Abstract
Periodontitis is a chronic inflammatory disease induced by Porphyromonas gingivalis (P. gingivalis) and other pathogens. P. gingivalis release various virulence factors including lipopolysaccharide (LPS). However, whether P. gingivalis–LPS inducing pyroptosis in human gingival fibroblasts (HGFs) remains unknown. In present study, P. gingivalis–LPS decreased the membrane integrity of HGFs, and pyroptosis-associated cytokines were upregulated at the mRNA level. In addition, pyroptosis proteins were highly expressed in gingival tissues of periodontitis. P. gingivalis–LPS induced gingivitis in the rat model, and the expression level of pyroptosis-associated proteins increased. Together, P. gingivalis–LPS can activate the pyroptosis reaction, which may be a pro-pyroptosis status in a relative low concentration.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Periodontitis is a chronic progressive disease developed from gingival inflammation to alveolar resorption, which is mainly caused by multiple pathogens [1], while several associated risk factors, such as smoking, defective prosthesis, and systemic diseases, may aggravate its progression [2]. Periodontitis has a high incidence worldwide [3], while the mechanism of periodontium destruction at the molecular level remains unclear. P. gingivalis is one of the main pathogens involved in periodontal diseases, and plays an important role in the development of the disease [4]. The bacteria and their metabolites activate multi-proteins oligomers, which may motivate immunoreaction and induce damage to the host cells [5].
Programmed cell death (PCD) is a regulated cell death mediated by a range of gene expression levels, including apoptosis, necroptosis, and pyroptosis [6]. Apoptosis is the active process that contributes to the elimination of injured cells and regeneration of healthy cells [7], and is regulated by caspase-3 and caspase-7 activation [8]. At present, apoptotic cells present in the periodontium are strongly correlated with periodontitis, confirming the role of apoptosis in periodontitis [9]. However, apoptosis may lead to pathogen elimination even before triggering host inflammation [10], which indicates the existence of other forms of PCD in periodontitis. Necroptosis presents as another form of regulated cell death found in cases of infection and sterile inflammation environments [11]. This involves inflammatory diseases correlated to interaction with receptor-interacting serine-threonine kinase (RIPKs) and mixed lineage kinase domain-like protein (MLKL) [12]. In contrast to apoptosis, necroptosis cells release intracellular substances into the extracellular environment for immune recognition, instead of being rapidly cleared by phagocytes [13]. Notably, necroptosis is also generated by P. gingivalis to exert a detrimental inflammatory response and impairment of the periodontium [14].
Pyroptosis, a newly discovered pro-inflammatory programmed cell death, which depends on caspase-1 activation, is characterized by cellular swelling and membrane pore formation [15]. Pyroptosis occurs after the activation of the nucleotide-binding domain and leucine-rich repeat-containing receptors (NLRs) [16]. In the canonical pyroptosis pathway, NLRs and other inflammasome complexes can activate and cleave caspase-1 after the formation and activation IL-18, IL-1β, and hydrolyzing gasdermin D (GSDMD), playing vital roles in the pore formation on plasma membranes [17], the imbalanced osmotic pressure of cells, and the upregulation of the response of other pro-inflammatory cytokines [18]. In the non-canonical pathway, pathogen-associated molecular patterns (PAMPs) can motivate caspase-4/5/11 and induce the destruction of cell membrane integrity [19]. Several studies have reported the higher expression of NLRs (NLRP3, NLRP6, and NLRC4) in periodontitis [20, 21], which implies pyroptosis activation.
Indeed, gram-negative bacteria and their endotoxins play an essential role in periodontitis development, and P. gingivalis has high pathogenicity [22]. Although P. gingivalis–LPS has been proven to be highly inflammatory to periodontal tissues, and trigger the secretion of various pro-inflammatory cytokines [23], literatures that describe the role of P. gingivalis–LPS in pyroptosis remains scarce. Considering the strong association of inflammation and pyroptosis, it was hypothesized that P. gingivalis–LPS may promote pyroptosis in the periodontium. In the present study, GFs were stimulated with P. gingivalis–LPS in vitro and in vivo, in order to determine the role of pyroptosis in the initiation and development of periodontitis. The aim of the present study was to evaluate the role of P. gingivalis–LPS in inducing pyroptosis in GFs. In addition, the regulation of various pyroptosis-associated cytokines was analyzed in healthy and chronic periodontitis (CP) gingival tissues.
MATERIALS AND METHODS
Experimental Ethics
The present study was approved by the Ethics Committee of the Hospital of Stomatology, Jilin University, China. The participants provided a signed informed consent before the study, and the clinical samples were collected according to the ethical standard of the Declaration of Helsinki. All animal experiments were conducted according to the ethical guidelines for animals.
