Abstract
Ongoing environmental changes may reduce the population size and geographic distribution of many ecologically sensitive species. Predicting where populations will (or will not) be likely to resist these changes is crucial for planning their protection. The blond titi monkey (Callicebus barbarabrownae) is listed as Critically Endangered by the International Union for Conservation of Nature because of its small and declining population due to deforestation and hunting. The species is endemic to the Brazilian Caatinga dry forest, which has undergone extensive deforestation and climate-induced desertification. We address the extinction risk of this species in a spatially explicit context by predicting the geographic distribution of suitable areas that may permit the persistence of potentially viable populations over the next 50 years. We combined species distribution modeling with population viability analysis under more optimistic and pessimistic climate change scenarios and simulated forest loss, assessing how different populations will likely cope with these changes during the coming decades. Future models did not differ between the scenarios, but changes in the climatic suitability of the species’ habitat predicted a reduction of one-third of the currently suitable area. Viable populations in large forest remnants represent only a quarter of all known populations, and they also face a decline in climatic suitability. Smaller populations may be extirpated, regardless of the level of forest recovery. This species’ long-term persistence entails improving habitat quality and the connectivity of the large remnants to neighbor areas. Finally, the integration of spatial and temporal models is feasible and helpful to address threats and aid the conservation of primates.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Habitat loss and fragmentation, combined with climate change, are the principal threats to biodiversity worldwide (Garcia et al., 2014; Travis, 2003). The isolation of populations in fragments limit their dispersal between adequate habitats, which may increase pressures on scant resources and reduce genetic viability through increased endogamy (Galán-Acedo et al., 2021). Ongoing climate change also may be forcing a shift in the distribution of many species as they track suitable conditions (Parmesan, 2006). As a result, local populations in increasingly fragmented landscapes will face a combination of threats that may limit their long-term persistence (Brook et al., 2008; Fischer & Lindenmayer, 2007). In this context, predicting where suitable habitats will persist and accommodate viable populations of a species will be critical for the development of effective long-term conservation measures (Brook et al., 2009).
Many different approaches have been adopted to address the geographic distribution of species and the viability of their populations. The spatial distribution of species, including hindcasts and forecasts, has generally been described using Species Distribution Modeling (SDM)—a technique designed to estimate a geographic distribution by combining the known occurrences of a species with layers of environmental variables and other constraints on species dispersal and its colonization potential (Araújo & Peterson, 2012). The assessment of populations’ viability and extinction risk is typically based on Population Viability Analysis (PVA) (Beissinger & McCullough, 2002; Gilpin & Soulé, 1986). This approach combines data on the species’ life history with extrinsic effects—such as changes in habitat size (and carrying capacity), hunting, and supplementation—to simulate the dynamics of individual populations over a predefined timespan or number of generations (Beissinger & McCullough, 2002; Boyce, 1992).
Although these approaches have become increasingly popular in conservation assessments, the application of only one approach limits the potential for the understanding of the spatiotemporal configuration that determines the vulnerability of a species. Estimating environmental suitability from spatial models alone may overlook the actual risk faced by some local populations, based on their demographic characteristics and extinction probabilities (Franklin, 2010). On the other hand, predicting population viability alone may overlook the ongoing environmental changes that may determine shifts in the geographic distribution of the species, which would alter the viability of some populations (Reed et al., 2002). Given this, the integration of spatial (i.e., SDM) and temporal (i.e., PVA) approaches should provide a more realistic evaluation of the temporal tendencies of populations in a spatially explicit context, in particular when threats, such as habitat loss and climate change, are combined (Keith et al., 2008). Despite its potential value for conservation planning, this integrative approach has been little explored in conservation studies (Brook et al., 2009), including plants (Keith et al., 2008) and birds (Andersen et al., 2017), but never, to our knowledge, for primates.
This integration should offer insights for conservation assessments of species undergoing spatiotemporal modifications, such as those imposed by habitat fragmentation and climate change (Brook et al., 2009). One example of species in this type of scenario is the blond titi monkey (Callicebus barbarabrownae)—the only primate endemic to the Brazilian xeric forest, the Caatinga (Printes et al., 2020). In recent years, the vegetation cover of this ecoregion has been transformed extensively by farming, cattle ranching, and firewood extraction (Castelletti et al., 2004). By the end of this century, the semi-arid Caatinga—one of the most climate-sensitive regions in South America, with a high risk of desertification (Brasil, 2004)—is predicted to suffer significant shifts in climate, in particular in precipitation, which may decrease by up to one third (Torres et al., 2017). This will likely exacerbate the situation faced by C. barbarabrownae, which is listed as Critically Endangered (CR) by the International Union for Conservation of Nature (IUCN) due to its small and declining remnant population (<250 mature individuals) and few subpopulations, each with fewer than 50 mature individuals (Printes et al., 2016, 2020). Hunting also is a constant threat to this species. Worse still, no viable C. barbarabrownae populations have been identified in protected areas (Printes et al., 2016, 2020). Given this scenario, strategies to better protect the species have been developed by the Brazilian National Action Plan for the Conservation of Northeastern Primates (PAN PRINE) since 2011 (, 2018; Brasil/ICMBio, 2011).
In this study, we assess the risk of extinction of C. barbarabrownae over the next 50 years, across its entire geographical distribution, under the combined threat of climate change and habitat loss. For this, we integrate SDM with temporal simulations of PVA. This study also will contribute to the PAN PRINE providing data-driven recommendations for the conservation of the species.
