Abstract
Primate responses to habitat alteration vary depending on the species’ dietary guild and forest type. Leaves from secondary vegetation can provide nutritious resources to folivorous primates, whereas frugivores, burdened with a scattered spatial and temporal distribution of fruiting resources, require larger home ranges, potentially limiting their ability to cope with altered landscapes. Within coastal southeastern Madagascar, we sought to determine whether two lemur species occupying contrasting ecological niches respond differently to the changing features of their degraded and fragmented habitat. We conducted behavioral observations between 2011 and 2013 on frugivorous collared brown lemurs (Eulemur collaris) and folivorous southern bamboo lemurs (Hapalemur meridionalis). To estimate the ability of lemurs to use pioneer species, we categorized all plants used for feeding and resting as fast growing, mid-growing, or slow growing. We fitted general linear mixed-effects models, one for each plant growth category with monthly proportional use rates as the dependent variable, and included species (E. collaris and H. meridionalis), activity (feeding and resting), and season (dry and wet) as fixed effects. Our results show that E. collaris used both slow- and mid-growing plant species most often, while H. meridionalis were more likely to use fast-growing plants, which indicated an ability to use secondary/disturbed vegetation. Frugivorous E. collaris appear more limited by climax plants, while folivorous H. meridionalis appear to be slightly more adaptable, a finding that is consistent with that for other primate folivores.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tropical deforestation is one of the primary threats to global biodiversity (Achard et al. 2002; Asner et al. 2009; Dirzo and Raven 2003; Gibson et al. 2011; Sala et al. 2000). The destruction, fragmentation, and degradation of remaining habitats threaten many species’ ability to survive (Oates 2013). Although forest fragments typically persist after deforestation, they effectively become islands within an anthropogenic landscape, most of which are unsuitable habitat for the majority of forest species (Broadbent et al. 2008; Laurance et al. 2009, 2011).
Ecological flexibility is loosely defined as the ability of an organism to adjust to changes, e.g., anthropogenic, gradual, and stochastic, within its environment (Isaac and Cowlishaw 2004; Nowak and Lee 2013; Wieczkowski 2003). In more specific terms, flexibility may encapsulate various behavioral modifications including the diet, i.e., exploitation of alternative food sources, as well as altering activity, ranging pattern, and vertical strata use in response to new dietary opportunities. This ability to expand niche breadth is key to withstanding the risks of anthropogenic and/or stochastic habitat modification (Lee 2003).
It is important to understand behavioral responses of forest-dwelling primates to habitat degradation and fragmentation because of the increasing rate of habitat alteration and limited ability of most species to move between forest fragments (Marsh 2003). How a primate responds to habitat degradation, however, seems to vary depending on species and type of forest (Boyle and Smith 2010; Chapman et al. 2000; Cowlishaw and Dunbar 2000; Harcourt and Doherty 2005; Onderdonk and Chapman 2000). Secondary growth may produce foods of higher dietary quality compared to foods available in mature forests, thus making folivorous, i.e., leaf-eating, primates less affected by habitat degradation (Chapman et al. 2002; Ganzhorn 1995; Ganzhorn et al. 1999b; Plumptre and Reynolds 1994). For example, populations of folivorous black howlers (Alouatta caraya and A. pigra) have been documented to use and rely heavily on fast-growing, exotic plant species, e.g., Eucalyptus and shaded cocoa plantations, for both occasional food and resting/sleeping within fragmented, anthropogenic landscapes (Bicca-Marques and Calegaro-Marques 1994; Bonilla-Sánchez et al. 2012; Zárate et al. 2014). Similarly, black-and-white colobus (Colobus guereza) appear to do well in some disturbed, i.e., previously logged, habitats (Chapman et al. 2000; Tutin et al. 1997b). Frugivorous, i.e., fruit-eating, primates, however, have to cope with the scattered spatial and temporal distribution of fruiting resources, thus often requiring larger home ranges (Estrada and Coates-Estrada 1996; Rode et al. 2006; cf. Tutin et al. 1997a). Many frugivorous primates have disappeared from forest fragments, e.g., gray-cheeked mangabeys (Lophocebus albigena) and Mexican spider monkeys (Ateles geoffroyi vellerosus), and appear to be restricted to continuous forests (Estrada and Coates-Estrada 1996; Tutin et al. 1997b). Despite these potential limitations, some frugivorous primates, such as chimpanzees (Pan troglodytes) and Sumatran orangutans (Pongo abelii), demonstrate an ability to survive within degraded, anthropogenic landscapes, foraging on a mixture of crops and wild fruits (Campbell-Smith et al. 2011; Hockings and McLennan 2012; McLennan and Hockings 2014). As frugivorous primates are important seed dispersers, their ability to cope within anthropogenic landscapes has major implications for the maintenance of forest diversity: they are fundamental in the regeneration of degraded habitats (Chapman 1995; Ganzhorn 1999a; Razafindratsima and Dunham 2014).
Frugivorous strepsirrhines from Madagascar contribute on a larger scale to their respective ecosystems, e.g., seed dispersal, compared to primates in the Neotropics or mainland Africa (Jernvall and Wright 1998). As frugivorous lemurs are essential to maintaining the unique forests of Madagascar, their demise would likely trigger extinction cascades (Federman et al. 2016; Ganzhorn et al. 1999a; Jernvall and Wright 1998; Razafindratsima and Dunham 2014). Within Madagascar, for example, >80% of forest area exists <1 km from an edge (Harper et al. 2007), and thus fragmentation is of great concern for the survival of forest fauna and flora species (Hannah et al. 2008; Waeber et al. 2015). This can be complicated further by introduced exotic and invasive species that threaten the preservation of endemic biodiversity as well as ecosystem restoration efforts (D’Antonio and Vitousek 1992). Although the limits of lemurs’ tolerance, i.e., coping strategies, to fragmented, secondary, and degraded habitats are poorly understood (Campera et al. 2014; Donati et al. 2011; Eppley et al. 2015a; Gardner 2009; Irwin et al. 2010; Lehman et al. 2006), it is imperative to understand the relationship between species and these altered habitats if we are to properly conserve primates and other species (Cristóbal-Azkarate and Arroyo-Rodríguez 2007; Isabirye-Basuta and Lwanga 2008; Onderdonk and Chapman 2000).
The Anosy region along the southeast coast of Madagascar provides a complex mosaic of heavily fragmented upland and swamp forest habitats, monodominant exotic species, old and new timber plantations, and a large-scale ilmenite ore mine and separation plant facility (Barthlott et al. 1996; Ganzhorn et al. 2007b; Ramanamanjato et al. 2002). This area provides an excellent model with which to explore the behavioral and feeding ecological flexibilities among the lemurs that inhabit it (Bollen and Donati 2006; Eppley et al. 2015a; Rabenantoandro et al. 2007), and how they cope with habitat disturbance. Within southeast Madagascar, sympatric collared brown lemurs (Eulemur collaris) and southern bamboo lemurs (Hapalemur meridionalis) occupy different ecological niches, the frugivorous and folivorous dietary guild, respectively. Previous research has shown that E. collaris is tolerant to habitat degradation and strong seasonal resource availability by flexibly modifying many aspects of its behavioral ecology, such as feeding strategies and home range use (Campera et al. 2014; Donati et al. 2011). Similarly, H. meridionalis display a flexible ecology, using three distinct habitats (littoral forest, littoral swamp, and Melaleuca-dominated swamp) for both resting and feeding purposes (Eppley et al. 2015a).
