Abstract
Selection of sleeping sites has consequences for individual fitness. Non-human primates often bias their selection towards arboreal sites, and the lemurs of Madagascar typically rest/sleep in trees, tree holes, and/or constructed nests. Three non-mutually exclusive hypotheses to explain sleeping site selection include protection from predators, avoidance of parasitic vectors, and improved thermoregulation. Here, we examine these hypotheses for the unusual sleeping site selections by the southern bamboo lemur (Hapalemur meridionalis). Within the Mandena littoral forest of southeast Madagascar, the southern bamboo lemur is known for its ecological flexibility compared to other bamboo lemur species, including a dietary niche expansion to feeding on the ground. Between October 2012 and December 2013, we observed bamboo lemurs from three social groups for 1778.67 h, conducting full-day focal follows on 11 adult individuals (five males, six females). During this period, all three groups were observed to sleep on the ground, with one of these groups also using an abandoned nest of a Madagascar crested ibis (Lophotibis cristata). We collected habitat and temperature data to examine whether selection was influenced by environmental variables. Terrestrial sleeping (N = 17) was observed in all individuals but one adult female, with individuals burrowing under thick vegetation more often during the hot austral summer. While difficult to rigorously test, it is possible that terrestrial sleep sites and/or sleeping in a bird nest may impair visual detection by some aerial and terrestrial predators. Neither of these sites (i.e., terrestrial sleeping or use of a bird nest), however, is likely to minimize exposure to parasites/vectors. Terrestrial sleeping appears to support a thermoregulatory strategy, whereas the use of a bird nest could not be empirically tested. Our observations of unique sleeping site locations used by southern bamboo lemurs further the complexity of their natural history and that of Malagasy strepsirrhines.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The choice of rest and sleep locations may have a profound impact on individual survival (Cowlishaw 1994). Primates spend roughly half of their lives at resting/sleeping sites, and their selection of these specific sites is a vital aspect of their behavioral ecology (Anderson 1998). Most primate species are arboreal, and those that forage and travel terrestrially often still ascend trees each evening to sleep, e.g., Guinea baboons (Papio papio; Anderson and McGrew 1984), patas monkeys (Erythrocebus patas), tantalus monkeys (Cercopithecus aethiops tantalus; Nakagawa 1999), and chimpanzees (Pan troglodytes; Pruetz et al. 2008; Samson et al. 2013). As sleeping site selection is integral to an individual’s fitness, multiple hypotheses have been postulated, including protection against terrestrial predators, thermoregulatory benefits, and anti-vector strategies (Anderson 1998, 2000; McGrew 2004).
The anti-predator hypothesis postulates that arboreal sleeping provides protection from terrestrial predators, hence it is more secure than terrestrial sleeping (Anderson 1998, 2000). Many primate studies have shown that sleeping sites are selected based on tree dimensions/traits (e.g., height, size, and density) to provide protection from potential terrestrial predators (Anderson 1998; Cheyne et al. 2012; Stewart and Pruetz 2013). Among chimpanzees (Pan troglodytes verus) and gorillas (Gorilla beringei graueri), it has been suggested that sleeping on the ground occurs when predation pressure is relaxed (Yamagiwa 2001; Koops et al. 2007).
Parasites such as mosquitoes and lice are thought to affect the sleeping habits of numerous primate species, whereby the selection of dense vegetation, tree holes, and nest tree species may decrease exposure to potential vectors and minimize zoonotic disease transmission (Heymann 1995; Fruth and Hohmann 1996; Anderson 1998). There are likely site-specific differences influencing this selection pressure. For example, chimpanzees (P. t. schweinfurthii) at Toro-Semliki, Uganda, preferentially selected to build their nests in a specific tree species (Cynometra alexandri), which was found to significantly reduce exposure to mosquitoes (Samson et al. 2013).