Methods
Study Populations
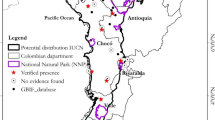
Callicebus barbarabrownae is endemic to the Caatinga vegetation of the Brazilian states of Bahia and Sergipe, with a known distribution of approximately 300,000 km2, at altitudes of 241–908 m above sea level (Printes et al., 2016). We obtained occurrence records primarily from the database of the National Center for Research and Conservation of Brazilian Primates (ICMBio/CPB) and recently published studies (Jerusalinsky, 2013; Marques et al., 2013; Printes, 2007; Printes, 2011; Printes et al., 2016), with a total of 154 records. We identified the remnants containing known populations of C. barbarabrownae based on the overlap between population records and the vegetation map. We also considered as valid occurrences the geographic coordinates within a radius of 1 km from a forest remnant and considered remnants distant less the 1.0 km as being the same remnant. This was based on known travel distances of Callicebus in open areas (Gouveia et al., 2016). Based on this procedure, 26 records were not related to forest remnants and were assumed to represent georeferencing errors or records of extinct populations in forests that no longer exist. We thus excluded them from all analyses. The final dataset consisted of 128 valid records of occurrence in 49 forest remnants (Fig. 1).
Known occurrence localities of Callicebus barbarabrownae and the associated remnants of Caatinga dry forest (dark gray). The localities are identified by their numbers in Table S1 (Electronic Supplementary Material).
Data Acquisition
We mapped the forest cover of the study region based on data from MAPBIOMAS (Projeto MapBiomas, 2020) at a spatial resolution of 1:100,000. We considered primary and secondary forests as relevant for the studied species. We then estimated the area of the remnants and used them to estimate their carrying capacity for the PVA. In this analysis, we set the initial population size at the carrying capacity (i.e., N = K). To estimate N and K, we used two different density estimates, 1.2 and 2.3 individuals/km2, both based on a survey of 91 C. barbarabrownae groups with a total area of 221 km2 at the centre of the species’ distribution (Coelho et al., 2020). Previous studies have reported larger (4.0 individuals/km2; Freitas et al., 2011) and smaller (1.15 individuals/km2; Corsini & Moura, 2014) estimates. However, we had no information on how population density varies spatially and which factors may drive this variation. Therefore, we decided that the values provided by Coelho et al. (2020) represent a more reliable estimate, because they cover a much larger proportion of the species distribution in the Caatinga and are midway between the extremes of the previous studies.
To obtain a species distribution model, we first build an ecological niche model (i.e., ENMs) that establishes the association between occurrences and climatic factors (Peterson et al., 2011). For the ENMs, we selected four climatic variables from WorldClim (Hijmans et al., 2005) at a 2.5’ (ca. 5.0 km) spatial resolution. These variables were (i) maximum temperature of the warmest month, (ii) minimum temperature of the coldest month, (iii) precipitation of the driest month, and (iv) precipitation seasonality. All of these factors presented minimal collinearity within the study region (based on a cross-correlation matrix, considering Pearson’s r < 0.70 as the threshold), and describe critical aspects of the climate of the Caatinga, which are expected to constrain the distribution of C. barbarabrownae (Gouveia et al., 2016). We obtained these climatic data for the current conditions (i.e., between 1970 and 2000) and projected future scenarios for 2061–2080 (hereafter referred to as 2070).
For future projections, we modeled the species’ distribution in two extreme climatic scenarios, one optimistic and one pessimistic, which correspond to the Representative Concentration Pathway (RCP) of 2.6 and 8.5 Watts per square meter of radiative forcing, respectively (Moss et al., 2008). These values correspond to the greenhouse gas concentrations that are predicted to increase mean temperatures within the range of the species by 1.4°C or 3.2°C, respectively (Hijmans et al., 2005). Different institutions have developed climate forecasts with slightly different outputs, known as General Circulation Models (GCMs). To reduce the uncertainties generated by the different GCMs, we used the MIROC6 and CNRM-CM6-1 models for each scenario (Hijmans et al., 2005). We choose these GCMs based on the availability of data at the resolution and scenarios used in the analyses and on the spatial resolution of the models (Torres et al., 2017).
Population viability analysis requires reliable data on the life-history traits of the species. Because these data are not available for most parameters of C. barbarabrownae, we used information available on the four congener species, as summarized by Bicca-Marques and Heymann (2013) (see Table S2, Electronic Supplementary Material). As most traits of the five Callicebus species are broadly similar, including body size, only negligible allometric variation is expected in the other traits. The biological and ecological attributes of most primates have a high level of phylogenetic conservatism (Kamilar & Cooper, 2013), which means that traits tend to be shared among closely related species, in particular, congeners. The only trait that exhibited variation was the age at first reproduction, with estimates of 30 to 39 months. In this case, we used a midpoint of 36 months, i.e., 3 years.
Data Analyses
Species Distribution Models
We generated the species distribution models and their projection for future scenarios by combining the species occurrence records with climatic factors and factors known to limit the occurrence of C. barbarabrownae. We included geographical barriers and habitat types as limiting factors. The distribution of the species is limited to the north by the São Francisco River, and its occurrence is otherwise restricted to forest habitats (Gouveia et al., 2016).