Our study sought to determine whether the dietary guilds of our two taxa predict their ability to use fast-growing, i.e., pioneer and exotic, plant species, and how this is a potential signal of ecological flexibility to altered habitats. Habitat edges often contain a higher abundance of pioneer species as compared to climax habitat (Laurance et al. 2006, 2007). Furthermore, climax plants struggle to regenerate in open habitats, such as edge areas and/or plantations (Benitez-Malvido 1998). The general observation that folivores are able to cope better within degraded environments led us to predict that Hapalemur meridionalis will use more forest edge habitat compared to Eulemur collaris. As fast-growing tropical plant species often provide a continuous, i.e., nonseasonal, and relatively large biomass presence of young leaves (Coley et al. 1985; Poorter 1999), which consequently produce more protein (Wasserman and Chapman 2003), we predict that H. meridionalis will use fast-growing tree species more often than sympatric frugivorous E. collaris. As these are the two largest lemur species within the southeast coastal landscape and this degraded littoral environment has a limited number of mature trees, we predict that they will use similar tree species for resting. We predict that E. collaris will use fewer feeding trees during the dry season, i.e., when there are fewer available food resources (Bollen and Donati 2005; Campera et al. 2014). Lastly, exotic plant species, e.g., introduced, nonendemic, are shown to incur lower levels of leaf herbivory compared to endemic plant species (Lake and Leishman 2004). Thus, we predict that the folivorous H. meridionalis would avoid exotic plant species.
Methods
Study Site
We conducted our study in the Mandena Conservation Zone (24°95′S 46°99′E; hereafter Mandena), along the southeast coast of Madagascar, ca. 10 km north of Fort Dauphin (Tolagnaro). Located <3 km from the coast and characterized by a low canopy growing on sandy substrate (Dumetz 1999), this protected area consists of ca. 82 ha of seasonally inundated swamp among 148 ha of degraded littoral forest fragments (Ganzhorn et al. 2007a). This littoral zone experiences less seasonality than the humid eastern forests (Bollen and Donati 2005), with a mean temperature of 22.5 °C (range: 9.5–35.0 °C) and total annual precipitation of 2808 mm, typically generating a wet season between November and April (Eppley et al. 2015a, 2016b). Compared to the less degraded littoral forests further north (Bollen and Donati 2006), the degree of anthropogenic degradation in Mandena resulted from the historical extraction of utilitarian timber species and charcoal production because of the close proximity of the Anosy region capital (Ingram and Dawson 2006; Vincelette et al. 2007b). The area immediately surrounding these fragmented forests is composed of monodominant timber plantations, an exposed sand-scrub matrix, and the large-scale ilmenite mining concession and associated administration and extraction/separation facilities (Ganzhorn et al. 2007b). In addition to the two cathemeral lemurids, i.e., Eulemur collaris and Hapalemur meridionalis, this littoral area is inhabited by four nocturnal strepsirrhines: Ganzhorn’s mouse lemur (Microcebus ganzhorni), eastern fat-tailed dwarf lemur (Cheirogaleus medius), greater dwarf lemur (C. major), and the southern woolly lemur (Avahi meridionalis).
Study Species
Our study focuses on two sympatric lemurs inhabiting Mandena: Eulemur collaris and Hapalemur meridionalis. Both are medium-sized lemurs, although E. collaris is considerably larger, with a mean body mass of 2.2 kg (Donati et al. 2011), compared to the mean body mass of H. meridionalis, which is 1.1 kg (Eppley et al. 2015b). Both of these lemurid species exhibit a cathemeral activity pattern (Donati et al. 2007; Eppley et al. 2015c). Species are classified according to dietary guild based on diets comprising ≥50% of a specific food category (Ganzhorn 1997). As the annual diet of E. collaris consists of ≥70% fruits, it is classified as frugivorous (Donati et al. 2007, 2011). The annual diet of H. meridionalis consists of ≥70% foliose matter; thus this species is classified as folivorous (Eppley et al. 2011, 2016a).
We captured lemur subjects via Telinject® blow darts (administered by an experienced Malagasy technician) containing a hypnotic anesthesic (4–5 mg/kg of ketamine hydrochloride or tiletamine hydrochloride). We captured and equipped four individuals (one for each group) of Eulemur collaris with radio-collars (TW-3, Biotrack, 29 g). We captured 10 individuals of Hapalemur meridionalis from four social groups, and radio-collared with data-logging tags (ARC400, Advanced Telemetry Systems; Isanti, MN, USA). We used radio-collars to expedite the amount of time it took to locate lemur groups each day; however, not all adult focal individuals were radio-collared. All subjects recovered from anesthesia within 1.5 h and were not moved from the capture area. Furthermore, we followed lemurs until they regained full mobility in trees. There were no injuries as a consequence of the captures. The collars were below the 5% threshold of the subjects’ weights. For more specific information on the capturing/collaring processes of E. collaris, see Campera et al. (2014), and for H. meridionalis, see Eppley et al. (2015c, 2016c).
Data Collection
We collected data for each species during different years. For Eulemur collaris, M. Balestri and M. Campera observed group AB from March 2011 to January 2012, and group C from June 2011 to January 2012. We conducted data collection on a focal individual from 06:00 to 18:00 h. We collected behavioral data in 5-min intervals via instantaneous sampling (Altmann 1974), specifically noting the tree species used for feeding and resting. Furthermore, we recorded the position of the focal E. collaris individual in 30-min intervals via a handheld GPS. For Hapalemur meridionalis, T. M. Eppley conducted full-day focal observations (from sunrise to sunset) with groups 1, 2, and 4 (we used group 3 exclusively for home range data collection) between January and December 2013. We identified all observed plant food items consumed by the focal individual, noting the plant species’ scientific name, and recorded feeding duration via continuous sampling (Altmann 1974). Furthermore, we recorded all instances ≥15 min for continuous resting. Lastly, we recorded H. meridionalis focal waypoint locations via GPS in 15-min intervals.
J. Rabenantoandro and F. Randriatafika identified all plant species used for feeding and resting by both lemur species, and we categorized these into three successive growth rates as they occur under natural conditions. As such, fast-growing plant species reached maturity in <2 yr, mid-growing plant species reached maturity between 2 and 5 yr, while slow-growing species reached maturity in >5 yr, with categories based on previous botanical assessments (cf. Vincelette et al. 2007a). Furthermore, J. Rabenantoandro identified exotic plant species, i.e., nonendemic, which we validated with an index of exotic and invasive species in Madagascar (Gérard et al. 2015).
Data Analyses
We entered all ranging data into ArcGIS 10.2 (ESRI) using the Geospatial Modeling Environment (GME) spatial ecology interface (Beyer 2012). Ranging and statistical analyses were conducted using R version 3.2.3 (R Development Core Team 2015). We determined each group home range with a 95% kernel density estimate, while core areas were determined as a 50% kernel density estimate (Worton 1989). We then created a forest edge polygon buffer 100 m inside the littoral forest boundary that allowed us to calculate the total amount of forest edge and nonedge habitat within each lemur species’ home range and core areas within Mandena (Laurance et al. 2007; Lehman et al. 2006).
