Abstract
Periodontitis is a dental plaque–induced chronic inflammatory disease. Long-term exposure of the host to periodontal pathogens leads to a hyporesponsive state to the following stimulations, which is described as endotoxin tolerance. Neutrophils are the most abundant innate immune cells in the body. To clarify the roles of endotoxin tolerance in periodontitis, inflammatory responses in Porphyromonas gingivalis (P. gingivalis) lipopolysaccharide (LPS)–tolerized neutrophils were explored in this study. Here, apoptosis and respiratory burst in neutrophils upon single or repeated P. gingivalis LPS stimulations were explored by flow cytometry. Cytokine production (TNF-α, IL-8, and IL-10) in tolerized neutrophils or neutrophils co-cultured with peripheral blood mononuclear cells was determined by ELISA. Phagocytosis of P. gingivalis by tolerized neutrophils was also assayed by flow cytometry. In addition, quality and quantitation of neutrophil extracellular trap (NET) formation were detected using immunofluorescence microscope and microplate reader, respectively. The protein expressions of extracellular signal–regulated kinase1/2 (ERK1/2), c-Jun N-terminal kinase (JNK), and p38 mitogen-activated protein kinase (p38 MAPK) were examined to identify possible mechanisms for the abovementioned changes. Tolerance induced by P. gingivalis LPS significantly suppressed apoptosis, reactive oxygen species (ROS) generation, and phagocytosis in neutrophils (p < 0.05). In both neutrophils alone and co-culture system, repeated P. gingivalis LPS stimulations significantly decreased TNF-α production, but increased IL-10 secretion (p < 0.05). Moreover, in tolerized neutrophils, NET formations were strengthened and there were more released extracellular DNA (p < 0.05). In P. gingivalis LPS-tolerized neutrophils, phosphorylation of ERK1/2 was suppressed compared with that in non-tolerized cells. Taken together, immune responses in neutrophils were reprogrammed by P. gingivalis LPS-induced tolerance, which might be related with the development of inflammation in periodontal tissues. Moreover, ERK1/2 might play important roles in endotoxin tolerance triggered by P. gingivalis LPS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Periodontitis is a plaque-induced chronic inflammatory disease, which eventually leads to alveolar bone resorption and tooth loss. Unfortunately, there is no excellent treatment so far. Porphyromonas gingivalis (P. gingivalis), a member of red complex, has been proven to be related with the development of periodontitis [1, 2]. Lipopolysaccharide (LPS), the major outer membrane constituent of P. gingivalis, can trigger immune and inflammatory responses in the host due to its potential toxicity and antigenicity. However, the roles and pathogenic mechanisms of P. gingivalis LPS still need to be fully investigated.
Neutrophils are the predominant innate immune cells, severing as the first leukocytes recruited to inflammatory sites. Over 95% of inflammatory cells in periodontal pockets are neutrophils [1, 3]. Numerical or functional abnormalities in neutrophils increase the susceptibility of periodontal infections and may lead to some hereditary diseases with severe periodontal destructions, such as Chediak-Higashi syndrome, Papillon-Lef’evre syndrome, and cyclic/permanent neutropenia [4]. Bactericidal or antibacterial strategies employed by neutrophils include phagocytosis, respiratory burst, formation of neutrophil extracellular traps (NETs), cytokine production, etc. [3, 5]. However, excessive and prolonged immune responses in neutrophils also contribute to the destruction in periodontal tissues.
Endotoxin tolerance refers to a refractory state to the second bacteria or their virulence factor stimulations after a primary challenge [6]. It can suppress the immune and inflammatory responses and prevent the possible tissue damage caused by excessive defensive responses. Therefore, it may be a potential defense strategy utilized by the host to maintain homeostasis [7]. It is well accepted that endotoxin tolerance is a reprogramming of the immune system, including the decreased secretion of pro-inflammatory cytokines TNF-α and IL-1β and the increased production of anti-inflammatory cytokines IL-10 [6, 8, 9]. It needs to point out that the suppressed inflammatory responses due to endotoxin tolerance might also contribute to inefficient removal of bacteria.
Periodontitis is a long and polymicrobial infectious disease. Therefore, endotoxin tolerance might develop. However, the effects and underlining mechanisms of tolerance in periodontal tissues are still unintelligible, especially the roles of neutrophils in the progression of periodontitis.
In this study, we hypothesized that endotoxin tolerance induced by P. gingivalis LPS might alter the inflammatory responses in neutrophils, including apoptosis, respiratory burst, cytokine production, phagocytosis, and NET formation, which might have some effects on the development of periodontitis. In addition, the possible involvement of mitogen-activated protein kinase (MAPK) signal pathway was explored to reveal the mechanisms of endotoxin tolerance induced by P. gingivalis LPS.
MATERIALS AND METHODS
Reagents
P. gingivalis ATCC 33277 LPS was obtained from InvivoGen (CA, USA). Escherichia coli (E. coli) O127:B8 LPS and 2′,7′-dichlorofluorescein diacetates (DCFH-DA) were provided by Sigma-Aldrich (MI, USA). Fluorescent Active Caspase 3 Staining Kit was purchased from Biovision (CA, USA). TNF-α, IL-8, and IL-10 ELISA kits were from R&D (MN, USA). Fluorescein-5-isothiocyanate (FITC Isomer I) was purchased from Molecular Probes (MA, USA). SYTOX Green was from Life Technologies (CA, USA). Anti-myeloperoxidase (MPO) antibody was gotten by Abcam (CA, UK). Antibodies to extracellular signal–regulated kinase1/2 (ERK1/2), phospho-ERK1/2 (p-ERK1/2), c-Jun N-terminal kinase (JNK), phospho-JNK (p-JNK), p38 MAPK, and phospho-p38 MAPK (p-p38 MAPK) were supplied by Cell Signaling Technology (MA, USA).
Cell Culture
This study was approved by the Ethical Committee of Nanjing Medical University in accordance with the principles of the Declaration of Helsinki (Permit Number: 20130204) and written informed consents were obtained from all recruits.
