Abstract
The Burmese snub-nosed monkey (Rhinopithecus strykeri) is one of the most recently discovered primate species, and occurs only along the border of Myanmar and China. Its ecology is largely unknown owing to its harsh and remote habitat. However, study of this new species can contribute to our understanding of how primates adapt to a high-altitude lifestyle. We here describe our preliminary study of a group of R. strykeri, using a mix of direct observation and camera traps, at Pianma, Yunnan, China. From May 2013 to May 2014, we conducted direct observation and deployed 30 camera traps to examine the social characteristics of R. strykeri, estimate group home range via the modified minimum convex polygon method, and estimate the vertical range used. We achieved direct observation on 8 days and obtained 222 camera trap images triggered by the passing of R. strykeri. The cameras captured five one-male, multifemale units and one all-male unit. We observed fusion of units without aggression during both direct observation and camera trapping, suggesting that R. strykeri lives in a multilevel society, similarly to the other members of the genus. The ratio of adults to immatures was high relative to stable populations of Rhinopithecus, suggesting the population is in decline. We estimated the group’s home range to be 22.9 km2 and found that R. strykeri occurred at 2400–3300 m. Our work shows that camera traps can be used effectively to survey rare primates.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Members of the genus Rhinopithecus inhabit a wide range of habitats, which vary from the tropical evergreen broadleaf forests of Vietnam in R. avunculus to the high-altitude conifer forests of China in R. bieti. Rhinopithecus species have been documented living at 200–4600 m above sea level (Kirkpatrick and Grueter 2010). Snub-nosed monkeys have larger home ranges than most other colobines do, as well as much larger group sizes (Kirkpatrick and Grueter 2010). These groups are organized as multilevel societies consisting of several one-male, multifemale units (OMUs) associated with one or several satellite all-male units (AMUs) to form a stable and cohesive group; groups exceeding 400 individuals have been documented (Grueter 2013; Kirkpatrick and Grueter 2010). This complex multilevel social organization is relatively rare across nonhuman primates (Grueter et al. 2012). It is likely that the multilevel societies in Rhinopithecus species evolved from an ancestral Asian colobine that lived in separate OMUs (Grueter et al. 2012; Qi et al. 2014).
The Burmese or black snub-nosed monkey, Rhinopithecus strykeri, is the most recently discovered member of genus Rhinopithecus, bringing the total number of snub-nosed monkey species to five (Geissmann et al. 2011). It is closely related to R. bieti, the black-and-white snub-nosed monkey, in both morphology and genetics, especially to a R. bieti haplogroup in the southern distribution (Geissmann et al. 2011; Liedigk et al. 2012; Zhou et al. 2014). Preliminary surveys suggest that R. strykeri occurs only in Myanmar and China between the N’mai Hka River to the west and the Salween River to the east (Geissmann et al. 2011; Long et al. 2012; Ma et al. 2014). This species dwells in extremely harsh, mountainous terrain; its habitat consists of cool temperate rain forest, mixed temperate forest, and bamboo forest 1720–3700 m above sea level (Geissmann et al. 2011; Ma et al. 2014; Li et al. 2014). There are either three or four groups totaling 260–330 individuals in Myanmar (Geissmann et al. 2011), and up to 10 such groups totaling 490–620 individuals in China (Ma et al. 2014). Although the detailed distribution and life history of R. strykeri are still unknown, rapid population decline and habitat loss, especially in Myanmar, have pushed the species to the edge of extinction. The International Union for Conservation of Nature considers this new primate species Critically Endangered (Geissmann et al. 2012); therefore, comprehensive field research and conservation action are imperative.
The black snub-nosed monkey dwells in extremely harsh, mountainous terrain, with steep slopes and dense forests, and no monkeys have been habituated (Geissmann et al. 2011; Ma et al. 2014), making it difficult to track and observe them in their natural habitat. We here describe preliminary data on the social characteristics and ecology of a group of R. strykeri obtained using direct observation and infrared camera traps at Pianma, Yunnan, China.