Clinical Samples Collection
The gingival specimens were collected at the Department of Periodontology and Oral and Maxillofacial Surgery, Hospital of Stomatology, Jilin University, China. The specimens were obtained from 16 donors (19–56 years), which were divided in to two study groups; healthy group included healthy individuals (n = 8) from gingiviectomy in third molars extraction and chronic periodontitis (CP) group that included patients with chronic periodontitis (n = 8) from periodontal flap therapy. A predefined inclusion criteria were shown in Table 1. The gingival specimens were cut into sections (4-μm thick) for immunohistochemistry after dehydration and paraffin embedding.
Cell Culture
HGFs were obtained from five healthy individuals (three males and two females), who underwent gingivectomy during the third molars extraction. The cell extraction was generated according to the steps previously described in a study. Briefly, the gingival tissues were gently washed with PBS and digested in 2.5 g/L of dispase II (Solarbio, Beijing, China). The connective tissue part was isolated before cutting into small fragments (1 mm3), and culturing in Dulbecco’s modified Eagle’s medium (HyClone, USA) containing 10% fetal bovine serum (Biological Industries Co., Haemek, Israel). Then, this was maintained at 37 °C in a humidified incubator with 5% CO2.
Cells were identified via the staining with the epithelial marker cytokeratin 8 (Wanleibio, Shenyang, China) and mesenchymal marker vimentin (Wanleibio). The cells at the logarithmic growth phase from third to sixth passages were used in the present study.
Cell Viability
The HGFs were collected in 96-well plates, and challenged with P. gingivalis–LPS (InvivoGen San Diego, CA, USA) at different concentrations. Then, these cells were washed with PBS, and refreshed with culture medium containing Cell Counting Kit-8 (CCK-8, Dojindo, Kyushu Island, Japan) after 48 h. Afterwards, the absorbance was measured at 450 nm (OD 450) using a microplate reader (Bole Life Medical Products Co, Ltd., Shanghai, China).
Caspase-1 Activation Detection
The activation of caspase-1 in HGFs infected with P. gingivalis–LPS was detected using a caspase-1 activity kit (Beyotime, Shanghai, China), according to manufacturer’s instructions, and the caspase-1 changing curve was explored. The caspase-1 production was determined based on the quantity of yellow colored p-nitroaniline (pNA) from the cleavage of acetyl-Tyr-Val-Ala-Asp p-nitroanilide (Ac-YVAD-pNA). Then, cells were harvested, lysed, and centrifuged at 4 °C for 15 min at 16,000×g. Afterwards, the supernatant was mixed with Ac-YVAD-pNA in a 96-well plate, and the OD value was measured at a wavelength of 405 nm.
Flow Cytometry Analysis
The cells were cultured in 24-well plates, and exposed to different concentrations of P. gingivalis–LPS (1 μg/mL for low and 10 μg/mL for high). The caspase-1 inhibitor Z-YVAD-FMK (YVAD, Abcam, UK) was added to detect the cell death. Cells were stained with Annexin V-FITC (Beyotime, Shanghai, China) for phosphatidylserine exposure, and with propidium iodide (PI) for the nucleus. Then, these stained cells were analyzed using a flow cytometer (BD Biosciences, San Diego, CA, USA). The rate of double-positive–stained cells was used for comparison.
Lactate Dehydrogenase Activity Assay
In order to assess the membrane integrity of these cells, the total LDH and LDH release activities of HGFs were detected. After challenging with P. gingivalis–LPS and YVAD in the 96-well plate, cells were washed with PBS and detected using a LDH Assay Kit (Beyotime, Shanghai, China). Then, the absorbance was measured at 490 nm.
Real-Time Quantitative Polymerase Chain Reaction
The total RNA from HGFs were isolated using the TRIeasy™ reagent (YEASEN, Shanghai, China), and synthesized to cDNA using a reverse transcription kit (YEASEN). The mRNAs were expanded using the SYBR-Green Real-time PCR master mix (YEASEN) in a CFX96 RT-PCR Detection System (Bio-Rad, Hercules, CA, USA). The primer sequences used in the present study are presented in Table 2. The qPCR cycling protocol consisted of the initial activation cycle at 95 °C for 5 min, followed by 40 cycles of denaturation at 95 °C for 10 s, annealing at 60 °C for 20 s, extension at 72 °C for 20 s, and a final extension at 72 °C for 5 min. The relative expression levels of genes were calculated using the comparative 2−ΔΔCT method. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an internal control.