Lemurs, the strepsirrhines of Madagascar, live in a highly seasonal environment with high climatic stochasticity that is assumed to have had a major influence on the evolution of lemur life history traits (Wright 1999; Dewar and Richard 2007). Many species of the lemur family Cheirogaleidae exhibit hibernation or torpor to conserve energy or water during the cold, dry and resource-deficient austral winter months. These mouse and dwarf lemurs (Microcebus spp. and Cheirogaleus spp., respectively) construct nests, or use trees holes or burrows, which may offset thermoregulatory risks presented by large temperature variations (Kappeler 1998; Dausmann et al. 2004; Lutermann et al. 2010; Blanco et al. 2013), but the use of these shelters by multiple individuals (Perret 1998) may not always lead to energetic benefits (Dausmann and Glos 2015) and could lead to increased parasite transfers (Zohdy et al. 2012; Kappeler et al. 2015). Constructing nests is not limited to cheirogaleids; for example, ruffed lemurs (Varecia spp.) also construct communal nests to provide protection for their infants (Morland 1990; Tecot et al. 2012; Baden et al. 2013). Thus far, this is the only genus of the family Lemuridae that are known to utilize nests. Resting and sleeping sites for lemurid genera are mostly confined to arboreal sites, although recent observations on ring-tailed lemurs (Lemur catta) have described their selection of cave sites, where they presumably sleep either on the ground and/or on rock ledges (Sauther et al. 2013). Similar to the Fongoli chimpanzees (Pruetz 2007), it is thought that these cave sleeping sites potentially provide safety from predation and thermoregulatory benefits (Sauther et al. 2013).
In this study, we report observations of two unusual resting/sleeping sites selected by an arboreal lemurid species, the southern bamboo lemur (Hapalemur meridionalis). We will discuss these observations in relation to the three non-mutually exclusive hypotheses (i.e., anti-predator strategy, thermoregulatory benefits, and anti-vector strategy).
Methods
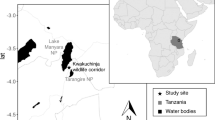
The Mandena littoral forest (24°95 S, 46°99 E) is located in southeast Madagascar, 10 km north of Fort Dauphin (Tolagnaro). This small protected area consists of 230 ha of fragmented littoral forest with seasonally-inundated littoral and Melaleuca-dominated swamps (Eppley et al. 2015a). Southern bamboo lemurs (H. meridionalis) are medium-sized lemurs with a mean ± SE body mass of 1.072 ± 0.107 kg (N = 15), and are characterized as folivores (Eppley et al. 2011, 2015b) that exhibit a cathemeral activity pattern (Eppley et al. 2015c). They live in small social groups [5.6 ± 1.5 individuals (mean ± SE), N = 5; Eppley et al. 2016], and are unusual for often feeding terrestrially in both the littoral forest and swamp, frequently spending multiple hours grazing on various graminoid species (Families Poaceae and Cyperaceae; Eppley et al. 2011). The inclusion of a terrestrial stratum in their feeding repertoire carries additional predation risks compared to an otherwise strictly arboreal lifestyle (Eppley et al. 2011). As part of a larger behavioral ecology study, we captured H. meridionalis from four social groups and radio-collared ten adult individuals with data-logging tags (Advanced Telemetry Systems, Inc.; Isanti, MN, USA). For information on group demographics and the capturing/collaring processes, see Eppley et al. (2015c, 2016).
From October 2012 to December 2013, we observed bamboo lemurs from three of the four social groups for 1778.67 h, conducting full-day focal follows on 11 adult individuals (five males, six females). We recorded all instances of continuous resting and/or sleeping ≥15 min, and marked all sites with flagging tape and a unique code. We collected tree scientific binomial and family names, diameter at breast height (DBH; cm), height (m), and the specific habitat (i.e., littoral forest, littoral swamp, or Melaleuca swamp). Furthermore, we categorized the locations of terrestrial sleep sites. We defined 'burrow' as the animal positioning itself under a thick substrate (e.g., under thick/matted vegetation or in a cavernous hole in the ground) so as to completely cover its body and obscure itself from visibility. We defined instances of when the animal was on the ground but positioned in such a way that it was obscured from sight (from ≤5 m) as 'not visible'. Examples of this include sleeping on the ground within an area of thick tree saplings and low leaves, under a fallen leaf from Ravenala madagascariensis, and under thick ground-covering ferns (Phymatosorus scolopendria). Lastly, we defined all instances of lemurs sleeping on the ground while visually exposed as 'open', that is, the individual(s) was visible from a distance ≥5 m. As an example, these instances included lemurs sleeping on the forest floor and visible from distances sometimes greater than 10 m.
Temperature (°C) was recorded in 30-min intervals with Lascar EL-USB-1 temperature data loggers (Lascar Electronics Inc., Erie, PA, USA), operated by custom software (EasyLog USB Version 5.45, Lascar Electronics). Four data loggers were positioned 1.5 m above the ground throughout the study site to account for differences between habitats, specifically with two placed in the littoral forest, one in the littoral swamp, and one in the Melaleuca swamp. We matched the sleeping site characteristics with the specific habitat temperature data logger closest to the observation, and averaged the recorded temperatures (°C) for the duration of time bamboo lemurs spent sleeping at the location.