We ran four different approaches for the SDM – Maxent, Generalized Linear Model (GLM), RandomForest (RF), and Boosted Regression Trees (BRT), as implemented in the “sdm” package, version1.0-89 (Naimi & Araújo, 2016) in the R environment (R Development Team, 2020). Because the MaxEnt method requires background points as input occurrence records, we obtained these by sampling 10,000 background points in the study region. These background points are points drawn at random to characterize the environmental domain of the study region (Phillips et al., 2009). The other methods (i.e., GLM, RF, and BRT) all require both occurrence and absence records. When absence data are lacking, as in our case, they can be replaced by pseudo-absences, which consist of locations where the species’ presence is not known. For this, we sampled 100 pseudo-absence records within the study area that were at least 50 km from known points of occurrence, an approach known as spatially stratified pseudo-absence (Barbet-Massin et al., 2012). The models were calibrated using tenfold cross-validation, based on random samples of 70% and 30% of the training and test datasets, respectively. We retained the most accurate method based on the ratio of the area under the curve (AUC), in this case, the receiving operating characteristic curve (ROC), and the AUC of the null expectation obtained by bootstrapping based on the z statistic. This method is referred to as the partial ROC statistic (Peterson et al., 2008), which has been shown to be a much better method for estimating the performance of ENMs compared with previous approaches based on the ROC (Peterson et al., 2011).
We converted the resulting suitability values into a binary classification of presence/absence using a threshold determined by the maximum model training sensitivity plus specificity. Because areas suitable for C. barbarabrownae are restricted to the primary and secondary forests found in the study region, we refined the resulting maps of environmental suitability according to the distribution of these vegetation formations.
To facilitate the comprehension of the results, we compiled two consensus models for each scenario (optimistic and pessimistic), considering the overlap of the two different GCMs. We used this consensus to identify which forest remnants are predicted to contain climatically suitable areas for C. barbarabrownae in the future under the different climate scenarios.
Population Viability Analysis
We set our PVA to the same period of the SDM, i.e., 2070, thus matching the timespan evaluated in the two approaches. We first implemented a baseline model (Boyce, 1992) with the trait values (Table S2, Electronic Supplementary Material) and then determined the minimum viable population (MVP) by simulating different hypothetical populations with varying initial sizes (N = K) from 4 to 300 individuals (Boyce, 1992), which were adequate for this purpose. The MVP is the estimated minimum population that has the highest chance of persistence over a given period. Because C. barbarabrownae is classified as Critically Endangered (Printes et al., 2020) and there is some uncertainty in the model parameters, we choose to use a more conservative criterion of viability. We assumed that a population is viable when it persists for 50 years with a minimum probability of persistence of 98% (i.e., an extinction probability of 2.0%) and minimum retention of genetic diversity (heterozygosity) of 95%. A 95% persistence probability is a standard value for PVA. However, the software used (Vortex v. 10.0.7.9; Lacy & Pollak, 2014) assumes that each population comprises unrelated individuals and has no more than second-degree endogamy. Because this condition is not typical of isolated primate populations in forest remnants, as in the present study, a more restrictive cutoff would be recommendable (Lacy, 1993). Based on the MVP and the estimates of C. barbarabrownae population density used in the present study, we estimated the minimum viable habitat (MVH), which defines the minimum area required for a viable population to persist over the long term (Brito & Grelle, 2006).
We also simulated populations under different scenarios of habitat loss. The most recent estimates point to a reduction in the vegetation cover of the Caatinga at rates of 0.19–0.44% yr–1, with a mean of 0.30% yr–1 between 1985 and 2017 (Projeto MapBiomas, 2020). We thus simulated scenarios of habitat loss for populations with different initial sizes by setting an annual reduction in the carrying capacity (K) of the remnants over the next 50 years. We used three scenarios based on the observed trends: i) an ideal scenario of 0.0% yr–1; ii) a mean scenario of 0.3% yr–1; and iii) a worst-case scenario of 0.5% yr–1.
We also simulated the population dynamics following the application of management strategies to recover unviable populations. For this, we simulated habitat recovery scenarios of between 0.5% and 50% of the proportion of existing forest per annum to evaluate the effects of increasing carrying capacity. This arbitrary but broad range of values allowed us to verify which management strategies would prevent different populations from extirpation.
We integrated the PVA and SDMs by first estimating the areas suitable for C. barbarabrownae in the different scenarios, including the predicted suitability of the known areas of its occurrence in the future. We then ran a PVA for the population sizes and carrying capacities as estimated from the size of the remnants found to be suitable in all scenarios. This procedure provided us with a broad picture of how changes in the suitable areas resulting from climate change and habitat loss would be expected to affect the viability of the populations over the long term.
We processed the spatial data using ArcGis 9.3 (ESRI, 2008) and R environment (R Development Team, 2020), using the raster, version 3.4-13 (Hijmans, 2014) and SDMTools, version 1.1-20 (VanDerWal et al., 2014) packages, respectively.
Ethical note
This study involved no capture or handling of living animals whatsoever. The study was based on the analysis of existing data on geographic localities and the life-history traits of the study genus, spatial layers of climatic variables, and computational tools for ecological analyses.
Data availability
All the data analyzed in this study are provided in the supplementary information files.
Results
The 49 forest remnants (Table S1, Electronic Supplementary Material) in which C. barbarabrownae has been recorded range in size from 0.002 km2 to 36,950 km2, with a total area of ca. 50,000 km2. Overall, 20 of these remnants are less than 1 km2 in area, and 36 are less than 10 km2. Most small populations occur in the eastern extreme of the species’ range, where they tend to occupy smaller remnants (<1.0 km2) than the average size (1,024 km2).
Species Distribution Models
Only the Maxent model was adequate for the prediction of the distribution C. barbarabrownae, and we thus retained it for the subsequent analyses. The mean AUC ratio of the Maxent model obtained from the PartialROC analyses was 1.42 (range: 1.15–1.63), which was significantly larger than expected (P < 0.001) based on the z distribution (for other models, see Table S3, Electronic Supplementary Material). The optimistic and pessimistic forecast scenarios were nearly identical in terms of their geographic distribution and predicted suitability (Fig. S1), and we will consider them together as the “future models” in the rest of this paper (Fig. 2).