Gingival Inflammation Model Establishment
A total of 20 male Sprague-Dawley (SD) rats (5–7 weeks old, 240–270 g) were provided by HFK BioScience, Beijing, China. These rats were fed under specific pathogen-free conditions, and were randomly divided into two groups (gingivitis and control groups). For the gingivitis group, after the intraperitoneal injections of 10% chloral hydrate (0.35 mL/100 g), P. gingivalis–LPS (15 μL, 1 mg/mL) was injected into the bottom of the gingival sulcus at the labial site of the maxillary central incisor on alternative days for 10 days.
For the control group, the rats were treated in exactly the same manner. However, these rats received normal saline instead of P. gingivalis–LPS. The bleeding on probing (BOP) and probing depth (PD) were recorded at the initial and final times of injection. After 10 days, the gingivae were excised into 4-μm sections, fixed in 10% formalin, and prepared after dehydration and paraffin embedding.
Immunohistochemistry
The clinical and animal sections were prepared and analyzed for IHC using a previously described protocol [24]. The clinical sections were stained for antibodies against NLRP3, caspase-1, caspase-4, caspase-5, IL-1β (ABclonal, Wuhan, China), and IL-18 (Biosynthesis, Beijing, China). The animal sections were stained for antibodies against NLRP3, caspase-1, IL-1β, IL-18, and caspase-11 (Abcam, Cambridge, MA, USA). Then, these sections were observed by light microscopy and calculated using an Image-Pro Plus software 6.0 (Media Cybernetics, Sarasota, USA) by the integrated optical density (IOD) value [25].
Statistical Analysis
The data analyses were performed using the SPSS v22.0 software (IBM, New York, USA). Comparisons among groups were assessed using analysis of variance (ANOVA). Dunnett’s t test was used to compare the differences among groups. A P value of < 0.05 was considered statistically significant.
RESULTS
Cell Characterization
The immunological staining revealed that the cells were typically spindle shape and negative for cytokeratin 8 and positive for vimentin (Fig. 1), which verified the fibroblast category. Combining with the resource, cells were confirmed as HGFs.
The High Concentration of LPS Promoted Cell Death in HGFs
After exposure to LPS for 48 h, HGF growth was significantly inhibited in a relatively high dose (≥ 50 μg/mL), while no significant changes were observed when the concentration was ≤ 10 μg/mL (Fig. 2).
LPS Changed the Caspase-1 Secretion in HGFs
Caspase-1 plays a key role during the process of pyroptosis. Thus, caspase-1 secretion was detected in HGFs. Under low LPS concentration (1 μg/mL), the caspase-1 level reached its peak at 12 h, gradual decreased with time, and tended to be stable at 48 h (Fig. 3a). A similar trend was observed in high concentration LPS (10 μg/mL). However, when the concentration was increased to the toxic level (50 μg/mL), a higher caspase-1 level was observed at 48 h, when compared to that at 12 h (Fig. 3b).
LPS Enhances the Membrane Permeability in HGFs
Pyroptotic cells exhibited changes in pore formation and swelling. The flow cytometry analysis revealed that the double positive (annexin V-FITC+/PI+) cell rate significantly increased, and these results reversed after the addition of caspase-1 inhibitor YVAD (Fig. 4a). Although the total LDH exhibited no changes, the LDH release quantity was promoted by LPS stimulation at a high concentration. However, YVAD suppressed this tendency (Fig. 4b). Noticeably, compared to the effects at 12 h, the effects were reinforced at 48 h. These results indicate that LPS may facilitate the membrane permeability of HGFs associated with caspase-1, and this effect was more distinct with time (Fig. 4).
P. gingivalis–LPS affected the membrane permeability in HGFs. a LPS increased the rate of double positive cells in HGFs, and YVAD limited this effect. The effect was more obvious at 48 h, when compared to that at 12 h. b The total LDH amount had no significant difference among the different LPS concentrations. The LDH release was greater at 10 μg/mL of LPS in HGFs, presenting an increase in membrane permeability (low, 1 μg/mL; high, 10 μg/mL; YVAD, 1 mM).
LPS Promoted the Permeability by Pyroptosis
P. gingivalis–LPS is a common substance that triggers inflammatory cytokine secretion. Both pyroptosis and necroptosis are induced by this inflammatory response. In order to determine the final direction, the associated genes expression was analyzed. The mRNA expression of NLRP3, caspase-5, IL-1β, and IL-18 were upregulated at different levels after LPS infection, while caspase-4 level exhibited insignificant changes. Furthermore, as the pore protein in pyroptosis, GSDMD gene expression increased at mRNA level, but was attenuated by the addition of YVAD (Fig. 5a). However, RIPK3 and the pore proteins in necroptosis MLKL did not exhibit any significant changes, and the mRNA levels were not interfered by the addition of YVAD (Fig. 5b). Those consequences demonstrate that LPS augments the membrane permeability through the activation of the pyroptosis reaction.