Neutrophils were freshly isolated from peripheral venous blood of healthy nonsmoking volunteers by density gradient centrifugation as previously prescribed [10]. Polymorphprep (Axis-Shielld, Norway) was employed in this cell preparation. After 600g centrifugation for 30 min, two leukocyte layers were available. Neutrophils were collected from the lower band rich in polymorphonuclear cells, while monocytes were further prepared from the top layer by cell sorting (FACSAira II, BD, USA) based on cell size and intracellular granules [11]. The purity and viability of isolated neutrophils were consistently greater than 95% and 90%, respectively, which were determined by flow cytometry, while the purity and viability of the yielded monocyte population was close to 98% and 90%. Both two kinds of cells were suspended in RPMI 1640 (Gibco, USA) supplemented with 10% fetal calf serum (Ausbian, Australia) at 37 °C in a humidified 5% CO2 atmosphere.
Endotoxin Tolerance Induction
Neutrophils or neutrophils co-cultured with peripheral blood mononuclear cells (PBMCs) at a ratio of 4:1 were cultured in 6-well plates at a density of 5 × 105 cells/mL. Cells were divided into 5 groups. Group 1 was incubated in medium alone. Groups 2 and 4 were incubated in medium for 12 h, washed for 2 h, and then stimulated with 1 μg/mL P. gingivalis LPS or 1 μg/mL E. coli LPS (served as positive control), respectively, for 3 h to preform apoptosis and respiratory burst assessment, 24 h for ELISA, and 30 min for western blot. Groups 3 and 5 were cultured in medium containing 1 μg/mL P. gingivalis LPS or 1 μg/mL E. coli LPS for 12 h, washed, and restimulated with 1 μg/mL P. gingivalis LPS or 1 μg/mL E. coli LPS, respectively, for the subsequent explorations as described in groups 2 and 4.
Apoptosis Assessment
Neutrophils were collected, washed twice, and then resuspended in 300 μL PBS with 1 μL FITC-DEVD-FMK at 37 °C for 40 min. The cells were washed again, resuspended in 500 μL PBS, and analyzed by flow cytometry using the FL-1 channel. The results were expressed as percentages relative to the values of group 1, which were normalized to 100%.
Reactive Oxygen Species Detection
The suspended neutrophils were collected and incubated with 1 μg/mL DCFH-DA for 40 min at 37 °C. DCFH-DA is a nonfluorescent probe, which can penetrate cell membranes and be oxidized to fluorescent 2′,7′-dichlorofluorescein (DCF) by intracellular reactive oxygen species (ROS) [12]. Then, the cells were washed, resuspended in PBS, and analyzed by FACSCalibur (BD Biosciences, USA). The results of each group were expressed as percentages relative to the fluoresce intensities of group 1, which were normalized to 100%.
Elisa
The levels of TNF-α, IL-8, and IL-10 in supernatants from neutrophils and the co-culture system were measured by ELISA kits according to the manufacturer’s protocol. The absorbance was detected in Spectramax 190 microplate reader (Molecular Devices, CA, USA) at 540 nm.
Phagocytosis Assay
P. gingivalis ATCC 33277 and E. coli ATCC 25922 were kindly supplied by Jiangsu Key Laboratory of Oral Diseases (Nanjing, China). P. gingivalis was cultured on brain heart infusion (BHI) agar plates containing 5% sheep blood, 5 mg/L hemin, 1 mg/L menadione, 75 μg/mL kanamycin, and 2 μg/mL vancomycin for 5 days anaerobically (75% N2, 10% CO2, 15% H2) at 37 °C and inoculated into BHI broth. E. coli was cultured on Luria-Bertani agar plates containing 10 g/L tryptone and 5 g/L yeast extract for 4 h aerobically at 37 °C and transferred into Luria-Bertani liquid medium for enrichment. Then, both two kinds of bacteria were harvested by centrifugation, washed, resuspended in PBS, and labeled with FITC at a concentration of 0.5 mg/mL for subsequent experiments.
The phagocytic activity of neutrophils was analyzed by flow cytometry. Briefly, freshly isolated neutrophils at a concentration of 5 × 105 cells/mL were cultured in 6-well plates and divided into 4 groups. P. gingivalis and E. coli were opsonized with 200 μg/mL human IgG (Sigma-Aldrich, USA) prior to incubation with neutrophils. Groups A and C were incubated with medium alone, while groups B and D were challenged with 1 μg/mL P. gingivalis LPS or 1 μg/mL E. coli LPS (served as positive control) for 12 h, respectively. Then, the cells were restimulated with opsonized FITC-labeled P. gingivalis (groups A and B) or E. coli (groups C and D) at a multiple of infection (MOI) of 20 in a shaking water bath at 37 °C. After 0 min, 30 min, 60 min, and 90 min, aliquots of 300 μL were taken from each well. Phagocytosis was stopped on ice and the extracellular fluorescence was quenched by 2 mg/mL trypan blue for 5 min. Fluorescence signal emitted by endocytosed bacteria was measured by FACSCalibur and results were expressed as percentages of fluorescent phagocytes relative to the values of the same group at 0 min.
NET Formation
Neutrophils were divided into 5 groups and pretreated as described in “Endotoxin Tolerance Induction.” After 12 h, cells were seeded on poly-l-lysine-coated slides in 12-well plates and rechallenged as before. Then, cells were fixed with 4% paraformaldehyde for 20 min, permeabilized with 0.2% Triton X-100 for 15 min, and blocked with 2% bovine serum albumin (BSA) for 30 min at room temperature. Subsequently, cells were incubated with antibody against MPO (1:250) overnight at 4 °C, followed by incubation with Alexa Fluor 488–conjugated anti-rabbit IgG (H+L) (Beyotime, China) for 2 h, washed and stained with DAPI (Beyotime, China) for 2 min. Finally, NETs were observed using a fluorescence microscope (Leica, Germany) and percentages of neutrophils forming NETs under 5 random fields of views were counted.
For NET quantification, grouping and pretreatment were performed as described before. Five micromoles of Sytox Green was added into the conditioned culture medium of each group during restimulations. After 1-h incubation at 37 °C, 100 μL of cell suspension (2 × 105 cells) was pipetted and analyzed by SpectraMax M2e microplate reader (Molecular Devices, München, Germany), using a 504-nm excitation and 523-nm emission filters. The fluorescent intensities were expressed as percentages relative to the values of the cells treated with blank culture medium, which were normalized to 100%.
