Abstract
The world’s number one cause of death is cardiovascular diseases. The pathogenesis of different disease entities in the cardiovascular disease spectrum is complicated and multifactorial. Inflammation in these complicated etiologies serves as a key position and is a significant cause of atherosclerosis, which contributes to the underlying pathology. Therefore, therapeutic targeting of inflammatory pathways in patients with cardiovascular diseases such as atherosclerosis enhances cardiovascular results. Inflammasomes are intracellular protein complexes engaged in atherosclerosis pathogenesis and activated by multiple danger signals. Emerging proof has revealed that Nod-like receptor protein 3 (NLRP3) inflammasome, which regulates caspase-1 activation and later pro-interleukin processing, triggers inflammatory reactions in the vascular wall and leads to atherosclerotic plaque formation. Inflammasome-mediated signaling interference could decrease inflammation and mitigate illness severity. In this section, we provide an overview of the present literature on the underlying mechanisms leading to the activation of NLRP3 inflammasome and the role of NLRP3 inflammasome in the progression of atherogenesis and highlight the possibility of therapeutic interventions due to mechanisms involved in the of inhibition of NLRP3 activation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Inflammation is the basis of a broad range of physiological and pathological processes. In particular, chronic inflammation for many modern human diseases constitutes a biochemical basis. Inflammasomes are protein structures that provide a platform for molecular signaling to activate caspase-1 and to regulate maturation of powerful pro-inflammatory cytokine interleukin-one beta, (IL-1 β) hence, contributing towards inflammatory cell death, in response to pathogen-associated and danger-associated molecular pattern molecules (PAMPs and DAMPs), reactive oxygen species (ROS), cholesterol crystals, and environmental irritants [1, 2]. Among various kinds of inflammasomes that are recognized so far, the best characterized is the nucleotide-binding oligomerization domain, leucine-rich-containing family, pyrin domain-containing-3 (NLRP3) inflammasome is well known, which recognize non-microbial danger signals and lead to sterile inflammatory reactions under different disease circumstances such as chronic obstructive pulmonary disease, asthma, gout, heart failure, and myocardial infarction [3, 4]. The anti-inflammatory treatment targeting interleukin-1β (IL-1β) pathway significantly reduced the rate of relapse of cardiovascular events that had little to do with lipid levels. It confirms directly the theory of inflammation in atherosclerosis and provides a theoretical basis for the clinical anti-inflammatory treatment of atherosclerosis [5]. The focus of this review is on the possible activation mechanisms, role, and regulatory mechanisms of the NLRP3 inflammasome in atherosclerosis, so that NLRP3 inflammasome may be therapeutically targeted in atherosclerosis.
OVERVIEW OF NLRP3 INFLAMMASOME
NLRP3 Inflammasome Structure
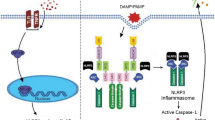
NLRP3 inflammasome is an innate immune receptor and comprises of three domains; C-terminal leucine-rich repeats, a key nucleotide-binding and oligomerization domain, and an N-terminal pyrin effector domain (PYD). NLRP3 inflammasome includes mainly a cytosolic NLRP3 inflammasome molecule, an adapter protein termed as apoptosis-associated speck-like (ASC), and an effector procaspase-1. ASC protein contains an N-terminal PYD and a C-terminal caspase recruitment (CARD) domain, also known as Pycard. Procaspase-1 which is a cysteine protease precursor consists of a CARD and a caspase domain. The PYD of NLRP3 inflammasome protein interacts with the PYD of ASC upon activation by DAMPs and PAMPs, and the ASC-CARD domain recruits the procaspase-1-CARD domain to form the NLRP3-ASC-procaspase-1 complex and therefore induces caspase-1 auto-activation. Caspase-1 processes pro-IL-18 to its bioactive mature form, which exerts a potent pro-inflammatory effect [6]. Furthermore, recent studies indicate that caspase-1 cleaves gasdermin D to induce pyroptosis, a type of inflammatory programmed cell death with increased plasma membrane permeability as shown in Fig. 1 [7].
