Abstract
Pulpitis is a complicated chronic inflammatory process which can be in a dynamic balance between damage and repair. The extracellular matrix plays an important regulatory role in wound healing and tissue repair. The aim of this study was to explore the role of the epigenetic mark, enhancer of zeste homolog 2 (EZH2) on the degradation of extracellular matrix during pulpitis. Quantitative polymerase chain reaction was used to assess the expression of matrix metalloproteinases (MMPs) and type I collagen in human dental pulp cells (HDPCs) upon EZH2 and EI1 (EZH2 inhibitor) stimulation. The mechanism of EZH2 affecting extracellular matrix was explored through quantitative polymerase chain reaction and Western blot. A rat model of dental pulp inflammation was established, and the expression of type I collagen in dental pulp under EZH2 stimulation was detected by immunohistochemical staining. EZH2 upregulated the expression of MMP-1, MMP-3, MMP-8, and MMP-10 and decreased the production of type I collagen in HDPCs, while EI1 had the opposite effect. EZH2 activated the nuclear factor-kappa B (NF-κB) and p38 signaling pathways in HDPCs, the inhibition of which reversed the induction of MMPs and the suppression of type I collagen. EZH2 can downregulate the type I collagen levels in an experimental model of dental pulpitis in rats. EZH2 promotes extracellular matrix degradation via nuclear factor-κB (NF-κB) and P38 signaling pathways in pulpitis. EZH2 can decrease the type I collagen levels in vivo and in vitro.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
The dental pulp is derived from ectodermal stroma and is a loose connective tissue rich in extracellular matrix (ECM). Type I and type III collagen is the primary constituent of the ECM of dental pulp [1]. Pulpitis is one of the common dental disorders associated with tooth pulp inflammation [2]. In pulpitis tissue, HDPCs, the main dental pulp component, can interact with immune cells to secrete significant amounts of inflammatory cytokines and chemokines locally. These cytokines and chemokines may promote pulpitis progression and tissue destruction or regulate inflammatory responses to eliminate pathogens [3, 4]. Recent studies have proved that ECM plays an important regulatory role in cell proliferation, differentiation, wound healing, and tissue repair [5]. ECM is closely related to the repair function of dental pulp tissue and participates in and regulates this process, such as mediating the migration and differentiation of dental pulp cells, becoming a solid support for newly generated dentin cells, and taking part in the initiation of dentin mineralization [6]. As extracellular matrix plays an important role in the progress and repair of pulpitis, studying the dynamic changes of extracellular matrix in pulpitis will help reveal the mechanism of pulpitis and provide a theoretical basis for the treatment of pulpitis.
Previous studies have demonstrated that the endopeptidase activity of matrix metalloproteinase (MMP) mediates the degradation of ECM, which may promote the progress of pulpitis [7]. The activity of MMP against extracellular matrix substrates is regulated at 4 “gates”: (1) by transcriptional regulation of MMP genes, (2) by precursor activation, (3) by differences in substrate specificity, and (4) by MMP inhibitors [8]. Existing research attempts to reveal the characteristics of transcription factors that control the active promoter of the MMP gene, but the transcription mechanism that regulates MMP during the progression of inflammatory diseases is unclear, such as epigenetic regulation [9]. In inflammatory diseases such as rheumatoid arthritis, tuberculosis, and diabetes, epigenetics (including DNA methylation, histone modifications, and non-coding RNA) can regulate the expression of MMP and affect the progress of inflammatory diseases [10].
At present, it has been reported that the histone methylation of H3K27 may be involved in dental pulp inflammation and repair process [11]. Studies have shown that the reduced expression of H3K27me3 in inflammatory dental pulp tissues and dental pulp cells may induce the regeneration process of dental pulp [11]. In addition, Jumonji domain protein 3 (JMJD3) is an enzyme that eliminates the histone H3K27 methylation marker and can regulate the expression and transcriptional activity of H3K27me3. Recently, it was discovered that Jmjd3 can regulate the differentiation and cell characteristics of macrophages, suggesting that it may provide a link between inflammation and epigenome reprogramming [12].