Methods
Study Area and Study Group
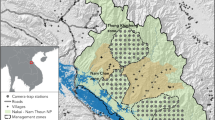
We conducted the survey at Pianma (26°2.337′N, 98°39.127′E), located on the west slope of the southern part of the Gaoligongshan National Nature Reserve (Fig. 1) in Lushui county, Nujiang Lisu Autonomous Prefecture, Yunnan, China. The survey area was ca. 80 km2, with an elevation of ca. 1900–3800 m. Annual average temperature is 14.6–2.2°C and annual average rainfall is 1200–3900 mm; both vary with altitude (Xue 1995a). Forest types found at the site included 1) mid-mountain humid evergreen broadleaf forest (1800–2800 m), with Quercus kongshanensis and Lithocarpus variolosus as the dominant species; 2) Yunnan hemlock forest (2700–3100 m) with Tsuga dumosa and Rhododendron protistum as the dominant species; and 3) bamboo-conifer mixed forest (>3100 m), dominated by bamboo but including other species such as Abies delavayi, Ribes himalense, Gaultheria cardiosepala, and Rhododendron mallotum (Xue 1995b).
Recent reports suggest that only one group of Rhinopithecus strykeri exists at Pianma (Li et al. 2014; Long et al. 2012; Ma et al. 2014). This group numbers ca. 80–100 individuals and uses an area of ca. 12 km2 in the central-southern part of Pianma (Li et al. 2014; Ma et al. 2014). We focused our study on this group based on recent reports and local knowledge.
Direct Observation and Population Counts
We conducted direct observation from 08:30 h to 18:00 h (GMT + 8) three to five times per week from May 5, 2013 to May 24, 2014. In addition to searching for monkeys themselves, we recorded all traces of activity of Rhinopithecus strykeri including feces, broken branches, and food litter. Once we found a monkey group or indirect evidence of their presence, we used a GPS receiver (South S720 GIS) to record the location and then attempted to follow, observe, and videorecord the monkeys. The GPS receiver used the WGS-84 reference coordinate system with single point accuracy to within 5 m of the true position.
Camera Trap Monitoring
We performed camera trap monitoring from November 18, 2013 to May 24, 2014. We deployed 30 camera traps (10 Ltl-Acorn 6210MC and 20 Ltl-Acorn 5210A) in the monkeys’ putative home range based on a 6-mo preliminary field survey that began on May 5, 2013. Both types of camera were equipped with a main passive infrared sensor and two side prep sensors to detect heat and motion. The infrared flash range was 25 m (infrared wavelength 850 nm). The sensors' maximum detection range was also 25 m (medium sensitive setting), and covered a 100° arc in front of the camera. Cameras operated 24 h per day and triggered when motion was detected. We attached cameras to tree trunks at a height of 30–50 cm facing animal tracks, water sources, mineral licking sites, and resting sites at altitudes ranging 2570–3240 m. We set cameras as follows: “Photo,” highest image quality (12 megapixels), medium trigger sensitivity, 1-s trigger interval, three shots per trigger, side prep sensors “on,” time and date stamp “on.” We recorded the geographic coordinates of each camera with a GPS during deployment. We used 8 GB Sandisk SDHC memory cards to record image data, and 8 (Ltl-Acorn 5210A) or 12 (Ltl-Acorn 6210MC) AA batteries to power the cameras. We checked cameras every 1–2 mo to replace SD cards or batteries as necessary.
Data Analysis
We examined each photo and cataloged each animal species, the number of individuals, their sex and age (if possible), and the date and time of the visit. We used Adobe Photoshop CC 2014 to enhance details of underexposed photos to assist in identification. We only used images in which individual Rhinopithecus strykeri were visible for further analysis. We recorded an independent visit of R. strykeri if one of the following three criteria were met: 1) consecutive photographs of different individuals, 2) consecutive photographs of individuals taken more than half an hour apart, or 3) nonconsecutive photos of individuals (O’Brien et al. 2003; Tan et al. 2013).