Remnants of the Caatinga dry forest in which Callicebus barbarabrownae is predicted to occur by 2070 according to optimistic (upper panel) and pessimistic (lower panel) scenarios (Representative Concentration Pathways – RCPs of 2.6 and 8.5 W/m2 of radiative forcing, respectively) based on the consensus of Maxent model from two Global Circulation Models – GCMs (MIROC6 and CNRM-CM6-1).
After accounting for forest cover, the future models predicted a contraction of 33% of climatic area suitable for C. barbarabrownae (i.e., from 54,410 km2 to 36,428 km2). Only five small remnants, distributed at the northeastern extreme of the species range (with a total of 98.2 km2), were predicted to lose all their climatic suitability (the red ellipsis in Fig. 2). Among them are the two largest remnants (36,950 km2 and 1,698 km2) with known populations, which are predicted to lose suitability in approximately half of their area.
Population Viability Analysis
The MVP of C. barbarabrownae, estimated from varying hypothetical initial population sizes, indicated that demographic viability could be reached with 60 individuals. However, the genetic viability required a population of at least 135 individuals (Fig. 3b), which we accepted as the MVP for the next 50 years (Table S4, Electronic Supplementary Material).
Based on the MVP estimated for a density of 1.2 or 2.3 individuals/km2, the MVH for C. barbarabrownae was 112.5 km2 or 58.7 km2, respectively. However, this variation did not substantially alter the proportion of the remnants that were smaller than the MVH. Forty-one remnants were smaller than the MVH at a density of 1.2 individuals/km2, while 42 remnants were smaller than the MVH at 2.3 individuals/km2. As a result, the estimated carrying capacity varied from less than one individual in the smaller patches (regardless of the density used) to 60,200 or 115,384 individuals in the largest remnant, respectively, based on the densities of 1.2 and 2.3 individuals/km2.
In the analyses of predicted effects of deforestation, we found that the extinction risk only increased for already demographically unviable populations (i.e., <53 individuals) at present, particularly those with a predicted size of 28 individuals or less. By contrast, viable populations do not become prone to extinction within 50 years (Figs. 4a, b). In the case of genetic diversity, deforestation was found to affect all populations negatively, but once again, with more substantial effects being predicted in populations that were genetically unviable over the long term, i.e., those with fewer than 109 individuals (Fig. 5; Table S5).
Remnants of the Caatinga dry forest in which populations of Callicebus barbarabrownae are predicted to be viable and nonviable up to 2070, based on the PVA. A – Serra da Vargem; B – Santa Brígida; C – Canudos; D – Euclides da Cunha/ Quijingue; E – Monte Santo; F – Itiúba; G – Andaraí, Campo Formoso, Contendas do Sincorá, Gentio do Ouro, Ibipeba, Lençóis, Miguel Calmon, Morro do Chapéu, Saúde, Ubiaí; H – Boa Vista do Tupim and Ruy Barbosa.
The reforestation simulations predicted a reduction of the mean probability of extinction, a slower mean time to extinction, and an increase in genetic diversity and final population size (Table S6, Electronic Supplementary Material). However, only populations larger than 53 individuals reached a probability of extinction of less than 2%, and only when we considered a forest recovery rate of at least 5% per annum. In relation to genetic viability, a shift from unviable to viable was only predicted for one population with 109 individuals (in Santa Brigida, Bahia), which was found to be marginally viable in the previous analyses, without reforestation. No level of reforestation (even 50% per annum) was found to prevent extinction in populations with nine individuals or less, with these extinctions being predicted to occur in less than 30 years.
Discussion
Our results show that the situation of nearly all the known C. barbarabrownae populations may deteriorate within this century. This trend includes the likely extirpation of the smallest populations and a decline in the large population inhabiting climatic suitable remnants. Perhaps most importantly, our findings were not affected in any major way by the population density estimate used in the analysis, although the density of C. barbarabrownae may vary even more across its range (Coelho et al., 2020; Freitas et al., 2011). Even though little is known of the geographic variation and the external drivers of the species’ population density, field observations suggest that habitat heterogeneity may play a critical role. Likely, heterogeneous habitats provide a highly diverse resource base throughout the year in the highly seasonal environment of the Caatinga (Freitas et al., 2011).
Our estimates of the total number of individuals for the species (60,200–115,384 individuals) were considerably larger than previous estimates (<250 mature individuals; Printes et al., 2016, 2020). These estimates derive from the available information and are based on the existing population ecology theory. However, they should be treated with caution, because they are likely to represent overestimates, especially considering that our initial population sizes were equal to the carrying capacity of the patches (i.e., N = K). This assumes that populations are at equilibrium with the environment in terms of resource use and demographic pressures, such as predation, hunting, and stochastic fluctuations, and that the entire region is homogeneous in terms of its habitat quality (Lacy, 2019). Nonetheless, hunting pressure, environmental fluctuations, and habitat heterogeneity vary considerably within the Caatinga, and the assumption that the whole of an occupied remnant is suitable for C. barbarabrownae often may be unrealistic.
Species Distribution
The moderate change from the current scenario to the future distribution pattern, combined with the similarities between the future scenarios, indicates that C. barbarabrownae is not especially susceptible to the effects of climate alone. This prospect is different from that found for primates in the Amazon region, to which climate alone can substantially reduce the species suitable areas (Sales et al., 2019). However, the trend predicted for C. barbarabrowne is similar to other groups of organisms in the Caatinga, such as plants (Silva et al., 2019) and invertebrates (Leal et al., 2017; Lira et al., 2020). However, considering the interaction between the predicted shift in distribution and the changes in the forest habitats, the suitable area is reduced further. Although C. barbarabrownae may be able to overcome these trends by dispersing into new areas, climate change is predicted to reduce its current range from the edges inward, thus limiting the potential of the species to disperse elsewhere. Furthermore, based on the dispersal capacity of congener species, of approximately 1.0-km across open areas, recent findings indicate that climate change also may decrease the functional connectivity within the range of C. barbarabrownae (Gouveia et al., 2016). In fact, the higher temperatures and lower humidity of the Caatinga imply that C. barbarabrownae might be even less tolerant of exposure in open areas in the Caatinga than in humid rainforest occupied by the other Callicebus species. These constraints may include overheating and dehydration, which may further reduce the dispersal potential of C. barbarabrownae in the Caatinga (Hill et al., 2004).