In addition, the methyltransferase of H3K27, enhancer of zeste homolog 2 (EZH2j), can be used as a regulator of pulp inflammation [11]. Our previous research found that during the development of dental pulp inflammation, the expression of EZH2 increased [11]. Existing studies suggest that EZH2 is involved in the repair process of dental pulp damage, and whether EZH2 can affect dental pulp inflammation by regulating MMPs remains to be studied.
It is known that nuclear factor-kappa B (NF-κB) and mitogen-activated protein kinase (MAPK) signaling pathways are required for the induction of histone modifiers [12]. NF-κB and MAPK signaling pathways are also known to be major vehicles for producing inflammation [13]. In this study, we addressed the following hypothesis: EZH2 affects the expression of MMP in dental pulp through the NF-κB pathway or MAPK pathway, thereby mediating the degradation of extracellular matrix and affecting the progress of dental pulp inflammation such as the collagen degradation.
MATERIALS AND METHODS
Cell Culture and Treatment
Primary HDPCs were collected from the caries-free third molars and the premolars that need to be extracted for orthodontics of healthy donors in the maxillofacial surgery clinic of Peking University School of Stomatology (ethics approval number: PKUSSIRB-201732003). Cells were maintained in α-MEM medium (Gibco, USA) with 10% fetal calf serum (FBS) (Excell Bio, Australia) and 100U/ml penicillin and 100μg/ml streptomycin sulfate, and passages 3–5 were used. Cells were treated with recombinant EZH2 (20ng/ml, Abnova, USA) or EI1 (2uMol/l APExBIO, Abnova, USA) for the given times. In the specified experiments, cells were pretreated with one of the following specific pathway inhibitors: BAY11-7082(an Iκ B phosphorylation inhibitor, 1 uM, Selleckchem, Houston, TX) or SB203580 (a p38 MAPK inhibitor, 20 uM, Cell Signaling Technology, Danvers, MA).
Characterization of HDPCs
The fourth-generation HDPCs was inoculated in a 12-well plate and grown in slides. After the cells grew close to confluence, they were fixed with 4% paraformaldehyde. Primary antibodies included vimentin (1:1000; Proteintech, USA) and cytokeratin 14 (1:1000; Proteintech, USA). A standard immunohistochemistry kit (Zhongshanjinqiao, Beijing, China) was used for immunohistochemistry. After counterstaining with hematoxylin and dehydration, the expression of related antibodies was observed.
RNA Isolation and Reverse Transcription and Real-Time PCR
Total RNA of the HDPCs was extracted by TRIzol reagent (Invitrogen, Carlsbad, CA). RNA concentrations were measured using a NanoDrop spectrophotometer (Thermo Fisher Scientific, USA). Complementary DNA was synthesized from 1000 ng RNA using a PrimeScript RT kit (TaKaRa, Dalian, China). The produced complementary DNA was prepared as templates for the polymerase chain reaction using SYBR Premix Ex Taq (TaKaRa) according to the manufacturer’s instruction. Conditions were applied as follows: 50 °C for 2 min, then 95 °C for 10 min, followed by 40 cycles of 94 °C for 15 s, and 60 °C for 1 min using an ABI PRISM 7500 Sequence Detection System (Applied Biosystems, Foster City, CA, USA). The Ct values obtained from different samples were compared using the 2–ΔΔCt method. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) served as an internal reference gene. Primers were designed to generate products of less than 200 bp for efficient analysis and were as follows: MMP-1,5′-AGATTCTACATGCGCACAAATC-3′ (forward) and 5′-CCTTTGAAAAACCGGACTTCAT-3′ (reverse); MMP-2,5′-CAACTACAACTTCTTCCCTCGCA-3′ (forward) and 5′-GGTCACATCGCTCCAGACTTG-3′ (reverse); MMP-3,5′-GAGGACACCAGCATGAACCT-3′ (forward) and 5′-CACCTCCAGAGTGTCGGAGT-3′ (reverse); MMP-8,5′-CCAACTATGCTTTCAGGGAAAC-3′ (forward) and 5′-GTTGGATAGGGTTGCTTGAAAG-3′ (reverse); MMP-10,5′-TTGCCCAGCAATACCTAGAAAA-3′ (forward) and 5′-GAACTTCTGCATTCCTTGGATT-3′ (reverse); MMP-13,5′-GCACTTCCCACAGTGCCAT-3′ (forward) and 5′-AGTTCTTCCCTTGATGGCCG-3′ (reverse); COL1A1,5′-GCTCGTGGAAATGATGGTGC-3′ (forward) and 5′-ACCCTGGGGACCTTCAGAG-3′ (reverse); and GAPDH,5′-TCAACAGCGACCCACTC-3′ (forward) and 5′-GCTCTAGCCAAATTCGTTGTC-3′ (reverse).
