Abstract
Multiple anthropogenic drivers of extinction risk in primates are increasing. The expansion of urban areas, road networks, and agricultural frontiers are threatening primates through habitat loss, fragmentation, and increased incidences of hunting. Man-made climate change is affecting habitat quality and availability, particularly in rare ecosystems. Three of Peru’s endemic primate species, the yellow-tailed woolly monkey (Lagothrix flavicauda), the San Martin titi monkey (Plecturocebus oenanthe Sensu, Byrne H, Rylands AB, Carneiro JC, Alfaro JWL, Bertuol F, da Silva MNF, Messias M et al. (2016) Phylogenetic relationships of the New World titi monkeys (Callicebus): first appraisal of taxonomy based on molecular evidence. Frontiers in Zoology 13:1–26) and the Peruvian night monkey (Aotus miconax), have naturally restricted distributions in the foothills of the country’s northeastern Andes. Montane forest habitat in this area not only suffers from the highest rates of deforestation in the country but is also predicted to be among the most at risk areas from the effects of man-made climate change. Using data from extensive published and unpublished field surveys, this study modeled the species’ historical, current, and future distributions. To best estimate the effects of multiple drivers of extinction risk, I used models of future climate change scenarios coupled with predications of expanding human settlement and hunting over multiple timescales. Results of these models predict a reduction in niche availability for A. miconax and L. flavicauda and an increase in niche availability for P. oenanthe. In all cases predicted habitat loss was less than in previous studies. However, when taking into account anthropogenic disturbance, habitat loss is much more severe. I suggest that predictive modeling is a useful tool for conservation, but should always use the most up-to-date data and results should be interpreted with caution based on expert knowledge of the species and area. Future climate change is predicted to increase threats to many species but deforestation and hunting will remain the major threats for many primates.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Species distribution model
- Maximum entropy
- Aotus miconax
- Callicebus oenanthe
- Lagothrix flavicauda
- Climate change
- Agricultural frontier
Introduction
Conservation in the twenty-first century is predicted to be dominated by mitigation of the effects of man-made climate changes and the ramifications to natural systems and the species that depend on them (Bonan 2013; Laurance and Williamson 2001; Lewis 2006; Thomas et al. 2004; van Aalst 2006). Some changes are already being observed in temperatures, precipitation levels, cloud formation, and secondary impacts such as changes in plant phenologies that are predicted to have drastic consequences for ecosystems and species (Bertin 2008; Dore 2005; Lenoir et al. 2008; McCarty 2001; van Aalst 2006; Walther et al. 2002). Of the world’s major biomes, tropical montane forests will be one of the most severely affected (Bubb et al. 2004; Foster 2001; Herzog 2011; Still et al. 1999), with many more localized climate changes seen in air temperatures, cloud formation, and cloud capture (Pielke et al. 2002). This will affect many primates and other species that have restricted distributions or specialized habitat requirements (Newbold et al. 2014). Currently, all but one primate species listed as Critically Endangered on the IUCN Redlist of Threatened Species have restricted distributions (IUCN 2013), 26 of which have distributions in Montane habitats, five of which are entirely restricted to montane and pre-montane areas (Shanee 2013).
Another major concern for conservationists is the continued expansion of agricultural frontiers to support a growing human population and its demand for food and other resources (Fearnside 1983; Garland 1995; Newbold et al. 2014; Perz et al. 2005; Sanchez-Cuervo and Aide 2013; Wyman and Stein 2009). As much of the worlds suitable lands have already been converted to agricultural production, new frontiers are opening up in areas less suited to clearance, including montane ecosystems (Cayuela et al. 2006; Hall et al. 2009). The clearance of montane areas for agriculture works in tandem with climate changes to intensify local scale effects, increasing air temperatures, which in turn slows cloud formation and lowers precipitation levels, slowing forest regeneration. Heavier downpours increase erosion on slopes, further limiting forest regeneration, lowering soil fertility, necessitating the clearance of more areas for cultivation, resulting in a dangerous cycle (Laurance and Williamson 2001; Pielke et al. 2002).
The montane and pre-montane forests of northern Peru lie at the heart of the Tropical Andes Biodiversity Hotspot and are among the most threatened forested areas in the world (Myers et al. 2000; Robles Gil et al. 2004). Peru’s northern regions of Amazonas and San Martin suffer from the highest immigration and deforestation rates in the country (INEI 2008; PROCLIM/CONAM 2005; Reategui and Martinez 2007) accounting for approximately 18 % of Amazonian forest loss in Peru in the year 2000 (INRENA 2005). The tropical Andes are home to incredible levels of biodiversity with ~30,000 vascular plant species, 50 % of which are endemic, and the highest number of vertebrate species of any “Biodiversity Hotspot” (Myers et al. 2000). This includes 584 species and 69 genera of endemic birds. Diversity and endemism of mammals is similarly high with at least 75 species and five monotypic genera endemic to the area (Myers 2003; Myers et al. 2000).
Peru’s cloud forests account for only 5 % of the country’s 700,000 km2 tropical forests (Bubb et al. 2004) but species diversity is comparable to that of the much more extensive eastern Amazonian lowlands (Pacheco et al. 2009). In particular, the area between the Marañón and Huallaga rivers, ~8000 km2, has very high levels of endemism but is also severely threatened by logging, slash and burn and industrialized agriculture (Schjellerup 2000; Shanee 2012a), subsistence and commercial hunting (Shanee 2012b), and the cultivation of illicit crops such as coca ( Erythroxylum coca ) and opium poppies ( Papaver somniferum ). The production of these illicit crops is a double threat to the environment. The production causes habitat loss and contamination whilst the measures employed to control them, such as defoliant sprays and forced crop clearances, not only remove the crops but also affect neighboring forests and increase deforestation when producers relocate to new areas (Dourojeanni 1989; Fjeldså et al. 1999, 2005; Young 1996).
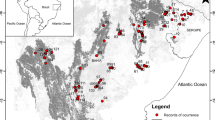
Three of Peru’s eight endemic primates (Boubli et al. 2012; Alfaro et al. 2012a, b; Marsh et al. 2013; Matauschek et al. 2011; Wilson et al. 2013) have distributions restricted to the Marañón-Huallaga landscape (Shanee et al. 2013b). The yellow-tailed woolly monkey (Lagothrix flavicauda), the San Martin titi monkey (Plecturocebus oenanthe), and the Peruvian night monkey (Aotus miconax) are all considered threatened by the IUCN (2013), with L. flavicauda and P. oenanthe considered Critically Endangered and A. miconax considered Endangered. Until recently, very little was known about these species, but recent conservation-based research programs have provided basic data on the distribution and ecology of these species (For example, Bóveda-Penalba et al. 2009; Buckingham and Shanee 2009; deLuycker 2007a, b; Shanee 2011; Shanee et al. 2011, 2013a, 2015). This information has been vital in providing a better understanding of threats, conservation need, and population trends for these species. The very restricted distributions of the three species are probably a result of the high levels of habitat heterogeneity in the area which is almost completely surrounded by the Maranon and Huallaga river valley’s (Fig. 1) divided by isolated areas of dry forests, high mountain ridges, and deep river valleys, impeding dispersion into new areas and creating localized bioclimatic zones (Shanee et al. 2013b, c, 2015).