Based on live video of the monkeys collected during the preliminary survey, we categorized individual Rhinopithecus strykeri into the following age/sex classes based on body size, coloration of fur, and other traits: Adult males were the largest individuals in the band, with black/dark gray/brown fur, more noticeably erect and divided black fur on their heads, thicker tails, and pale pink faces and testes; adult females were also large but with less noticeably erect head fur, plus visible labia and vagina; juveniles were medium-sized individuals with deep gray to black fur; infants were the smallest individuals with dark to light gray fur and light gray faces. We considered all individuals that were carried by other group members to be infants. We logged individuals who could not be assigned to an age/sex class as uncertain.
We recorded the presence of a one-male, multifemale unit (OMU) when one adult male and several adult females and infants appeared in images. We also considered a single female to be a member of an OMU. We recorded different OMUs when different units appeared in images after an interval of time had elapsed and/or there was space between multiple OMUs. We identified all-male unit (AMU) by the presence of multiple males in one image or different males in consecutive images. We did not assume that a single male was an AMU member, because solitary males have been observed in another Rhinopithecus species, R. roxellana (Qi et al. 2014; Xiang et al. 2014; Zhao and Li 2009).
We estimated the group’s vertical range based on both location data collected during observation and camera site elevations. Because the minimum convex polygon (MCP) method can result in overestimates of total home range area when places that the animals do not use occur within the polygon (Grueter et al. 2009), we used the concave polygon method to “modify” MCP (Harris et al. 1990), excluding areas such as farmland, meadows, and roads that monkeys never visited. We entered all geographic coordinates obtained from camera traps and direct surveys into Google Earth Pro 7.1.
Ethical Note
Before conducting this study, we gained approval from the State Forestry Administration of China, the Gaoligongshan National Nature Reserve, the local government, and the Institutional Animal Care and Use Committee of Central South University of Forestry & Technology. Data collected were purely observational.
Results
We obtained 51 geographic coordinates for sites of Rhinopithecus strykeri from direct observation. We contacted the monkeys for 8 d and counted >100 individuals in a large cohesive group on October 17, 2013, when they crossed an open area. We found most traces of monkey activity between 2700 m and 2900 m (70.6 %, N = 51, Fig. 2), with the lowest site at 2469 m and the highest at 3076 m.
We obtained 8990 images with a total of 18 species of mammals and 15 species of birds. Rhinopithecus strykeri triggered only 222 of these photographs. R. strykeri visited camera traps a total of 16 times at 7 different locations, and 14 of these visits (88 %) occurred at altitudes >2900 m (Table I, Fig. 2). We identified a total of 90 individual monkeys with 31 classed as adult males, 29 adult females, 7 juveniles, 7 infants, and 16 as uncertain. The ratio of adult males to adult females was 1.1, adult females to infants 4.1, and adult individuals to immature individuals 4.3 (Table I, Fig. 3).
Rhinopithecus strykeri photographed by camera traps at Pianma, Yunnan, China from November 18, 2013 to May 24, 2014. (A) An adult male. The arrow points to its testes. (B) An adult female. The arrow shows the labia and vagina. (C) An adult female carrying an infant. The arrow shows the infant. (D) Uncertain individual, where vegetation and body orientation precluded age/sex classification. (E) Two juveniles playing (indicated by white circles). (F) An all-male unit (AMU) of seven individuals. (G) A one-male, multifemale unit (OMU) including one adult male, three adult females, and one infant. (H) An incomplete OMU, including two adult females and one infant. (I) One adult male and one adult female at a water source. The adult female is hidden by vegetation, but was identified in consecutive images and was drinking water on the ground.
We obtained group counts of five OMUs and one AMU when individuals passed in front of cameras consecutively (Table II, Fig. 3) and also identified discrete behaviors such as drinking water and playing (Fig. 3). In two cases, the monkeys visited two different camera traps on the same day (Table I). On the morning of January 10, 2014, at 09:23 h, they visited camera no. 0007, which was deployed near a water source in a valley. At 16:58 h on the same day they visited no. 0004, which was deployed on a ridge ca. 1026 m away from the no. 0007 to the south. On April 22, 2014, the group visited camera no. 0011 which was deployed on a ridge at 10:21 h and later visited no. 0025, deployed on a nearby ridge, ca. 827 m to the south at 12:24 (Figs. 3 and 4).
