 5000 W·mK−1) in a plane at room temperature. Therefore, is of special interest to use graphene for thermal properties enhancement [10, 14, 15].
Most of the literature, is found to be based in the study of nanofluids’ thermal conductivity. It has been reported that the thermal conductivity increases with the increase in the concentration and size of the nanoparticles for nanofluids. For example, it has been found that the thermal conductivity of nanofluids with metal oxide nanoparticles for concentrations higher than 1 % is remarkable increased compared to the base fluid [16]. In addition, in many of the works on the thermal conductivity of nanofluids, different particles such as CNTs and metal oxide particles have been used measuring their thermal properties at room temperature. Ahammed et al. [16] showed that the thermal conductivity of graphene in water is influenced by temperature. The percentage of enrichment of thermal conductivity was around 18 %V for 0.17 %V concentration at room temperature measured by hot wire technique. Esfahani et al., [17] reported an enrichment of GO nanosheets of 19.9 % for a concentration of 0.5 wt% in water as the base liquid. In this work, the thermal diffusivity of graphene oxide (GO) in water was measured using the thermal lens technique at room temperature for high concentrations. Until now, no works have been found in the literature, for which this thermal property of GO is reported for higher concentrations. Possible applications of this work lies on of high-performance chillers in solar cells [12] micro-electronics [18], nuclear energy and systems for enhancing forced convective heat transfer [13].
2 Experimental Part
2.1 Samples Preparation
The GO was prepared using the methodology Hummers with some modifications [19]. For the synthesis, 1 g of graphite was added into 23 mL of 98 % H2SO4 and stirred at room temperature for 24 h. After that, 100 mg of NaNO3 was added to the mixture and stirred for 30 min. Subsequently, the mixture was kept below 5 °C using an ice bath, and 3 g of KMnO4 was slowly added. After heating to 35 °C–40 °C, the mixture was stirred for another 30 min. Then, 46 mL of water was added for 25 min. Then, 140 mL of water and 10 mL of 30 % H2O2 were added to stop the reaction. Then, the sample was centrifuged and the synthesized GO at a concentration of 0.5 mg·mL−1 was dispersed by sonication into individual sheets in distilled water. With the TL technique, it is important to highlight that the samples were sonicated for 50 min to avoid agglomeration of the nanoparticles and the measurement was taken in less than one second. An aqueous dispersion of GO was prepared by chemical reduction of GO using benzylamine. Then, 2 mL of GO aqueous dispersion was added into 4 mL of H2O and 200 mL of benzylamine were added and the sample was stirred for 30 min at room temperature. After that, the mixture was heated to 90 °C for 90 min. Finally, a dark brown GO dispersion was obtained. The GO nanofluids were stable for 3 months without significant change in the spectral pattern (not shown). Electrostatic interactions and hydrogen bonding between GO and water play a significant role in extraordinary properties of stabilizing in the solution [20].
Concentrations of graphene/water used in this work were 0.82 %V, 1.63 %V, 2.43 %V, and 3.22 %V, 4 %V (Fig. 1). Due to the GO samples are contained in water, the calibration was done with the base liquid. From the expression tc = we2/4D, it is possible to obtain the adjustment parameter tc to determine the thermal diffusivity. The errors for each concentration, which vary between 1 and 4%, are typical errors in the fit of the theoretical thermal lens equation to the experimental data. Before the measurements, a previous calibration is made to see if similar values are obtained. 10 measurements of the samples were taken and to find the uncertainty of the thermal diffusivity value | ΔD | = D |Δtc/tc|, the uncertainty of data depending on one variable procedure was used [21].
Preparation of graphene oxide (GO) for different concentrations 0.82 %V, 1.63 %V, 2.43 %V, and 3.22 %V, 4 %V by the method of Hummers [19]
2.2 Experimental Set-up
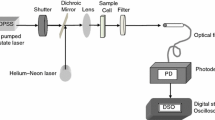
The basic principle of the thermal lens technique (TL) consists to expose a sample to an excitation laser with a Gaussian distribution. Then, the sample absorbs the incident photons and the energy is transformed into heat, generating temperature gradient. This results in a variation of the refractive index of the sample. A second probe laser of lower energy passes through this medium creating a divergent or convergent. This effect is called TL. This signal provides information on the thermal properties of the sample and is widely used for the measurement of the thermal diffusivity of semitransparent samples [22].