From our behavioral sampling of Eulemur collaris and Hapalemur meridionalis, we calculated monthly proportional use rates for all feeding and resting trees. For each of the three plant species growth categorizations, we fitted general linear mixed-effects models (LMMs) using the lmer function of the lme4 package developed for R (Bates et al. 2012). For each LMM, our dependent response variable was the monthly proportion of plant species used, i.e., fast-growing plants, mid-growing plants, and slow-growing plants, while our fixed effects were the lemur species (E. collaris and H. meridionalis), activity (feeding and resting), and season (dry and wet). We included lemur social group as random effect to control for repeated sampling. We then used the ANOVA function to calculate likelihood ratio tests for model comparison, allowing us to determine which model had the best explanatory power by comparing Akaike’s information criterion (AIC) values for all possible models. P-values were obtained with a likelihood ratio test using the afex package (Singmann 2014) developed for R, with significance considered at P < 0.05. Residuals from the analyses did not deviate from normality according to the Kolmogorov–Smirnov test.
To determine which factors are linked to the use of exotic plants within Mandena, we fitted generalized linear mixed-effects models (GLMM) using the glmer function of the lme4 package developed for R (Bates et al. 2012), with the monthly use of an exotic plant as a binomial dependent variable, as opposed to endemic plants. As with the LMMs, our fixed effects were lemur species, activity, and season, with group included as the random effect to control for repeated sampling. We then used the ANOVA function to calculate likelihood ratio tests for model comparison and determined which model had the most explanatory power by comparing the AIC values for all possible models.
Ethical Note
Our research protocols were approved and permits authorized by the Commission Tripartite of the Direction des Eaux et Forêts de Madagascar (Autorisation de Recherché n. 29/11/MEF/ SG/DGF/DCB.SAP/SCB du 20/01/11 and n. 240/12/MEF/SG/DGF/DCB.SAP/SCB du 17/09/12), adhering to the legal requirements of Madagascar. We conducted research under the collaboration agreement between the Department of Animal Biology of the University of Antananarivo and the Department of Animal Ecology and Conservation of the University of Hamburg, and QIT Madagascar Minerals (QMM).
Results
Ranging
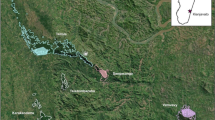
We observed Eulemur collaris for 962 h and Hapalemur meridionalis for 1762 h. Both lemurid species’ home ranges were within the central to northern portions of Mandena, and were not limited to only littoral forest areas, but rather encompassed a mixture of both littoral forest and swamp (Fig. 1). Considering species’ home ranges, E. collaris used considerably larger areas than H. meridionalis (Table I). The proportions of edge habitat used by both species within their home range were similar, with forest edge comprising a mean of 37.4% of the home ranges of E. collaris (N = 2), and 45.6% of the home ranges of H. meridionalis home ranges (N = 4) (Table I). Considering only the core areas, forest edge comprised similar mean percentages of E. collaris (50.6%) and H. meridionalis (42.6%) habitat.
Location of Eulemur collaris and Hapalemur meridionalis group home ranges (95% kernel density estimates) within the Mandena littoral forest and swamp in Madagascar. Portions of the swamp are composed of monodominant strands of exotic Melaleuca, while lighter gray areas to the east are a sand-scrub matrix and those to the west are a matrix of sand-scrub and Eucalyptus plantations. We collected data on E. collaris between March 2011 and January 2012, and on H. meridionalis between January and December 2013.
Diet
We identified 105 different plant species used by Eulemur collaris and 112 species used by Hapalemur meridionalis for feeding and resting (Table II). Twenty-four plant species were eaten by both lemurs. More specifically, E. collaris food resources comprised 16.9% fast-growing, 28.6% mid-growing, and 54.6% slow-growing plants. For H. meridionalis, food resources comprised 38.0% fast-growing, 16.9% mid-growing, and 45.1% slow-growing plants. Twenty-seven plant species were used for resting by both lemur species. For E. collaris, we categorized 14.3% of all resting plants as fast-growing, 24.8% as mid-growing, and 61.0% as slow-growing, while for H. meridionalis, we categorized 27.7% of their used plants as fast-growing, 14.3% as mid-growing, and 58.0% as slow-growing. Both E. collaris and H. meridionalis displayed large differences in their use of these plant growth categories between activity (Fig. 2) and season (Fig. 3).
Comparison of monthly proportional medians (including interquartiles and ranges) between Eulemur collaris and Hapalemur meridionalis on their selection of (a) fast-growing, (b) mid-growing, and (c) slow-growing plants for feeding and resting. We collected data between March 2011 and January 2012 on E. collaris, and between January and December 2013 on H. meridionalis in Mandena, Madagascar.
Comparison of monthly proportional medians (including interquartiles and ranges) between Eulemur collaris and Hapalemur meridionalis on their selection of (a) fast-growing, (b) mid-growing, and (c) slow-growing plants during the dry and wet seasons. We collected data between March 2011 and January 2012 on E. collaris, and between January and December 2013 on H. meridionalis in Mandena, Madagascar.
The model with the best predictive value for fast-growing plants (AIC = –93.06, χ2 = 21.59, df = 1, P < 0.001) showed that both lemur species and activity were likely to influence their use (Table III) whereas season had no effect. Specifically, Hapalemur meridionalis were most likely to use fast-growing plants, and most often for feeding (Fig. 2a). Season was not significantly predictive (Fig. 3a). The model with the best predictive value for mid-growing plants (AIC = –163.11, χ2 = 9.29, df = 1, P < 0.01) showed that all fixed-effects, i.e., species, activity, and season, influenced use of these plants (Table III). Specifically, Eulemur collaris was most likely to use mid-growing plants. Furthermore, these plants were more likely to be used for feeding (Fig. 2b), and to be used in the dry season (Fig. 3b). The model with the best predictive value for slow-growing plants (AIC = –63.90, χ2 = 21.87, df = 1, P < 0.001) showed again that all fixed-effects, i.e., species, activity, and season, influenced use of these plants (Table III). E. collaris was most likely to use slow-growing plants, with these plants most often used for resting (Fig. 2c), specifically during the wet season (Fig. 3c).
Exotic Species in Mandena
There were five plant species in Mandena classified as exotics, likely the consequence of human activities and then dispersed in various ways, e.g., wind. These were broad-leaved paperbark tree (Melaleuca quinquenervia), guava (Psidium spp.), Pemba grass (Stenotaphrum dimidiatum), Polynesian arrowroot (Tacca leontopetaloides), and soapbush (Clidemia hirta). We observed Hapalemur meridionalis feeding on flowers of M. quinquenervia and resting in this species, while they fed on the leaves (grass blades) of S. dimidiatum. We observed Eulemur collaris using four exotic species, feeding on the ripe fruits of C. hirta, Psidium spp., and T. leontopetaloides, and resting in M. quinquenervia. H. meridionalis used exotics in 33 of 36 total months (6.6 ± 1.5% of plants used monthly), whereas E. collaris used exotic plant species only in 4 of 19 total months (0.3 ± 0.2% of plants used monthly). The model with the best predictive value (AIC = 105.91, χ2 = 1.33, df = 1, P < 0.001) showed that exotic plants were most likely to be used by H. meridionalis, and most often for feeding (Table IV). Season was not included in the best-fit model.