Analysis of consecutive images captured by no. 0025 on April 22 and April 28, 2014, and no. 0017 on April 17, 2014, showed that different OMUs and the AMU moved in the same direction but that there was always a space between the units (Table I, Fig. 3). We also observed multiple OMUs forming a large group without aggression from October 15 to October 17, 2013, during direct observation.
Combining all geographic coordinates, we estimated the vertical range of this group of Rhinopithecus strykeri to be 2400–3300 m (Fig. 2). Using the modified MCP method, our preliminary estimate of the home range was 22.9 km2 (Fig. 4).
Discussion
Our findings suggest that camera traps can be deployed successfully to survey Rhinopithecus strykeri. This contributes to a body of work using camera traps to investigate and monitor arboreal primates (Bezerra et al. 2014; Easton et al. 2011; Galvis et al. 2014; Huang et al. 2014; Kierulff et al. 2004; Olson et al. 2012). As a noninvasive, quantitative technique, infrared camera traps can collect data on relative abundance, distribution, social organization, population dynamics, behavior, and the reaction of primates to other species with minimum human disturbance; this is especially important when subjects are elusive, occur at low densities, are not distributed in a predictable manner, are not habituated, or are located in remote areas (Gerber et al. 2014; O’Connell et al. 2011). Researchers can deploy camera traps at designated locations to capture specific and rare behavior, or use camera traps in combination with other methods to learn more about primate life history (Bezerra et al. 2014; Blake et al. 2010; Boyer-Ontl and Pruetz 2014; Pebsworth et al. 2012; Tan et al. 2013).
Our results suggest that Rhinopithecus strykeri exhibits a multilevel social organization, comprising several OMUs and at least one AMU (Fig. 3), similar to other snub-nosed monkeys (R. bieti: Kirkpatrick et al. 1998; R. roxellana: Qi et al. 2014; R. brelichi: Xiang et al. 2009a; R. avunculus: Boonratana and Le 2013). The ratios of adult females to infants and adults to immatures of R. strykeri at Pianma based on photographic evidence are much higher than the ratios of 2 and 1 in stable populations of R. bieti and R. roxellana (R. bieti: Kirkpatrick et al. 1998; Xiang et al. 2013; R. roxellana: Tan et al. 2007). In line with the ratios of 4.7 and 2.5 reported earlier for our study group (Li et al. 2014), we suggest that the group is in decline. Hunting and habitat destruction or degradation may be to blame (Li et al. 2014; Ma et al. 2014). However, we could not monitor the entire group owing to the technical limitations of the camera traps (Swann et al. 2004), and it was difficult to identify the age and sex of every individual, especially when some images were blurred or not captured in proper body orientation. Differences in time spent on the ground between age/sex classes may also create bias in the observed ratios of females to infants. For example, female R. bieti without clinging infants spend more time on the ground than expected, while females with clinging infants spend less time on the ground than expected (Xiang et al. 2009b). Thus, we should be cautious when using data from camera traps alone to evaluate population parameters or population structure.
Our preliminary estimate of the total area used by Rhinopithecus strykeri, 22.9 km2, may be more reasonable than the previous estimate of 12 km2 (Li et al. 2014), as it is closer to the home range area of groups of R. bieti, R. roxellana, and R. brelichi, which usually exceed 20 km2 (R. bieti: Kirkpatrick et al. 1998; Ren et al. 2009; Xiang et al. 2013; R. roxellana: Li et al. 2000; Su et al. 1998; R. brelichi: Z. F. Xiang unpubl. data). The altitudinal range at Pianma, where R. strykeri ranged between ca. 2400 m and 3300 m, is consistent with previous work, as the reported vertical range of R. strykeri is 1720–3700 m above sea level in both Myanmar and China, with the greatest amount of activity at 2600–3100 m (Geissmann et al. 2011; Li et al. 2014; Ma et al. 2014). However, we did not find evidence that R. strykeri ranged below 2400 m or above 3300 m. We suggest that R. strykeri may not range frequently at lower or higher altitudes. The lower bound is likely due to human disturbance and forest degradation; little potential habitat exists at lower altitudes, especially beyond the boundary of the Gaoligongshan National Nature Reserve (Li et al. 2014; Ma et al. 2014). The upper bound may be attributed to extremely dense bamboo and a lack of large trees for sleeping, traveling and feeding in areas above 3300 m (Xue 1995b).