Population Viability
The PVA indicated a minimum viable population of 135 individuals, which would require a forest patch of ca. 60–110 km2, depending on the population density. This area is similar to that estimated (ca. 78 km2) for Sapajus xanthosternos (da Silva et al., 2016), a second critically endangered primate, whose geographic range partially overlaps with that of C. barbarabrownae. However, the much larger-bodied S. xanthosternos was predicted to achieve viability—considering no threats, over 100 years—with a much smaller population of only 5–6 individuals (Silva et al., 2016). Based on our findings, approximately 85% of all the remnants known to contain C. barbarabrownae will be unable to maintain viable populations. Our projections point to the occurrence of extirpation events in around 30 years, which will lead to further loss of genetic diversity and the potential of the species to resist other threats, such as climate change (Frankham et al., 2008).
We also predicted that neither deforestation nor reforestation appears to alter the current status of most populations, except for one population close to a viable size that may become viable after reforestation. In general, deforestation would accelerate the extirpation of habitats that are already unviable, and reforestation would not make them viable, reflecting the enormous variation in the size of the remnants. Most remnants are either too small to ensure the viability of their populations, even following reforestation, or large enough to avoid extinction, even if deforestation continues at current rates of 0.3% yr–1, which has remained virtually constant over the past 30 years (Projeto MapBiomas, 2020). However, the deforestation rate in the Caatinga has increased to an alarming 11.5% in the past 3 years (Projeto MapBiomas, 2020). If this trend is not reversed very shortly, even more extinctions than predicted and increasing pressure on the large populations may occur.
Our integrated approach offers an overview of the trends expected for C. barbarabrownae. Further research initiatives to improve our findings are needed, especially regarding the reliability of the population viability analysis, to refine conservation strategies for this species. In particular, field data are needed to overcome the extensive knowledge gaps in the life-history traits of C. barbarabrownae, which will improve the reliability of the extinction risk assessments. Nearly all the data used in our simulations were obtained from congener species, and it is still unclear to what extent these reproductive traits and mortality rates represent C. barbarabrownae. Some variation would be likely, given the need to adapt to the relatively arid and seasonal Caatinga. More comprehensive inventories of C. barbarabrownae populations will be relevant to elucidate the factors that determine the variation in the population density of this species, which will be important for planning management strategies. Systematic field monitoring, combined with genetic studies, will provide more accurate data on the genetic variability of large and small populations, providing critical insights into metapopulation structure and the potential of the species to resist climate change through adaptation (Andersen et al., 2017; Keith et al., 2008). These and other integrative approaches would considerably enhance our understanding of this critically endangered species, which will be increasingly important in the context of the significant ongoing threats to its survival, such as habitat loss and climate change.
Conservation guidelines
Our results hint at measures that may enhance the survival potential of the remaining C. barbarabrownae populations. In remnants with populations close to viability, reforestation may reduce extinction risk at the time scale investigated in the present study. However, as the size of the remnant is not the main issue, connectivity to larger remnants in the local landscape may be the principal priority. This would enhance gene flow and mitigate the inbreeding depression (Arroyo-Rodríguez & Mandujano, 2009; Marsh, 2003). This recommendation appears appropriate for the remnant at Monte Santo, Bahia (Fig. 5, remnant E).
While potentially maintaining a large quantity of habitat, many large remnants may be constrained by climate shifts, which may reduce existing habitat considerably, especially in the western and northern extremes of the study region. In these cases, the prevention of hunting, logging, and other impacts could be enough to ensure habitat quality and population densities. These measures also may enhance the chances of the populations to adapt to climate change over the long term through a process of evolutionary rescue (Diniz-Filho et al., 2019), given that larger populations and higher densities favor genetic diversity and adaptability (Moritz & Agudo, 2013). This strategy should be appropriate to the remnants in Canudos (Fig. 5, remnant C) and in the region that encompasses Andaraí, Campo Formoso, Contendas do Sincorá, Gentio do Ouro, Ibipeba, Lençóis, Miguel Calmon, Morro do Chapéu, Saúde, and Ubiaí (Fig. 5, remnant G; Table S1, Electronic Supplementary Material). To achieve these goals, expanding the existing network of protected areas in these areas would be a priority.
The small remnants are in a more critical situation, however. The populations of these remnants may not only lack demographic viability but may also lose climatic suitability. Even on a massive scale, reforestation may not be sufficient to prevent the extirpation of these populations. In addition to being small, these remnants are typically isolated by distances between 4 km and 23 km. Given the dispersal capacity of the species, even enhancing landscape connectivity is likely to be ineffective in these cases (Battin, 2004). Therefore, these populations will likely require close monitoring and eventually translocation if the local genetic pool is to be preserved. This scenario applies to the remnants in Sergipe state and the municipality of Candeal in Bahia.