Discussion
We found that Eulemur collaris and Hapalemur meridionalis used similar proportions of forest edge habitat within their home ranges and core areas; thus our prediction that H. meridionalis would use greater edge habitat was not supported. As predicted, the frugivorous E. collaris was more likely to use both slow- and mid-growing plant species, while the folivorous H. meridionalis was more likely to use fast-growing plants in Mandena. In terms of activity, slow-growing trees were particularly important for E. collaris resting, in line with our prediction, whereas H. meridionalis used a similarly large amount of slow-growing trees for resting. As predicted, fast-growing plants (comprising mostly herbs and scrubs) seem to be preferred by H. meridionalis, which exhibited greater ability to include pioneer species in its diet, a finding that is consistent with other studies of folivorous primates (Bicca-Marques and Calegaro-Marques 1994; Bonilla-Sánchez et al. 2012; Ganzhorn et al. 1999b). However, the use of exotic (nonendemic) plant species for feeding by H. meridionalis did not support our prediction, as these small-bodied folivores consumed items from these nonnative plants nearly every month.
Although bamboo lemurs are folivores, they are often considered to be dietary specialists because of the large proportion of their feeding focused on bamboos (Ballhorn et al. 2016; Tan 1999). However, when there are alternative habitats adjacent to a degraded habitat, e.g., mangrove swamp, monodominant plantation, even dietary specialists can adapt and exploit them (Galat-Luong and Galat 2005; Grimes and Paterson 2000; Nowak 2008). Such is the case with bamboo lemurs, which have been observed to use alternative and/or degraded habitats (Eppley et al. 2015a; Grassi 2006; Martinez 2008; Wright et al. 2008). Furthermore, the occasional use of wetland habitat by primates may become obligate if preferred upland habitat becomes increasingly disturbed (Nowak 2008, 2013; Quinten et al. 2010); however, when species are highly selective within their habitat, the loss of key resources may result in their ultimate demise (Lee and Hauser 1998). In contrast, low selectivity may enhance a species’ chances for survival, even in heavily disturbed habitats (Guo et al. 2008).
In general, bamboo lemurs (Hapalemur spp. / Prolemur simus) appear less susceptible to habitat degradation than more frugivorous species, i.e., Propithecus spp., Eulemur spp., Varecia spp. (Arrigo-Nelson 2006; Dehgan 2003; Irwin et al. 2010; Schwitzer et al. 2007). Despite this, there appears to be some variation in bamboo lemur responses to degraded habitats. For example, H. occidentalis have been observed to feed on invasive Clidemia hirta and crop forage on rice (Oryza sativa) in agricultural fields adjacent to Masoala National Park (Martinez 2008), while H. griseus have been observed to shift their diet to exotic guava (Psidium cattleianum) during fruiting periods in a previously selectively logged area of Ranomafana National Park (Grassi 2006). Furthermore, the greater bamboo lemur (P. simus) is known to inhabit shaded coffee plantations (Wright et al. 2008). Similar to these fragment-tolerant bamboo lemurs, H. meridionalis displayed an ability to adjust across various habitats, i.e., littoral forest, littoral swamp, and an invasive Melaleuca-dominated swamp, and though this was slightly seasonal, they were able to feed and rest for large portions of time in each habitat in all seasons (Eppley et al. 2015a). Additionally, they exhibited the highest dietary diversity recorded for a bamboo lemur species (Eppley et al. 2016a). In addition to the flexible activity pattern exhibited by H. meridionalis in Mandena, these lemurs are also able to adjust flexibly to contrasting floristic and structural habitats, exploiting resources that are specific to each environment (Eppley et al. 2015a, 2016a).
Two previous studies on Eulemur collaris in Mandena indicate that these lemurs in the fragmented littoral forest tend to remain highly frugivorous but they expand their home range when compared to less disturbed forests (Campera et al. 2014; Donati et al. 2011). This flexible strategy differs from other brown lemur populations that seem to be able to shift seasonally to a more folivorous diet, e.g., E. macaco macaco (Colquhoun 1997), E. mongoz (Curtis 2004), E. rufifrons (Sussman 1977); for a detailed meta-analysis, see Sato et al. (2016). The feeding preference of E. collaris for mid- and slow-growing species, which tend to represent large trees rather than herbs/scrubs and thus are rarer in highly fragmented areas than in pristine forest, is in line with an expansion of the threshold of area requirement. Our results show a preference of E. collaris for mid-growing species in the dry seasons while slow-growing, usually climax trees, are selected more often in the wet season. This is an indication that E. collaris may tend to use pioneer species more frequently during periods of low resource abundance, e.g., the dry season in Mandena, when climax trees show phenological bottlenecks. This hypothesis is worth exploring in future studies matching fine-grained phenological data with lemur seasonal feeding.
The preference for fruiting trees does not mean that Eulemur collaris is not capable of using pioneer or exotic species growing in edge areas both for feeding and for resting, as indicated by the similar values of edge use and their use of four exotic plant species. In Mandena, E. collaris have been seen to move in the periphery of forest fragments to feed on fruits of the exotic Psidium spp. (Campera et al. 2014; Donati et al. 2011) and domestic lychee (Litchi chinensis; Donati pers. obs.). In Ste. Luce (20 km north of Mandena), E. collaris have also been observed to move to the forest edge, or even outside of it, to feed on the fruits of exotic and/or pioneer species, e.g., the fruits of the pioneer meramaintso (Sarcolaena multiflora: Campera et al. 2014). This pattern does not seem to be unusual for brown lemurs even in less disturbed forests, as migrations from familiar areas to feed on exotic Psidium spp. have also been recorded in E. rufifrons in Ranomafana (Overdorff 1993; Wright 1999).
In areas more heavily affected by habitat alteration, the genus Eulemur may rely heavily on exotic trees, in most cases for fruits or for resting/sleeping. In the gallery forest fragment of Berenty, during specific periods of the year the hybrids E. rufifrons × E. collaris base the majority of their diet on fruits of the exotic Manilla tamarind (Pithecellobium dulce; Donati, unpubl. data). In Ampasikely, a 50-ha coastal private landholding located in northwestern Madagascar, E. macaco feed on 23 exotic plant species that were introduced as cash crops, such as coffee (Coffea spp.), papaya (Carica papaya), mango (Mangifera indica), and lebbeck or woman’s tongue (Albizia lebbeck: Simmen et al. 2007). Thus, the low level of reliance on exotic species by E. collaris recorded in our study seems to be more the consequence of the low frequency of suitable exotic species than the lack of flexibility of these collared brown lemurs to include unusual food species in their diet.
Habitat disturbance may benefit folivorous lemurs in several ways. It can increase the heterogeneity of a forest and therefore increase the amount or density of food resources (Oates 1996). Disturbance can increase the relative abundance of certain plant species that may be preferred food sources, such as pioneer and light-gap species, and terrestrial herbaceous vegetation (Oates 1996). Light gaps created by tree falls and/or selective felling may help to maintain floristic diversity by harboring a higher density of tree stems (Brokaw and Busing 2000). These gaps can also increase the number of early successional specialists, which tend to have leaves with increased protein, less fiber, and lower phenolic content, as well as increasing the quantity of young leaves and improving the quality of mature leaves (Chapman et al. 2002; Ganzhorn 1992, 1995; Oates 1996). Our finding that Hapalemur meridionalis exhibit a flexible behavioral and feeding ecology is not all that surprising. Bamboo lemur congeners exploit bamboo, which is highly prevalent in their habitat and thrives particularly well in slightly disturbed areas. The increased sunlight reaching both the canopy and forest floor further increases the quantity and quality of staple foods (bamboo and leaves) and provides higher quality supplemental foods (light-gap species and introduced species). Furthermore, similar to our H. meridionalis results, H. griseus in Ranomafana National Park exhibit a tolerance to forest edge (Lehman et al. 2006). Ultimately, the ability to use forest edge may have future benefits, in that altered landscapes with habitat matrices could provide potential conservation value as vital refuges (Chapman and Lambert 2000; Riley 2007).