Data were tested for normality using the Kolmogorov–Smirnov one-sample test. As they deviated from normality in several cases, we used the nonparametric Mann–Whitney test to evaluate whether temperature (°C) and sleep duration (min) differed between the specific sites selected for terrestrial sleeping. We then combined burrow and not visible sites as ‘protected’ locations as they likely provided similar protection from climatic variables, unlike those sites categorized as 'open'. Based on these sleeping location categories, we again used the nonparametric Mann–Whitney test to determine whether there was a relationship between ambient temperature and sleep duration. All analyses were performed using PASW v. 21.0 and statistical significance was set at P < 0.05.
Results
Terrestrial resting sites
We recorded 346 sleeping bouts greater than 15 min by the southern bamboo lemurs in Mandena, totaling 362.00 h during the study period. Of these, we recorded 17 resting/sleeping bouts on the ground, totaling 19.17 h. While the average (±SD) arboreal sleeping bout was 62.67 ± 50.77 min, the average ground sleeping bout was 67.65 ± 49.34 min, and the longest terrestrial sleeping bout recorded was approximately 180 min (Table 1). Furthermore, terrestrial sleeping bouts were heavily concentrated around midday when temperatures would be at their highest (Fig. 1). Most of these bouts took place in Melaleuca swamp (68.26 %, N = 11), and the remaining terrestrial sleeping bouts took place in the littoral forest (31.74 %, N = 6). Over the study period, the mean temperature was 22.54 °C (range 9.5–35 °C). While there was a tendency to sleep in the forest when it was warmer (Mann–Whitney: Z = −1.76, N 1 = 6 forest sites, N 2 = 11 Melaleuca sites, P = 0.078), the duration of sleeping was not significantly influenced by a specific habitat (Mann–Whitney: Z = −0.71, N 1 = 6 forest sites, N 2 = 11 Melaleuca sites, P = 0.478). Six (35.3 %) of the terrestrial sleeping bouts consisted of individuals burrowing themselves under thick mats of vegetation (e.g., tall grasses and reeds), while another six bouts involved the lemurs completely obscuring themselves from sight by squeezing under densely packed tree saplings and young screw pine (Pandanus spp.) leaves, creating obscured visual detection similar to the burrowing behavior. The remaining five bouts (29.41 %) were located in the open in the littoral forest. Ambient temperature was significantly lower when bamboo lemurs slept in protected sites compared to open sites (Mann–Whitney: Z = −2.48, N 1 = 12 protected sites, N 2 = 5 open sites, P = 0.013; Table 2). Sleeping duration, however, did not significantly differ between these locations (Mann–Whitney: Z = −1.06, N 1 = 12 protected sites, N 2 = 5 open sites, P = 0.287).
Avian nest resting site
Individuals from group 4 were twice found sleeping in a previously occupied nest constructed by a Madagascar crested ibis (Lophotibis cristata). The nest was located at an approximate height of 12 m in a large Poupartia chapelieri tree (Family: Lauraceae). The tree had a DBH of 54 cm and a height of 15 m, an emergent tree in this portion of the littoral forest. On June 6th, 2013, all seven H. meridionalis (two adult females, two adult males, and 3 juveniles) and the adult female Lemur catta that had joined the group (Eppley et al. 2015b) slept in a huddle in the crested ibis nest for approximately 70 min (from 10:05 to 11:15 am), with an ambient temperature of 20.6 °C. A few days later, on June 9th, 2013, we located group 4 at the same ibis nest, where we found five H. meridionalis (one adult female, 2 adult males, and 2 juveniles) huddled and sleeping. Not all group members were present, one adult female and juvenile were absent, as was the female L. catta. Those in the nest emerged and left the nest at 07:15 am after approximately 60 min when the temperature remained at 12.5 °C. It should be further noted that the nest began to deconstruct and had nearly completely fallen apart by November 2013.
Discussion
Our data provide puzzling natural history observations for bamboo lemurs. To our knowledge, neither resting/sleeping on the ground nor in a bird’s nest had been previously observed. We will discuss the bamboo lemurs’ sleeping site selection in terms of the three potential strategies, for both unusual sleeping site selections: terrestrial and avian nest.