Western Blot Analysis
The cells were lysed using RIPA buffer (Solarbio, China) supplemented with protease inhibitors (Solarbio, China). Cell homogenates were obtained by gently scraping the cells from each well, and the protein concentrations from the lysates were determined by using the BCA Protein Assay Kit (Thermo Scientific, Rockford, IL, USA). Prior to loading, total protein samples were denatured by heating at 95°C for 10min in 5x SDS-PAGE sample loading buffer (Applygen, China). Thirty micrograms of the protein samples was separated by SDS-polyacrylamide gel electrophoresis at 110V for 90min and transferred to 0.45-μm polyvinylidene fluoride (PVDF) membranes at a constant current of 300mA for 60min. After blocking in 5% nonfat dry milk (Bioruler, China) at room temperature, the membranes were incubated with rabbit anti-phospho-p65 (catalog no. 3033, Cell Signaling Technology, Danvers, MA), rabbit anti-p65 (catalog no. 8242, Cell Signaling Technology), rabbit anti-phospho-p38 (catalog no. 4511, Cell Signaling Technology), rabbit anti-p38(catalog no. 9212, Cell Signaling Technology), and rabbit anti-GAPDH (catalog no. 10494-1-AP, Proteintech, USA) overnight at 4°C. The membrane was incubated for 1 h at room temperature with horseradish peroxidase (HRP)-conjugated anti-rabbit immunoglobulin (SA00001-2, Proteintech, USA) based on the source of the corresponding primary antibody, and the immunoblots were detected using a Western enhanced chemiluminescence blotting kit (ECL, SOLIBRO, China).
Construction of Rat Experimental Pulp Infection Model
Twelve male 6-week-old SD rats (Weitong Lihua Company, China) were subdivided into 4 groups of 3 rats each. Rats were classified according to the sealing medicine: LPS (10mg/ml, L2880, Sigma, USA) for group 1, LPS (10mg/ml) + EZH2 (20ng/ml) for group 2, LPS (10mg/ml) + EI1 (20ng/ml) for group 3, and group 4 was not operated on as the control. Animals were sacrificed 1 and 3 days after surgery and compared to a control group. Under general intraperitoneal anesthesia, all animals except the control group underwent pulp exposure on the occlusal face of the mandibular first molar using a 1/4-size spherical bur at high speed. The gelatin sponge with sealing medicine is placed in the perforated hole, and the cavity was sealed with glass ionomer (Fuji, GC Corporation, Tokyo, Japan). Upon decapitation, the jaws were immersed promptly in 4% formaldehyde, embedded in paraffin, and sectioned at 5-mm thickness.
Immunohistochemical Staining
Immunostaining was performed on formalin-fixed, paraffin-embedded tissue. For immunohistochemistry, paraffin sections were dewaxed in xylene, rehydrated with distilled water, and then subjected to antigen retrieval for 30 min at 95°C. The slides were subsequently incubated overnight at 4°C with rabbit anti-collagen I antibody (1:400, Bioss, Beijing, China). Slides were then treated with an anti-rabbit secondary antibody (Zhongshanjinqiao, Beijing, China) and developed using avidin-conjugated HRP with diaminobenzidine as a substrate (Zhongshanjinqiao) followed by hematoxylin counter staining.