Computer-based modeling of species distributions and ecological niches has become popular in recent years with better access to more powerful processors and dedicated software (Bocedi et al. 2014; Brown 2014; de Souza Muñoz et al. 2011; Goodchild et al. 1996; Guo and Liu 2010; Phillips et al. 2006; Skidmore 2004; Thuiller et al. 2009). Computer-based modeling is particularly useful when field surveys are made difficult by the physical impediments of the terrain or sociopolitical factors limiting researchers’ access to some areas within a species distribution. Many different modeling techniques exist, each with its own advantages and disadvantages (Elith and Graham 2009; Elith et al. 2006; Guisan et al. 2007; Guo and Liu 2010; Thuiller et al. 2009), in recent years ecological niche modeling with Maxent program (Phillips et al. 2006) has proven to be a robust presence-only modeling technique that balances accuracy with limitations on data availability, time, and model complexity. In addition, many tools and recommendation on how best to use Maxent for modeling have been developed to further robustness of models (Brown 2014; Warren et al. 2010). Similarly, computer modeling provides the best option for predicting the effects of future climate changes. These predictions are constantly being refined with many models now freely available to researchers (Hijmans et al. 2005; Kriticos et al. 2012).
For this chapter, I aim to model the possible effects of future climate changes on the distributions of three of Peru’s endemic and most endangered primate species. Building on this, I will model the effect of various simple thresholds as proxies to simulate expansion of the agricultural frontier and hunting pressure . This is done to highlight the challenges and opportunities climate changes may present for conservation for these species. Also, to examine the utility of GIS-based predicative modeling, balancing complexity with robustness.
Methods
Species and Distributions
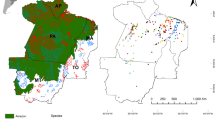
Lagothrix flavicauda, Plecturocebus oenanthe, and Aotus miconax are endemic to a small area of northern Peru in the departments of Amazonas and San Martin (Shanee et al. 2015; Aquino and Encarnación 1994; Bóveda-Penalba et al. 2009; Shanee 2011). Lagothrix flavicauda and A. miconax are sympatric throughout the majority of their distributions (Fig. 2a, c) on the eastern slopes of the Andes in a thin band of montane cloud forest between approx. S78° 12′30″ and S75°24′55″ at altitudes ranging from 1500 to 2800 m above sea level, (MSL) although in some areas they are found at slightly higher or lower altitudes (Allgas et al. 2014; Campbell 2011; Shanee et al. 2013b, c). Plecturocebus oenanthe is restricted to the pre-montane area of the Rio Mayo valley south to the west of the Rio Huallaga as far as the Rio Huyallabamba (Fig. 2b) in lowland terra firme and tropical dry forests at elevations between 200 and 1200 MSL (Bóveda-Penalba et al. 2009). This species has been also been reported in small areas outside of these boundaries (Bóveda-Penalba et al. 2009; Shanee et al. 2013b; Vermeer et al. 2011).
Map showing the estimated distributions of the focal species in northern Peru, (a) A. miconax, (b) P. oenanthe, and (c) L. flavicauda, with insets showing location of study area. Distribution maps adapted from Rowe and Myers (2012)
Data Collection and Preparation
I used point data for each species from confirmed presence localities in previously published studies and my own fieldwork (Shanee et al. 2015; Bóveda-Penalba et al. 2009; Shanee 2011). This gave a total of 48 points for Lagothrix flavicauda, 110 points for Plecturocebus oenanthe, and 73 points for Aotus miconax. To remove spatially non-independent localities and clusters of points, I spatially rarefied occurrence data using three natural breaks between 5 and 25 km2. For bioclimatic variables layers, I used freely available data sets from Worldclim (Hijmans et al. 2005). This provided me with 19 bioclimatic layers representing different environmental variables (Table 1). All layers were clipped to the bounds of a polygon layer of Peru’s national borders. I then carried out a principle component analysis (PCA) of climate heterogeneity, variable layers that showed high levels of homogeneity were then removed from subsequent analyses. I then made bias files for selection of locations for background and pseudo-absence points for use in predictions to limit errors of commission (e.g., overprediction of the model) (Anderson and Raza 2010; Phillips et al. 2009). Bias files were created using a minimum convex polygon (MCP) buffered to 200 km outside of sample presence points. I then carried out a spatial jackknife to evaluate which model performed best and used this for final modeling (Boria et al. 2014; Radosavljevic and Anderson 2014; Shcheglovitova and Anderson 2013).
For future predictions, I downloaded four sets of layers representing the same bioclimatic variables produced by the International Panel on Climate Change fifth assessment (Raper 2012; Rogelj et al. 2012). I chose the models from the NASA Goddard space institute, these layers are freely available from Worldclim (Hijmans et al. 2005). The future bioclimatic layers represented predictions of conditions under different greenhouse gas representative concentration pathways (RCP) in different years (Moss et al. 2010): RCP = 26 and 85 for 2050 and 2070. These layers had the same spatial resolution as the layers used in the initial analysis and were also clipped within the bounds of the Peru polygon. The final model, based on the results of spatial jackknifing, was then projected onto these climate scenarios. As standard, ROC (Receiver Operating Characteristic) curve and AUC (Area Under Curve) were used as measures of the predictive power and fit of the models (Peterson et al. 2007; Merckx et al. 2011).
Final Modeling
Even using bias files to limit the possible extent of niche predictions the Maxent models can over predict possible niche area when compared to known geographical barriers limiting the species’ distributions, often including areas outside of a species’ actual or historical distribution. To rectify this all model outputs for A. miconax and L. flavicauda were clipped to areas within the bounds of a polygon, representing the area between the Maranon and Huallaga rivers (Fig. 1). Similarly, outputs for P. oenanthe were clipped within a polygon representing the area between the Maranon and Huallaga rivers north of the Huyllabamba River and south of the eastern cordillera that forms the margin of the Mayo River Valley (Fig. 1). I then divided model predictions into ten equally sized classes representing different probability levels of species presence, the two lowest classes (0–9.9 and 10–19.9 %) were then removed to reduce errors of commision. The remaining eight classes were then divided into two subclasses representing two levels of probability (Good and Very Good). I then calculated the area of each subclass and overall as a measure of original habitat extension for each species (Table 2). To calculate the current area of occupancy of each species, I overlaid a forest cover layer (Hansen et al. 2013) to the outputs, removing areas with less than 50 % forest cover from predictions as areas without suitable habitat (Shanee et al. 2015; Wyman et al. 2011) and calculated the area in km2 of each subclass and overall to estimate the current distribution of the species. I repeated this for the four future climate scenario predictions. To predict the effect of future expansion of the agricultural frontier and the effect of hunting on the species, I created two thresholds of moderate and high hunting pressure/possibility of deforestation within the 35- and 55-year time frames used in the climate change analysis. Thresholds were set at areas <1 km (high pressure) and <5 km (moderate pressure) away from human settlement and infrastructure for high and moderate pressure, respectively. Additionally, I remodeled these thresholds including only habitat outside of protected areas to see if the current protected area system will be sufficient to support viable populations of the three species taking into account possible changes in niche occurrence with future climate changes.