Thermoregulation strategy
Ambient temperatures were significantly lower when bamboo lemurs slept in burrows and other sites that provided them protection from climatic variables. However, the temperatures recorded during terrestrial sleeping bouts were neither excessively hot nor extremely cold (Table 1). Terrestrial sleeping bouts were most often observed midday during the austral summer, and involved the lemurs burrowing or positioning themselves so that they were hidden from sight. Due to the high occurrence during the austral summer, it is possible that terrestrial sleeping provided a thermoregulatory benefit as lemurs would presumably be cooler lying on the ground. While we are unable to comment on whether the ground was actually cooler than an arboreal resting position, the tendency to rest in the littoral forest when it was warmer could be due to the greater canopy cover here compared to the Melaleuca swamp (Eppley et al. 2015a). It should be noted that humidity may play an important role in sleeping site selection (Koops et al. 2012); however, neither humidity nor wind speed were collected as potential climatic variables, a shortcoming of the study.
Many species of Cheirogaleus are known to construct and use nest sites (Kappeler 1998; Lutermann et al. 2010; Thorén et al. 2010), although these sites are often used for long torpor and/or hibernation bouts. Ruffed lemurs (Varecia spp.) are the only lemurid species known to construct nests, although these nests are typically limited to seasons when infants and young juveniles are present in the group (Morland 1990; Tecot et al. 2012; Baden et al. 2013). In contrast, the use of an abandoned bird nest by H. meridionalis provided a structure that was large enough to support all individuals within the group, allowing them to all huddle together. Though our observations occurred during the austral winter when the use of a nest may have provided additional thermoregulatory benefits at night (Thorén et al. 2010), the ambient temperatures during these observations were not exceptionally cold.
Anti-vector strategy
While more fine-grained data (e.g., ecto- and/or endo-parasite loads) are needed to specifically test this hypothesis, the utilization of specific sleeping site characteristics (e.g., tree holes, dense vegetation) has been suggested to reduce parasite exposure (Heymann 1995). While the bamboo lemurs of Mandena spend a substantial amount of time on the ground (Eppley et al. 2011), increased terrestriality may increase exposure to unfamiliar pathogens and, thus, increase parasite loads compared to sympatric arboreal species (Loudon and Sauther 2013). Therefore, the use of terrestrial resting sites, regardless of the habitat type, are likely not supportive of an anti-vector strategy, and would presumably increase the risk of acquiring zoonotic pathogens.
On the other hand, building nests has been postulated to act as an anti-vector strategy by providing protection from parasite infection (Stewart 2011; Samson et al. 2013; but see Koops et al. 2012). However, H. meridionalis were not observed to make their own nests, rather they utilized an abandoned bird nest that provided a similar structure. Unlike primate nests, bird nests have been shown to host myriad parasites which can potentially be transmitted between individuals utilizing the same nest (Savage et al. 2009). This potential for zoonotic transmission between birds and mammals, however, is likely dependent on the length of time the nest was unoccupied before the bamboo lemurs began utilizing it.
Anti-predation strategy
Bamboo lemurs (Hapalemur spp.) have a grayish-brown pelage which, when coupled with their ability to maintain silence and vertically cling to a tree trunk, allows them to stay practically hidden from sight and potential predators (Tan 2006). The Mandena site presents a suite of habitats, each with contrasting tree diversity, density, and structure (Eppley et al. 2015a). The fact that H. meridionalis chose to rest on the ground in both the littoral forest (continuous canopy cover) and the Melaleuca swamp (sparse canopy cover; Eppley et al. 2015a) seems to suggest that the risk from terrestrial predators in both habitats may have been relaxed. Even so, both aerial and terrestrial predators are present within Mandena. These include three aerial raptor species (i.e., Madagascar harrier-hawk Polyboroides radiates, Henst’s goshawk Accipiter henstii, and Madagascar buzzard Buteo brachypterus) known to prey on small- to medium-sized lemurs (Karpanty 2006), as well as arboreal/terrestrial Eupleridae carnivores, e.g., fossa Cryptoprocta ferox (Donati et al. 2007), and large boid snakes (Goodman et al. 1993; Eppley and Ravelomanantsoa 2015). It is possible that the open resting locations on the ground provided an open visual field that would allow for the early detection of potential predators (Treves 2002), while both burrows and visually obscured terrestrial sites would make detection by a predator difficult. Despite this speculation, we cannot conclusively state that sleeping on the ground fulfilled an anti-predator strategy.