In sum, this study has demonstrated that camera traps can be a useful tool to study snub-nosed monkey ecology. Further research into the behavioral ecology and conservation biology of Rhinopithecus strykeri is essential.
References
Bezerra, B. M., Bastos, M., Souto, A., Keasey, M. P., Eason, P., Schiel, N., et al. (2014). Camera trap observations of nonhabituated Critically Endangered wild blonde capuchins, Sapajus flavius (formerly Cebus flavius). International Journal of Primatology, 35(5), 895–907.
Blake, J. G., Guerra, J., Mosquera, D., Torres, R., Loiselle, B. A., & Romo, D. (2010). Use of mineral licks by white-bellied spider monkeys (Ateles belzebuth) and red howler monkeys (Alouatta seniculus) in eastern Ecuador. International Journal of Primatology, 31(3), 471–483.
Boonratana, R., & Le, X. C. (2013). Coping with fragmented forests: The Critically Endangered Tonkin snub-nosed monkeys (Rhinopithecus avunculus) in Viet Nam. In L. K. Marsh & C. A. Chapman (Eds.), Primates in fragments: Complexity and resilience (pp. 313–327). New York: Springer.
Boyer-Ontl, K. M., & Pruetz, J. D. (2014). Giving the forest eyes: The benefits of using camera traps to study unhabituated chimpanzees (Pan troglodytes verus) in southeastern Senegal. International Journal of Primatology, 35(5), 881–894.
Easton, J., Chao, N., Mulindahabi, F., Ntare, N., Rugyerinyange, L., & Ndikubwimana, I. (2011). Status and conservation of the only population of the Vulnerable owl-faced monkey Cercopithecus hamlyni in Rwanda. Oryx, 45(3), 435–438.
Galvis, N., Link, A., & Fiore, A. D. (2014). A novel use of camera traps to study demography and life history in wild animals: A case study of spider monkeys (Ateles belzebuth). International Journal of Primatology, 35(5), 908–918.
Geissmann, T., Lwin, N., Aung, S. S., Aung, T. N., Aung, Z. M., Hla, T. H., et al. (2011). A new species of snub-nosed monkey, genus Rhinopithecus Milne-Edwards, 1872 (Primates, Colobinae), from northern Kachin State, northeastern Myanmar. American Journal of Primatology, 73(1), 96–107.
Geissmann, T., Momberg, F., & Whitten, T. (2012). Rhinopithecus strykeri. IUCN Red List of Threatened Species. v. 2014.3. http://www.iucnredlist.org (Accessed 27 November 27, 2014).
Gerber, B. D., Williams, P. J., & Bailey, L. L. (2014). Primates and cameras: Noninvasive sampling to make population-level inferences while accounting for imperfect detection. International Journal of Primatology, 35(5), 841–858.
Grueter, C. C. (2013). The biology of snub-nosed monkeys, douc langurs, proboscis monkeys and simakobu. Hauppauge: Nova.
Grueter, C. C., Chapais, B., & Zinner, D. (2012). Evolution of multilevel social systems in nonhuman primates and humans. International Journal of Primatology, 33(5), 1002–1037.
Grueter, C. C., Li, D. Y., Ren, B. P., & Wei, F. W. (2009). Choice of analytical method can have dramatic effects on primate home range estimates. Primates, 50(1), 81–84.
Harris, S., Cresswell, W. J., Forde, P. G., Trewhella, W. J., Woollard, T., & Wray, S. (1990). Home-range analysis using radio-tracking data—a review of problems and techniques particularly as applied to the study of mammals. Mammal Review, 20(2–3), 97–123.