Activation of NLRP3 Inflammasome
Activation of the NLRP3 inflammasome is managed at several levels and generally involves two independent signals in macrophages: a priming signal, which is the first signal for activation of the NLRP3 inflammasome and an activation signal as shown in Fig. 2. The priming phase positively regulates NLRP3 inflammasome, involving expression of NLRP3 inflammasome and pro-IL-β at the transcriptional level via activation of nuclear factor kappa-light-chain-enhancer of activated B cells, i.e., NF-κB pathway, in response to microbial components such as toll-like receptor (TLR) ligands, endogenous molecules such as tumor necrosis factor, or IL-β [8, 9]. Studies have shown recently that FAS-associated death domain protein (FADD) and caspase-8 also control NLRP3 inflammasome expression induction during priming [10]. A deeper understanding of molecular mechanisms involved in the activation of NLRP3 inflammasome suggested that myeloid differentiation primary response MyD88 adapter, the IL-1 receptor-associated kinase (IRAK1), and IRAK4 downstream kinases were implicated in the transcriptional regulation of NLRP3 inflammasome. Besides, NLRP3 inflammasome is deubiquitinase during priming, which results in decreased proteasomal degradation and thus extend the protein life period. Lysine-63-specific deubiquitinase BRCC36, a JAMM (JAB1/MPN/Mov34) domain-containing Zn2+ metalloprotease, promotes NLRP3 inflammasome deubiquitinating during priming and regulates NLRP3 inflammasome activation [11]. A regulatory ribonucleic acid (RNA) such as micro-RNA-233 is a negative regulator of NLRP3 inflammasome expression, suppresses its activation by binding to a conserved region in its sequence, and hence modulates post-transcriptional NLRP3 inflammasomes activation [12]. Also, the latest evidence points to the role of the RNA-binding tris-tetraprolin protein, which by binding to NLRP3 inflammasome, three-primed untranslated region acts as a negative NLRP3 inflammasome regulator in human macrophages [13].
Activation of NLRP3 inflammasome by dual-signaling model. Left side: priming signal is induced by endogenous cytokines molecules or which results in upgradation of NLRP3 and pro-interleukin-1 beta (IL-1β) via activation of the transcription factor NF-κB. CASP-8 and FAS-associated death domain protein (FADD) are associated with priming through regulation of activation of NF-κB pathway. Lys-63-specific deubiquitinase BRCC36 (BRCC3) and IL-1 receptor-associated kinase 1 (IRAK1) control, independently of transcription, activation of NLRP3. Other activation on the right side: signal 2 is delivered through a multitude of stimuli, including pore-forming toxins, ATP, viral RNA, and particulate matter, and activation of NLRP3 inflammatory molecules. A number of signaling mechanisms underlying NLRP3 activation have been proposed. K+ efflux is induced by NLRP3, essential for NLRP3 activation for signaling of Ca2+ is suggested for mitochondrial dysfunction, and involves activation of NLRP3. Also suggested to mediate NLRP3 activation were mitochondrial dysfunction-derived signals such as oxidized mtDNA or externalization of phospholipid cardiolipin, reactive oxygen species (mtROS). The MAVS mitochondrial adaptor mediates RNA virus-induced NLRP3 activation. Minute particles activates NLRP3 through K+ efflux induction by lysosomal rupture and possibly by releasing cathepsins. Nek7 is an important inflammatory regulator of NLRP3. IL-1R, receptor IL-1β; TLR, toll-like receptor; TNFR, receptor factor of tumor necrosis.
Furthermore, rapid post-translational changes of the pre-synthesized NLRP3 inflammasome have been described. However, the impact of post-translational changes on NLRP3 inflammasome activation is rather complex and incomplete, but it appears to rely on the precise place and type of post-translational modification. For example, phosphorylation at serine 5 within the PYD of NLRP3 inflammasome inhibits its activation, most probably by interfering with a PYD–PYD interaction interface, thus preventing receptor oligomerization [14]. Nevertheless, protein phosphatase PP2A may de-phosphorylate NLRP3 inflammasome leading to the activation. Consistently, nitrosylation modification of NLRP3 inflammasome occurs when interferon-gamma receptor undergoes activation and has a suppressive effect on NLRP3 inflammasome protein, thus impeding oligomerization [15].