Results
After multi-distance spatial rarefying of the original species occurrence points (48 for Lagothrix flavicauda, 110 for Plecturocebus oenanthe , and 73 points for Aotus miconax) to remove spatially non-independent localities and clusters, the number of data points for L. flavicauda, P. oenanthe, and A. miconax used in subsequent analyses were 34, 45, and 39, respectively. The PCA for climatic heterogeneity showed high autocorrelation of variables in 11 of the 19 bioclimatic layers; therefore, I used only eight in the final model, Annual Mean Temperature, Mean Diurnal Range, Isothermality, Temperature Seasonality, Annual Precipitation, Precipitation Seasonality, Precipitation of Warmest Quarter, and Precipitation of Coldest Quarter.
Model Results
The results from models projected onto the future bioclimatic layers showed no significant differences between predictions for years or RCP levels (All p > 0.001); therefore, results presented here are averages across the four different year/RCP combinations for each species.
Aotus miconax
The final ecological niche model for Aotus miconax gave an ROC curve AUC of 0.913 for training data. Minimum training presence was 0.473. Results of the jackknife test showed the environmental variable with highest gain when used alone was annual precipitation. The environmental variable that decreased gain the most when omitted was annual mean temperature.
When clipped to within known geographical boundaries and excluding cells in the lowest two probability levels, the total original possible extent of occurrence of A. miconax was ~37,220 km2. After reclassification into two subclasses representing Good and Very Good probabilities of species presence (20–59.9 and 60–100 %), estimated original niche sizes were 22,640 and 14,580 km2 for each subclass. After deforested areas were removed from these predications (areas <50 % forest cover) the current maximum possible extent of occurrence is ~29,990 km2 of which 17,930 km2 was classed as Good and 12,060 km2 was classed as Very Good. Future climate changes are predicted to reduce niche availability for A. miconax by a further 9 %, the most affected probability subclass is predicted Very Good category with a further 16 % loss. Including the 1 and 5 km future deforestation/hunting pressure buffers there is a 16 and 53 % loss of niche availability. This is reduced to 15 and 44 % for the two respective buffer thresholds when assuming no future habitat loss or hunting within protected areas.
Plecturocebus oenanthe
The final ecological niche model for Callicebus oenanthe gave an ROC curve AUC of 0.951 for training data. Minimum training presence was 0.370. Results of the jackknife test showed the environmental variable with highest gain when used alone was precipitation of the coldest quarter. The environmental variable that decreased gain the most when omitted was annual mean temperature.
When clipped to within known geographical boundaries and excluding cells in the lowest two probability levels, the total original possible extent of occurrence of C. oenanthe was ~6,992 km2. After reclassification into two subclasses representing Good and Very Good probabilities of species presence (20–59.9 and 60–100 %), estimated original niche sizes were 4,335 and 2,657 km2 for each subclass. After deforested areas were removed from these predications (areas <50 % forest cover), the current maximum possible extent of occurrence is ~5,547 km2 of which 3,628 km2 was classed as Good and only 1,919 km2 was classed as Very Good. Future climate changes are predicted to increase niche availability for C. oenanthe by almost 24 %, the largest increase is predicted to be in the Very Good category with an increase of over 100 % in niche availability. Including the 1 and 5 km future deforestation/hunting pressure buffers there is a loss of 6 and 72 % of niche availability. This was reduced to 26 and 50 % for each respective buffer threshold when assuming no future habitat loss or hunting within protected areas.
Lagothrix flavicauda
The final ecological niche model for Lagothrix flavicauda gave an ROC curve AUC of 0.910 for training data. Minimum training presence was 0.387. Results of the jackknife test showed the environmental variable with highest gain when used alone was precipitation of the warmest quarter. The environmental variable that decreased gain the most when omitted was precipitation seasonality.
When clipped to within known geographical boundaries, and excluding cells in the lowest two probability levels, the total original possible extent of occurrence of L. flavicauda was ~57,910 km2. After reclassification into two subclasses representing Good and Very Good probabilities of species presence (20–59.9 and 60–100 %), estimated original niche sizes were 37,150 and 20,760 km2 for each subclass. After deforested areas were removed from these predications (areas <50 % forest cover), the current maximum possible extent of occurrence is ~39,060 km2 of which 22,460 km2 was classed as Good and 16,600 km2 was classed as Very Good. Future climate changes are predicted to reduce niche availability for L. flavicauda by a further 7 %, the most affected of the probability subclasses is predicted Good category with a further 18 % loss, the Very Good category is predicted to increase by 7 %. Including the 1 and 5 km future deforestation/hunting pressure buffers there is an additional predicted 16 and 54 % loss of niche availability. This is reduced to 12 and 46 % for each respective buffer threshold when assuming no future habitat loss or hunting within protected areas.
Discussion
The areas of the original ecological niches modeled here for A. miconax and L. flavicauda are similar to those from previous GIS-based studies (Shanee et al. 2015; Buckingham and Shanee 2009). The largest difference found was in the estimated original niche size for P. oenanthe, which is much smaller than previous studies have estimated (Ayres and Clutton-Brock 1992; Shanee et al. 2011). Similarly, future climate changes are predicted to reduce the available niche area for A. miconax and L. flavicauda, whereas niche area for C. oenanthe is actually predicted to increase with future climate changes, even when taking into account expansion of the agricultural frontier. Actual levels of habitat loss for all three species are estimated here to be much lower than previous predictions (Buckingham and Shanee 2009; Shanee et al. 2011). The use of a 50 % forest cover threshold for species habitat does not include the effect of hunting pressure, which is high for all three species, particularly L. flavicauda (Shanee 2012b), nor does it take into account the effect of habitat fragmentation on the species’ dispersal ability. Using the <1 and <5 km thresholds may give a truer picture of actual presence of species, as many available areas which have the correct bioclimatic conditions may not currently hold populations of these species.
As with all modeling, the predictions presented here are only as good as the data available. I am confident that I have used the most complete data sets for species presence points, including results from several recently published exhaustive field studies (Shanee et al. 2013b, 2015; Bóveda-Penalba et al. 2009; Shanee 2011). By using only published data and localities from my own recent field surveys and those of researchers whose methods are known, I have avoided problems of unreliability of data downloaded from internet databases, museum collections, and other sources where accuracy of species data points is uncertain (Chan et al. 2011; Graham et al. 2008).
The resolution of data layers used in modeling effect the robustness of results, with finer resolutions generally producing better results (Vale et al. 2014). The bioclimatic data sets I used have a resolution of ~1 km which allow for the models to include all but micro-scale gradients in niche presence (Elith and Graham 2009). Comparing the Maxent outputs and the distribution maps for A. miconax and L. flavicauda given by Rowe and Myers (2012) (Fig. 2a, c), this limitation can be seen clearly in central Amazonas, where the species are not present (Shanee et al. 2015; Shanee 2011) but the correct bioclimatic conditions exist (Figs. 3, 4, and 5). Even so, when the deforestation layer was applied to models the corresponding area is largely removed from the resulting distribution predictions. Scale is another factor that can influence applicability of models (Guisan and Thuiller 2005; Suárez-Seoane et al. 2014). By using bias files to limit gain and results of commission models were improved (Guisan and Thuiller 2005). I corrected problems of over prediction by clipping model outputs to known geographic barriers. Other problems in accuracy of modeling can occur from spatial autocorrelation of point data, inflating measures of accuracy (Veloz 2009), and spatial heterogeneity of bioclimatic layers. By carrying out a PCA of climate variables and spatially rarefying locality data, I was able to limit the possible effect of these problems on model results (Boria et al. 2014; Veloz 2009).