Various folivorous primates, such as Alouatta spp., are able to inhabit anthropogenically disturbed habitats, likely owing to a broad range of behavioral adaptations (Bonilla-Sánchez et al. 2012; Zárate et al. 2014). Howlers in these habitats increase their dietary breadth (Bicca-Marques 2003) and we found a similar pattern in Hapalemur meridionalis in Mandena (Eppley et al. 2016a). By comparison, arboreal frugivores such as brown spider monkeys (Ateles hybridus) are not as flexible, and have been shown to be adversely affected by the constraints of living in an anthropogenic, degraded forest (Marsh et al. 2016). This is not always the case, however, as even frugivorous primates, e.g., red-bellied lemurs (Eulemur rubriventer), display an ability to use and be tolerant of forest edge (Lehman et al. 2006). Although E. collaris and H. meridionalis displayed differences in the degree of pioneer exotic plant species they used, they used similar proportions of forest edge within their home ranges and core areas.
The further fragmentation of remaining forests is of great concern if forest species of Madagascar are to persist (Ganzhorn et al. 2014). Although the fate of all lemur species should be considered precarious because of increasing habitat destruction, the knowledge that some lemurs are able to cope with this degradation (to a certain degree) should be seen as positive. Some primate species adapted to narrow ecological specializations may be sensitive to natural or anthropogenic habitat perturbations (Harcourt et al. 2005; Kamilar and Paciulli 2008), whereas others have been shown to adjust to changing environments (Anderson et al. 2007; Nowak and Lee 2013). Our study on two lemurids living in the highly disturbed littoral forest fragments shows that both lemurs are able to use pioneer and exotic species for feeding and resting. However, whereas frugivorous Eulemur collaris appear more limited by climax plants, folivorous Hapalemur meridionalis show a wider range of adaptability, probably favored by its diet and smaller body size.
References
Achard, F., Eva, H. D., Stibig, H. J., Mayaux, P., Gallego, J., et al. (2002). Determination of deforestation rates of the world's humid tropical forests. Science, 297, 999–1002.
Altmann, J. (1974). Observational study of behavior: Sampling methods. Behaviour, 49, 227–266.
Anderson, J., Rowcliffe, J. M., & Cowlishaw, G. (2007). Does the matrix matter? A forest primate in a complex agricultural landscape. Biological Conservation, 135, 212–222.
Arrigo-Nelson, S. J. (2006). The impact of habitat disturbance on the feeding ecology of the Milne-Edwards’ sifaka (Propithecus edwardsi) in Ranomafana National Park, Madagascar. Ph.D. dissertation, Stony Brook University.
Asner, G. P., Rudel, T. K., Aide, T. M., Defries, R., & Emerson, R. (2009). A contemporary assessment of change in humid tropical forests. Conservation Biology, 23, 1386–1395.
Ballhorn, D. J., Rakotoarivelo, F. P., & Kautz, S. (2016). Coevolution of cyanogenic bamboos and bamboo lemurs on Madagascar. PLoS ONE, 11, e0158935.
Barthlott, W., Lauer, W., & Placke, A. (1996). Global distribution of species diversity in vascular plants: Towards a world map of phytodiversity. Erdkunde, 50, 317–327.
Bates, D., Maechler, M., & Bolker, B. (2012). lme4: Linear mixed-effects models using S4 classes (2011). R package version 0.999375-42.
Benitez-Malvido, J. (1998). Impact of forest fragmentation on seedling abundance in a tropical rain forest. Conservation Biology, 12, 380–389.
Beyer, H. L. (2012). In Geospatial modelling environment. 0.7.2.0. http://www.spatialecology.com/gme
Bicca-Marques, J. C. (2003). How do howler monkeys cope with habitat fragmentation? In L. K. Marsh (Ed.), Primates in fragments: Ecology and conservation (pp. 283–303). New York: Springer Science + Business Media.
Bicca-Marques, J. C., & Calegaro-Marques, C. (1994). Exotic plant species can serve as staple food sources for wild howler populations. Folia Primatologica, 63, 209–211.
Bollen, A., & Donati, G. (2005). Phenology of the littoral forest of Sainte Luce, southeastern Madagascar. Biotropica, 37, 32–43.
Bollen, A., & Donati, G. (2006). Conservation status of the littoral forest of southeastern Madagascar: A review. Oryx, 40, 57–66.
Bonilla-Sánchez, Y. M., Serio-Silva, J. C., Pozo-Montuy, G., & Chapman, C. A. (2012). Howlers are able to survive in Eucalyptus plantations where remnant and regenerating vegetation is available. International Journal of Primatology, 33, 233–245.
Boyle, S. A., & Smith, A. T. (2010). Can landscape and species characteristics predict primate presence in forest fragments in the Brazilian Amazon? Biological Conservation, 143, 1134–1143.
Broadbent, E. N., Asner, G. P., Keller, M., Knapp, D. E., Oliveira, P. J., & Silva, J. N. (2008). Forest fragmentation and edge effects from deforestation and selective logging in the Brazilian Amazon. Biological Conservation, 141, 1745–1757.
Brokaw, N., & Busing, R. T. (2000). Niche versus chance and tree diversity in forest gaps. Trends in Ecology & Evolution, 15, 183–188.
Campbell-Smith, G., Campbell-Smith, M., Singleton, I., & Linkie, M. (2011). Apes in space: Saving an imperilled orangutan population in Sumatra. PLoS ONE, 6, e17210.
Campera, M., Serra, V., Balestri, M., Barresi, M., Ravaolahy, M., et al. (2014). Effects of habitat quality and seasonality on ranging patterns of collared brown lemur (Eulemur collaris) in littoral forest fragments. International Journal of Primatology, 35, 957–975.
Chapman, C. A. (1995). Primate seed dispersal: Co-evolution and conservation implications. Evolutionary Anthropology, 4, 74–82.
Chapman, C. A., & Lambert, J. E. (2000). Habitat alteration and the conservation of African primates: Case study of Kibale National Park, Uganda. American Journal of Primatology, 50, 169–185.
Chapman, C. A., Balcomb, S. R., Gillespie, T. R., Skorupa, J. P., & Struhsaker, T. T. (2000). Long-term effects of logging on African primate communities: A 28-year comparison from Kibale National Park, Uganda. Conservation Biology, 14, 207–217.
Chapman, C. A., Chapman, L. J., Bjorndal, K., & Onderdonk, D. A. (2002). Application of protein to fiber ratios to predict colobine abundance on different spatial scales. International Journal of Primatology, 23, 283–310.
Coley, P. D., Bryant, J. P., & Chapin, F. S. (1985). Resource availability and plant antiherbivore defense. Science, 230, 895–899.