The second stage of activation mechanism is the focus of current research and a variety of mature theories have developed.
Ion Flux Theory
A reduction in the concentration of intracellular potassium ions (K+) was first recognized as the prevalent cause of activation of NLRP3 inflammasome [16]. A newly recognized NLRP3 inflammasome element, i.e., never in mitosis gene-a, NIMA-related kinase 7 (NEK7) also needs K+ efflux for NLRP3 inflammasome assembly. K+ efflux is mediated by extracellular adenosine triphosphate (ATP), leading to the activation of non-selective purinergic receptor P2X7 cationic channel, and resulting in localized K+ efflux from macrophages as shown in Fig. 3 [17, 18]. Various agonists also contribute to K+ efflux, such as bacterial pore-forming toxins, bacterial ionophore nigericin, and certain antibiotics including gramicidin, neomycin, tyrothricin, and polymyxin B. By inhibiting K+ efflux, activation of NLRP3 inflammasome and IL-1β maturation is impaired [19]. Inhibition of calcium ion (Ca2+) mobilization also reduces NLRP3 inflammasome activation. Interaction of inositol 1,4,5-trisphosphate (IP3) and its IP3R receptor on endoplasmic reticulum (ER) encourages the mobilization of Ca2+ and therefore, the enormous release of Ca2+ from ER results in mitochondrial Ca2+ overload, resulting in mitochondrial damage, and leads to the mitochondrial ROS formation. Mitochondrial ROS act as a central trigger for the activation of NLRP3 inflammasome. Other studies on the contrary have shown that signaling of Ca2+ is unessential for activation of NLRP3 inflammasome. Further studies are required to clarify the Ca2+ signaling processes involved in the activation of NLRP3 inflammasome [20, 21].
Activators of NLRP3-induced K+-efflux results in mtROS production and mitochondrial damage, leading to enrichment of CLICs in plasma membrane with Cl− efflux promotion, through CLIC-linked Cl− efflux, which helps in NEK7–NLRP3 interaction and ensuring NLRP3 inflammasome assembly. ER release Ca2+ causes mitochondrial Ca2+overload and ultimately mitochondrial damage, releasing mtROS production, which activates NLRP3 inflammasome. NEK7, component of NLRP3 inflammasome, after binding to NLRP3 protein, along with K+ efflux, production of ROS, and efflux of Cl− for NLRP3 inflammasome assembly.
Another significant ion engaged in inflammatory activation of NLRP3 inflammasome is sodium ion (Na+) influx. A report by Schorn et al. shows that stimulation by monosodium urate crystals increases the Na+ load and then cells balance it by the passive influx of water, which reduces K+ below the threshold, resulting in the activation of NLRP3 inflammasome [22].
Researcher Verhoef initially investigated the role of chloride (Cl−) efflux in the activation of NLRP3 inflammasome. Findings demonstrated that declined extracellular Cl− concentration would allow the Cl− intracellular efflux and encourages the activation of caspase-1 and the production of IL-1β. Investigations have shown that indanyloxyacetic acid; i.e., IAA-94, natriuretic peptide B, and flufenamic acid, which are inhibitors of Cl− channels, may inhibit NLRP3 inflammasome activation [23].
The volume-regulated anion channel (VRAC) was initially identified as a critical anion channel for controlling the activation of NLRP3 inflammasome. The most compelling evidence was that non-steroidal anti-inflammatory drugs prevent the activation of the NLRP3 inflammasome by suppressing Cl− efflux via VRAC. Moreover, researchers Tang et al. showed that intracellular chloride channel (CLIC), which is another anion channel, could work as an activator of VRAC. Furthermore, chloride efflux mediated by CLIC can encourage communication between NEK-7-NLRP3 inflammasome and later ASC oligomerization. However, the VRAC-CLIC connection requires further confirmation [24, 25].