Though it has been postulated that nests may provide protection from potential predators (Fruth and Hohmann 1993; Pruetz et al. 2008), this hypothesis has received little support (Koops et al. 2012). In particular, the Madagascar harrier-hawk has a special adaptation to reach even into tree holes used as sleeping sites by lemurs (e.g., Schülke and Ostner 2000). Aerial raptors were typically encountered scanning the canopy above the littoral forest and surrounding forest edge, but never in the littoral swamp or Melaleuca swamp (Eppley, personal observation). When considering the use of an ibis nest, this sleeping site was located under the canopy of the littoral forest; however, the risk of detection by aerial forest raptors would likely still be considerably high (Csermely 1996; Karpanty 2006). While the nest probably offered little protection from aerial predators, the use of the nest by the entire group would visually obscure detection by terrestrial predators.
Conclusion
Given the limited number of observations, we are unable to test each of the hypotheses presented conclusively; however, it would appear that use of terrestrial and bird nest sleeping sites by H. meridionalis may potentially lend support to thermoregulation strategies. Though the risk of predation for bamboo lemurs may be relaxed in Mandena, we cannot conclusively state whether sleeping terrestrially or in a nest provided support as an anti-predator strategy. Furthermore, neither of these sleep sites appears to act as an anti-vector strategy, rather they are both more likely to increase pathogen transmissions. Whether or not the use of a nest fulfills any of these hypotheses, it may have an adaptive value by increasing sleep quality (Videan 2006), and, thus, lead to increased fitness (Fruth and Hohmann 1996). Our observations present unique sleeping site locations utilized by southern bamboo lemurs, furthering the complexity of their natural history and potentially providing insight into the evolution of Malagasy strepsirrhines.
References
Anderson JR (1998) Sleep, sleeping sites, and sleep-related activities: awakening to their significance. Am J Primatol 46:63–75
Anderson JR (2000) Sleep-related behavioural adaptations in free-ranging anthropoid primates. Sleep Med Rev 4:355–373
Anderson JR, McGrew WC (1984) Guinea baboons (Papio papio) at a sleeping site. Am J Primatol 6:1–14
Baden AL, Wright PC, Louis EE Jr, Bradley BJ (2013) Communal nesting, kinship, and maternal success in a social primate. Behav Ecol Sociobiol 67:1939–1950
Blanco MB, Dausmann KH, Ranaivoarisoa JF, Yoder AD (2013) Underground hibernation in a primate. Sci Rep 3:1768
Cheyne SM, Höing A, Rinear J, Sheeran LK (2012) Sleeping site selection by agile gibbons: the influence of tree stability, fruit availability and predation risk. Folia Primatol 83:299–311
Cowlishaw G (1994) Vulnerability to predation in baboon populations. Behaviour 131:293–304
Csermely D (1996) Antipredator behavior in lemurs: evidence of an extinct eagle on Madagascar or something else? Int J Primatol 17:349–354
Dausmann KH, Glos J (2015) No energetic benefits from sociality in tropical hibernation. Funct Ecol 29:498–505
Dausmann KH, Glos J, Ganzhorn JU, Heldmaier G (2004) Physiology: hibernation in a tropical primate. Nature 429:825–826
Dewar RE, Richard AF (2007) Evolution in the hypervariable environment of Madagascar. Proc Natl Acad Sci 104:13723–13727
Donati G, Ramanamanjato JB, Ravoahangy AM, Vincelette M (2007) Translocation as a conservation measure for a threatened species: the case of Eulemur collaris in the Mandena littoral forest, south-eastern Madagascar. In: Ganzhorn JU, Goodman SM, Vincelette M (eds) Biodiversity, ecology, and conservation of the littoral ecosystems in southeastern Madagascar, Tolagnaro (Fort Dauphin). Smithsonian Institution Press, Washington, DC, pp 237–243
Eppley TM, Ravelomanantsoa R (2015) Predation of an adult southern bamboo lemur Hapalemur meridionalis by a Dumeril’s boa Acrantophis dumerili. Lemur News 19:2–3