Conclusions
Overall, by integrating extensive field data with spatial (SDM) and temporal (PVA) modeling approaches, we have provided an overview of the extinction risks faced by the blond titi monkey, Callicebus barbarabrownae, a Critically Endangered primate endemic to the Brazilian Caatinga. Within the next half century, this species will likely face a decline in climatic suitability, population losses, and genetic deterioration—a process already observed in its sister species: Callicebus coimbrai (Hilário et al., 2017). Over the long term, these changes may constrain the species to a few last refuges of occurrence. This prognosis relies on the assessment of two crucial threats (Garcia et al., 2014)—habitat loss and climate change—which may be exacerbated by other factors, such as predation pressure, hunting, epidemics, and endogamy (Keller & Waller, 2002). In addition, large-scale infrastructure projects in the region, such as power plants (hydro, solar, and wind), roads, and power lines, also should be planned in such a way as to minimize further habitat loss and fragmentation. Despite the uncertainties, in particular the need for more comprehensive data on the ecological characteristics of the species and its populations, adequate measures, supported by the findings of the present study, may ensure the survival of C. barbarabrownae in the coming decades. This integrated approach can be valuable for informing management measures to avert the local extirpation of declining populations of primate species.
References
Andersen, L. H., Sunde, P., Pellegrino, I., Loeschcke, V., & Pertoldi, C. (2017). Using population viability analysis, genomics, and habitat suitability to forecast future population patterns of Little Owl Athene noctua across Europe. Ecology and Evolution, 7(24), 10987–11001.
Araújo, M. B., & Peterson, A. T. (2012). Uses and misuses of bioclimatic envelope modeling. Ecology, 93(7), 1527–1539.
Arroyo-Rodríguez, V., & Mandujano, S. (2009). Conceptualization and measurement of habitat fragmentation from the primates’ perspective. International Journal of Primatology, 30(3), 497–514.
Barbet-Massin, M., Jiguet, F., Albert, C. H., & Thuiller, W. (2012). Selecting pseudo-absences for species distribution models: how, where and how many? Methods in Ecology and Evolution, 3(2), 327–338.
Battin, J. (2004). When good animals love bad habitats: Ecological traps and the conservation of animal populations. Conservation Biology, 18(6), 1482–1491.
Beissinger, S. R., & McCullough, D. R. (2002). Population viability analysis. University of Chicago Press.
Bicca-Marques, J. C., & Heymann, E. H. (2013). Ecology and behaviour of titi monkeys (genus Callicebus). In L. M. Veiga, A. Barnett, S. F. Ferrari, & M. Norconk (Eds.), Evolutionary biology and conservation of titis, sakis, and uacaris (pp. 196–207). Cambridge University Press.
Boyce, M. S. (1992). Population viability analysis. Annual Review of Ecology and Systematics, 23(1), 481–506.
Brasil. (2004). Programa de Ação Nacional de Combate à Desertificação e Mitigação dos Efeitos da Seca/PAN-Brasil. Brasília (DF): Ministério do Meio Ambiente/Secretaria de Recursos Hídricos. 214 pp.
Brasil/ICMBio (Instituto Chico Mendes de Conservação da Biodiversidade). (2011). Plano de Ação Nacional para a Conservação dos Primatas do Nordeste: Sumário Executivo. Available at: http://www.icmbio.gov.br/portal/images/stories/docs-plano-de-acao/pan-primatas-caatinga/sumario-primatas-nordeste-web.pdf.
Brasil/ICMBio (Instituto Chico Mendes de Conservação da Biodiversidade). (2018). Plano de Ação Nacional para a Conservação dos Primatas do Nordeste: Sumário Executivo. Available at: https://www.icmbio.gov.br/portal/faunabrasileira/plano-de-acao-nacional-lista/865-pan-primatas-do-nordeste
Brito, D., & Grelle, C. E. V. (2006). Estimating minimum area of suitable habitat and viable population size for the northern muriqui (Brachyteles hypoxanthus). Biodiversity & Conservation, 15(13), 4197–4210.
Brook, B. W., Sodhi, N. S., & Bradshaw, C. J. (2008). Synergies among extinction drivers under global change. Trends in Ecology & Evolution, 23(8), 453–460.
Brook, B. W., Akçakaya, H. R., Keith, D. A., Mace, G. M., Pearson, R. G., & Araújo, M. B. (2009). Integrating bioclimate with population models to improve forecasts of species extinctions under climate change. Biology Letters, 5, 723–725.
Castelletti, C. H. M., Silva, J. M. C., Tabarelli, M., & Santos, A. M. M. (2004). Quanto ainda resta da Caatinga? Uma estimativa preliminar. In J. M. C. Silva, M. Tabarelli, & M. F. e. L. Lins (Eds.), Biodiversidade da Caatinga: áreas e ações prioritárias para a conservação (pp. 91–100). Ministério do Meio Ambiente.
Coelho, I. P., Collins, S. J., Santos Júnior, E. M., Valença-Montenegro, M. M., Jerusalinsky, L., & Alonso, A. C. (2020). Playback point counts and N-mixture models suggest higher than expected abundance of the critically endangered blond titi monkey in northeastern Brazil. American Journal of Primatology, 82(5), e23126.
Corsini, C. F., & Moura, A. C. A. (2014). Census of the Blond Titi Monkey Callicebus barbarabrownae (Pitheciidae) in the Semi-Deciduous Atlantic Forest of Chapada Diamantina. Brazil. Neotropical Primates, 21(2), 177–182.
da Silva, F. A., Canale, G. R., Kierulff, M. C. M., Duarte, G. T., Paglia, A. P., & Bernardo, C. S. (2016). Hunting, pet trade, and forest size effects on population viability of a critically endangered Neotropical primate, Sapajus xanthosternos (Wied-Neuwied, 1826). American Journal of Primatology, 78(9), 950–960.