P. gingivalis–LPS induced the pyroptosis, but not the necroptosis, in HGF. a The NLRP3, IL-1β, IL-18, and GSDMD mRNA levels increased under LPS stimulation. YVAD slightly decreased the effect, while a slight change was observed for caspase-4 and caspase-5. b RIPK3 and MLKL slightly changed, and YVAD had no effect on this expression (low, 1 μg/mL; high, 10 μg/mL; YVAD, 1 mM).
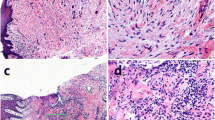
The Upregulation of Pyroptosis in Gingival Tissues with Periodontitis
In order to assess the pyroptosis activity in the gingival tissues of periodontitis, samples obtained from the CP and healthy groups were collected and stained with pyroptosis-associated proteins (Fig. 6). Compared with the healthy group, NLRP3, caspase-1, caspase-4, IL-18, and IL-1β expression levels were higher in CP group, while caspase-5 exhibited no significant differences between the two groups (Table 3, Fig. 6).
The expression of different proteins in the human gingival samples. The expressions of NLRP3 (P < 0.01), caspase-1 (P = 0.023), caspase-4 (P = 0.041), IL-18 (P = 0.045), and IL-1β (P = 0.022) in the CP samples were significantly higher, when compared to those obtained from healthy subjects, while caspase-5 had no difference (P = 0.365).
LPS Triggered Gingivitis in the Rat Experimental Model
After 10 days, all the rats were healthy, and survived without presenting any sign of systemic disease. The gingivae of rats treated with LPS turned red and swollen. Among these 10 rats, eight rats presented with BOP (+), while merely one rat was similar to that of rats in the control group. In addition, the PD significantly increased after the LPS injection (P < 0.01), indicating that P. gingivalis–LPS may promote gingivitis development (Table 4).
LPS Facilitated the Pyroptosis of Gingivitis In vivo
It was further analyzed whether P. gingivalis–LPS can promote gingival pyroptosis in vivo. The results revealed that NLRP3, caspase-1, caspase-11, and IL-1β expression levels significantly increased in the gingivitis group, while IL-18 did not exhibit any significant differences (Fig. 7, Table 5). These findings demonstrate that P. gingivalis–LPS induced gingivitis existed after the pyroptosis activation.
The expression of different proteins in rat gingival samples. The expression of NLRP3 (P = 0.029), caspase-1 (P = 0.029), caspase-11 (P = 0.04), and IL-1β (P = 0.031) in the CP samples were significantly higher, when compared to those obtained from healthy subjects, while IL-18 (P = 0.657) had no difference.
DISCUSSION
The present study investigated the role of P. gingivalis–LPS in inducing pyroptosis in gingival fibroblasts, and regulating the associated cytokines at the molecular level. A rat model was used to induce gingivitis by injecting P. gingivalis–LPS, and the role of pyroptosis in the initiation and development of periodontal diseases was determined. The result of the present study demonstrated that P. gingivalis–LPS inhibits HGF growth and facilitates the membrane permeability of HGF associated with caspase-1. P. gingivalis motivates the host’s immune responses to bacterial challenges, and manipulates a series of immune escapes, resulting in the destruction of periodontal tissues [26, 27]. P. gingivalis–LPS possesses immunobiological activities, which include the secretion of various kinds of inflammatory cytokines and stromal degradation [28]. Cytokines attract immune cells and facilitate the destruction of periodontal tissues [29]. In contrast to gingivitis, periodontitis leads to irreversible bone resorption and attachment loss [30]. Although pyroptosis exists in periodontal diseases, the role of P. gingivalis–LPS in inducing pyroptosis is still not fully understood. Therefore, it would be beneficial to explore the relationship between P. gingivalis–LPS and pyroptosis in GFs.
P. gingivalis–LPS exhibited hypotoxicity and inhibited the growth of HGFs at 50 μg/mL within 48 h. The host can manipulate the inflammation by regulating the pro-inflammatory or anti-inflammatory system, in order to maintain the balance to a certain extent [31]. In the present study, caspase-1 production was at a higher level at 12 h, when compared to that at 48 h (for 1 or 10 μg/mL of LPS). However, this tendency vanished when the LPS reached a toxic dose. The immune tolerance of HGFs is likely to be regulated, while the LPS dose would remain within a safe concentration, and the tolerance would disappear after this exceeds the threshold [32].