Colquhoun, I. C. (1997). A predictive socioecological study of the black lemur (Eulemur macaco macaco) in northwestern Madagascar. Ph.D. dissertation, Washington University.
Cowlishaw, G., & Dunbar, R. (2000). Primate conservation biology. Chicago: University of Chicago Press.
Cristóbal-Azkarate, J., & Arroyo-Rodríguez, V. (2007). Diet and activity pattern of howler monkeys (Alouatta palliata) in Los Tuxtlas, Mexico: Effects of habitat fragmentation and implications for conservation. American Journal of Primatology, 69, 1013–1029.
Curtis, D. J. (2004). Diet and nutrition in wild mongoose lemurs (Eulemur mongoz) and their implications for the evolution of female dominance and small group size in lemurs. American Journal of Physical Anthropology, 124, 234–247.
D’Antonio, C. M., & Vitousek, P. M. (1992). Biological invasions by exotic grasses, the grass/fire cycle, and global change. Annual Review of Ecological Systems, 23, 63–87.
Dehgan, A. (2003). The behavior of extinction: Predicting the incidence and local extinction of lemurs in fragmented habitats of southeastern Madagascar. Ph.D. dissertation, University of Chicago.
Dirzo, R., & Raven, P. H. (2003). Global state of biodiversity and loss. Annual Review of Environment and Resources, 28, 137–167.
Donati, G., Bollen, A., Borgognini-Tarli, S. M., & Ganzhorn, J. U. (2007). Feeding over the 24-h cycle: Dietary flexibility of cathemeral collared lemurs (Eulemur collaris). Behavioral Ecology and Sociobiology, 61, 1237–1251.
Donati, G., Kesch, K., Ndremifidy, K., Schmidt, S. L., Ramanamanjato, J. B., et al. (2011). Better few than hungry: Flexible feeding ecology of collared lemurs Eulemur collaris in littoral forest fragments. PLoS ONE, 6, e19807.
Dumetz, N. (1999). High plant diversity of lowland rainforest vestiges in eastern Madagascar. Biodiversity and Conservation, 8, 273–315.
Eppley, T. M., Verjans, E., & Donati, G. (2011). Coping with low-quality diets: A first account of the feeding ecology of the southern gentle lemur, Hapalemur meridionalis, in the Mandena littoral forest, southeast Madagascar. Primates, 52, 7–13.
Eppley, T. M., Donati, G., Ramanamanjato, J.-B., Randriatafika, F., Andriamandimbiarisoa, L. N., et al. (2015a). The use of an invasive species habitat by a small folivorous primate: Implications for conservation. PLoS ONE, 10, e0140981.
Eppley, T. M., Hall, K., Donati, G., & Ganzhorn, J. U. (2015b). An unusual case of affiliative association of a female Lemur catta in a Hapalemur meridionalis social group. Behaviour, 152, 1041–1061.
Eppley, T. M., Ganzhorn, J. U., & Donati, G. (2015c). Cathemerality in a small, folivorous primate: Proximate control of diet activity in Hapalemur meridionalis. Behavioral Ecology and Sociobiology, 69, 991–1002.
Eppley, T. M., Donati, G., & Ganzhorn, J. U. (2016a). Determinants of terrestrial feeding in an arboreal primate: The case of the southern bamboo lemur (Hapalemur meridionalis). American Journal of Physical Anthropology, 161, 328–342.
Eppley, T. M., Donati, G., & Ganzhorn, J. U. (2016b). Unusual sleeping site selection by southern bamboo lemurs. Primates, 57, 167–173.
Eppley, T. M., Ganzhorn, J. U., & Donati, G. (2016c). Latrine behaviour as a multimodal communicatory signal station in wild lemurs: The case of Hapalemur meridionalis. Animal Behaviour, 111, 57–67.
Estrada, A., & Coates-Estrada, R. (1996). Tropical rain forest fragmentation and wild populations of primates at Los Tuxtlas. International Journal of Primatology, 5, 759–783.
Federman, S., Dornburg, A., Daly, D. C., Downie, A., Perry, G. H., et al. (2016). Implications of lemuriform extinctions for the Malagasy flora. Proceedings of the National Academy of Sciences of the United States of America, 113, 5041–5046.
Galat-Luong, A., & Galat, G. (2005). Conservation and survival adaptations of Temminck’s red colobus (Procolobus badius temmincki), in Senegal. International Journal of Primatology, 26, 585–603.
Ganzhorn, J. U. (1992). Leaf chemistry and the biomass of folivorous primates in tropical forests. Oecologia, 91, 540–547.
Ganzhorn, J. U. (1995). Low-level forest disturbance effects on primary production, leaf chemistry, and lemur populations. Ecology, 76, 2084–2096.
Ganzhorn, J. U. (1997). Test of Fox’s assembly rule for functional groups in lemur communities in Madagascar. Journal of Zoology, 241, 533–542.
Ganzhorn, J. U., Fietz, J., Rakotovao, E., Schwab, D., & Zinner, D. (1999a). Lemurs and the regeneration of dry deciduous forest in Madagascar. Conservation Biology, 13, 794–804.
Ganzhorn, J. U., Wright, P. C., & Ratsimbazafy, J. (1999b). Primate communities: Madagascar. In J. G. Fleagle, C. H. Janson, & K. E. Reed (Eds.), Primate communities (pp. 75–89). Cambridge: Cambridge University Press.
Ganzhorn, J. U., Andrianasolo, T., Andrianjazalahatra, T., Donati, G., Fietz, J., et al. (2007a). Lemurs in evergreen littoral forest fragments. In J. U. Ganzhorn, S. M. Goodman, & M. Vincelette (Eds.), Biodiversity, ecology, and conservation of the littoral ecosystems in Southeastern Madagascar, Tolagnaro (Fort Dauphin) (pp. 223–225). Washington, DC: Smithsonian Institution Press.
Ganzhorn, J. U., Goodman, S. M., & Vincelette, M. (2007b). Biodiversity, ecology and conservation of littoral ecosystems in southeastern Madagascar, Tolagnaro (Fort Dauphin). Washington, DC: Smithsonian Institution Press.
Ganzhorn, J. U., Wilmé, L., & Mercier, J.-L. (2014). Explaining Madagascar’s biodiversity. In I. R. Scales (Ed.), Conservation and environmental management in Madagascar (pp. 17–43). New York: Routledge.
Gardner, C. J. (2009). A review of the impacts of anthropogenic habitat change on terrestrial biodiversity in Madagascar: Implications for the design and management of new protected areas. Malayan Nature Journal, 2, 2–29.
Gérard, A., Ganzhorn, J. U., Kull, C. A., & Carrière, S. M. (2015). Possible roles of introduced plants for native vertebrate conservation: The case of Madagascar. Restoration Ecology, 23, 768–775.
Gibson, L., Lee, T. M., Koh, L. P., Brook, B. W., Gardner, T. A., et al. (2011). Primary forests are irreplaceable for sustaining tropical biodiversity. Nature, 478, 378–381.
Grassi, C. (2006). Variability in habitat, diet, and social structure of Hapalemur griseus in Ranomafana National Park, Madagascar. American Journal of Physical Anthropology, 131, 50–63.
Grimes, K., & Paterson, J. D. (2000). Colobus guereza and exotic plant species in the Entebbe Botanical Gardens. American Journal of Primatology, 51, 59–60.