(a) Prediction of the original ecological niche area of A. miconax, (b) Current habitat availability for A. miconax, original niche area minus current deforestation, (c) Predicted future habitat availability for A. miconax based on modeling results, with areas of current deforestation removed, and (d) Predicted future habitat availability for A. miconax, including 1 and 5 km thresholds of predicted deforestation and hunting
(a) Prediction of the original ecological niche area of P. oenanthe, (b) Current habitat availability for P. oenanthe, original niche area minus current deforestation, (c) Predicted future habitat availability for P. oenanthe based on modeling results, with areas of current deforestation removed, and (d) Predicted future habitat availability for P. oenanthe, including 1 and 5 km thresholds of predicted deforestation and hunting
(a) Prediction of the original ecological niche area of L. flavicauda, (b) Current habitat availability for L. flavicauda, original niche area minus current deforestation, (c) Predicted future habitat availability for L. flavicauda based on modeling results, with areas of current deforestation removed, and (d) Predicted future habitat availability for L. flavicauda, including 1 and 5 km thresholds of predicted deforestation and hunting
As expected future climate changes are predicted to reduce niche availability for A. miconax and L. flavicauda. This is because of general and localized changes in temperatures, precipitation levels, and cloud formations, all of which will in turn have drastic consequences on plant phenologies affecting habitat availability and quality (Bubb et al. 2004; Foster 2001; Herzog 2011; Pielke et al. 2002; Still et al. 1999). Interestingly, my models predicted a large increase in niche availability for P. oenanthe with future climate changes. These very different results could stem from the different habitat requirements of the species. Aotus miconax and L. flavicauda are restricted to higher elevation montane forests, which are predicted to be very sensitive to climate changes (Bubb et al. 2004; Herzog 2011; Still et al. 1999), whereas C. oenanthe is restricted to lower elevation pre-montane and tropical dry forests. Increased temperatures and reduced precipitation may account for the predicted increase in niche availability, particularly in dry forest areas. These differences in the predicted effects of future climate changes on niche availability for these species demonstrates the complexities involved in modeling such changes (Newbold et al. 2014). Caution needs to be used when interpreting this result as the increase in area is mainly outside of the species current distribution. In this case the species, or habitat, may not be able to adapt quickly enough to the geographic shift in niche location (Feeley and Silman 2010), and this could therefore constitute a significant decrease in the actual niche availability. When I applied the two thresholds of predicted future land use change and anthropogenic hunting pressure, the models all predicted reductions in niche availability for all species.
Natural inaccessibility and socioeconomic instability played major roles in protecting A. miconax and L. flavicauda, and to a lesser extent P. oenanthe, from anthropogenic pressures for many years (deLuycker 2007b; Ellenbogen 1999; Kent 1993; Shanee 2011; Young 1996). Since the paving of the main highway from Peru’s Pacific coast to the Amazonian lowlands, increased immigration from the high mountain sierras of Peru’s interior has caused widespread deforestation and substantial increases in hunting rates (deLuycker 2007b; Dreyfus 1999; Morales 1986; Shanee 2012a). From remote, unsettled regions, this area now has the highest immigration and deforestation rates in Peru (INEI 2008; PROCLIM/CONAM 2005; Reategui and Martinez 2007). This has caused severe fragmentation of habitat for all three species (Shanee et al. 2011, 2015; Bóveda-Penalba et al. 2009; Leo Luna 1987; Shanee 2011). As in all areas, deforestation, fragmentation, and the presence of livestock and waste products have many negative impacts on populations of wildlife (Newbold et al. 2014) including, increased competition for resources (Andrén 1994; Estrada and Coates-Estrada 1996), increased hunting pressure (Jerozolimski and Peres 2003; Michalski and Peres 2005; Peres 2001), increased zoonotic infections (Chapman et al. 2006; Fahrig 2003; Gillespie et al. 2005; Goldberg et al. 2008; Sanchez-Larranega and Shanee 2012), and reduced connectivity between populations, reducing genetic fitness (Bergl et al. 2008; Brenneman et al. 2012; Marsh et al. 2013).
Protected area networks have been a mainstay of conservation for many years but have been criticized for shortfalls in effectiveness in protecting species (Cantú-Salazar et al. 2013; Geldmann et al. 2013; Rodrigues et al. 2003; Seiferling et al. 2012), increasing the need for landscape level solutions that include local communities in gap areas (Gálvez et al. 2013; Porter-Bolland et al. 2012). Community-managed forests provide a solution for conservation in highly populated areas and often perform better then protected areas (Porter-Bolland et al. 2012). The inclusion of conservation programs in gap areas is of particular importance as levels of land development around protected areas has a direct influence on their effectiveness as conservation units (Durán et al. 2013; Leroux and Kerr 2013). In northern Peru, the inclusion of communities is of particular importance as human populations are relatively high and increasing (PROCLIM/CONAM 2005; Shanee et al. 2014). The protected area network in northern Peru covers a fairly large area of forests including areas of current and future habitat for A. miconax and L. flavicauda but provides little protection for P. oenanthe. As with other areas in the Andes, protected areas in northern Peru may not be enough to safeguard these species from anthropogenic development activities (Swenson et al. 2012). Including the predicted increase in niche area for P. oenanthe, anthropogenic activities will still reduce total available area for the species, even assuming no more habitat loss within protected areas.
The results presented here show that multiple drivers of extinction risk combine to threaten species (Newbold et al. 2014) and that future man-made climate changes will have variable effects depending on a species’ habitat and ecological needs (Newbold et al. 2014). Although climate change is predicted to dominate conservation during this century (Bonan 2013; Laurance and Williamson 2001; Lewis 2006; Lewis et al. 2011; van Aalst 2006; Veech and Crist 2007), other anthropogenic activities are still and, in many cases, will continue to be the major drivers of extinctions (Feeley and Silman 2010; Hurtt et al. 2011; Krausmann et al. 2013; Newbold et al. 2014; Peres et al. 2010; Tilman et al. 2001). Future conservation actions should not only concentrate on mitigating the effects of climate change but should also concentrate on reducing other anthropogenic pressures which are driving species to extinction. This is particularly true for species with limited geographic ranges and habitat specializations (Newbold et al. 2014) that are intrinsically more at risk of extinction (Cardillo et al. 2005; Purvis et al. 2000a, b) but that also may not be able to adapt to changing climates and habitats in the near future.
References
Alfaro, J. W. L., Boubli, J. P., Olson, L. E., Di Fiore, A., Wilson, B., Gutiérrez-Espeleta, G. A., et al. (2012a). Explosive Pleistocene range expansion leads to widespread Amazonian sympatry between robust and gracile capuchin monkeys. Journal of Biogeography, 39(2), 272–288.