Eppley TM, Verjans E, Donati G (2011) Coping with low-quality diets: a first account of the feeding ecology of the southern gentle lemur, Hapalemur meridionalis, in the Mandena littoral forest, southeast Madagascar. Primates 52:7–13
Eppley TM, Donati G, Ramanamanjato J-B, Randriatafika F, Andriamandimbiarisoa LN, Rabehevitra D, Ravelomanantsoa R, Ganzhorn JU (2015a) The use of an invasive species habitat by a small folivorous primate: implications for conservation. PLoS One 10:e0140981
Eppley TM, Hall K, Donati G, Ganzhorn JU (2015b) An unusual case of affiliative association of a female Lemur catta in a Hapalemur meridionalis social group. Behaviour 152:1041–1061
Eppley TM, Ganzhorn JU, Donati G (2015c) Cathemerality in a small, folivorous primate: proximate control of diel activity in Hapalemur meridionalis. Behav Ecol Sociobiol 69:991–1002
Eppley TM, Ganzhorn JU, Donati G (2016) Latrine behaviour as a multimodal communicatory signal station in wild lemurs: the case of Hapalemur meridionalis. Anim Behav 111:57–67
Fruth B, Hohmann G (1993) Ecological and behavioral aspects of nest building in wild bonobos (Pan paniscus). Ethology 94:113–126
Fruth B, Hohmann G (1996) Nest building behavior in the great apes: The great leap forward? In: McGrew WC, Marchant LF, Nishida T (eds) Great ape societies. Cambridge University Press, Cambridge, pp 225–240
Goodman SM, O’Connor S, Langrand O (1993) A review of predation on lemurs: implications for the evolution of social behavior in small nocturnal primates. In: Kappeler PM, Ganzhorn JU (eds) Lemur social systems and their ecological basis. Plenum Press, New York, pp 51–66
Heymann EW (1995) Sleeping habits of tamarins, Saguinus mystax and Saguinus fuscicollis (Mammalia; Primates; Callitrichidae), in north-eastern Peru. J Zool 237:211–226
Kappeler PM (1998) Nests, tree holes, and the evolution of primate life histories. Am J Primatol 46:7–33
Kappeler PM, Cremer S, Nunn CL (2015) Sociality and health: impacts of sociality on disease susceptibility and transmission in animal and human societies. Philos T R Soc B 370:20140116
Karpanty SM (2006) Direct and indirect impacts of raptor predation on lemurs in southeastern Madagascar. Int J Primatol 27:239–261
Koops K, Humle T, Sterck EHM, Matsuzawa T (2007) Ground-nesting by the chimpanzees of the Nimba Mountains, Guinea: environmentally or socially determined? Am J Primatol 69:407–419
Koops K, McGrew WC, de Vries H, Matsuzawa T (2012) Nest-building by chimpanzees (Pan troglodytes verus) at Seringbara, Nimba Mountains: antipredation, thermoregulation, and antivector hypotheses. Int J Primatol 33:356–380
Loudon JE, Sauther ML (2013) Verreaux’s sifaka (Propithecus verreauxi) and ring-tailed lemur (Lemur catta) endoparasitism at the Bezà Mahafaly special reserve. Madag Conserv Dev 8:21–28
Lutermann H, Verburgt L, Rendigs A (2010) Resting and nesting in a small mammal: sleeping sites as a limiting resource for female grey mouse lemurs. Anim Behav 79:1211–1219
McGrew WC (2004) The cultured chimpanzee: reflections on cultural primatology. Cambridge University Press, Cambridge
Morland HS (1990) Parental behavior and infant development in ruffed lemurs (Varecia variegata) in a northeast Madagascar rain forest. Am J Primatol 20:253–265
Nakagawa N (1999) Differential habitat utilization by patas monkeys (Erythrocebus patas) and tantalus monkeys (Cercopithecus aethiops tantalus) living sympatrically in northern Cameroon. Am J Primatol 49:243–264
Perret M (1998) Energetic advantage of nest-sharing in a solitary primate, the lesser mouse lemur (Microcebus murinus). J Mammal 79:1093–1102
Pruetz JD (2007) Evidence of cave use by savanna chimpanzees (Pan troglodytes verus) at Fongoli, Senegal: implications for thermoregulatory behavior. Int J Primatol 48:316–319
Pruetz JD, Fulton SJ, Marchant LF, McGrew WC, Schiel M, Waller M (2008) Arboreal nesting as anti-predator adaptation by savanna chimpanzees (Pan troglodytes verus) in southeastern Senegal. Am J Primatol 70:393–401
Samson DR, Muehlenbein MP, Hunt KD (2013) Do chimpanzees (Pan troglodytes schweinfurthii) exhibit sleep related behaviors that minimize exposure to parasitic arthropods? A preliminary report on the possible anti-vector function of chimpanzee sleeping platforms. Primates 54:73–80