Guo, S., Ji, W., Li, B., & Li, M. (2008). Response of a group of Sichuan snub-nosed monkeys to commercial logging in the Qinling Mountains, China. Conservation Biology, 22, 1055–1064.
Hannah, L., Dave, R., Lowry, P. P., II, Andelman, S., Andrianarisata, M., et al. (2008). Climate change adaptation for conservation in Madagascar. Biology Letters, 4, 590–594.
Harcourt, A. H., & Doherty, D. A. (2005). Species–area relationships of primates in tropical forest fragments: A global analysis. Journal of Applied Ecology, 42, 630–637.
Harcourt, A. H., Coppeto, S. A., & Parks, S. A. (2005). The distribution–abundance (density) relationship: Its form and causes in a tropical mammal order, primates. Journal of Biogeography, 32, 565–579.
Harper, G. J., Steininger, M. K., Tucker, C. J., Juhn, D., & Hawkins, F. (2007). Fifty years of deforestation and forest fragmentation in Madagascar. Environmental Conservation, 34, 1–9.
Hockings, K. J., & McLennan, M. R. (2012). From forest to farm: Systematic review of cultivar feeding by chimpanzees: Management implications for wildlife in anthropogenic landscapes. PLoS ONE, 7, e33391.
Ingram, J. C., & Dawson, T. P. (2006). Forest cover, condition, and ecology in human-impacted forests, south-eastern Madagascar. Conservation and Society, 4, 194–230.
Irwin, M. T., Wright, P. C., Birkinshaw, C., Fisher, B. L., Gardner, C. J., et al. (2010). Patterns of species change in anthropogenically disturbed forests of Madagascar. Biological Conservation, 143, 2351–2362.
Isaac, N. J., & Cowlishaw, G. (2004). How species respond to multiple extinction threats. Proceedings of the Royal Society of London B: Biological Sciences, 271, 1135–1141.
Isabirye-Basuta, G. M., & Lwanga, J. S. (2008). Primate populations and their interactions with changing habitats. International Journal of Primatology, 29, 35–48.
Jernvall, J., & Wright, P. C. (1998). Diversity components of impending primate extinctions. Proceedings of the National Academy of Sciences of the United States of America, 95, 11279–11283.
Kamilar, J. M., & Paciulli, L. M. (2008). Examining the extinction risk of specialized folivores: A comparative study of colobine monkeys. American Journal of Primatology, 70, 816–827.
Lake, J. C., & Leishman, M. R. (2004). Invasion success of exotic plants in natural ecosystems: The role of disturbance, plant attributes and freedom from herbivores. Biological Conservation, 117, 215–226.
Laurance, W. F., Nascimento, H. E. M., Laurance, S. G., Andrade, A., Fearnside, P. M., et al. (2006). Rain forest fragmentation and the proliferation of successional trees. Ecology, 87, 469–482.
Laurance, W. F., Nascimento, H. E. M., Laurance, S. G., Andrade, A., Ewers, R. M., et al. (2007). Habitat fragmentation, variable edge effects, and the landscape-divergence hypothesis. PLoS ONE, 2, e1017.
Laurance, W. F., Goosem, M., & Laurance, S. G. (2009). Impacts of roads and linear clearings on tropical forests. Trends in Ecology & Evolution, 24, 659–669.
Laurance, W. F., Camargo, J. L., Luizão, R. C., Laurance, S. G., Pimm, S. L., et al. (2011). The fate of Amazonian forest fragments: A 32-year investigation. Biological Conservation, 144, 56–67.
Lee, P. C. (2003). Innovation as a behavioural response to environmental challenges: A cost and benefit approach. In S. M. Reader (Ed.), Animal innovation (pp. 261–276). Oxford: Oxford University Press.
Lee, P. C., & Hauser, M. D. (1998). Long-term consequences of changes in territory quality on feeding and reproductive strategies of vervet monkeys. Journal of Animal Ecology, 67, 347–358.
Lehman, S. M., Rajaonson, A., & Day, S. (2006). Edge effects and their influence on lemur density and distribution in southeast Madagascar. American Journal of Physical Anthropology, 129, 232–241.
Marsh, L. K. (2003). Primates in fragments: Ecology and conservation. New York: Kluwer Academic/Plenum Publishers.
Marsh, C., Link, A., King-Bailey, G., & Donati, G. (2016). Effects of fragment and vegetation structure on the population abundance of Ateles hybridus, Alouatta seniculus and Cebus albifrons in Magdalena Valley, Colombia. Folia Primatologica, 87, 17–30.
Martinez, B. (2008). Occurrence of bamboo lemurs, Hapalemur griseus occidentalis, in an agricultural landscape on the Masoala peninsula. Lemur News, 13, 11–14.
McLennan, M. R., & Hockings, K. J. (2014). Wild chimpanzees show group differences in selection of agricultural crops. Scientific Reports, 4, 5956.
Nowak, K. (2008). Frequent water drinking by Zanzibar red colobus (Procolobus kirkii) in a mangrove forest refuge. American Journal of Primatology, 70, 1081–1092.
Nowak, K. (2013). Mangrove and peat swamp forests: Refuge habitats for primates and felids. Folia Primatologica, 83, 361–376.
Nowak, K., & Lee, P. C. (2013). “Specialist” primates can be flexible in response to habitat alteration. In L. K. Marsh & C. A. Chapman (Eds.), Primates in fragments: Complexity and resilience (pp. 199–211). Developments in Primatology: Progress and Prospects. New York: Springer Science+Business Media.
Oates, J. F. (1996). Habitat alteration, hunting and the conservation of folivorous primates in African forests. Australian Journal of Ecology, 21, 1–9.
Oates, J. F. (2013). Primate conservation: Unmet challenges and the role of the International Primatological Society. International Journal of Primatology, 34, 235–245.
Onderdonk, D. A., & Chapman, C. A. (2000). Coping with forest fragmentation: The primates of Kibale National Park, Uganda. International Journal of Primatology, 21, 587–611.
Overdorff, D. J. (1993). Similarities, differences, and seasonal patterns in the diets of Eulemur rubriventer and Eulemur fulvus rufus in the Ranomafana National Park, Madagascar. International Journal of Primatology, 14, 721–753.
Plumptre, A. J., & Reynolds, V. (1994). The effect of selective logging on the primate populations in the Budongo Forest Reserve, Uganda. Journal of Applied Ecology, 31, 631–641.
Poorter, L. (1999). Growth responses of 15 rain-forest tree species to a light gradient: The relative importance of morphological and physiological traits. Functional Ecology, 13, 396–410.
Quinten, M. C., Waltert, M., Syamsuri, F., & Hodges, J. K. (2010). Peat swamp forest supports high primate densities on Siberut Island, Sumatra, Indonesia. Oryx, 44, 147–151.
Rabenantoandro, J., Randriatafika, F., & Lowry, P. P. (2007). Floristic and structural characteristics of remnant littoral forest sites in the Tolagnaro area. In J. U. Ganzhorn, S. M. Goodman, & M. Vincelette (Eds.), Biodiversity, ecology, and conservation of the littoral ecosystems in southeastern Madagascar, Tolagnaro (Fort Dauphin) (pp. 65–77). Washington, DC: Smithsonian Institution Press.