Diniz-Filho, J. A. F., Souza, K. S., Bini, L. M., Loyola, R., Dobrovolski, R., Rodrigues, J. F. M., ... & Freitas, R. (2019). A macroecological approach to evolutionary rescue and adaptation to climate change. Ecography, 42(6), 1124-1141.
ESRI – Environmental Systems Research Institute. (2008). Inc. ArcGIS Professional GIS for the desktop, version 9.3. Software.
Fischer, J., & Lindenmayer, D. B. (2007). Landscape modification and habitat fragmentation: a synthesis. Global Ecology and Biogeography, 16, 265–280.
Frankham, R., Ballou, J. D., & Briscoe, D. A. (2008). Fundamentos de genética da conservação. Sociedade Brasileira de Genética.
Franklin, J. (2010). Moving beyond static species distribution models in support of conservation biogeography. Diversity and Distributions, 16, 321–330.
Freitas, E. B., De-Carvalho, C. B., & Ferrari, S. F. (2011). Abundance of Callicebus barbarabrownae (Hershkovitz 1990), (Primates: Pitheciidae) and other nonvolant mammals in a fragment of arboreal Caatinga in Northeastern Brazil. Mammalia, 75, 339–343.
Galán-Acedo, C., Arroyo-Rodríguez, V., & Chapman, C. A. (2021). Beyond patch size: The impact of regional context and habitat quality on three endangered primates. Perspectives in Ecology and Conservation, 19(2), 207–215.
Garcia, R. A., Cabeza, M., Rahbek, C., & Araújo, M. B. (2014). Multiple dimensions of climate change and their implications for biodiversity. Science, 344(6183).
Gilpin, M. E., & Soulé, M. E. (1986). Minimum viable populations: processes of extinction. In M. E. Soulé (Ed.), Conservation Biology: The Science of Scarcity and Diversity (pp. 19–34). Sinauer, Sunderland.
Gouveia, S. F., Souza-Alves, J. P., Rattis, L., Dobrovolski, R., Jerusalinsky, L., Beltrão-Mendes, R., & Ferrari, S. F. (2016). Climate and land use changes will degrade the configuration of the landscape for titi monkeys in eastern Brazil. Global Change Biology, 22(6), 2003–2012.
Hijmans, R. J. (2014). Raster: Geographic data analysis and modeling. R package, version 2.3-12. http://CRAN.R-project.org/package=raster.
Hijmans, R. J., Cameron, S. E., Parra, J. L., Jones, P. G., & Jarvis, A. (2005). Very high resolution interpolated climate surfaces for global land areas. International Journal of Climatology, 25(15), 1965–1978.
Hilário, R. R., Jerusalinsky, L., Santos, S., Beltrão-Mendes, R., & Ferrari, S. F. (2017). A primate at risk in Northeast Brazil: local extinctions of Coimbra Filho’s titi (Callicebus coimbrai). Primates, 58(2), 343–352.
Hill, R. A., Weingrill, T., Barrett, L., & Henzi, S. P. (2004). Indices of environmental temperatures for primates in open habitats. Primates, 45(1), 7–13.
Jerusalinsky, L. (2013). Distribuição geográfica e conservação de Callicebus coimbrai Kobayashi & Langguth, 1999 (Primates – Pitheciidae) na Mata Atlântica do nordeste do Brasil. PhD. Dissertation, Graduate Program in Biological Sciences (Zoology). Federal University of Paraíba. 228p.
Kamilar, J. M., & Cooper, N. (2013). Phylogenetic signal in primate behaviour, ecology and life history. Philosophical Transactions of the Royal Society B: Biological Sciences, 368(1618), 20120341.
Keith, D. A., Akçakaya, H. R., Thuiller, W., Midgley, G. F., Pearson, R. G., Phillips, S. J., ... & Rebelo, T. G. (2008). Predicting extinction risks under climate change: coupling stochastic population models with dynamic bioclimatic habitat models. Biology Letters, 4(5), 560-563.
Keller, L. F., & Waller, D. M. (2002). Inbreeding effects in wild populations. Trends in Ecology & Evolution, 17(5), 230–241.
Lacy, R. C. (1993). VORTEX: a computer simulation model for population viability analysis. Wildlife Research, 20(1), 45–65.
Lacy, R. C. (2019). Lessons from 30 years of population viability analysis of wildlife populations. Zoo biology, 38(1), 67–77.
Lacy, R. C., & Pollak, J. P. (2014). Vortex: A Stochastic Simulation of the Extinction Process. Version 10.0. Chicago Zoological Society.
Leal, I. R., Ribeiro-Neto, J. D., Arnan, X., Oliveira, F. M., Arcoverde, G. B., Feitosa, R. M., & Andersen, A. N. (2017). Ants of the Caatinga: diversity, biogeography, and functional responses to anthropogenic disturbance and climate change. In Caatinga - The Largest Tropical Dry Forest Region in South America (pp. 65–95). Springer.
Lira, A. F. D. A., Badillo-Montaño, R., Lira-Noriega, A., & de Albuquerque, C. M. R. (2020). Potential distribution patterns of scorpions in northeastern Brazil under scenarios of future climate change. Austral Ecology, 45(2), 215–228.
Marques, E. L. N., Beltrão-Mendes, R., & Ferrari, S. F. (2013). Primates, Pitheciidae, Callicebus barbarabrownae Hershkovitz, 1990: new localities for the critically endangered titi monkey in the São Francisco basin, state of Sergipe, Brazil [with erratum]. Check List, 9(1), 113–115.
Marsh, L. K. (2003). The nature of fragmentation. In L. K. Marsh (Ed.), Primates in fragments (pp. 1–10). Springer.
Moritz, C., & Agudo, R. (2013). The future of species under climate change: Resilience or decline? Science, 341(6145), 504–508.