Statistical Analysis
Results are presented as means ± SD of at least 3 independent biological experiments. For each experiment, we have at least 3 technical repeats. Significance was determined via one-way analysis of variance test. The difference was considered statistically significant at the P < 0.05 level.
RESULTS
Immunohistochemistry to Identify Dental Pulp Cells
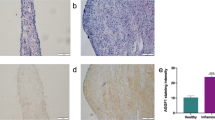
The dental papilla is the original tissue of the dental pulp. The undifferentiated mesenchymal cells of the dental papilla differentiate into astrocyte-forming fibroblasts, namely, dental pulp cells [14]. The HDPCs were fusiform in shape (Fig. 1a, b) and showed positive immunostaining for vimentin (Fig. 1b), but negative immunostaining for cytokeratin 14 (Fig. 1a).
Expression of cytokeratin 14 and vimentin in human dental pulp cells. (a) Immunocytochemical staining using cytokeratin 14 in HDPCs showed negative staining. (b) Immunocytochemical staining using vimentin in HDPCs showed positive staining. Data are representative of 3 independent experiments. Scale bar: 10X, 100um; 20X, 50um; 40X, 20um.
EZH2 Influenced ECM Balance of HDPCs
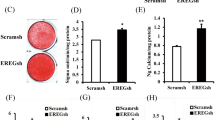
To investigate the effect of EZH2 on MMPs and type I collagen gene expression, HDPCs were treated with EZH2 for 2h, 4h, and 24h. Real-time RT-qPCR analysis demonstrated that in response to EZH2, the expressions of MMP-1, MMP-3, MMP-8, and MMP-10 were increased than the control group (P<0.05) (Fig. 2a-d), while the expression of MMP-2 and MMP-13 both had decreased (P<0.001) (Fig. 2e, f). There was a significant decrease in the expression of type I collagen (P<0.01) (Fig. 2h).
The effects of the EZH2 on the expression of MMPs and type I collagen in HDPCs. a The effect of EZH2 on expression of MMP3; b The effect of EZH2 on expression of MMP-8; c The effect of EZH2 on expression of MMP-1; d The effect of EZH2 on expression of MMP-10; e The effect of EZH2 on expression of MMP-2; f The effect of EZH2 on expression of MMP-13; h the effect of EZH2 on expression of type I collagen.The effects of EZH2 on MMP-1, MMP-2, MMP-3, MMP-8, MMP-9, MMP-10, MMP-13, and type I collagen expression in HDPCs were evaluated by quantitative polymerase chain reaction. Asterisk indicates significant difference from control. *P < .05, **P < .01. ***P < .001.
EZH2 Promotes ECM Degradation via NF-κB and p38 Signaling Pathways in HDPCs
To gain further insight into the mechanism underlying EZH2-induced MMPs expression in HDPCs, we examined the NF-κB and MAPK pathways, which have been suggested by previous reports to be involved in modulating cytokine/chemokine expression [15, 16]. EZH2 treatment led to the activation of the MAPK and NF-κB pathways, as demonstrated by phosphorylation of those signaling molecules after 15–45 min of stimulation (Fig. 3A). When we treated the HDPC with NF-κB inhibitor (BAY11-7082) or specific p38inhibitor (SB203580), the expressions of phosphorylation of those signaling molecules were decreased (Fig. 3b, c). The EZH2-mediated induction of MMP-1, MMP-3, MMP-8, and MMP-10 expression decreased significantly by treatment with an NF-κB inhibitor (BAY11-7082) or p38 (SB203580) inhibitors in 2 h (Fig. 3d-g). On the contrary, EZH2-mediated reduction of type I collagen, MMP-2, and MMP-13 expression increased significantly by treatment with an NF-kB inhibitor (BAY11-7082) or p38 (SB203580) inhibitors in 2 h (Fig. 3h).