Alfaro, J. W. L., Silva, J. D. E., & Rylands, A. B. (2012b). How different are robust and gracile capuchin monkeys? An argument for the use of Sapajus and Cebus. American Journal of Primatology, 74(4), 273–286.
Allgas, N., Shanee, S., Peralta, A., & Shanee, N. (2014). Yellow-tailed woolly monkey (Oreonax flavicauda: Humboldt 1812) altitudinal range extension, Uchiza, Peru. Neotropical Primates, 21(2), 207–207.
Anderson, R. P., & Raza, A. (2010). The effect of the extent of the study region on GIS models of species geographic distributions and estimates of niche evolution: Preliminary tests with montane rodents (genus Nephelomys) in Venezuela. Journal of Biogeography, 37, 1378–1393.
Andrén, H. (1994). Effects of habitat fragmentation on birds and mammals in landscapes with different proportions of suitable habitat: A review. Oikos, 71(3), 355–366.
Aquino, R., & Encarnación, F. (1994). Los primates del Peru. Primate Report, 40, 1–130.
Ayres, J. M., & Clutton-Brock, T. H. (1992). River boundaries and species range size in Amazonian primates. The American Naturalist, 140(3), 531–537.
Bergl, R. A., Bradley, B. J., Nsubuga, A., & Vigilant, L. (2008). Effects of habitat fragmentation, population size and demographic history on genetic diversity: The cross river gorilla in a comparative context. American Journal of Primatology, 70(9), 848–859.
Bertin, R. I. (2008). Plant phenology and distribution in relation to recent climate change. The Journal of the Torrey Botanical Society, 135(1), 126–146.
Bocedi, G., Palmer, S. C. F., Pe’er, G., Heikkinen, R. K., Matsinos, Y. G., Watts, K., et al. (2014). RangeShifter: A platform for modelling spatial eco-evolutionary dynamics and species’ responses to environmental changes. Methods in Ecology and Evolution, 5(4), 388–396.
Bonan, G. B. (2013). Forests and climate change: Forcings, feedbacks, and the climate benefits of forests. Science, 320(5882), 1444–1449.
Boria, R. A., Olson, L. E., Goodman, S. M., & Anderson, R. P. (2014). Spatial filtering to reduce sampling bias can improve the performance of ecological niche models. Ecological Modelling, 275, 73–77.
Boubli, J. P., Rylands, A. B., Farias, I. P., Alfaro, M. E., & Alfaro, J. L. (2012). Cebus phylogenetic relationships: A preliminary reassessment of the diversity of the untufted capuchin monkeys. American Journal of Primatology, 74(4), 381–393.
Bóveda-Penalba, A., Vermeer, J., Rodrigo, F., & Guerra-Vásquez, F. (2009). Preliminary report on the distribution of (Callicebus oenanthe) on the eastern feet of the Andes. International Journal of Primatology, 30(3), 467–480.
Brenneman, R. A., Johnson, S. E., Bailey, C. A., Ingraldi, C., Delmore, K. E., Wyman, T. M., et al. (2012). Population genetics and abundance of the endangered grey-headed lemur Eulemur cinereiceps in south-east Madagascar: Assessing risks for fragmented and continuous populations. Oryx, 46(2), 298–307.
Brown, J. L. (2014). SDMtoolbox: A python-based GIS toolkit for landscape genetic biogeographic and species distribution model analyses. Methods in Ecology and Evolution, 5(7), 694–700.
Bubb, P., May, I., Miles, L., & Sayer, J. (2004). Cloud forest agenda. Cambridge, England: United Nations Environment Programme—World Conservation Monitoring Centre.
Buckingham, F., & Shanee, S. (2009). Conservation priorities for the Peruvian yellow-tailed woolly monkey (Oreonax flavicauda): A GIS risk assessment and gap analysis. Primate Conservation, 24(1), 65–71.
Campbell, N. (2011). The Peruvian night monkey, Aotus miconax, a comparative study of occupancy between Cabeza del Toro and Cordillera de Colan. Oxford, England: Oxford Brookes University.
Cantú-Salazar, L., Orme, C. D., Rasmussen, P., Blackburn, T., & Gaston, K. (2013). The performance of the global protected area system in capturing vertebrate geographic ranges. Biodiversity and Conservation, 22(4), 1033–1047.
Cardillo, M., Mace, G. M., Jones, K. E., Bielby, J., Bininda-Emonds, O. R. P., Sechrest, W., et al. (2005). Multiple causes of high extinction risk in large mammal species. Science, 309(5738), 1239–1241.
Cayuela, L., Benayas, J. M. R., & Echeverría, C. (2006). Clearance and fragmentation of tropical montane forests in the highlands of Chiapas, Mexico (1975–2000). Forest Ecology and Management, 226(1–3), 208–218.
Chan, L. M., Brown, J. L., & Yoder, A. D. (2011). Integrating statistical genetic and geospatial methods brings new power to phylogeography. Molecular Phylogenetics and Evolution, 59(2), 523–537.
Chapman, C. A., Speirs, M. L., Gillespie, T. R., Holland, T., & Austad, K. M. (2006). Life on the edge: Gastrointestinal parasites from the forest edge and interior primate groups. American Journal of Primatology, 68(4), 397–409.
de Souza Muñoz, M. E., De Giovanni, R., de Siqueira, M. F., Sutton, T., Brewer, P., Pereira, R. S., et al. (2011). OpenModeller: A generic approach to species’ potential distribution modelling. GeoInformatica, 15(1), 111–135.
deLuycker, A. M. (2007). Notes on the yellow-tailed woolly monkey (Oreonax flavicauda) and its status in the protected forest of Alto Mayo, Northern Peru. Primate Conservation, 22(1), 41–47.
deLuycker, A. M. (2007). The ecology and behavior of the Rio Mayo titi monkey (Callicabus oenanthe) in the Alto Mayo, Northern Peru. Unpublished Ph.D. dissertation, Washington University in St Louis.
Dore, M. H. I. (2005). Climate change and changes in global precipitation patterns: What do we know? Environment International, 31(8), 1167–1181.
Dourojeanni, M. (1989). Environmental impact of coca cultivation and cocaine production in the amazon region of Peru. In F. R. Len & R. Castro de la Mata (Eds.), Pasta bisica de cocaina: Un estudio multidisciplinario (pp. 281–300). Lima, Peru: CEDRO.
Dreyfus, P. G. (1999). When all the evils come together. Journal of Contemporary Criminal Justice, 15(4), 370–396.
Durán, A. P., Rauch, J., & Gaston, K. J. (2013). Global spatial coincidence between protected areas and metal mining activities. Biological Conservation, 160, 272–278.
Elith, J., & Graham, C. H. (2009). Do they? How do they? Why do they differ? On finding reasons for differing performances of species distribution models. Ecography, 32(1), 66–77.
Elith, J., Graham, C. H., Anderson, R. P., Dudík, M., Ferrier, S., Guisan, A., et al. (2006). Novel methods improve prediction of species’ distributions from occurrence data. Ecography, 29(2), 129–151.
Ellenbogen, G. G. (1999). The shining path: A history of the millenarian war in Peru. Chapell Hill: University of North Carolina Press.
Estrada, A., & Coates-Estrada, R. (1996). Tropical rain forest fragmentation and wild populations of primates at Los Tuxtlas, Mexico. International Journal of Primatology, 17(5), 759–783.