The TL experimental setup is shown in Fig. 2. An Ar+Xe excitation laser with a wavelength of 534 nm and power of 40 mW is focused by a converging lens 1 of 10 cm, a shutter with a time of less than 1 ms, passing through a cuvette (1 cm) where the sample is contained. A second probe He–Ne laser with a wavelength of 632 nm and a power of 1 mW is slightly defocused by a 25 cm lens 2 and passed through the sample [9]. The waist of the probe and the excitation lasers are ωe = 49 μm and ωp = 181 μm. The signal obtained from the sample is collected using a photodiode (Thorlabs) using a spatial filter and a pinhole to ensure that only the signal from the probe laser light is detected and to concentrate the signal in the center of the detector. The signal obtained is recorded and decoded by a Labview software.
In Fig. 3, the typical spectra of the TL signal is shown. The system was calibrated with distilled water with a thermal diffusivity of 14.08 ± 0.09 × 10–4 cm2/s.
From Eq. 1 the TL signal is fitted and the adjustment parameters θ and tc associated with the photothermal energy and the characteristic time of the formation of the TL are obtained. The photothermal signal as a function of time is given by [22]:
where:
where I(t) and Io are the intensities in the center of the beam at time t and for I (t = 0) at the initial time, respectively. Pe is the power of the excitation laser, A, L, k, λp and dn/dT are the absorption of the sample, the length of the quartz cuvette, the thermal conductivity, the wavelength of the probe laser and the coefficient of the refractive index as function of the temperature, respectively; ω1p and ωe are the ratio of the probe and excitation beam of the sample, respectively. The constant optical parameters were m = 13.69 and V = 1.22. The time of TL formation for tc, is calculated as follows:
3 Results and Discussion
A Genesis 10 S UV–vis spectrophotometer was used to obtain the UV–visible spectrum of the samples in the range of 190–500 nm, with a step of 5 nm. The GO structural characterization and the morphology and particle size were determined by transmission electron microscopy (TEM). The measurements were made with a JEOL JEM1010 electron microscope with an accelerating voltage of 60 kV. For TEM characterization, a droplet of the nanofluids was placed on carbon-coated copper grid and dried at room temperature. Fourier Transform infrared spectroscopy (FTIR) was used to study the chemical structure and functional groups presents in a range of 400–4000 cm−1 with a step of 2 cm−1 with a Perkin Elmer spectrometer [23].
UV–Vis spectroscopy spectrum of the graphene oxide nanoparticles dispersions at different concentrations of graphene/water: 0.82 %V, 1.63 %V, 2.43 %V, 3.22 %V, and 4.00 %V is shown in Fig. 4. There are two main absorption peaks, one at 230 nm and another at 303 nm in the UV–visible. The peak at 230 nm is due to π–π* transition of C–C aromatic bonds for GO and the peak at 303 nm correspond to an n–π* transition. A shift of the peaks in the visible region is observed. Also, the absorption value increase when increased the concentration of the graphene oxide nanoparticles [24, 25].
TEM images of the particles with a uniform particle size distribution and spherical in shape are shown in Fig. 5 with diameters on the nanometric scale. Based on measurements taken from the TEM micrographs, the size distribution histogram for the samples was obtained (Fig. 5). A Gaussian adjustment with a narrow distribution of diameters with an average particle diameter of 7.4 nm and its size distribution with a standard deviation of 0.8 nm show the homogeneity of the diameter of the graphene oxide nanoparticles. The images were analyzed by ImageJ software.
The TL signal for different concentrations of GO is shown in Fig. 6. The continuous line shows the best fit of the Eq. 1 to the experimental data. The adjustment parameters characteristic time tc and θ were obtained. Using tc, the thermal diffusivity value was obtained from Eq. 4. θ is proportional to the variation of the refractive index as a function of the temperature (dn/dT) using Eq. 3. For each concentration 0.82 %V, 1.63 %V, 2.43 %V, 3.22 %V and 4.0 %V, the thermal diffusivity values were obtained: 15.02 ± 0.16 cm2·s−1, 17.07 ± 0.11 cm2·s−1, 20.53 ± 0.25 cm2·s−1, 22.65 ± 0.26 cm2·s−1 and 27.59 ± 0.51 × 10–4 cm2·s−1, respectively. Distilled water was used for TL system calibration. A thermal diffusivity value of 14.08 ± 0.09 × 10–4 cm2·s−1 was obtained in agreement with the values reported in the literature [26, 27]. To measure the thermal diffusivity of the water for calibration, a small concentration of R6G (rhodamine) at 0.0024 mg·L−1 was used to obtain an optimal window in the visible region. Water thermal diffusivity is similar in magnitude to common solvents showing poor thermal blooming effect. A dye such a R6G added to water improves the light absorption without altering the thermal diffusivity of the medium [28, 29]. Table 1 summarizes the results of the concentrations in %V, tc, θ and the diffusivities obtained by thermal lens.