Western Blot
Neutrophils were harvested in an ice-cold lysis buffer containing 1 mmol/L phenylmethylsulfonyl fluoride (PMSF). Protein extracts were separated by electrophoresis in 10% SDS polyacrylamide gel and then transferred to PVDF membranes. The membranes were blocked with 5% nonfat milk and incubated with primary antibodies against ERK1/2, p-ERK1/2, JNK, p-JNK, p38 MAPK, and p-p38 MAPK overnight at 4 °C. After incubation with appropriate secondary antibodies for 1 h, immunoreactive proteins were detected by ECL chemiluminescence kit (Millipore, USA) and semiquantification analyzed by Image J software version k 1.45 (National Institutes of Health, MD, USA). All results (ERK1/2, p-ERK1/2, JNK, p-JNK, p38 MAPK, and p-p38 MAPK) were normalized to the expression levels of GAPDH, and the levels of p-ERK1/2, p-JNK, or p-p38 MAPK were expressed as the relative gray value to ERK1/2, JNK, or p38 MAPK, respectively.
Statistical Analysis
All data are expressed as mean ± SD. Statistical analysis was performed by one-way ANOVA and differences between groups were compared by LSD test. The level of significance was set at P < 0.05.
RESULTS
Decreased Apoptosis in Neutrophils After Repeated LPS Stimulations
Caspase 3, a member of caspase family, is activated to initiate a protease cascade and then to trigger cell apoptosis. To evaluate the levels of apoptosis in neutrophils, caspase 3 activities in neutrophils stimulated with or without LPS were explored.
After a single challenge with either 1 μg/mL P. gingivalis LPS or 1 μg/mL E. coli LPS, there were no significant differences in caspase 3 activities compared with those in the blank control group (group 1, p > 0.05). However, there were significant decreases in caspase 3 activities in neutrophils retreated with 1 μg/mL P. gingivalis LPS or 1 μg/mL E.coli LPS compared with those in the cells challenged with the same LPS only once (p < 0.05, Fig. 1).
a, b Influences of endotoxin tolerance on the expressions of active caspase 3 in neutrophils. Neutrophils were pretreated with medium, 1 μg/mL P. gingivalis LPS, or 1 μg/mL E. coli LPS for 12 h, washed and then incubated with medium, 1 μg/mL P. gingivalis LPS, or 1 μg/mL E. coli LPS for additional 3 h. Levels of active caspase 3 were measured by flow cytometry. Data are expressed as mean ± SD (n = 5 per group). *p < 0.05. One representative result of five independent experiments is shown in a.
Decreased ROS Production in LPS-Tolerized Neutrophils
Using flow cytometry, ROS generation in neutrophils was quantified to investigate the effects of endotoxin tolerance on neutrophil respiratory burst. Compared with neutrophils without stimulations, levels of ROS were significantly increased in the cells treated with 1 μg/mL E. coli LPS (p < 0.05), but not 1 μg/mL P. gingivalis LPS (p > 0.05). After restimulation with the same LPS, ROS production was significantly decreased compared with those in the corresponding non-tolerized group (p < 0.05, Fig. 2).
a, b Effects of endotoxin tolerance on ROS production in neutrophils. Neutrophils were stimulated as described in Fig. 1. ROS production was measured by flow cytometry. Data are expressed as mean ± SD (n = 3 per group). *p < 0.05. One representative result of three independent experiments is shown in a.
Cytokine Secretion in Neutrophils and Co-culture System
Primary stimulation with 1 μg/mL P. gingivalis LPS or 1 μg/mL E. coli LPS resulted in significant increased levels of TNF-α, IL-10, and IL-8 in neutrophils compared with those in the cells without challenge (p < 0.05). Moreover, the amounts of the abovementioned cytokines induced by P. gingivalis LPS were obviously less than those induced by E. coli LPS (p < 0.05, Fig. 3), which disclosed the differences in biological characteristics between these two kinds of LPSs. After repeated challenge with the same LPS, levels of TNF-α and IL-8 were decreased compared with those stimulated with P. gingivalis LPS and E. coli LPS only once (p < 0.05, Fig. 3a, c). Meanwhile, IL-10 secretion was enhanced in neutrophils retreated with P. gingivalis LPS or E. coli LPS compared with that in the cells with only one stimulation (p < 0.05, Fig. 3b).
Cytokine production in neutrophils stimulated with LPS. Neutrophils were stimulated as described in Fig. 1. After 20 h, levels of TNF-α (a), IL-10 (b), and IL-8 (c) in the cultured supernatants were measured by ELISA. Data are expressed as mean ± SD (n = 3 per group). *p < 0.05.
Similar with the cytokine production in neutrophils, P. gingivalis LPS or E. coli LPS induced significantly increased production of TNF-α, IL-8, and IL-10 in co-culture system compared with that without any stimulations (p < 0.05, Fig. 4). After restimulation with the same LPS for additional 24 h, reduction of TNF-α levels and increase of IL-10 levels could also be observed in P. gingivalis LPS and E. coli LPS–tolerized co-culture system (p < 0.05, Fig. 4a, b). However, significant decreases in IL-8 levels were only observed in E. coli LPS–tolerized co-culture system (p < 0.05), but not in P. gingivalis LPS–tolerized group (p > 0.05, Fig. 4c).
Influences of endotoxin tolerance on cytokine production in neutrophils co-cultured with PBMCs. Freshly isolated neutrophils and monocytes from peripheral venous blood of healthy volunteers were co-cultured at ratio of 4:1. Then, the co-culture system was stimulated as described in Fig. 3. Levels of TNF-α (a), IL-10 (b), and IL-8 (c) in conditioned medium from co-culture system were measured by ELISA. Data are expressed as mean ± SD (n = 3 per group). *p < 0.05.
Decreased Phagocytosis in LPS-Tolerized Neutrophils
The kinetics of phagocytosis in neutrophils throughout a 90-min infection is shown in Fig. 5. Without pretreatment with P. gingivalis LPS or E. coli LPS, phagocytosis of FITC-labeled P. gingivalis or E. coli was enhanced in neutrophils at 60 min and 90 min compared with that at 0 min (p < 0.05). After pre-stimulation with P. gingivalis LPS or E. coli LPS, phagocytosis of the same bacteria in tolerized neutrophils were significantly decreased at 60 min and 90 min (p < 0.05, Fig. 5).
Kinetics of phagocytosis of in neutrophils. Neutrophils were pretreated with medium, 1 μg/mL P. gingivalis LPS, or 1 μg/mL E. coli LPS for 12 h, washed, and then challenged with opsonized FITC-labeled P. gingivalis or E. coli (MOI 20:1) at various time point (0 min, 30 min, 60 min, and 90 min). Fluorescence intensity was measured by flow cytometry. Data are expressed as mean ± SD (n = 3 per group). *p < 0.05.