EZH2 activates the MAPK pathway and NF-κB pathway. (a) HDPCs were incubated for the indicated time points with EZH2, and p38 and NF-κB activation was assessed by Western blot analysis of total and phosphorylated signaling proteins. (b and c) HDPCs were incubated for the indicated time points with EZH2 and SB203580 (a p38 inhibitor) or BAY-11-7082 (an NF-κB inhibitor), and p38 and NF-κB inhibition was assessed by Western blot analysis of total and phosphorylated signaling proteins. GAPDH and a-tubulin was used as a control. pp65, phosphop65; pp38, phospho-p38. (d–j) HDPCs were preincubated with BAY11-7082 or SB203580 before treatment with EZH2. Real-time PCR of MMPs and Col-1 expression are also shown.
EZH2 and EI1 Affect the Development of Pulpitis
EI1 is a competitive inhibitor of S-adenosyl-methionine (SAM), which serves as a universal methyl donor for HMT [17]. So EI1 can inhibit the enzyme activity of EZH2. After EI1 stimulated HDPCs for 2h, 4h, and 24h, the change of MMP-3 had no statistical difference in the first two groups but declined after 24h (P<0.0001) (Fig. 3A); the expression of MMP-8 was declined (P<0.001) (Fig. 3B); the expression of MMP-2 began to decrease after 24h (P<0.5) (Fig. 3C). The expressions of MMP-1, MMP-10, and MMP-13 all increased after EI1 stimulation (P<0.001) (Fig. 3d-f). The mRNA levels of type I collagen had a remarkable increase at 2h and 24h (Fig. 3h).
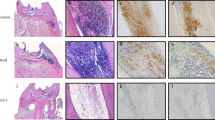
To examine Col-1 change during pulpitis in vivo, we established a pulpitis model in rats. Pulp tissue samples were stained with hematoxylin-eosin stain to verify the infection. We detected inflammatory cell infiltration in our previous study [18]. Immunohistochemistry staining of Col-1 further revealed that EZH2 can reduce the expression of Col-1 in inflammatory dental pulp tissue (Fig. 4j, k, m, and n). However, EI1 did not increase the expression of Col-1 significantly (Fig. 4l and o).
The effect of EZH2 and EI1 on the development of pulpitis. (a–h) The effects of EI1 on MMP-1, MMP-2, MMP-3, MMP-8, MMP-9, MMP-10, MMP-13, and type I collagen expression in HDPCs were evaluated by quantitative polymerase chain reaction. Asterisk indicates significant difference from control. *P < .05, **P < .01. ***P < .001. Immunohistochemical staining showed expression of Col-1 in dental pulp in the different groups (i–o). Scale bar: 10X, 100um; 20X, 50um; 40X, 20um.
DISCUSSION
In the experiment, HDPCs were further subjected to immunocytochemical detection of cell sources. Postnatal expression of vimentin is restricted to fibroblasts, endothelial cells, lymphocytes, and several specialized cells of the thymus and the brain [19]. Cytokeratin (CK) is abundant in keratinized cells, particularly CK14 and CK19, which are expressed in stratified squamous epithelial cells [20]. In our experiment, we showed that the cells were positive for vimentin and negative for cytokeratin 14, which proved that the cells were mesoderm derived and there were no epithelial cells mixed, which was consistent with the biological characteristics of HDPCs.