Fahrig, L. (2003). Effects of habitat fragmentation on biodiversity. Annual Review of Ecology, Evolution, and Systematics, 34, 487–515.
Fearnside, P. M. (1983). Land-use trends in the Brazilian Amazon region as factors in accelerating deforestation. Environmental Conservation, 10(02), 141–148.
Feeley, K. J., & Silman, M. R. (2010). Land-use and climate change effects on population size and extinction risk of Andean plants. Global Change Biology, 16(12), 3215–3222.
Fjeldså, J., Álvarez, M. D., Lazcano, J. M., & León, B. (2005). Illicit crops and armed conflict as constraints on biodiversity conservation in the Andes region. AMBIO, 34(3), 205–211.
Fjeldså, J., Lambin, E., & Mertens, B. (1999). Correlation between endemism and local ecoclimatic stability documented by comparing Andean bird distributions and remotely sensed land surface data. Ecography, 22(1), 63–78.
Foster, P. (2001). The potential negative impacts of global climate change on tropical montane cloud forests. Earth-Science Reviews, 55(1–2), 73–106.
Gálvez, N., Hernández, F., Laker, J., Gilabert, H., Petitpas, R., Bonacic, C., et al. (2013). Forest cover outside protected areas plays an important role in the conservation of the Vulnerable guiña Leopardus guigna. Oryx, 47(02), 251–258.
Garland, E. B. (1995). The social and economic causes of deforestation in the Peruvian Amazon basin: Natives and colonists. In M. Painter & W. H. Durham (Eds.), The social causes of environmental destruction in Latin America (pp. 217–246). Ann Arbor: University of Michigan Press.
Geldmann, J., Barnes, M., Coad, L., Craigie, I. D., Hockings, M., & Burgess, N. D. (2013). Effectiveness of terrestrial protected areas in reducing habitat loss and population declines. Biological Conservation, 161, 230–238.
Gillespie, T. R., Chapman, C. A., & Greiner, E. C. (2005). Effects of logging on gastrointestinal parasite infections and infection risk in African primates. Journal of Applied Ecology, 42(4), 699–707.
Goldberg, T. L., Gillespie, T. R., Rwego, I. B., Estoff, E. L., & Chapman, C. A. (2008). Forest fragmentation as cause of bacterial transmission among nonhuman primates, humans, and livestock, Uganda. Emerging Infectious Diseases, 14(9), 1375–1382.
Goodchild, M. F., Steyaert, L. T., Parks, B. O., & Johnston, C. (1996). GIS and environmental modeling: Progress and research issues. New York: Wiley.
Graham, C. H., Elith, J., Hijmans, R. J., Guisan, A., Peterson, A. T., Loiselle, B. A., et al. (2008). The influence of spatial errors in species occurrence data used in distribution models. Journal of Applied Ecology, 45(1), 239–247.
Guisan, A., & Thuiller, W. (2005). Predicting species distribution: Offering more than simple habitat models. Ecology Letters, 8(9), 993–1009.
Guisan, A., Zimmermann, N. E., Elith, J., Graham, C. H., Phillips, S., & Peterson, A. T. (2007). What matters for predicting the occurrences of trees: Techniques, data, or species’ characteristics? Ecological Monographs, 77(4), 615–630.
Guo, Q., & Liu, Y. (2010). ModEco: An integrated software package for ecological niche modeling. Ecography, 33(4), 637–642.
Hall, J., Burgess, N. D., Lovett, J., Mbilinyi, B., & Gereau, R. E. (2009). Conservation implications of deforestation across an elevational gradient in the Eastern Arc Mountains, Tanzania. Biological Conservation, 142(11), 2510–2521.
Hansen, M. C., Potapov, P. V., Moore, R., Hancher, M., Turubanova, S. A., Tyukavina, A., et al. (2013). High-resolution global maps of 21st-century forest cover change. Science, 342(6160), 850–853.
Herzog, S. K. (2011). Climate change and biodiversity in the tropical Andes. São José dos Campos, Brazil: Inter-American Institute for Global Change Research.
Hijmans, R. J., Cameron, S. E., Parra, J. L., Jones, P. G., & Jarvis, A. (2005). Very high resolution interpolated climate surfaces for global land areas. International Journal of Climatology, 25(15), 1965–1978.
Hurtt, G. C., Chini, L. P., Frolking, S., Betts, R. A., Feddema, J., Fischer, G., et al. (2011). Harmonization of land-use scenarios for the period 1500–2100: 600 years of global gridded annual land-use transitions, wood harvest, and resulting secondary lands. Climatic Change, 109(1-2), 117–161.
INEI. (2008). Instituto Nacional de Estadistica e Informatica (INEI). Retrieved November 20, 2009, from http://www.inei.gob.pe/.
INRENA. (2005). Mapa de deforestacion de la Amazonia Peruana—2000. Lima, Peru: Instituto Nacional de recursos Naturales.
IUCN. (2013). IUCN Red List of Threatened Species. Retrieved March 5, 2014, from www.redlist.org.
Jerozolimski, A., & Peres, C. A. (2003). Bringing home the biggest bacon: A cross-site analysis of the structure of hunter-kill profiles in neotropical forests. Biological Conservation, 111(3), 415–425.
Kent, R. B. (1993). Geographical dimensions of the shining path insurgency in Peru. Geographical Review, 83(4), 441–454.
Krausmann, F., Erb, K.-H., Gingrich, S., Haberl, H., Bondeau, A., Gaube, V., et al. (2013). Global human appropriation of net primary production doubled in the 20th century. Proceedings of the National Academy of Sciences, 110(25), 10324–10329.
Kriticos, D. J., Webber, B. L., Leriche, A., Ota, N., Macadam, I., Bathols, J., et al. (2012). CliMond: Global high-resolution historical and future scenario climate surfaces for bioclimatic modelling. Methods in Ecology and Evolution, 3(1), 53–64.
Laurance, W. F., & Williamson, G. B. (2001). Positive feedbacks among forest fragmentation, drought, and climate change in the Amazon. Conservation Biology, 15(6), 1529–1535.
Lenoir, J., Gégout, J. C., Marquet, P. A., de Ruffray, P., & Brisse, H. (2008). A significant upward shift in plant species optimum elevation during the 20th century. Science, 320(5884), 1768–1771.
Leo Luna, M. (1987). Primate conservation in Peru: A case study of the yellow-tailed woolly monkey. Primate Conservation, 8(1), 122–123.
Leroux, S. J., & Kerr, J. T. (2013). Land development in and around protected areas at the Wilderness Frontier. Conservation Biology, 27(1), 166–176.
Lewis, O. T. (2006). Climate change, species–area curves and the extinction crisis. Philosophical Transactions of the Royal Society, B: Biological Sciences, 361(1465), 163–171.
Lewis, S. L., Brando, P. M., Phillips, O. L., van der Heijden, G. M. F., & Nepstad, D. (2011). The 2010 Amazon drought. Science, 331(6017), 554.
Marsh, L. K., Chapman, C. A., Arroyo-Rodríguez, V., Cobden, A. K., Dunn, J. C., Gabriel, D., et al. (2013). Primates in fragments 10 years later: Once and future goals. In L. K. Marsh & C. A. Chapman (Eds.), Primates in fragments (pp. 503–523). New York: Springer.