Increased NET Formation in LPS-Tolerized Neutrophils
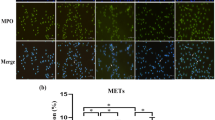
Next, NET formation was explored by immunofluorescence and quantificated by fluorescence microplate reader. Under fluorescence microscope, lobular nuclei of neutrophils were stained with DAPI and visualized in blue color, while MPO was presented as green (Fig. 6a). Unstimulated neutrophils were round-like cells without extracellular web-like structure; only a very small amount of extracellular DNA was detected and NET formations were occasionally observed. After stimulation with P. gingivalis LPS or E. coli LPS, neutrophils were characterized by nuclear expansion, chromosome depolymerization, extracellular web-like DNA strands, and positive staining of MPO, which indicated the formation of NETs (Fig. 6a). Furthermore, after repeated stimulation with P. gingivalis LPS or E. coli LPS, the percentage of NET-positive cells to the total cells counted was increased significantly compared with that following single challenge (p < 0.05, Fig. 6b).
NET formation in P. gingivalis LPS–tolerized neutrophils. Freshly isolated neutrophils were treated with medium, 1 μg/mL P. gingivalis LPS, or 1 μg/mL E. coli LPS for 12 h, washed, seeded onto cover slides precoated with 0.001% poly-l-lysine, and then restimulated with medium, 1 μg/mL P. gingivalis LPS, or 1 μg/mL E. coli LPS for additional 1 h. Neutrophils were stained for DNA (blue) and MPO (green), and then visualized by fluorescence microscopy. One representative result of five independent experiments is shown in a. Scale bar was 50 μm. The percentage of NET-positive cells to total cells counted was calculated (b). Quantity of extracellular DNA was determined by Sytox Green assays (c). Data are expressed as mean ± SD (n = 5 per group). *p < 0.05.
The quantity of extracellular DNA determined by a fluorescence microplate reader was consistent with NET visualization using immunofluorescent microscopy. After stimulation with P. gingivalis LPS or E. coli LPS, there were higher DNA levels than those in supernatants from unstimulated cells (p < 0.05). Moreover, compared with extracellular DNA from neutrophils treated with P. gingivalis LPS or E. coli LPS only once, the quantity of DNA released from the cells with repeated LPS stimulations was increased (p < 0.05, Fig. 6c).
Depressed ERK1/2 Activity in P. gingivalis LPS–Tolerized Neutrophils
Compared with blank controls, both P. gingivalis LPS and E. coli LPS increased the phosphorylation of ERK1/2 in neutrophils (p < 0.05). In P. gingivalis LPS and E. coli LPS–tolerized neutrophils, ERK1/2 phosphorylation was obviously suppressed compared with those in the cells with only one challenge (p < 0.05, Fig. 7b). The roughly similar trends were also found in JNK and p38 MAPK, but only statistically significant decrease of JNK phosphorylation between P. gingivalis LPS–tolerized and non-tolerized neutrophils was confirmed (p < 0.05, Fig. 7c). A representative result of three independent detections is shown in Fig. 7 a.
Depressed ERK1/2 activity in P. gingivalis LPS–tolerized neutrophils. Neutrophils were stimulated as described in Fig. 1. After 30 min, the expressions of ERK1/2, p-ERK1/2, JNK, p-JNK, p38 MAPK, and p-p38 MAPK were detected by western blots. One representative result of three independent experiments is shown in a. Relative levels of p-ERK1/2/ERK1/2, p-JNK/JNK, and p-p38 MAPK/p38 MAPK in neutrophils were determined by density analysis. Statistical significance was shown as mean ± SD (n = 3 per group). *p < 0.05.
DISCUSSION
Immune responses of the host to invasive periodontal pathogens are the major contributors to periodontal destructions. As the most abundant innate immune cells, neutrophils play a crucial role in the development of periodontal inflammation and destruction. In the present study, we found the complex effects of endotoxin tolerance induced by P. gingivalis LPS on apoptosis, ROS production, and cytokine production in neutrophils. More importantly, enhanced NET formation and suppressed phagocytosis in P. gingivalis LPS–tolerized neutrophils, and the changes in cytokine production in tolerized neutrophils co-cultured with PBMCs, were disclosed for the first time. Moreover, phosphorylation of ERK1/2 might participate in the abovementioned changes in tolerized neutrophils.
LPS, one of the most important virulence factors of gram-negative bacteria P. gingivalis, is a potent activator of immune responses. Lipid A of P. gingivalis LPS contains an unusual amount of heterogeneity, including tetra- and penta-acylated structures, and is quite different from that of E. coli LPS, the classic gram-negative bacterial LPS. It is not surprising that differences in biological potencies and pathogenicities do exist between these two kinds LPSs. E. coli LPS is a Toll-like receptor 4 (TLR4) agonist, while P. gingivalis LPS may function as a TLR2 agonist. Furthermore, it is controversial whether P. gingivalis LPS is an agonist or an antagonist of TLR4 [9, 13]. Therefore, E. coli LPS was chosen as a positive control in this study.
As a tightly regulated process which leads to programmed cell death, apoptosis can help the host to escape from the uncontrolled inflammation and tissue destruction. A case-control study reported that a larger number of apoptotic neutrophils were found in periodontitis patients [14]. Caspase 3, a marker of apoptosis, can be activated in execution pathway downstream to both extrinsic and intrinsic apoptosis pathways, then clave hundreds of cellular substrates to trigger apoptosis [15]. In François’ report, it was disclosed that the longer stimulations, the more expressions of myeloid cell leukemia-1 (Mcl-1), an antiapoptotic B cell leukemia/lymphoma 2 (Bcl-2) family member, alongside with the suppressed apoptosis [16]. Murray found that P. gingivalis LPS could inhibit HL60-derived neutrophil apoptosis through TLR2. Moreover, apoptosis in P. gingivalis LPS–stimulated group could be suppressed for a longer time and remained at a sustained lower level compared with that in E. coli LPS–stimulated group [17]. Our previous work found that 5 h after incubation with the supernatants from P. gingivalis LPS–tolerized THP-1, the levels of caspase 3 in neutrophils were downregulated. It was assumed that cytokines in the supernatants from P. gingivalis–tolerized THP-1 might have some effects on neutrophils to induce the abovementioned changes [18]. Therefore, tolerance induced by P. gingivalis LPS might prevent apoptosis and prolong neutrophil life span, which might contribute to resisting invading bacteria, but lead to prolonged inflammation and exacerbation of immune damages.