Huang, Z. P., Qi, X. G., Garber, P. A., Jin, T., Guo, S. T., Li, S., et al. (2014). The use of camera traps to identify the set of scavengers preying on the carcass of a golden snub-nosed monkey (Rhinopithecus roxellana). PLoS ONE, 9(2), e87318.
Kierulff, M. C. M., dos Santos, G. R., Canale, G., Guidorizzi, C. E., & Cassano, C. (2004). The use of camera-traps in a survey of the buff-headed capuchin monkey, Cebus xanthosternos. Neotropical Primates, 12(2), 56–59.
Kirkpatrick, R. C., & Grueter, C. C. (2010). Snub-nosed monkeys: Multilevel societies across varied environments. Evolutionary Anthropology: Issues, News, and Reviews, 19(3), 98–113.
Kirkpatrick, R. C., Long, Y. C., Zhong, T., & Xiao, L. (1998). Social organization and range use in the Yunnan snub-nosed monkey Rhinopithecus bieti. International Journal of Primatology, 19(1), 13–51.
Li, B. G., Chen, C., Ji, W. H., & Ren, B. P. (2000). Seasonal home range changes of the Sichuan snub-nosed monkey (Rhinopithecus roxellana) in the Qinling Mountains of China. Folia Primatologica, 71(6), 375–386.
Li, G. S., Chen, Y. X., Sun, W. M., Wang, X. W., Huang, Z. P., Li, Y. P., et al. (2014). Preliminary observation of population status and social organization of Rhinopithecus strykeri in Pianma Town, Nujiang County, China. Acta Theriologica Sinica, 34(4), 323–328 [in Chinese text with an English abstract].
Liedigk, R., Yang, M. Y., Jablonski, N. G., Momberg, F., Geissmann, T., Lwin, N., et al. (2012). Evolutionary history of the odd-nosed monkeys and the phylogenetic position of the newly described Myanmar snub-nosed monkey Rhinopithecus strykeri. PLoS ONE, 7(5), e37418.
Long, Y. C., Momberg, F., Ma, J., Wang, Y., Luo, Y. M., Li, H. S., et al. (2012). Rhinopithecus strykeri found in China! American Journal of Primatology, 74(10), 871–873.
Ma, C., Huang, Z. P., Zhao, X. F., Zhang, L. X., Sun, W. M., Scott, M. B., et al. (2014). Distribution and conservation status of Rhinopithecus strykeri in China. Primates, 55(3), 377–382.
O’Brien, T. G., Kinnaird, M. F., & Wibisono, H. T. (2003). Crouching tigers, hidden prey: Sumatran tiger and prey populations in a tropical forest landscape. Animal Conservation, 6(2), 131–139.
O’Connell, A. F., Nichols, J. D., & Karanth, K. U. (2011). Camera traps in animal ecology: Methods and analyses. New York: Springer Science+Business Media.
Olson, E. R., Marsh, R. A., Bovard, B. N., Randrianarimanana, H. L. L., Ravaloharimanitra, M., Ratsimbazafy, J. H., et al. (2012). Arboreal camera trapping for the Critically Endangered greater bamboo lemur, Prolemur simus. Oryx, 46(4), 593–597.
Pebsworth, P. A., Bardi, M., & Huffman, M. A. (2012). Geophagy in chacma baboons: patterns of soil consumption by age class, sex, and reproductive state. American Journal of Primatology, 74(1), 48–57.
Qi, X. G., Garber, P. A., Ji, W. H., Huang, Z. P., Huang, K., Zhang, P., et al. (2014). Satellite telemetry and social modeling offer new insights into the origin of primate multilevel societies. Nature Communication, 5, 5296.
Ren, B. P., Li, M., Long, Y. C., & Wei, F. W. (2009). Influence of day length, ambient temperature, and seasonality on daily travel distance in the Yunnan snub-nosed monkey at Jinsichang, Yunnan, China. American Journal of Primatology, 71(1), 233–241.