Pyroptosis is a cascade reaction from inflammasome complexes to executive proteins regulated by caspase-1, and cell death is triggered through membrane channels [33]. The flow cytometry analysis revealed that the double positive cell proportion (annexin V-FITC+/PI+) increased, while few annexin V-FITC+/PI- cells were detected. Similarly, the LDH release was augmented after the addition of 10 μg/mL of LPS, implying that the integrity of the membrane was altered in HGFs. Both results were reversed by the extra addition of YVAD. Although the LDH release contradicts with the CCK-8 assay, the total LDH outcome was more in line with the CCK-8 results, which ensured the reliability. In contrast to the pyroptosis, the apoptotic signals may induce membrane turnover, and present with annexin V-FITC+/PI- at an early stage and annexin V-FITC+/PI+ a later stage, finally turning into a shrinkage and fracture status [34]. In addition, the occurrence of apoptosis relies on the activation of caspase-3/7/9, instead of caspase-1 [35]. These findings confirm that the LPS induced pathological state is not apoptosis.
Furthermore, qPCR was used to validate these results. After LPS infection, the mRNA levels of NLRP3, caspase-5, IL-1β, and IL-18 were upregulated. Furthermore, the GSDMD expression significantly increased, which was consistent with the changes in the membrane. NLRP is a promoter of pyroptosis, and caspase-4/5 belongs to the non-canonical pyroptosis pathway, which is independent of caspase-1 [36]. This may restrict any significant changes in NLRP3 and caspase-4/5 levels induced by YVAD. However, LPS would directly activate the non-canonical pathway to trigger these effects [37]. In the present study, caspase-5 was upregulated under 10 μg/mL of LPS. GSDMD, IL-1β, and IL-18 were associated with pyroptosis. GSDMD belongs to the gasdermin family, which is capable of pore formation, changing the membrane structure, and misbalancing the osmotic pressure [18, 38]. IL-1β and IL-18 are cleaved from pro-IL-1β and pro-IL-18 to cause damage. IL-1β and IL-18 may recruit other immunocytes to strengthen the inflammatory response [39]. Precisely, IL-1β, IL-18, and GSDMD can also function in the non-canonical pathway, even though YVAD inhibits caspase-1 and the downstream reactions [40]. In the present study, caspase-5 activation may regulate IL-1β, IL-18, and GSDMD. These results present the distinction of these two pathways that exist in the upstream activation, while sharing similar ultimate molecules.
However, for PCD, membrane permeability was not only influenced by pyroptosis, but also necroptosis. This is another kind of inflammatory cell death that presents an annexin V-FITC+/PI+ in the flow cytometry and membrane rupture [41], which is driven by RIPK3 and executed by MLKL. Usually, caspase-8 is closely correlated to necroptosis. Nevertheless, a recent study revealed the molecular switching of caspase-8 between pyroptosis and necroptosis, while pyroptosis may alter the necroptosis level [42]. These present results demonstrated that there were no changes in the MLKL level, and slight changes in RIPK3 at a high concentration in the presence of YVAD. The gene levels presented no differences. These results indicate that the process is mainly driven by pyroptosis, but not necroptosis, and that these two kinds of PCDs are independent of each other.
In addition, the IHC analysis of the gingival samples revealed that the expression of NLRP3, caspase-1/4, IL-1β, and IL-18 was higher in the CP group, when compared to the healthy group. In the animal model, the BOP, NLRP3, caspase-1/11, and IL-1β expression levels increased in the gingivitis group, when compared to the control group, suggesting that P. gingivalis–LPS induced the gingivitis. These findings also indicate that the pyroptosis in GFs is activated during inflammation.
The 1–10 μg/mL P. gingivalis–LPS is usually selected as phlogogenic concentrations for cells in oral environment, such as macrophage, epithelial cell, and periodontal ligament cell. HGFs account for the most abundant cells in periodontal tissues. Therefore, healthy fibroblasts are likely to enhance periodontal tissue regeneration and healing [43, 44]. The phlogogenic LPS concentration could not make a lethal effect to cells in the short term; however, the killing results might appear at a long-time exposure and cell phenotype was modified [45]. In present study, even though the cascade reactions were totally activated, cell death still not happened when LPS was increased to 10 μg/mL within 48 h, which seemed like a tolerance in HGF. Recently, endotoxin tolerance induced by P. gingivalis–LPS was found in neutrophils. This feature could contribute to limiting immune damage, but have some adverse effects on the bacterial restriction [46]. Considering the cell death could also happen at a long-term stimulation, we supposed this might appear as a “pro-pyroptosis” state at the stimulation of practical conditions in HGF within 48 h because of endotoxin tolerance, and if the status was reversible when removing the stimulation remained unknown.