Moss, R. H., Babiker, M., Brinkman, S., Calvo, E., Carter, T., Edmonds, J. A., ... & Zurek, M. (2008). Towards new scenarios for analysis of emissions, climate change, impacts, and response strategies. Intergovernmental Panel on Climate Change. p. 132.
Naimi, B., & Araújo, M. B. (2016). sdm: a reproducible and extensible R platform for species distribution modelling. Ecography, 39(4), 368–375.
Parmesan, C. (2006). Ecological and evolutionary responses to recent climate change. Annual Review of Ecology, Evolution, and Systematics, 37, 637–669.
Peterson, A. T., Papes, M., & Soberón, J. (2008). Rethinking receiver operating characteristic analysis applications in ecological niche modeling. Ecological Modelling, 213(1), 63–72.
Peterson, A. T., Soberón, J., Pearson, R. G., Anderson, R. P., Martínez-Meyer, E., Nakamura, M., & Araújo, M. B. (2011). Ecological niches and geographic distributions (MPB-49). Princeton University Press.
Phillips, S. J., Dudík, M., Elith, J., Graham, C. H., Lehmann, A., Leathwick, J., & Ferrier, S. (2009). Sample selection bias and presence-only distribution models: implications for background and pseudo-absence data. Ecological Applications, 19, 181–197.
Printes, R. C. (2007). Avaliação taxonômica, distribuição e status do guigó-da-caatinga Callicebus barbarabrownae Hershkovitz, 1990 (Primates: Pitheciidae). PhD. Dissertation, Federal University of Minas Gerais, Belo Horizonte, Brazil.
Printes, R. C. (2011). Etnoprimatologia e conservação de Callicebus barbarabrownae. In J. M. D. Miranda & Z. M. B. Hirano (Eds.), A primatologia no Brasil – Volume 12 (pp. 15–29). UFPR.
Printes, R. C., Alonso, A. C. & Jerusalinsky, L. (2016). Processo de avaliação do risco de extinção da fauna brasileira. Instituto Chico Mendes de Conservação da Biodiversidade ICMBio. http://www.icmbio.gov.br/portal/faunabrasileira/lista-de-especies/8035-mamiferos-callicebus-barbarabrownae-guigo-da-caatinga.
Printes, R., Alonso, A. C., Jerusalinsky, L. & Mittermeier, R. A. (2020). Callicebus barbarabrownae. The IUCN Red List of Threatened Species 2020. https://doi.org/10.2305/IUCN.UK.2020-2.RLTS.T39929A17974834.en. Accessed 04 August 2020.
Projeto MapBiomas – v. 5.0. Sistema de Validação e Refinamento de Alertas de Desmatamento com Imagens de Alta Resolução. Available at: https://mapbiomas.org/. Accessed June 2020.
R Development Team (2020). R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing.
Reed, J. M., Mills, L. S., Dunning Jr, J. B., Menges, E. S., McKelvey, K. S., Frye, R., ... & Miller, P. (2002). Emerging issues in population viability analysis. Conservation Biology, 16, 7-19.
Sales, L. P., Ribeiro, B. R., Pires, M. M., Chapman, C. A., & Loyola, R. (2019). Recalculating route: dispersal constraints will drive the redistribution of Amazon primates in the Anthropocene. Ecography, 42, 1789–1801.
Silva, J. L. S. E., Cruz-Neto, O., Peres, C. A., Tabarelli, M., & Lopes, A. V. (2019). Climate change will reduce suitable Caatinga dry forest habitat for endemic plants with disproportionate impacts on specialized reproductive strategies. PloS one, 14(5), e0217028.
Torres, R. R., Lapola, D. M., & Gamarra, N. L. R. (2017). Future Climate Change in the Caatinga. In J. M. D. da Silva, I. R. Leal, & M. Tabarelli (Eds.), Caatinga – The Largest Tropical Dry Forest Region in South America (pp. 383–410). Springer.
Travis, J. M. J. (2003). Climate change and habitat destruction: a deadly anthropogenic cocktail. Proceedings of the Royal Society B: Biological Sciences, 270(1514), 467–473.
VanDerWal, J., Falconi, L., Januchowski, S., Shoo, L. & Storlie, C. (2014). MDSTools: tools for processing data associated with species distribution modelling exercises. – R package, ver. 1.1-20. http://www.rforge.net/MDSTools/.
Acknowledgments
HFB thanks the Instituto Chico Mendes de Conservação da Biodiversidade (ICMBio) for an undergraduate scholarship granted through the Programa de Bolsas de Iniciaico Científica (PIBIC) and Coordenção de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for a graduate scholarship (DS). ACA was supported by a stipend granted by ICMBio-CNPq covenant, RMB by a CAPES-PNPD post-doctoral fellowship (88887.320996/2019-00), SFG and SFF by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq 302854/2019-3 and 310852/2017-0, respectively). This project was also supported by CAPES/FAPITEC grants (#88881.157451/2017-01 and #88881.157961/2017-01) provided to SFF and SFG. SFG is a member of the National Institute of Science and Technology - Ecology, Evolution, and Conservation of Biodiversity (INCT-EECBio, CNPq/FAPEG, grant 380733/2017-0).
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Joanna Setchell
Supplementary Information
ESM 1
(DOCX 371 kb)
Rights and permissions
About this article
Cite this article
Barreto, H.F., Jerusalinsky, L., Eduardo, A.A. et al. Viability meets suitability: distribution of the extinction risk of an imperiled titi monkey (Callicebus barbarabrownae) under multiple threats. Int J Primatol 43, 114–132 (2022). https://doi.org/10.1007/s10764-021-00259-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10764-021-00259-7