Su, Y. J., Ren, R. M., Yan, K. H., Li, J. J., Yin, Z., Zhu, Z. Q., et al. (1998). Preliminary survey of the home range and range behavior of golden monkeys (Rhinopithecus roxellana) in Shennongjia National Natural Reserve, Hubei, China. In N. G. Jablonski (Ed.), The natural history of the doucs and snub-nosed monkeys (pp. 255–277). Singapore: World Scientific Press.
Swann, D. E., Hass, C. C., Dalton, D. C., & Wolf, S. A. (2004). Infrared-triggered cameras for detecting wildlife: An evaluation and review. Wildlife Society Bulletin, 32(2), 357–365.
Tan, C. L., Guo, S. T., & Li, B. G. (2007). Population structure and ranging patterns of Rhinopithecus roxellana in Zhouzhi National Nature Reserve, Shaanxi, China. International Journal of Primatology, 28(3), 577–591.
Tan, C. L., Yang, Y. Q., & Niu, K. F. (2013). Into the night: Camera traps reveal nocturnal activity in a presumptive diurnal primate, Rhinopithecus brelichi. Primates, 54(1), 1–6.
Xiang, Z. F., Huo, S., Xiao, W., Quan, R. C., & Grueter, C. C. (2009a). Terrestrial behavior and use of forest strata in a group of black-and-white snub-nosed monkeys Rhinopithecus bieti at Xiaochangdu, Tibet. Current Zoology, 55(3), 180–187.
Xiang, Z. F., Nie, S. G., Lei, X. P., Chang, Z. F., Wei, F. W., & Li, M. (2009b). Current status and conservation of the gray snub-nosed monkey Rhinopithecus brelichi (Colobinae) in Guizhou, China. Biological Conservation, 142(3), 469–476.
Xiang, Z. F., Xiao, W., Huo, S., & Li, M. (2013). Ranging pattern and population composition of Rhinopithecus bieti at Xiaochangdu, Tibet: Implications for conservation. Chinese Science Bulletin, 58(18), 2212–2219.
Xiang, Z. F., Yang, B. H., Yu, Y., Yao, H., Grueter, C. C., Garber, P. A., et al. (2014). Males collectively defend their one-male units against bachelor males in a multi-level primate society. American Journal of Primatology, 76(7), 609–617.
Xue, J. R. (1995a). Climate of Gaoligong Mountain. In J. R. Xue, J. S. Tang, Z. H. Xu, Y. M. Yang, Y. S. Chen, & J. H. Wang (Eds.), Gaoligong Mountain National Nature Reserve (pp. 14–30). Beijing: China Forestry Publishing House [in Chinese text].
Xue, J. R. (1995b). The vegetation distribution and composition. In J. R. Xue, J. S. Tang, Z. H. Xu, Y. M. Yang, Y. S. Chen, & J. H. Wang (Eds.), Gaoligong Mountain National Nature Reserve (pp. 59–115). Beijing: China Forestry Publishing House [in Chinese text].
Zhao, D. P., & Li, B. G. (2009). Do deposed adult male Sichuan snub-nosed monkey Rhinopithecus roxellana roam as solitary bachelors or continue to interact with former band members? Current Zoology, 55(3), 235–237.
Zhou, X. M., Wang, B. S., Pan, Q., Zhang, J. B., Kumar, S., Sun, X. Q., et al. (2014). Whole-genome sequencing of the snub-nosed monkey provides insights into folivory and evolutionary history. Nature Genetics, 46(12), 1303–1310.
Acknowledgments
This study was supported by the State Forestry Administration of China and Conservation International. We thank the Administration Nujiang Bureau and Lushui Bureau of Gaoligongshan National Nature Reserve for their support; P. Liu, S. C. Pu, H. Ran, and J. W. Su for field assistance; Ali Krtzon for her suggestion of the paper and English editing; and the editor-in-chief, Joanna Setchell, and two anonymous reviewers for their valued suggestions for revisions.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Chen, Y., Xiang, Z., Wang, X. et al. Preliminary Study of the Newly Discovered Primate Species Rhinopithecus strykeri at Pianma, Yunnan, China Using Infrared Camera Traps. Int J Primatol 36, 679–690 (2015). https://doi.org/10.1007/s10764-015-9848-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10764-015-9848-y