There were some limitations in the present study. The complex periodontal environment that contained multiple pathogens and exposed to various environmental changes could not be simulated. In addition to the LPS, various other secretions or structures may result in pyroptosis [47, 48]. Similarly, local factors, including hypoxia [49] and biofilm features [50], can also heighten the function, which require further investigation. Although HGFs established a “caspase-1 tolerance” from 12 to 48 h, the downstream cytokines expressed higher at 48 h, suggesting the delayed reaction of the signal transduction. However, whether HGF existing endotoxin tolerance and the specific state between inflammation and death require further research.
In summary, the present study demonstrated that P. gingivalis–LPS can activate the pyroptosis reaction, which may be a pro-pyroptosis status in a relatively low concentration.
References
Hiranmayi, K.V., K. Sirisha, M.V. Ramoji Rao, and P. Sudhakar. 2017. Novel pathogens in periodontal microbiology. Journal of Pharmacy & Bioallied Sciences 9 (3): 155–163.
Genco, R.J. 1996. Current view of risk factors for periodontal diseases. Journal of Periodontology 67 (10s): 1041–1049.
Dye, B.A. 2011. Global periodontal disease epidemiology. Periodontology 2000 58 (1): 10–25.
Kolenbrander, P.E., R.N. Andersen, D.S. Blehert, P.G. Egland, J.S. Foster, and R.J. Palmer Jr. 2002. Communication among oral bacteria. Microbiology and Molecular Biology Reviews 66 (3): 486–505.
Silva, N., L. Abusleme, D. Bravo, N. Dutzan, J. Garcia-Sesnich, R. Vernal, et al. 2015. Host response mechanisms in periodontal diseases. Journal of Applied Oral Science 23 (3): 329–355.
Kroemer, G., P. Petit, N. Zamzami, J.L. Vayssière, and B. Mignotte. 1995. The biochemistry of programmed cell death. The FASEB Journal 9 (13): 1277–1287.
Elmore, S. 2007. Apoptosis: a review of programmed cell death. Toxicologic Pathology 35 (4): 495–516.
Sgorbissa, A., R. Benetti, S. Marzinotto, C. Schneider, and C. Brancolini. 1999. Caspase-3 and caspase-7 but not caspase-6 cleave Gas2 in vitro: implications for microfilament reorganization during apoptosis. Journal of Cell Science 112 (Pt 23): 4475–4482.
Song, B., T. Zhou, W.L. Yang, J. Liu, and L.Q. Shao. 2017. Programmed cell death in periodontitis: recent advances and future perspectives. Oral Diseases 23 (5): 609–619.
Nagata, S. 2018. Apoptosis and clearance of apoptotic cells. Annual Review of Immunology 36 (1): 489–517.
Vanden Berghe, T., A. Linkermann, S. Jouan-Lanhouet, H. Walczak, and P. Vandenabeele. 2014. Regulated necrosis: the expanding network of non-apoptotic cell death pathways. Nature Reviews. Molecular Cell Biology 15 (2): 135–147.
Someda, M., S. Kuroki, H. Miyachi, M. Tachibana, and S. Yonehara. 2020. Caspase-8, receptor-interacting protein kinase 1 (RIPK1), and RIPK3 regulate retinoic acid-induced cell differentiation and necroptosis. Cell Death and Differentiation 27 (5): 1539–1553.
Li, J., X.J. Ke, F. Yan, L. Lei, and H. Li. 2018. Necroptosis in the periodontal homeostasis: signals emanating from dying cells. Oral Diseases 24 (6): 900–907.
Ke, X., L. Lei, H. Li, H. Li, and F. Yan. 2016. Manipulation of necroptosis by porphyromonas gingivalis in periodontitis development. Molecular Immunology 77: 8–13.
Awad, F., E. Assrawi, C. Louvrier, et al. 2018. Inflammasome biology, molecular pathology and therapeutic implications. Pharmacology & Therapeutics 187: 133–149.
Brodsky, I.E., and D. Monack. 2009. NLR-mediated control of inflammasome assembly in the host response against bacterial pathogens. Seminars in Immunology 21 (4): 199–207.
De Vasconcelos, N.M., N. Van Opdenbosch, H. Van Gorp, E. Parthoens, and M. Lamkanfi. 2019. Single-cell analysis of pyroptosis dynamics reveals conserved GSDMD-mediated subcellular events that precede plasma membrane rupture. Cell Death and Differentiation 26 (1): 146–161.
Liu, X., Z. Zhang, J. Ruan, Y. Pan, V.G. Magupalli, H. Wu, and J. Lieberman. 2016. Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature 535 (7610): 153–158.