Theory of Reactive Oxygen Species Production
Mitochondrial ROS production is one of the first NLRP3 inflammasome activation triggers recognized via a mechanism involving NEK7, which itself is a ROS sensor. Multiple agonists causing death of cell and dysfunction of mitochondria enhance oxidation of mitochondrial deoxyribonucleic acid (DNA), and thereby, activating the NLRP3 inflammasome. Imiquimod, a TLR7 agonist, may induce ROS-mediated activation of NLRP3 inflammasome; however, in response to the imiquimod stimulation, cells deficient in NEK7 do not produce IL-1β [26, 27]. ROS production is stimulated by different factors including ER stress, ATP, silica, and asbestos. This pathway is regulated by autophagy and mitophagy by eradicating damaged mitochondria. Furthermore, mitophagy impairment is recommended to stimulate inflammasomes activation through ROS species accumulation [28].
Lysosomal Destabilization Theory
In lysosomal destabilization, phagocytosis cannot process adequately, this phenomenon is referred to as frustrated phagocytosis in which phagocytosed molecules could not be swallowed or digested, ultimately leading to phagolysosomal destabilization and rupture. Amyloid β was recognized initially for the activation of NLRP3 inflammasome via lysosomal destabilization [29]. Insufficient clearance of large particulate activators (such as silica, cholesterol, and monosodium urate crystals), phagocytosed by macrophages, trigger phagolysosomal destabilization, hence contributes to rupturing of the lysosome, and ultimately release of cathepsin B, causing activation of NLRP3 inflammasome [30].
NLRP3 INFLAMMASOME IN ATHEROSCLEROSIS
Expression of NLRP3 Inflammasome in Atherosclerosis
Chronic and sterile inflammation owing to the innate immune system plays a major part in the development and advancement of atherosclerosis. It is a chronic inflammatory disorder with deposition of lipid, infiltration of leukocytes, and proliferation of vascular smooth muscle cells [31]. NLRP3 inflammasome expression has found in endothelial cells, smooth muscle cells, macrophages, dendritic cells, T cells, and monocytes. However, most work in atherosclerosis focused on the activation of inflammasomes in monocytes and macrophages. Enhanced concentration of NLRP3 inflammasome expression was found in the aorta of coronary atherosclerotic patients, which was correlated with the severity of coronary artery stenosis, along with the contribution of associated risk factors such as hypertension, smoking, diabetes, elevated lipoprotein (a), low high-density lipoprotein cholesterol, and high low-density lipoprotein (LDL) cholesterol [32, 33]. In addition, expression studies indicate that protein levels of the NLRP3 inflammasome, ASC and caspase1, IL-1 β, and IL-18 in carotid plaques were significantly high relative to healthy arteries, being higher in unstable than in stable plague [34, 35].
NLRP3 Inflammasome in Atherosclerotic Mice Model
Duewell et al. first showed the essential function of NLRP3 inflammasome in atherosclerosis. They used LDL receptor-deficient mice with NLRP3−/−, ASC−/−, and IL-1α/β−/− bone marrow. Low concentration of IL-18, along with weak atherosclerotic lesions, was observed after providing a high-fat diet for 8 weeks in this group [36]. In addition, in the bone marrow of LDLR-deficient mice, reduced caspase-1/11 levels showed a significant reduction in the atherosclerotic plaque [37]. NLRP3 inflammasome silencing also reduced hyperhomocysteinemia-induced macrophage infiltration and atherosclerotic lesions in such models [38].
The lectin-like receptor known as ox-LDL-1 (LOX-1) is an important ox-LDL receptor that contributes to the process of lipid accumulation in atherosclerosis. Various studies have shown that in vivo deletion of LOX-1 in LDLR−/− mice fed with a high-fat diet for 18 weeks resulted in increased collagen deposition and weakened atherosclerosis; however, in macrophages, in vitro silencing of LOX-1 resulted in decreased mitochondrial DNA (mtDNA) damage, ROS accumulation, and reduced NLRP3 inflammasome activation [39, 40].