Our experiment verified that EZH2 could increase the expression of MMP-1, MMP-3, MMP-8, and MMP-10 (Fig. 2). MMP-1(collagenase 1, fibroblast collagenase) is an essential enzyme responsible for degrading type I and type III collagen since the initial breakdown of these fibrillar collagen networks is mediated primarily by MMP-1 [21, 22]. In Su-Jung Shin’s experiment, the concentrations of MMP-1 in acute-inflamed pulps and chronic-inflamed pulps groups were significantly higher than in the control [7]. According to Bergenholtz G, MMP-8 in pulpal inflammatory lesions is mainly of polymorphonuclear neutrophils (PMN) origin [23]. PMNs are the main cells of pulp abscesses, and the main MMP-8 positivity was accumulated around the pulp abscess suggesting that it also contributes to the tissue destruction of pulp necrosis and abscesses. Previous studies have revealed that PMN leucocytes, macrophages, and plasma cells produced MMP-8 in pulp and periapical granulomas [24]. Evrosimovska B et al. confirmed that degradation of the collagen of the organic matrix from pulp tissue during chronic inflammation is an enzyme process and that collagenases (MMP-1, MMP-8) are involved in these destructive processes [25]. Rhim EM et al. found that the expression of MMP-3 and MMP-10 was upregulated in the pulp cells after 24 h of stimulation with TNF-α [26]. In our study, EZH2 suppressed the production of type I collagen in HDPCs. Type I and III collagens are the most abundant collagens in dental pulp, and collagen type I expression is thought to have a direct effect on osteogenic differentiation and mineralization [27]. During the process of inflammation, reduced collagen synthesis and increased degradation led to disintegration of ECM and remodeling of dental pulp [28]. Our experiment suggested that EZH2 may affect the development of pulpitis by regulating the expression of MMPs, but the specific mechanism is not clear, so we did further experiment.
In our study, the EZH2-induced inflammation response in HDPCs appears to represent a collaboration of the NF-κB, and p38 pathways. EZH2 stimulated the phosphorylation of p38 and NF-κB, whereas inhibition of p38 or NF-κB by specific inhibitors induced a dramatic reduction in EZH2-induced MMPs production, suggesting that they all have some roles in EZH2-mediated inflammation (Fig. 3). These results are in accordance with previous studies showing that ASH1L (absent, small, or homeotic 1-like, an H3K4 methyltransferase that can antagonize EZH2) significantly upregulates the expression of MMP-1, MMP-2, and MMP-13 through the MAPK signaling pathway [16]. In the rat experimental pulp infection model, EZH2 decreased the expression of Col-1, but EI1 could not upregulate the expression of Col-1 in rat pulpitis. However, EI1 could increase the expression of Col-1 in vitro. These results suggested that the development of pulpitis might be a multifactor regulatory process, and inhibition of EZH2 alone could not enough promote the repair of collagen fibers.
During pulpitis, pulp can be in a dynamic balance between damage and repair response [29, 30]. In recent years, studies have shown that ECM plays an important regulatory role in wound healing and tissue repair [5]. Exploring the dynamic changes of extracellular matrix in dental pulp inflammation will provide a theoretical basis for the treatment of pulpitis. Early studies have found that epigenetic regulatory factor EZH2 is involved in the process of dental pulp inflammation and repair [11]. Whether this process involves extracellular matrix degradation is not clear. This study verified that EZH2 affects the expression of MMP in dental pulp through the NF-κB pathway and p38 pathway (Fig. 3), thereby mediating the degradation of ECM and affecting the progress of dental pulp inflammation.
CONCLUSIONS
Our research suggested that EZH2 may promote extracellular matrix degradation via nuclear factor-κB (NF-κB) and P38 signaling pathways in pulpitis. It can decrease the type I collagen levels in vivo and in vitro. The downregulation of EZH2 might promote the inflammatory pulp to repair reaction in vitro, which might provide new ideas for the treatment of pulpitis and establish the theoretical basis for further research.
Data Availability
All data generated or analyzed during this study are included in this published article.
Abbreviations
- CK:
-
Cytokeratins
- ECM:
-
Extracellular matrix
- HDPC:
-
Human dental pulp cell
- JMJD3:
-
Jumonji domain protein 3
- MMPs:
-
Matrix metalloproteinases
- MAPK:
-
Mitogen-activated protein kinase
- NF-κB:
-
Nuclear factor-κB
- q-PCR:
-
Quantitative polymerase chain reaction
- EZH2:
-
Zeste homolog 2
References
van Amerongen, J.P., I.G. Lemmens, and G.J. Tonino. 1983. The concentration, extractability and characterization of collagen in human dental pulp. Archives of Oral Biology 28: 339–345.
Haug, S.R., and M.C. Marthinussen. 2019. Acute dental pain and salivary biomarkers for stress and inflammation in patients with pulpal or periapical inflammation. Journal of Oral & Facial Pain and Headache 33: 227–233.