There was an increase in thermal diffusivity with the concentration in %V of the nanoparticles as seen in Fig. 7. For example, this increase in thermal diffusivity was around 60 % for a 3.22 %V concentration of graphene compared to water at room temperature. Esfahani [17] reported an enrichment of GO nanosheets of 18.9 % for a concentration of 0.1 wt% in water as the base liquid. When increasing the concentration to 0.5 wt%, the enhancement was 19.9%, observing little variation of the enrichment, different to this work. According to Esfahani, the concentration of graphene is not relevant in thermal conductivity measurement. Therefore, in this work it was demonstrated that the concentration of GO is important for diffusivity thermal determination this was relevant, opposite to Esfahani's work. It is known that by the relation D = k/ρCp the thermal diffusivity is proportional to the thermal conductivity. If there is an increase in conductivity, the thermal diffusivity increases. Although the density and the heat capacity are involved in the equation, none of them were measured in this work. The product of ρCp have been defined as the specific volumetric heat capacity of the nanofluid [30]. Authors like Zhou et al., have demonstrated that this specific volumetric heat capacity is almost constant as a function of the volume of the fraction in the nanofluid [31]. The increase in conductivity with an increase in the volume of the fraction have been well demonstrated in the literature [32]. Afrand et al. [33] where it reported for nanoparticles dispersed in different base fluids, an enrichment of 90 % in nanofluids containing Fe3O4 nanoparticles in water for a concentration of 3 V%. From the experimental results it can be seen that thermal conductivity is enhanced with an increase in the volume fraction as shown in this work with a value of 95%. Although increasing concentration of nanostructures is known to be effective in enhancing the thermal conductivity of nanofluids, the mechanisms related to this enhancement must be elucidated.
The increase in thermal diffusivity of graphene oxide suspended in water with concentration has been explained due to the following factors which influence the enhancement of thermal diffusivity such as phonons, free electrons, collisions, and molecular diffusivity. If there is an increase in the temperature in the nanofluid, the heat transfer increases due to a greater vibration (phonons), a greater energy of free electrons and a rapid collision and molecular diffusion induces a higher thermal conductivity, due to the suspension of solid nanoparticles [16, 34].
4 Conclusions
Colloidal suspensions of graphene nanoparticles were prepared using the microwave-assisted hydrothermal method. The thermal diffusivity of nanofluids such as water containing graphene oxide (GO) nanoparticles, was observed to be strongly dependent on the presence of the nanoparticle concentration. The results show that the nanofluid had a substantially increased thermal diffusivity with respect to the base fluid. The diffusivity had an enrichment of 7 % to 95 % for concentrations of 0.82 %V to 4.0 %V. Possible factors for the enrichment of thermal diffusivity are due to phonons, free electrons, collisions, and molecular diffusion of the sample. Possible applications of this work is related to high cooling for devices such as solar cells and in micro-electronics.