Sauther ML, Cuozzo FP, Jacky IY, Fish KD, LaFleur M, Ravelohasindrazana LA, Ravoavy JF (2013) Limestone cliff-face and cave use by wild ring-tailed lemurs (Lemur catta) in southwestern Madagascar. Madag Conserv Dev 8:73–80
Savage AF, Robert V, Goodman SM, Raharimanga V, Raherilalao MJ, Andrianarimisa A, Ariey F, Greiner EC (2009) Blood parasites in birds from Madagascar. J Wildlife Dis 45:907–920
Schülke O, Ostner J (2000) Predation on Lepilemur by a harrier hawk and implications for sleeping site quality. Lemur News 6:5
Stewart FA (2011) Brief communication: why sleep in a nest? Empirical testing of the function of simple shelters made by wild chimpanzees. Am J Phys Anthropol 146:313–318
Stewart FA, Pruetz JD (2013) Do chimpanzee nests serve an anti-predatory function? Am J Primatol 75:593–604
Tan CL (2006) Behavior and ecology of gentle lemurs (genus Hapalemur). In: Gould L, Sauther ML (eds) Lemurs: ecology and adaptation. Springer, New York, pp 369–381
Tecot SR, Baden AL, Romine NK, Kamilar JM (2012) Infant parking and nesting, not allomaternal care, influence Malagasy primate life histories. Behav Ecol Sociobiol 66:1375–1386
Thorén S, Quietzsch F, Radespiel U (2010) Leaf nest use and construction in the golden-brown mouse lemur (Microcebus ravelobensis) in the Ankarafantsika National Park. Am J Primatol 72:48–55
Treves A (2002) Predicting predation risk for foraging, arboreal monkeys. In: Miller LE (ed) Eat or be eaten: predator sensitive foraging among primates. Cambridge University Press, Cambridge, pp 222–241
Videan EN (2006) Sleep in captive chimpanzee (Pan troglodytes): the effects of individual and environmental factors on sleep duration and quality. Behav Brain Res 169:187–192
Wright PC (1999) Lemur traits and Madagascar ecology: coping with an island environment. Yearb Phys Anthropol 42:31–72
Yamagiwa J (2001) Factors influencing the formation of ground nests by eastern lowland gorillas in Kahuzi-Biega National Park: some evolutionary implications of nesting behavior. J Hum Evol 40:99–109
Zohdy S, Kemp AD, Durden LA, Wright PC, Jernvall J (2012) Mapping the social network: tracking lice in a wild primate (Microcebus rufus) population to infer social contacts and vector potential. BMC Ecol 12:4
Acknowledgments
We thank the Direction du Système des Aires Protégées, and the Ministère de l’Environnement et Forêts of Madagascar for permission to conduct research. Special thanks to Jacques Rakotondranary and Tolona Andrianasolo, for their logistical assistance, and to Katie Hall for assistance in the field. We also thank the Environment Team at QIT Madagascar Minerals (QMM) for their assistance and provision of logistical support onsite, and acknowledge their helpful staff, especially Jean-Baptiste Ramanamanjato, Johny Rabenantoandro, Faly Randriatafika, Laza Andriamandimbiarisoa, David Rabehevitra, and Robertin Ravelomanantsoa. This field research was result of generous financial and in-kind support provided by the American Society of Primatologists, Conservation International’s Primate Action Fund, Idea Wild, Mohamed bin Zayed Species Conservation Fund (project number: 11253008), Primate Conservation Inc., and the Primate Society of Great Britain/Knowsley Safari Park.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
This study was conducted under the Accord de Collaboration between the University of Antananarivo, the University of Hamburg, and QMM. Research protocols were approved by all institutions and permits authorized by the Commission Tripartite of the Direction des Eaux et Forêts de Madagascar (Autorisation de recherché n.240/12/MEF/SG/DGF/DCB.SAP/SCB du 17/09/2012), adhering to the legal requirements of Madagascar.
About this article
Cite this article
Eppley, T.M., Donati, G. & Ganzhorn, J.U. Unusual sleeping site selection by southern bamboo lemurs. Primates 57, 167–173 (2016). https://doi.org/10.1007/s10329-016-0516-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10329-016-0516-4