As the first line of defense in innate immunity, neutrophils engulf bacteria and then kill them by non-oxidative and oxidative mechanisms. The generation of ROS, including H2O2, hypochlorite, hydroxyl radicals, and singlet oxygen, was an oxygen-dependent way to eliminate bacteria. Unfortunately, bactericidal activity of ROS is non-specificity and ROS released into extracellular matrix might damage surrounding tissues [19, 20]. Our experiment found that there was an increase of detectable ROS production in neutrophils challenged with E. coli LPS, but not P. gingivalis LPS, which were consistent with Gölz’s study [21] and might be related with the biological differences between these two kinds of LPSs. Moreover, accompanied by a suppressed apoptosis, a decreased ROS production was detectable in both P. gingivalis LPS– and E. coli LPS–tolerized neutrophils in this study, which implied the restrained inflammatory responses and immune damages.
Production of various kinds of cytokines, including inflammatory cytokine TNF-α, anti-inflammatory cytokine IL-10, and chemokine IL-8, is one of the most critical strategies employed by the host to resist invading periodontal pathogens. It is well known that neutrophils represent a first line of defense against invading periodontal pathogens [3]. Neutrophils in periodontal tissues produce chemoattractant to attract additional neutrophils and macrophages, and then regulate their activities. Cytokines secreted by macrophages also contribute to further migrations of neutrophils [3, 5]. A cytokine cascade is then formed to amplify inflammatory signals. Thus, in this study, neutrophil isolations and neutrophils co-cultured with PBMCs were employed to investigate the cross-talk between neutrophils and macrophages in endotoxin tolerance.
Our present results indicated the decreased production of TNF-α and increased secretion of IL-10 in both neutrophil isolations and co-culture system after repeated LPS stimulations, which was consisted with our previous experiment concerning THP-1 cells and Fadok’s research [9, 22]. Our previous experiment also disclosed the altered cytokine profiles in P. gingivalis LPS–tolerized THP-1 cells, including downregulated cytokines IL-12 p40 and IL-12 p70 and upregulated cytokines death receptor 6 (DR6) and type 2 IL-1 receptor (IL-1 R2) [18]. Inconsistent changes in different cytokines implied that endotoxin tolerance was not the global decline of all cytokines, but a selective reprogramming aimed at limiting inflammatory damage [23]. In general, quantities and categories of cytokines secreted by monocytes/macrophages are much more than those from neutrophils. Therefore, higher levels of TNF-α and IL-10 were observed in co-culture system. Moreover, suppressed IL-8 production was only confirmed in P. gingivalis LPS–tolerized neutrophil isolations, but not in co-culture system. As a distinctive endotoxin, tolerance induced by P. gingivalis LPS might be impaired in monocytes/macrophages [9, 13, 24]. Similar to our research, Zaric found sustained levels of IL-8 and decreased secretion of TNF-α in THP-1 cells after repeated P. gingivalis LPS challenge [25].
Previous researches indicated that pro-inflammatory cytokine TNF-α and chemokine IL-8 were involved in the development of periodontitis [26, 27]. Importantly, TNF-α is capable to promote the differentiation and activation of osteoclasts and plays a pivotal role in inflammatory bone resorption [28]. It is well accepted that TNF-α is a hallmark of endotoxin tolerance [29]. Therefore, decreased levels of TNF-α in both neutrophil isolations and co-culture system implied the establishment of endotoxin tolerance. IL-10 is a potent anti-inflammatory cytokine, which inhibits the activation of Th1 cells and monocytes/macrophages. Although the categories and quantities of cytokines secreted by neutrophils are much less than those from macrophages, neutrophils can produce a large amount of IL-10 responding to Gram-negative bacteria, thus playing a regulatory role in immune responses [30]. Endotoxin tolerance does not simply inhibit immune responses. Changes in cytokine pattern due to endotoxin tolerance favor limit excessive inflammatory responses and immune damages. On the other hand, it might also weaken the ability to resist invading periodontal pathogens.
Neutrophils express Fc receptors, phagocytose opsonized P. gingivalis and E. coli, and then form mature phagosomes to kill these bacteria [31]. Given that neutrophils are suspended in cultured medium, it is impossible to thoroughly separate neutrophils from P. gingivalis/E. coli during the progress of washing. Therefore, in this study, P. gingivalis LPS/E. coli LPS was used as the first stimulation instead of P. gingivalis/E. coli, while opsonized FITC-labeled P. gingivalis/E. coli was employed for the second time. In our study, P. gingivalis/E. coli stimulation induced active phagocytosis by non-tolerized neutrophils, which was in consistent with the abovementioned reports. Interestingly, phagocytosis of P. gingivalis and E. coli was severely inhibited in tolerized neutrophils. McLeish reported that phosphorylation of ERK1/2 and p38 MAPK was involved in the phagocytosis of opsonized Staphylococcus aureus by neutrophils [32]. Furthermore, Rossi found adiponectin inhibited phagocytosis of E.coli by neutrophil via blocking phosphoinositide 3-kinase (PI3K)/protein kinase B (PKB) and ERK1/2 activation [33]. Therefore, in this study, the depressed expressions of p-ERK1/2 in tolerized neutrophils might be the reason for the downregulated phagocytosis.