Hahn, C.L., and F.R. Liewehr. 2007. Update on the adaptive immune responses of the dental pulp. Journal of Endodontia 33: 773–781.
Staquet, M.J., S.H. Durand, E. Colomb, A. Romeas, C. Vincent, F. Bleicher, et al. 2008. Different roles of odontoblasts and fibroblasts in immunity. Journal of Dental Research 87: 256–261.
Frangogiannis, N.G. 2017. The extracellular matrix in myocardial injury, repair, and remodeling. The Journal of Clinical Investigation 127: 1600–1612.
Jain, A., and R. Bahuguna. 2015. Role of matrix metalloproteinases in dental caries, pulp and periapical inflammation: an overview. Journal of Oral Biology and Craniofacial Research 5: 212–218.
Shin, S.J., J.I. Lee, S.H. Baek, and S.S. Lim. 2002. Tissue levels of matrix metalloproteinases in pulps and periapical lesions. Journal of Endodontia 28: 313–315.
Birkedal-Hansen, H. 1993. Role of matrix metalloproteinases in human periodontal diseases. Journal of Periodontology 64: 474–484.
Labrie, M., and Y. St-Pierre. 2013. Epigenetic regulation of mmp-9 gene expression. Cellular and Molecular Life Sciences 70: 3109–3124.
Moores, R.C., S. Brilha, F. Schutgens, P.T. Elkington, and J.S. Friedland. 2017. Epigenetic regulation of matrix metalloproteinase-1 and -3 expression in mycobacterium tuberculosis infection. Frontiers in Immunology 8: 602.
Hui, T., A. Peng, Y. Zhao, C. Wang, B. Gao, P. Zhang, et al. 2014. EZH2, a potential regulator of dental pulp inflammation and regeneration. Journal of Endodontia 40: 1132–1138.
De Santa, F., M.G. Totaro, E. Prosperini, S. Notarbartolo, G. Testa, and G. Natoli. 2007. The histone H3 lysine-27 demethylase Jmjd3 links inflammation to inhibition of polycomb-mediated gene silencing. Cell 130: 1083–1094.
Thompson, W.L., and L.J. Van Eldik. 2009. Inflammatory cytokines stimulate the chemokines CCL2/MCP-1 and CCL7/MCP-3 through NFkB and MAPK dependent pathways in rat astrocytes [corrected]. Brain Research 1287: 47–57.
Morsczeck, C., and T.E. Reichert. 2018. Dental stem cells in tooth regeneration and repair in the future. Expert Opinion on Biological Therapy 18: 187–196.
Zhao, Y., C.L. Wang, R.M. Li, T.Q. Hui, Y.Y. Su, Q. Yuan, X.D. Zhou, and L. Ye. 2014. Wnt5a promotes inflammatory responses via nuclear factor kappaB (NF-kappaB) and mitogen-activated protein kinase (MAPK) pathways in human dental pulp cells. The Journal of Biological Chemistry 289: 21028–21039.
Bei, Y., H. Tianqian, Y. Fanyuan, L. Haiyun, L. Xueyang, Y. Jing, et al. 2017. ASH1L suppresses matrix metalloproteinase through mitogen-activated protein kinase signaling pathway in pulpitis. Journal of Endodontics 43: 306–314.e2.
Liu, T.P., H.L. Lo, L.S. Wei, H.H. Hsiao, and P.M. Yang. 2015. S-Adenosyl-L-methionine-competitive inhibitors of the histone methyltransferase EZH2 induce autophagy and enhance drug sensitivity in cancer cells. Anti-Cancer Drugs 26: 139–147.
Chen, Y.Y., Z.Q. Hu, T.Q. Hui, and H. Liu. 2020. Enhancer of zeste homolog 2 affects dental pulp inflammation by regulating macrophage chemotaxis. Beijing Da Xue Xue Bao. Yi Xue Ban 52: 18–23.