Matauschek, C., Roos, C., & Heymann, E. W. (2011). Mitochondrial phylogeny of tamarins (Saguinus, Hoffmannsegg 1807) with taxonomic and biogeographic implications for the S. nigricollis species group. American Journal of Physical Anthropology, 144(4), 564–574.
McCarty, J. P. (2001). Review: Ecological consequences of recent climate change. Conservation Biology, 15(2), 320–331.
Merckx, B., Steyaert, M., Vanreusel, A., Vincx, M., & Vananerbeke, J. (2011). Null models reveal preferential sampling, spatial autocorrelation and overfitting in habitat suitability modelling. Ecological Modelling, 222(3), 588–597.
Michalski, F., & Peres, C. A. (2005). Anthropogenic determinants of primate and carnivore local extinctions in a fragmented forest landscape of southern Amazonia. Biological Conservation, 124(3), 383–396.
Morales, E. (1986). Coca and cocaine economy and social change in the Andes of Peru. Economic Development and Cultural Change, 35(1), 143–161.
Moss, R. H., Edmonds, J. A., Hibbard, K. A., Manning, M. R., Rose, S. K., van Vuuren, D. P., et al. (2010). The next generation of scenarios for climate change research and assessment. Nature, 463(7282), 747–756.
Myers, N. (2003). Biodiversity hotspots revisited. BioScience, 53(10), 916–917.
Myers, N., Mittermeier, R. A., Mittermeier, C. G., da Fonseca, G. A. B., & Kent, J. (2000). Biodiversity hotspots for conservation priorities. Nature, 403(6772), 853–858.
Newbold, T., Hudson, L. N., Phillips, H. R. P., Hill, S. L. L., Contu, S., Lysenko, I., et al. (2014). A global model of the response of tropical and sub-tropical forest biodiversity to anthropogenic pressures. Proceedings of the Royal Society of London. Series B, 281(1805), 1–10.
Pacheco, V., Cadenillas, R., Salas, E., Tello, C., & Zeballos, H. (2009). Diversity and endemism of Peruvian mammals. Revista Peruana de Biología, 16(1), 5–32.
Peres, C. A. (2001). Synergistic effects of subsistence hunting and habitat fragmentation on Amazonian forest vertebrates. Conservation Biology, 15(6), 1490–1505.
Peres, C. A., Gardner, T. A., Barlow, J., Zuanon, J., Michalski, F., Lees, A. C., et al. (2010). Biodiversity conservation in human-modified Amazonian forest landscapes. Biological Conservation, 143(10), 2314–2327.
Perz, S. G., Aramburú, C., & Bremner, J. (2005). Population, land use and deforestation in the Pan Amazon basin: A comparison of Brazil, Bolivia, Colombia, Ecuador, Perú and Venezuela. Environment, Development and Sustainability, 7(1), 23–49.
Peterson, A. T., Papeş, M., & Eaton, M. (2007). Transferability and model evaluation in ecological niche modeling: A comparison of GARP and Maxent. Ecography, 30(4), 550–560.
Phillips, S. J., Anderson, R. P., & Schapire, R. E. (2006). Maximum entropy modeling of species geographic distributions. Ecological Modelling, 190(3–4), 231–259.
Phillips, S. J., Dudík, M., Elith, J., Graham, C. H., Lehmann, A., Leathwick, J., et al. (2009). Sample selection bias and presence-only distribution models: Implications for background and pseudo-absence data. Ecological Applications, 19, 181–197.
Pielke, R. A., Marland, G., Betts, R. A., Chase, T. N., Eastman, J. L., Niles, J. O., et al. (2002). The influence of land-use change and landscape dynamics on the climate system: Relevance to climate-change policy beyond the radiative effect of greenhouse gases. Philosophical Transactions of the Royal Society of London, Series A: Mathematical, Physical and Engineering Sciences, 360(1797), 1705–1719.
Porter-Bolland, L., Ellis, E. A., Guariguata, M. R., Ruiz-Mallén, I., Negrete-Yankelevich, S., & Reyes-García, V. (2012). Community managed forests and forest protected areas: An assessment of their conservation effectiveness across the tropics. Forest Ecology and Management, 268, 6–17.
PROCLIM/CONAM. (2005). Informe del proyecto proclim—Conam. Lima, Peru: INRENA.
Purvis, A., Gittleman, J. L., Cowlishaw, G., & Mace, G. M. (2000a). Predicting extinction risk in declining species. Proceedings of the Royal Society of London. Series B: Biological Sciences, 267(1456), 1947–1952.
Purvis, A., Jones, K. E., & Mace, G. M. (2000b). Extinction. BioEssays, 22(12), 1123–1133.
Radosavljevic, A., & Anderson, R. P. (2014). Making better Maxent models of species distributions: Complexity, overfitting, and evaluation. Journal of Biogeography, 41, 629–643.
Raper, S. (2012). Climate modelling: IPCC gazes into the future. Nature Climate Change, 2(4), 232–233.
Reategui, F., & Martinez, P. (2007). Feorestal. Zonificacion ecologica economica del departamento de Amazonas. Lima, Peru: Instituto de Investigaciones de la Amazonia Peruana (IIAP).
Robles Gil, P., Seligmann, P. A., Ford, H., & Mittermeier, R. A. (2004). Hotspots revisited. Mexico City, Mexico: CEMEX.
Rodrigues, A. S. L., Andelman, S. J., Bakarr, M. I., Boitani, L., Brooks, T. M., Cowling, R. M., et al. (2003). Global gap analysis: Towards a representative network of protected areas. Advances in Applied Biodiversity Science, 5, 100.
Rogelj, J., Meinshausen, M., & Knutti, R. (2012). Global warming under old and new scenarios using IPCC climate sensitivity range estimates. Nature Climate Change, 2(4), 248–253.
Rowe, N., & Myers, M. (2012). All the worlds' primates. Retrieved May 28, 2012. from www.alltheworldsprimates.org.
Sanchez-Cuervo, A. M., & Aide, T. M. (2013). Identifying hotspots of deforestation and reforestation in Colombia (2001–2010): Implications for protected areas. Ecosphere, 4(11), 143.
Sanchez-Larranega, J., & Shanee, S. (2012). Parásitos gastrointestinales en el mono choro cola amarilla (Oreonax flavicauda) y el mono nocturno Andino (Aotus miconax) en Amazonas, Perú. Neotropical Primates, 19(1), 38–41.
Schjellerup, I. (2000). La Morada. A case study on the impact of human pressure on the environment in the Ceja de Selva, northeastern Peru. AMBIO, 29, 451–454.
Seiferling, I. S., Proulx, R., Peres‐Neto, P. R., Fahrig, L., & Messier, C. (2012). Measuring protected-area isolation and correlations of isolation with land-use intensity and protection status [Medición del Aislamiento de Áreas Protegidas y Correlaciones del Aislamiento con la Intensidad de Uso del Suelo y el Estatus de Protección]. Conservation Biology, 26(4), 610–618.