Ramanamanjato, J.-B., Mcintyre, P. B., & Nussbaum, R. A. (2002). Reptile, amphibian, and lemur diversity of the Malahelo Forest, a biogeographical transition zone in southeastern Madagascar. Biodiversity and Conservation, 11, 1791–1807.
Razafindratsima, O. H., & Dunham, A. E. (2014). Assessing the impacts of nonrandom seed dispersal by multiple frugivore partners on plant recruitment. Ecology, 96, 24–30.
R Development Core Team (2015). R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. http://www.R-project.org
Riley, E. P. (2007). Flexibility in diet and activity patterns of Macaca tonkeana in response to anthropogenic habitat alteration. International Journal of Primatology, 28, 107–133.
Rode, K. D., Chapman, C. A., McDowell, L. R., & Stickler, C. (2006). Nutritional correlates of population density across habitats and logging intensities in redtail monkeys (Cercopithecus ascanius). Biotropica, 38, 625–634.
Sala, O. E., Chapin, F. S., III, Armesto, J. J., Berlow, E., Bloomfield, J., et al. (2000). Global biodiversity scenarios for the year 2100. Science, 287, 1770–1774.
Sato, H., Santini, L., Patel, E. R., Campera, M., Yamashita, N., et al. (2016). Dietary flexibility and feeding strategies of Eulemur: A comparison with Propithecus. International Journal of Primatology, 37, 109–129.
Schwitzer, N., Randriatahina, G. H., Kaumanns, W., Hoffmeister, D., & Schwitzer, C. (2007). Habitat utilization of blue-eyed black lemurs, Eulemur macaco flavifrons (Gray, 1867), in primary and altered forest fragments. The Journal of Privacy and Confidentiality, 22, 79–87.
Simmen, B., Bayart, F., Marez, A., & Hladik, A. (2007). Diet, nutritional ecology, and birth season of Eulemur macaco in an anthropogenic forest in Madagascar. International Journal of Primatology, 28, 1253–1266.
Singmann, H. (2014). afex: Analysis of factorial experiments. R package (version 0.9-109).
Sussman, R. W. (1977). Feeding behaviour of Lemur catta and Lemur fulvus. In T. H. Clutton-Brock (Ed.), Primate ecology: Studies of feeding and ranging behaviour in lemurs, monkeys, and apes (pp. 1–36). New York: Academic Press.
Tan, C. L. (1999). Group composition, home range size, and diet of three sympatric bamboo lemur species (genus Hapalemur) in Ranomafana National Park, Madagascar. International Journal of Primatology, 20, 547–566.
Tutin, C. E. G., Ham, R. M., White, L. J. T., & Harrison, M. J. S. (1997a). The primate community of the Lopé Reserve, Gabon: Diets, responses to fruit scarcity, and effects on biomass. American Journal of Primatology, 42, 1–24.
Tutin, C. E. G., White, L. J. T., & Mackanga-Missandzou, A. (1997b). The use by rain forest mammals of natural forest fragments in an equatorial African savanna. Conservation Biology, 11, 1190–1203.
Vincelette, M., Rabenantoandro, J., Randrihasipara, L., Randriatafika, F., & Ganzhorn, J. U. (2007a). Results from ten years of restoration experiments in the southeastern littoral forests of Madagascar. In J. U. Ganzhorn, S. M. Goodman, & M. Vincelette (Eds.), Biodiversity, ecology, and conservation of the littoral ecosystems in southeastern Madagascar, Tolagnaro (Fort Dauphin) (pp. 337–354). Washington, DC: Smithsonian Institution Press.
Vincelette, M., Théberge, M., & Randrihasipara, L. (2007b). Evaluations of forest cover at regional and local levels in the Tolagnaro region since 1950. In J. U. Ganzhorn, S. M. Goodman, & M. Vincelette (Eds.), Biodiversity, ecology, and conservation of the littoral ecosystems in southeastern Madagascar, Tolagnaro (Fort Dauphin) (pp. 49–58). Washington, DC: Smithsonian Institution Press.
Waeber, P. O., Wilmé, L., Ramamonjisoa, B., Garcia, C., Rakotomalala, D., et al. (2015). Dry forests in Madagascar: Neglected and under pressure. International Forestry Review, 17, 127–148.
Wasserman, M. D., & Chapman, C. A. (2003). Determinants of colobine monkey abundance: the importance of food energy, protein and fibre content. Journal of Animal Ecology, 72, 650–659.
Wieczkowski, J. A. (2003). Aspects of the ecological flexibility of the Tana River mangabey (Cercocebus galeritus) in its fragmented habitat, Tana River, Kenya. Ph.D. dissertation, University of Georgia.
Worton, B. J. (1989). Kernel methods for estimating the utilization distribution in home-range studies. Ecology, 70, 164–168.
Wright, P. C. (1999). Lemur traits and Madagascar ecology: Coping with an island environment. American Journal of Physical Anthropology, 110, 31–72.
Wright, P. C., Johnson, S. E., Irwin, M. T., Jacobs, R., Schlichting, P., et al. (2008). The crisis of the critically endangered greater bamboo lemur (Prolemur simus). Primate Conservation, 23, 5–17.
Zárate, D. A., Andresen, E., Estrada, A., & Seri-Silva, J. C. (2014). Black howler monkey (Alouatta pigra) activity, foraging and seed dispersal patterns in shaded cocoa plantations versus rainforest in southern Mexico. American Journal of Primatology, 76, 890–899.
Acknowledgments
We thank the Direction du Système des Aires Protégées, the Ministère de l’Environnement et Forêts of Madagascar, and the Mandena Management Committee (COGEMA) for permission to conduct research. We also thank the QMM Rio Tinto Environmental Team for their assistance and provision of logistical support on site and acknowledge their helpful staff, especially Manon Vincelette, Laza Andriamandimbiarisoa, David Rabehevitra, Christophe Rambolamanana, and Claude Soanary. Thanks to Shauna Mora for assistance with the GIS analysis. T. M. Eppley is grateful to Jacques Rakotondranary and Tolona Andrianasolo for obtaining research permits, and to Robertin Ravelomanantsoa, Katie Hall, and Natalie Breden for assistance in the field. T. M. Eppley also thanks the following organizations for their generous financial and in-kind support: American Society of Primatologists, Conservation International Primate Action Fund, Idea Wild, Mohamed bin Zayed Species Conservation Fund (Project Number: 11253008), Primate Conservation Inc., and the Primate Society of Great Britain/Knowsley Safari Park. M. Campera and M. Balestri thank Aristide Andrianarimisa, Germain, and Crescent for their assistance in the field. M. Campera and M. Balestri were supported by the Rufford Small Grant Foundation, and thank the Madagascar Institute for the Conservation of Tropical Environments (MICET). Many thanks to the guest editors, Matt McLennan, Noemi Spagnoletti, and Kim Hockings for the invitation to contribute to this special issue, and to Joanna Setchell and two anonymous reviewers for helpful comments.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest and no competing financial interests.
Additional information
Handling Editor: Noemi Spagnoletti
Rights and permissions
About this article
Cite this article
Eppley, T.M., Balestri, M., Campera, M. et al. Ecological Flexibility as Measured by the Use of Pioneer and Exotic Plants by Two Lemurids: Eulemur collaris and Hapalemur meridionalis . Int J Primatol 38, 338–357 (2017). https://doi.org/10.1007/s10764-016-9943-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10764-016-9943-8