Kartenbeck, J., K. Schwechheimer, R. Moll, and W.W. Franke. 1984. Attachment of vimentin filaments to desmosomal plaques in human meningiomal cells and arachnoidal tissue. The Journal of Cell Biology 98: 1072–1081.
Yoshida, K., K. Sato, M. Tonogi, Y. Tanaka, G.Y. Yamane, and A. Katakura. 2015. Expression of cytokeratin 14 and 19 in process of oral carcinogenesis. The Bulletin of Tokyo Dental College 56: 105–111.
Krane, S.M. 1994. Clinical importance of metalloproteinases and their inhibitors. Annals of the New York Academy of Sciences 732: 1–10.
Birkedal-Hansen, H., W.G. Moore, M.K. Bodden, L.J. Windsor, B. Birkedal-Hansen, A. DeCarlo, et al. 1993. Matrix metalloproteinases: a review. Critical Reviews in Oral Biology and Medicine 4: 197–250.
Bergenholtz, G. 2000. Evidence for bacterial causation of adverse pulpal responses in resin-based dental restorations. Critical Reviews in Oral Biology and Medicine 11: 467–480.
Wahlgren, J., T. Salo, O. Teronen, H. Luoto, T. Sorsa, and L. Tjaderhane. 2002. Matrix metalloproteinase-8 (MMP-8) in pulpal and periapical inflammation and periapical root-canal exudates. International Endodontic Journal 35: 897–904.
Evrosimovska, B., C. Dimova, I. Kovacevska, and S. Panov. 2012. Concentration of collagenases (MMP-1, -8, -13) in patients with chronically inflamed dental pulp tissue. Prilozi 33: 191–204.
Rhim, E.M., S.J. Ahn, J.Y. Kim, K.H. Kim, H.W. Lee, E.C. Kim, K.Y. Kim, and S.H. Park. 2013. Stimulation of matrix metalloproteinases by tumor necrosis factor-alpha in human pulp cell cultures. Journal of Endodontia 39: 795–800.
Helder, M.N., A.L. Bronckers, and J.H. Woltgens. 1993. Dissimilar expression patterns for the extracellular matrix proteins osteopontin (OPN) and collagen type I in dental tissues and alveolar bone of the neonatal rat. Matrix 13: 415–425.
Luo, H., C. Wang, M. Liu, B. Yin, A. Peng, D. Huang, et al. 2018. Inhibition of SOX9 promotes inflammatory and immune responses of dental pulp. Journal of Endodontia 44: 792–799.
Goldberg, M., A. Njeh, and E. Uzunoglu. 2015. Is pulp inflammation a prerequisite for pulp healing and regeneration? Mediators of Inflammation 2015: 347649.
Cooper, P.R., M.J. Holder, and A.J. Smith. 2014. Inflammation and regeneration in the dentin-pulp complex: a double-edged sword. Journal of Endodontia 40: S46–S51.
Funding
This work was supported by the Natural Science Foundation of China (NSFC) (grant # 81800959).
Author information
Authors and Affiliations
Contributions
Jie He, Tianqian Hui, and Man Qin designed the study; Jie He and Yingyi Chen performed the research. Jie He, Yingyi Chen, and Ziqi Hu analyzed the data and contributed to the search and collation of literature. Jie He and Ling Ye wrote the manuscript. Ling Ye, Man Qin, and Tianqian Hui contributed to the revision of the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The entire study was approved by the human research committee of Peking University School of Stomatology (ethics approval number: PKUSSIRB-201732003) and performed after written informed consent from patients was obtained. This study has been verified by the Peking University Biomedical Ethics Committee Approved by the Animal Welfare Ethics Committee (Approval Number: LA2018044).
Consent for publication
The participant has consented to the submission of the manuscript to the journal.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
He, J., Qin, M., Chen, Y. et al. EZH2 Promotes Extracellular Matrix Degradation via Nuclear Factor-κB (NF-κB) and p38 Signaling Pathways in Pulpitis. Inflammation 44, 1927–1936 (2021). https://doi.org/10.1007/s10753-021-01470-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-021-01470-7