The aforementioned results disclosed the decreased phagocytosis, ROS production, and pro-inflammatory cytokine secretion in P. gingivalis LPS–tolerized neutrophils, all of which inhibited eradication of P. gingivalis. Then, how do tolerized neutrophils resist invading pathogens? In 2004, Brinkmann reported a novel anti-microbial mechanism of neutrophils, which could kill bacteria efficiently after the cells die [34]. This mechanism called NET is distinctly different from apoptosis and necrosis. They are mainly composed of uncondensed extracellular chromatin decorated with multiple anti-microbial granules with high local concentration, including elastase, MPO, cathepsin G, DNA, and histone. Then, bacteria, fungi, viruses, and parasites can be trapped, neutralized, and killed efficiently [35]. Phorbol myristate acetate (PMA), LPS, and Gram-positive and Gram-negative bacteria can induce the formation of NETs [34]. It has been realized that NETosis includes suicidal NETosis and vital NETosis. The former is a classic NET involving MPO-dependent nuclear envelope disintegration and the death of neutrophils [36], while the latter is a non-lytic form of NETosis involving the secretion of nuclear chromatin accompanied by granule proteins independently of cell death [37]. Recently, a study about gout, a kind of acute inflammatory reaction, proved that aggregated NETs limited the severity of inflammation and promoted inflammation resolution by degrade inflammatory mediators [38]. On the surface of oral mucosa, neutrophils from saliva rapidly released NETs to bind and kill bacteria, which was pivotal for host defenses [39]. However, excessive NET formation or reduced NET removal in periodontal tissues might play an important role in the pathogenesis of periodontitis [40].
Our results indicated that in P. gingivalis LPS–tolerized neutrophils, NET formation was augmented significantly accompanied with increased levels of extracellular MPO. Although phagocytosis, ROS production, and pro-inflammatory cytokine secretion were weakened, a new balance in immune responses would be set up to maintain homeostasis in periodontal tissues.
It has been demonstrated that nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, ROS, MPO, Drosophila Eph kinase (DEK), peptidylarginine deiminase 4 (PAD4), and autophagy were involved in the formation of NETs [36, 41, 42]. Originally, ROS was considered indispensable in NETosis [41]. Later, Remijsen found ROS was insufficient to induce NETs in PMA-stimulated neutrophils, while both autophagy and NADPH oxidase were involved in NETosis [36]. Moreover, Mor-Vaknin found that NETs were completely failed to be formed in neutrophils from Dek-deficient mice, which proved that DEK was crucial to the formation of NETs in inflammatory arthritis [42]. In our present study, ROS production was increased in non-tolerized neutrophils, which was in line with the change of NETs in these cells. However, in P. gingivalis LPS–tolerized neutrophils, ROS production was decreased, while the formation of NETs was increased, which was contradictory with the commonly referred ROS-dependent NET formations. The specific underlying mechanisms concerning tolerized neutrophils still need to be further illustrated.
P. gingivalis and E. coli LPS activate neutrophils via TLR4 and/or TLR2 [33]. Subsequently, myeloid differentiation protein 88 (MyD88), tumor necrosis factor receptor–associated factor 6 (TRAF6), and transforming growth factor-activated kinase-1 (TAK-1) were recruited to activate IL-1 receptor–associated kinases (IRAKs) and trigger p38 MAPK/JNK pathways through MAP kinase kinase (MKK) family [5]. It was found that release of resistin in neutrophils stimulated with P. gingivalis LPS and E. coli LPS depended on PI3K, JNK, and p38 MAPK [43]. Nguyen disclosed that TNF-α and GM-CSF induced ROS production in neutrophils via p38 MAPK and ERK1/2, respectively [44]. In addition, it was also reported that E. coli LPS could activate neutrophils via p38 MAPK and JNK, and then induced macrophage chemoattractant protein-1 (MCP-1) expression, actin assembly, and respiratory burst [45, 46]. Furthermore, E. coli LPS could induce ROS production and NETosis in neutrophils via JNK-dependent pathway [47]. Given the important roles of MAPKs in the activation of innate immune cells, their roles in endotoxin tolerance were explored in this experiment. Decreased phosphorylation of ERK1/2 and JNK was observed in P. gingivalis LPS–tolerized neutrophils, which suggested the possible involvement of ERK1/2 and JNK in P. gingivalis LPS–induced tolerance. TLR-PI3K cross-talk independent of MyD88 was disclosed to activate ERK1/2, JNK, and p38 and be involved the inhibition of phagocytosis in neutrophils [48]. Therefore, the activation of PI3K may be related to the downregulation of ERK1/2 and JNK phosphorylation in P. gingivalis LPS–tolerized neutrophils and need to be explored in the future.
CONCLUSION
These results suggest that P. gingivalis LPS–induced tolerance reprograms the immune responses in neutrophils, including the decreased secretion of pro-inflammatory cytokines, the increased production of anti-inflammatory cytokines, the suppressions of phagocytosis, ROS generation and apoptosis, and the augmentation of NET formation. These changes might contribute to restrict immune damage, but have some negative effects on the resistance of invading bacteria. ERK1/2 might be involved in the development of endotoxin tolerance induced by P. gingivalis LPS in neutrophils. Regulations and mechanisms of NETs in the development of periodontitis still need to be further explored. In addition, an animal model also needs to be developed to confirm the roles of endotoxin tolerance induced by P. gingivalis LPS in vivo in the future.
Founding Information
This work was supported by the National Natural Science Foundation of China through project 81771075 and a project funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (2018-87).
References
Hajishengallis, G. 2014. Immuno-microbial pathogenesis of periodontitis: Keystones, pathobionts, and the host response. Trends in Immunology 35: 3–11.
How, K.Y., K.P. Song, and K.G. Chan. 2016. Porphyromonas gingivalis: An overview of periodontopathic pathogen below the gum line. Frontiers in Microbiology 7: 53.
Cortés-Vieyra, R., C. Rosales, and E. Uribe-Querol. 2016. Neutrophil functions in periodontal homeostasis. Journal of Immunology Research 2016: 1396106.
Papayannopoulos, V. 2018. Neutrophil extracellular traps in immunity and disease. Nature Reviews. Immunology 18: 134–147.
Hajishengallis, G. 2000. New developments in neutrophil biology and periodontitis. Periodontol 2020 (82): 78–92.
Pena, O.M., J. Pistolic, D. Raj, C.D. Fjell, and R.E. Hancock. 2011. Endotoxin tolerance represents a distinctive state of alternative polarization (M2) in human mononuclear cells. Journal of Immunology 186: 7243–7254.
Soares, M.P., R. Gozzelino, and S. Weis. 2014. Tissue damage control in disease tolerance. Trends in Immunology 35: 483–494.
Sun, Y., H. Li, M.F. Yang, W. Shu, M.J. Sun, and Y. Xu. 2012. Effects of aging on endotoxin tolerance induced by lipopolysaccharides derived from Porphyromonas gingivalis and Escherichia coli. PLoS One 7: e39224.