Yang, J., Y. Zhao, and F. Shao. 2015. Non-canonical activation of inflammatory caspases by cytosolic LPS in innate immunity. Current Opinion in Immunology 32: 78–83.
Liu, J., J. Duan, Y. Wang, and X. Ouyang. 2014. Intracellular adhesion molecule-1 is regulated by Porphyromonas gingivalis through nucleotide binding oligomerization domain-containing proteins 1 and 2 molecules in periodontal fibroblasts. Journal of Periodontology 85 (2): 358–368.
Liu, W., J. Liu, W. Wang, Y. Wang, and X. Ouyang. 2018. NLRP6 induces pyroptosis by activation of caspase-1 in gingival fibroblasts. Journal of Dental Research 97 (12): 1391–1398.
Lamont, R.J., and H.F. Jenkinson. 1998. Life below the gum line: pathogenic mechanisms of Porphyromonas gingivalis. Microbiology and Molecular Biology Reviews 62 (4): 1244–1263.
Fitzsimmons, T.R., S. Ge, and P.M. Bartold. 2017. Compromised inflammatory cytokine response to P. gingivalis LPS by fibroblasts from inflamed human gingiva. Clinical Oral Investigations 22 (2): 919–927.
Li, Y., J. Li, J. Sun, Y. Liu, D. Liu, L. Du, et al. 2020. Expression of RAD51 and its clinical impact in oral squamous cell carcinoma. Analytical Cellular Pathology 2020: 1827676.
Li, Y., Z. Xu, J. Li, S. Ban, C. Duan, and W. Liu. 2018. Interleukin-18 expression in oral squamous cell carcinoma: its role in tumor cell migration and invasion, and growth of tumor cell xenografts. FEBS Open Bio 8 (12): 1953–1963.
Zenobia, C., and G. Hajishengallis. 2015. Porphyromonas gingivalis virulence factors involved in subversion of leukocytes and microbial dysbiosis. Virulence 6 (3): 236–243.
Makkawi, H., S. Hoch, E. Burns, K. Hosur, G. Hajishengallis, C.J. Kirschning, and G. Nussbaum. 2017. Porphyromonas gingivalis stimulates tlr2-pi3k signaling to escape immune clearance and induce bone resorption independently of myd88. Frontiers in Cellular and Infection Microbiology 7: 359.
Derradjia, A., H. Alanazi, H.J. Park, R. Djeribi, A. Semlali, and M. Rouabhia. 2015. α-Tocopherol decreases interleukin-1β and -6 and increases human β-defensin-1 and -2 secretion in human gingival fibroblasts stimulated with porphyromonas gingivalis lipopolysaccharide. Journal of Periodontal Research 51 (3): 295–303.
Márton, I.J., and C. Kiss. 2014. Overlapping protective and destructive regulatory pathways in apical periodontitis. Journal of Endodontia 40 (2): 155–163.
Novak, M.J., H.M. Albather, and J.M. Close. 2008. Redefining the biologic width in severe, generalized, chronic periodontitis: Implications for therapy. Journal of Periodontology 79 (10): 1864–1869.
Graham-Engeland, J.E., N.L. Sin, J.M. Smyth, D.R. Jones, E.L. Knight, M.J. Sliwinski, and C.G. Engeland. 2018. Negative and positive affect as predictors of inflammation: timing matters. Brain, Behavior, and Immunity 74: 222–230.
Hu, J., X. Liu, J. Zhao, et al. 2019. Identification of pyroptosis inhibitors that target a reactive cysteine in gasdermin D. Cancer Immunology Research 7 (2): A132.
Shi, J., W. Gao, and F. Shao. 2017. Pyroptosis: gasdermin-mediated programmed necrotic cell death. Trends in Biochemical Sciences 42 (4): 245–254.
Mulhall, H.J., A. Cardnell, K.F. Hoettges, F.H. Labeed, and M.P. Hughes. 2015. Apoptosis progression studied using parallel dielectrophoresis electrophysiological analysis and flow cytometry. Integrative Biology 7 (11): 1396–1401.
Brentnall, M., L. Rodriguez-Menocal, R.L. De Guevara, E. Cepero, and L.H. Boise. 2013. Caspase-9, caspase-3 and caspase-7 have distinct roles during intrinsic apoptosis. BMC Cell Biology 14 (1): 32.
Xiang, H., F. Zhu, Z. Xu, and J. Xiong. 2020. Role of inflammasomes in kidney diseases via both canonical and non-canonical pathways. Frontiers in Cell and Development Biology 27 (8): 106.