Shanee, S. (2011). Distribution survey and threat assessment of the yellow-tailed woolly monkey (Oreonax flavicauda, Humboldt 1812), Northeastern Peru. International Journal of Primatology, 32(3), 691–707.
Shanee, N. (2012a). The dynamics of threats and conservation efforts for the tropical Andes hotspot in Amazonas and San Martin, Peru. Canterbury, England: Kent University.
Shanee, N. (2012b). Trends in local wildlife hunting, trade and control in the Tropical Andes Hotspot, Northeastern Peru. Endangered Species Research, 19(2), 177–186.
Shanee, S. (2013). Conservation and ecology of Andean primates in Peru. Unpublished Ph.D. dissertation, Oxford Brookes University, Oxford.
Shanee, S., Allgas, N., & Shanee, N. (2013a). Preliminary observations on the behavior and ecology of the Peruvian night monkey (Aotus miconax: Primates) in a remnant cloud forest patch, north eastern Peru. Tropical Conservation Science, 6(1), 138–148.
Shanee, S., Allgas, N., Shanee, N., & Campbell, N. (2015). Distribution, ecological niche modelling and conservation assessment of the Peruvian Night Monkey (Mammakia: Primates: Aotidae: Aotus miconax Thomas, 1927) in Northeastern Peru, with notes on the distributions of Aotus spp. Journal of Threatened Taxa, 7(3), 6947–6964.
Shanee, S., Shanee, N., & Allgas-Marchena, N. (2013b). Primate surveys in the Maranon-Huallaga landscape, Northern Peru with notes on conservation. Primate Conservation, 27, 3–11.
Shanee, S., Shanee, N., Campbell, N., & Allgas, N. (2013c). Biogeography and conservation of Andean primates in Peru. In N. B. Grow, S. Gursky-Doyen, & A. Krzton (Eds.), High altitude primates (pp. 63–83). New York: Springer.
Shanee, N., Shanee, S., & Horwich, R. H. (2014). Effectiveness of locally run conservation initiatives in north-east Peru. Oryx, 49, 239–247.
Shanee, S., Tello-Alvarado, J. C., Vermeer, J., & Boveda-Penalba, A. J. (2011). GIS risk assessment and GAP analysis for the Andean titi monkey (Callicebus oenanthe). Primate Conservation, 26(1), 17–23.
Shcheglovitova, M., & Anderson, R. P. (2013). Estimating optional complexity for ecological niche models: A jackknife approach for species with small sample sizes. Ecological Modelling, 269, 9–17.
Skidmore, A. (2004). Environmental modelling with GIS and remote sensing. London: Taylor and Francis.
Still, C. J., Foster, P. N., & Schneider, S. H. (1999). Simulating the effects of climate change on tropical montane cloud forests. Nature, 398(6728), 608–610.
Suárez-Seoane, S., Virgós, E., Terroba, O., Pardavila, X., & Barea-Azcón, J. M. (2014). Scaling of species distribution models across spatial resolutions and extents along a biogeographic gradient. The case of the Iberian mole Talpa occidentalis. Ecography, 37(3), 279–292.
Swenson, J. J., Young, B. E., Beck, S., Comer, P., Cordova, J. H., Dyson, J., et al. (2012). Plant and animal endemism in the eastern Andean slope: Challenges to conservation. BMC Ecology, 12(1), 1–19.
Thomas, C. D., Cameron, A., Green, R. E., Bakkenes, M., Beaumont, L. J., Collingham, Y. C., et al. (2004). Extinction risk from climate change. Nature, 427(6970), 145–148.
Thuiller, W., Lafourcade, B., Engler, R., & Araujo, M. B. (2009). BIOMOD—A platform for ensemble forecasting of species distributions. Ecography, 32, 369–373.
Tilman, D., Reich, P. B., Knops, J., Wedin, D., Mielke, T., & Lehman, C. (2001). Diversity and productivity in a long-term grassland experiment. Science, 294(5543), 843–845.
Vale, C. G., Tarroso, P., & Brito, J. C. (2014). Predicting species distribution at range margins: Testing the effects of study area extent, resolution and threshold selection in the Sahara–Sahel transition zone. Diversity and Distributions, 20(1), 20–33.
van Aalst, M. K. (2006). The impacts of climate change on the risk of natural disasters. Disasters, 30(1), 5–18.
Veech, J. A., & Crist, T. O. (2007). Habitat and climate heterogeneity maintain beta-diversity of birds among landscapes within ecoregions. Global Ecology and Biogeography, 16(5), 650–656.
Veloz, S. D. (2009). Spatially autocorrelated sampling falsely inflates measures of accuracy for presence-only niche models. Journal of Biogeography, 36(12), 2290–2299.
Vermeer, J., Tello-Alvarado, J. C., Moreno-Moreno, S., & Guerra-Vásquez, F. (2011). Extension of the geographical range of White-browed Titi monkeys (Callicebus discolor) and evidence for sympatry with San Martin Titi monkeys (Callicebus oenanthe). International Journal of Primatology, 32(4), 924–930.
Walther, G., Post, E., Convey, P., Menzel, A., Parmesan, C., Beebee, T. J. C., et al. (2002). Ecological responses to recent climate change. Nature, 416(6879), 389–395.
Warren, D. L., Glor, R. E., & Turelli, M. (2010). ENMTools: A toolbox for comparative studies of environmental niche models. Ecography, 33(3), 607–611.
Wilson, D. E., Mittermeier, R. A., Ruff, S., Martinez-Vilalta, A., & Llobet, T. (2013). Handbook of the mammals of the world: Primates. Arrington, VA: Buteo Books.
Wyman, M. S., & Stein, T. V. (2009). Modeling social and land-use/land-cover change data to assess drivers of smallholder deforestation in Belize. Applied Geography, 30(3), 329–342.
Wyman, M. S., Stein, T. V., Southworth, J., & Horwich, R. H. (2011). Does population increase equate to conservation success? Forest fragmentation and conservation of the black howler monkey. Conservation and Society, 9(3), 216–228.
Young, K. R. (1996). Threats to biological diversity caused by coca/cocaine deforestation in Peru. Environmental Conservation, 23(1), 7–15.
Acknowledgements
I wish to thank Noga Shanee, Nestor Allgas, Alejandra Zamora, and everyone at Neotropical Primate Conservation for their help in preparing this study. Also Noe Rojas, Ana Peralta, and Fernando Guerra for their help in the field as well as Julio Tello, Antonio Boveda, and Jan Vermeer from Proyecto Mono Tocon, for their previous field studies without which modeling would not have been possible. Data used for this work was gathered, thanks to funding from Neotropical Primate Conservation and from Community Conservation, Primate Conservation Inc, Margot Marsh Biodiversity Foundation, National Geographic Society, International Primate Protection League, Primate Society of Great Britain, and American Society of Primatologists.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Shanee, S. (2016). Predicting Future Effects of Multiple Drivers of Extinction Risk in Peru’s Endemic Primate Fauna. In: Waller, M. (eds) Ethnoprimatology. Developments in Primatology: Progress and Prospects. Springer, Cham. https://doi.org/10.1007/978-3-319-30469-4_18
Download citation
DOI: https://doi.org/10.1007/978-3-319-30469-4_18
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-30467-0
Online ISBN: 978-3-319-30469-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)