Sun, Y., H. Li, M.J. Sun, Y.Y. Zheng, D.J. Gong, and Y. Xu. 2014. Endotoxin tolerance induced by lipopolysaccharides derived from Porphyromonas gingivalis and Escherichia coli: Alternations in Toll-like receptor 2 and 4 signaling pathway. Inflammation 37: 268–276.
Thomas, H.B., R.J. Moots, S.W. Edwards, and H.L. Wright. 2015. Whose gene is it anyway? The effect of preparation purity on neutrophil transcriptome studies. PLoS One 10: e0138982.
Ren, L., S. Yang, P. Zhang, Z. Qu, Z. Mao, P.H. Huang, Y. Chen, M. Wu, L. Wang, P. Li, and T.J. Huang. 2018. Standing surface acoustic wave (SSAW)-based fluorescence-activated cell sorter. Small 14: e1801996.
Ribeiro, A.L., A.L. Shimada, C.B. Hebeda, T.F. de Oliveira, A.P. de Melo Loureiro, R. Filho Wdos, A.M. Santos, W.T. de Lima, and S.H. Farsky. 2011. In vivo hydroquinone exposure alters circulating neutrophil activities and impairs LPS-induced lung inflammation in mice. Toxicology 288: 1–7.
Ding PH, Darveau RP, Wang CY, and Jin L. 2017. 3LPS-binding protein and its interactions with P. gingivalis LPS modulate pro-inflammatory response and Toll-like receptor signaling in human oral keratinocytes. PLoS One 12: e0173223.
Nicu, E.A., P. Rijkschroeff, E. Wartewig, K. Nazmi, and B.G. Loos. 2018. Characterization of oral polymorphonuclear neutrophils in periodontitis patients: A case-control study. BMC Oral Health 18: 149–162.
Nagata, S., and M. Tanaka. 2017. Programmed cell death and the immune system. Nature Reviews. Immunology 17: 333–340.
Zhao, H., Y. Ma, and L. Zhang. 2018. Low-molecular-mass hyaluronan induces pulmonary inflammation by up-regulation of Mcl-1 to inhibit neutrophil apoptosis via PI3K/Akt1 pathway. Immunology 155: 387–395.
Murray, D.A., and J.M. Wilton. 2003. Lipopolysaccharide from the periodontal pathogen Porphyromonas gingivalis prevents apoptosis of HL60-derived neutrophils in vitro. Infection and Immunity 71: 7232–7235.
Zhu, X.Q., W. Lu, Y. Chen, X.F. Cheng, J.Y. Qiu, Y. Xu, and Y. Sun. 2016. Effects of Porphyromonas gingivalis lipopolysaccharide tolerized monocytes on inflammatory responses in neutrophils. PLoS One 11: e0161482.
Liu, C., L. Mo, Y. Niu, X. Li, X. Zhou, and X. Xu. 2017. The role of reactive oxygen species and autophagy in periodontitis and their potential linkage. Frontiers in Physiology 8: 439–459.
Ziltener, P., T. Reinheckel, and A. Oxenius. 2016. Neutrophil and alveolar macrophage-mediated innate immune control of legionella pneumophila lung infection via TNF and ROS. PLoS Pathogens 12: e1005591.
Gölz L, Memmert S, Rath-Deschner B, Jäger A, Appel T, Baumgarten G, Götz W, and Frede S. 2014. LPS from P. gingivalis and hypoxia increases oxidative stress in periodontal ligament fibroblasts and contributes to periodontitis. Mediators Inflamm 2014: 986264.
Fadok, V.A., D.L. Bratton, A. Konowal, P.W. Freed, J.Y. Westcott, and P.M. Henson. 1998. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. The Journal of Clinical Investigation 101: 890–898.
Novakovic B, Habibi E, Wang SY, Arts RJW, Davar R, Megchelenbrink W, Kim B, Kuznetsova T, Kox M, Zwaag J, Matarese F, van Heeringen S.J, Janssen-Megens E.M, Sharifi N, Wang C, Keramati F, Schoonenberg V, Flicek P, Clarke L, Pickkers P, Heath S, Gut I, Netea M.G, Martens J.H.A, Logie C, and Stunnenberg H.G. 2016. β-Glucan reverses the epigenetic state of LPS-induced Immunological Tolerance. Cell 167: 1354–1368.
Martin M, Katz J, Vogel S. N, and Michalek S. M. 2001. Differential induction of endotoxin tolerance by lipopolysaccharides derived from Porphyromonas gingivalis and Escherichia coli. Journal of Immunology 167: 5278–5285.
Zaric, S., C. Shelburne, R. Darveau, D.J. Quinn, S. Weldon, C.C. Taggart, and W.A. Coulter. 2010. Impaired immune tolerance to Porphyromonas gingivalis lipopolysaccharide promotes neutrophil migration and decreased apoptosis. Infection and Immunity 78: 4151–4156.
Romero-Castro, N.S., M. Vázquez-Villamar, J.F. Muñoz-Valle, S. Reyes-Fernández, V.O. Serna-Radilla, S. García-Arellano, and N. Castro-Alarcón. 2020. Relationship between TNF-α, MMP-8, and MMP-9 levels in gingival crevicular fluid and the subgingival microbiota in periodontal disease. Odontology 108: 25–33.
Xiong, G., W. Ji, F. Wang, F. Zhang, P. Xue, M. Cheng, Y. Sun, X. Wang, and T. Zhang. 2019. Quercetin inhibits inflammatory response induced by LPS from Porphyromonas gingivalis in human gingival fibroblasts via suppressing NF-κB signaling pathway. BioMed Research International 2019: 6282635.
Hienz, S.A., S. Paliwal, and S. Ivanovski. 2015. Mechanisms of bone Resorption in periodontitis. Journal of Immunology Research 2015: 615486.
Tanabe, S.I., and D. Grenier. 2008. Macrophage tolerance response to Aggregatibacter actinomycetemcomitans lipopolysaccharide induces differential regulation of tumor necrosis factor-alpha, interleukin-1 beta and matrix metalloproteinase 9 secretion. Journal of Periodontal Research 43: 372–377.
Zhang, X., L. Majlessi, E. Deriaud, C. Leclerc, and R. Lo-Man. 2009. Coactivation of Syk kinase and MyD88 adaptor protein pathways by bacteria promotes regulatory properties of neutrophils. Immunity 31: 761–771.