Vande, W.L., and M. Lamkanfi. 2016. Pyroptosis. Curr Biol, 2016 26 (13): R568–R572.
Sborgi, L., S. Rühl, E. Mulvihill, J. Pipercevic, R. Heilig, H. Stahlberg, and S. Hiller. 2016. GSDMD membrane pore formation constitutes the mechanism of pyroptotic cell death. The EMBO Journal 35 (16): 1766–1778.
He, W.T., H. Wan, L. Hu, P. Chen, X. Wang, Z. Huang, Z.H. Yang, C.Q. Zhong, and J. Han. 2015. Gasdermin D is an executor of pyroptosis and required for interleukin-1β secretion. Cell Research 25 (12): 1285–1298.
Kayagaki, N., I.B. Stowe, B.L. Lee, K. O’Rourke, K. Anderson, S. Warming, T. Cuellar, B. Haley, M. Roose-Girma, Q.T. Phung, P.S. Liu, J.R. Lill, H. Li, J. Wu, S. Kummerfeld, J. Zhang, W.P. Lee, S.J. Snipas, G.S. Salvesen, L.X. Morris, L. Fitzgerald, Y. Zhang, E.M. Bertram, C.C. Goodnow, and V.M. Dixit. 2015. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature 526 (7575): 666–671.
Polito, L., M. Bortolotti, M. Pedrazzi, D. Mercatelli, M.G. Battelli, and A. Bolognesi. 2016. Apoptosis and necroptosis induced by stenodactylin in neuroblastoma cells can be completely prevented through caspase inhibition plus catalase or necrostatin-1. Phytomedicine 23 (1): 32–41.
Fritsch, M., and Saskia D Günther, Schwarzer R, et al. 2019. Caspase-8 is the molecular switch for apoptosis, necroptosis and pyroptosis. Nature 575 (7784): 1–5.
Chiquet, M., C. Katsaros, and D. Kletsas. 2015. Multiple functions of gingival and mucoperiosteal fibroblasts in oral wound healing and repair. Periodontology 2000 68 (1): 21–40.
Tipton, D.A., A.A. Hatten, J.P. Babu, and MKh Dabbous. 2015. Effect of glycated albumin and cranberry components on interleukin-6 and matrix metalloproteinase-3 production by human gingival fibroblasts. Journal of Periodontal Research 51 (2): 228–236.
Bozkurt, S.B., S.S. Hakki, E.E. Hakki, Y. Durak, and A. Kantarci. 2016. Porphyromonas gingivalis lipopolysaccharide induces a pro-inflammatory human gingival fibroblast phenotype. Inflammation 40 (1): 144–153.
Jian-Yu, Gu, Yu-Jie Liu, Xiang-Qing Zhu, Jia-Ying Qiu, and Ying Sun. 2020. Effects of endotoxin tolerance induced by Porphyromonas gingivalis lipopolysaccharide on inflammatory responses in neutrophils. Inflammation 43 (5): 1692–1706.
Jung, Y.J., H.K. Jun, and B.K. Choi. 2015. Contradictory roles of Porphyromonas gingivalis gingipains in caspase-1 activation. Cellular Microbiology 17 (9): 1304–1319.
Fleetwood, A.J., M.K.S. Lee, W. Singleton, A. Achuthan, M.C. Lee, N.M. O'Brien-Simpson, et al. 2017. Metabolic remodeling, Inflammasome activation, and pyroptosis in macrophages stimulated by Porphyromonas gingivalis and its outer membrane vesicles. Frontiers in Cellular and Infection Microbiology 4 (7): 351.
Cheng, R., W. Liu, R. Zhang, Y. Feng, N.A. Bhowmick, and T. Hu. 2017. Porphyromonas gingivalis-derived lipopolysaccharide combines hypoxia to induce caspase-1 activation in periodontitis. Frontiers in Cellular and Infection Microbiology 14 (7): 474.
Li, Y.Y., B.S. Li, W.W. Liu, Q. Cai, H.Y. Wang, Y.Q. Liu, Y.J. Liu, and W.Y. Meng. 2020. Effects of D-arginine on Porphyromonas gingivalis biofilm. Journal of Oral Science 62 (1): 57–61.
Funding
The study was supported by the grant from the Finance Department of Jilin Province (JCSZ2019378-3; JCSZ2020304-24) and the grants from the Department of Science and Technology of Jilin Province (20180101121JC; 20200404108YY).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, YY., Cai, Q., Li, BS. et al. The Effect of Porphyromonas gingivalis Lipopolysaccharide on the Pyroptosis of Gingival Fibroblasts. Inflammation 44, 846–858 (2021). https://doi.org/10.1007/s10753-020-01379-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-020-01379-7