References
M.V. Marquezini, N. Cella, A.M. Mansanares, H. Vargas, L.C.M. Miranda, Meas. Sci. Technol. 2, 396 (1991)
J. Caereles, C. Glorieux, J. Thoen, Rev. Sci. Ins. 69, 2452 (1998)
S.M. Shibli, A.L.L. Dantas, A. Bee, Braz. J. Phys. 31(3), 418 (2001)
M. Chirtoc, G. Milhailescu, Phys. Rev. B 40, 9606 (1980)
R. Carbajal Valdez, J.L. Jiménez-Pérez, A. Cruz Orea, Z.N. Correa Pacheco, M.L. Alvarado Noguez, I.C. RomeroIbarra, J.G. Mendoza Álvarez, Thermochim. Acta 657, 66 (2017)
G. López-Gamboa, J.L. Jiménez-Pérez, Z.N. Correa-Pacheco, M.L. Alvarado-Noguez, M. Amorin Lima, A. Cruz-Orea, J.G. Mendoza Alvarez, Int. J. Thermophys. 41(10), 1 (2020)
N. Sezer, M.A. Atieh, M. Koç, Powder Technol. 344, 404 (2019)
X. Liu, Z. Rao, Thermochim. Acta 647, 15 (2017)
J.L. Jimenez-Perez, G. Lopez Gamboa, J.F. Sanchez Ramirez, Z.N. Correa-Pacheco, V.E. Lopez Lopez, L. Tepech-Ccarrillo, Appl. Phys. A 122, 925 (2016)
M. Gresil, Z. Wang, Q.A. Poutrel, C. Soutis, Sci. Rep. 7, 1 (2017)
Y. Wang, C. Zou, W. Li, Y. Zou, H. Huang, Int. J. Heat Mass Tran. 156(119735), 1 (2020)
L.O. Usoltseva, M.V. Korobov, M.A. Proskurnin, J. Appl. Phys. 128(19), 190901 (2020)
C. Liu, M. Chen, W. Yu, Y. He, ES Energy Environ. 2, 31 (2018)
M. Potenza, A. Cataldo, G. Bovesecchi, S. Corasaniti, P. Coppa, S. Belluci, AIP Adv. 7(075214), 1 (2017)
M.R. Rodríguez-Laguna, A. Castro-Alvarez, M. Sledzinska, J. Maire, F. Costanzo, B. Ensing, M. Pruneda, P. Ordejón, C.M. Sotomayor Torres, P. Gómez-Romero, E. Chávez-Ángel, Nanoscale 10, 15402 (2018)
N. Ahammed, L. Godson Asirvatham, J. Titus, J. Raja Bose, S. Wongwises, Int. Commun. Heat Mass 70, 66 (2016)
M.R. Esfahani, E.M. Languri, M.R. Nunna, Int. Commun. Heat Mass Transf. 76, 308 (2016)
S. Vishnuprasad, K. Haribabu, V.T. Perarasu, Heat Mass Transf. 55, 2221 (2019)
W. Hummers Jr., R. Offeman, J. Am. Chem. Soc. 80(6), 1339 (1958)
H.S. Kim, T.J. Oweida, Y.G. Yingling, J. Mater. Sci. 53, 5766 (2008)
M. Drosg, Dealing with Uncertainties, A Guide to Error Analysis (Springer, Berlin, 2007).
J. Shen, R.D. Lowe, R.D. Swook, Chem. Phys. 165, 385 (1992)
J.L. Jiménez-Pérez, R. Gutiérrez-Fuentes, G. López-Gamboa, J.F. Sánchez-Ramírez, Z.N. Correa-Pacheco, R. Carbajal-Valdéz, Opt. Mater. 84, 236 (2018)
Q. Lai, S. Zhu, X. Luo, M. Zou, S. Huang, AIP Adv. 2(031146), 1 (2012)
T. Emiru, D. Ayele, Egypt J. Basic Appl. Sci. 4, 74 (2017)
P.R.B. Pedreira, L. Hirsch, J.R.D. Pereira, A.N. Medina, A.C. Bento, M.L. Baesso, Rev. Sci. Inst. 74, 808 (2003)
R.C. Weast, Handbook of Chemistry and Physics (CRC Press, Boca Raton, 1987).
V.P. Zharov, K.E. Mercer, E.N. Galitovskaya, M.S. Smeltzer, Biophys. J. 90, 619 (2006)
V.P. Zharov, D.O. Lapotko, IEEE J. Sel. Top. Quant. Electron. 11, 733 (2005)
V.M. Lenart, N.G.C. Astrath, R.F. Turchiello, G.F. Goya, S.L. Gómez, J. Appl. Phys. 123, 085107 (2018)
L.P. Zhou, B.X. Wang, X.F. Peng, X.Z. Du, Y.P. Yang, Adv. Mech. Eng. 2, 172085 (2010)
R. Bakhtiari, B. Kamkari, M. Afrand, A. Abdollahi, Powder Technol. (2021)
M. Afrand, D. Toghraie, N. Sina, Int. Commun. Heat Mass Transf. 75, 62 (2016)
T. Hong, H. Yang. C. J. Choi, J. Appl. Phys. 97(064311), 1 (2005)
Acknowledgments
Authors would like to thank CONACYT, COFAA, and CGPI-IPN, Mexico, for their partial financial support. Also, to the Red de Nanofotónica.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jiménez-Pérez, J.L., López-Gamboa, G., Sánchez-Ramírez, J.F. et al. Thermal Diffusivity Dependence with Highly Concentrated Graphene Oxide/Water Nanofluids by Mode-Mismatched Dual-Beam Thermal Lens Technique. Int J Thermophys 42, 107 (2021). https://doi.org/10.1007/s10765-021-02861-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10765-021-02861-6