Herrera, B.S., H. Hasturk, A. Kantarci, M.O. Freire, O. Nguyen, S. Kansal, and T.E. Van Dyke. 2015. Impact of Resolvin E1 on murine neutrophil phagocytosis in type 2 diabetes. Infection and Immunity 83: 792–801.
McLeish, K.R., J.B. Klein, P.Y. Coxon, K.Z. Head, and R.A. Ward. 1998. Bacterial phagocytosis activates extracellular signal-regulated kinase and p38 mitogen-activated protein kinase cascades in human neutrophils. Journal of Leukocyte Biology 64: 835–844.
Rossi, A., and J. Lord. 2013. Adiponectin inhibits neutrophil phagocytosis of Escherichia coli by inhibition of PKB and ERK 1/2 MAPK signalling and mac-1 activation. PLoS One 8: e69108.
Brinkmann, V., U. Reichard, C. Goosmann, B. Fauler, Y. Uhlemann, D.S. Weiss, Y. Weinrauch, and A. Zychlinsky. 2004. Neutrophil extracellular traps kill bacteria. Science 303: 1532–1535.
Bruns, S., O. Kniemeyer, M. Hasenberg, V. Aimanianda, S. Nietzsche, A. Thywissen, A. Jeron, J.P. Latgé, A.A. Brakhage, and M. Gunzer. 2010. Production of extracellular traps against Aspergillus fumigatus in vitro and in infected lung tissue is dependent on invading neutrophils and influenced by hydrophobin RodA. PLoS Pathogens 6: e1000873.
Remijsen, Q., T. Vanden Berghe, E. Wirawan, B. Asselbergh, E. Parthoens, R. De Rycke, S. Noppen, M. Delforge, J. Willems, and P. Vandenabeele. 2011. Neutrophil extracellular trap cell death requires both autophagy and superoxide generation. Cell Research 21: 290–304.
Kusunoki, Y., D. Nakazawa, H. Shida, F. Hattanda, A. Miyoshi, S. Masuda, S. Nishio, U. Tomaru, T. Atsumi, and A. Ishizu. 2016. Peptidylarginine deiminase inhibitor suppresses neutrophil extracellular trap formation and MPO-ANCA production. Frontiers in Immunology 7: 227.
Schauer, C., C. Janko, L.E. Munoz, Y. Zhao, D. Kienhöfer, B. Frey, M. Lell, B. Manger, J. Rech, E. Naschberger, R. Holmdahl, V. Krenn, T. Harrer, I. Jeremic, R. Bilyy, G. Schett, M. Hoffmann, and M. Herrmann. 2014. Aggregated neutrophil extracellular traps limit inflammation by degrading cytokines and chemokines. Nature Medicine 20: 511–517.
Mohanty, T., J. Sjögren, F. Kahn, A.H. Abu-Humaidan, N. Fisker, K. Assing, M. Mörgelin, A.A. Bengtsson, N. Borregaard, and O.E. Sørensen. 2015. A novel mechanism for NETosis provides antimicrobial defense at the oral mucosa. Blood 126: 2128–2137.
White, P.C., I.J. Chicca, P.R. Cooper, M.R. Milward, and I.L. Chapple. 2016. Neutrophil extracellular traps in periodontitis: A web of intrigue. Journal of Dental Research 95: 26–34.
Fuchs, T.A., U. Abed, C. Goosmann, R. Hurwitz, I. Schulze, V. Wahn, Y. Weinrauch, V. Brinkmann, and A. Zychlinsky. 2007. Novel cell death program leads to neutrophil extracellular traps. The Journal of Cell Biology 176: 231–241.
Mor-Vaknin, N., A. Saha, M. Legendre, C. Carmona-Rivera, M.A. Amin, B.J. Rabquer, M.J. Gonzales-Hernandez, J. Jorns, S. Mohan, S. Yalavarthi, D.A. Pai, K. Angevine, S.J. Almburg, J.S. Knight, B.S. Adams, A.E. Koch, D.A. Fox, D.R. Engelke, M.J. Kaplan, and D.M. Markovitz. 2017. DEK-targeting DNA aptamers as therapeutics for inflammatory arthritis. Nature Communications 8: 14252.
Furugen, R., H. Hayashida, and T. Saito. 2013. Porphyromonas gingivalis and Escherichia coli lipopolysaccharide causes resistin release from neutrophils. Oral Diseases 19: 479–483.
Nguyen, G.T., E.R. Green, and J. Mecsas. 2017. Neutrophils to the ROScue: Mechanisms of NADPH oxidase activation and bacterial resistance. Frontiers in Cellular and Infection Microbiology 7: 373.
Yan, S.R., W. Al-Hertani, D. Byers, and R. Bortolussi. 2002. Lipopolysaccharide-binding protein- and CD14-dependent activation of mitogen-activated protein kinase p38 by lipopolysaccharide in human neutrophils is associated with priming of respiratory burst. Infection and Immunity 70: 4068–4074.
Zemans, R.L., and P.G. Arndt. 2009. Tec kinases regulate actin assembly and cytokine expression in LPS-stimulated human neutrophils via JNK activation. Cellular Immunology 258: 90–97.
Khan, M.A., A. Farahvash, D.N. Douda, J.C. Licht, H. Grasemann, N. Sweezey, and N. Palaniyar. 2017. JNK activation turns on LPS- and gram-negative bacteria-induced NADPH oxidase-dependent suicidal NETosis. Scientific Reports 7: 3409.
Makkawi, H., S. Hoch, E. Burns, and K. Hosur. 2017. Porphyromonas gingivalis stimulates TLR2-PI3K signaling to escape immune clearance and induce bone resorption independently of MyD88. Frontiers in Cellular and Infection Microbiology 7: 359.
Author information
Authors and Affiliations
Contributions
The experiments were designed by YS; performed by JG, YL, XZ, and JQ; and analyzed by JG and YL. The manuscript was written by JG and YL, and reviewed and edited by YS. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
This study was approved by the Ethical Committee of Nanjing Medical University in accordance with the principles of the Declaration of Helsinki (Permit Number: 20130204) and written informed consents were obtained from all recruits.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gu, Jy., Liu, Yj., Zhu, Xq. et al. Effects of Endotoxin Tolerance Induced by Porphyromonas gingivalis Lipopolysaccharide on Inflammatory Responses in Neutrophils. Inflammation 43, 1692–1706 (2020). https://doi.org/10.1007/s10753-020-01243-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-020-01243-8