Since enrichment of cytoplasm with mtDNA is deleterious and contribute to atherosclerosis, Tumurkhuu et al. discovered an association between oxy-guanine DNA glycosylase (OGG1), a DNA glycosylate enzyme that removes oxidized DNA, with atherosclerosis process involving NLRP3 inflammasome activation [41]. The OGG1−/−LDLR−/− mice showed increased mtDNA accumulation, severe inflammatory reaction, and larger atherosclerotic plaques as compared with LDLR−/− mice fed with a Western diet [42]. However, by silencing NLRP3 inflammasome, such a phenomenon could be overturned, suggesting that OGG1 is an adverse regulator of atherosclerosis. In addition, micro-RNA-9 was also recognized to deactivate NLRP3 inflammasome and decrease its inflammatory response towards the atherosclerotic process [43].
NLRP3 Inflammasome and Macrophages
Macrophages play a key role in atherosclerosis during early or late plaque formation and plaque rupture. In early atherosclerosis, macrophage-derived NLRP3 inflammasome shows involvement in inflammatory anti-injury reactions, which are beneficial for plaque stability. Relatively, in late atherosclerosis, the NLRP3 inflammasome induces premature macrophage death and a significant amount of lipid release, which increases plaque vulnerability [44, 45]. Several studies have shown in recent years that oxidized low-density lipoprotein (ox-LDL) and cholesterol crystals can activate the NLRP3 inflammasome and caspase-1, triggering macrophages into pyroptosis and leading to increase in the release of IL-1β and IL-18. The above factors cause a reduction in the stability of the plaque [46]. Suppressing the NLRP3 inflammasome gene in mice inhibited inflammatory response, which hindered the atherosclerotic progress. Specifically, silencing the caspase-1/11 gene of the mouse bone marrow, which will greatly decrease the plaque’s necrotic lipid core. MCC950, an NLRP3 inflammasome inhibitor, has shown to improve the stability of mouse platelets because it suppresses the inflammatory response by macrophages. In addition, MCC950 prevents macrophages transformation into foam cells via inhibiting ox-LDL uptake and promoting cholesterol outflow; therefore, atherosclerosis progression is prevented.
NLRP3 Inflammasome and Vascular Endothelial and Smooth Muscle Cells
Endothelial cells (ECs) have close communication with the blood, and EC dysfunction is an important link in forming and developing atherosclerosis. The ox-LDL can induce heat shock of vascular endothelial cells, through the ROS mechanism [47]. Nicotine, being the most common risk factor, activates the NLRP3 inflammasome to initiate inflammation as well as cell apoptosis of ECs, thus worsening atherosclerosis [48]. The levels of IL-1β, IL-18, P-selectin, and vascular cell adhesion molecule-l increases during the inflammatory process of atherosclerosis, triggering the adhesion of the mononuclear phagocyte system, thus making atherosclerosis vulnerable [49]. In addition, activation of caspase-1 induced by NLRP3 inflammasome promotes the expression of CXCL16 chemokine, and its receptor CXCR6, leading to the recruitment of T lymphocytes into the subcutaneous tissues, thereby, facilitating and promoting the inflammatory reaction of ECs [50]. Hemodynamic abnormalities have been found to promote the activation of NLRP3 inflammasome together with the secretion and release of IL-1β in endothelial cells by activating the sterol regulatory element-binding protein 2 (SREBP2). SREBP is the main regulator of cholesterol synthesis and provides both signals 1 and 2 for the formation of NLRP3 inflammasome. Similarly, when the activated form of SREBP in an apolipoprotein E (Apoe protein involve in fats metabolism)-deficient mice was overexpressed, increased arteriosclerosis was observed [51].
Vascular smooth muscle cells (VSMCs) are essential mid-membrane cells in coronary arteries [52]. The activated VSMCs have a good proliferation and migration capability at the early stage of AS, which migrate from the middle membrane to the inner membrane. The fibrous cap is stabilized by secreting an extracellular layer, playing a significant function in stopping the degradation of the plaque. However, in the late stage of atherosclerosis owing to a significant volume of lipid deposition in the plaque, cholesterol stimulates several pro-inflammatory genes in vascular smooth muscle cells, resulting in the activation of NLRP3 inflammasome response and exacerbating the inflammatory reaction, ultimately resulting in plaque necrotic lipid nuclear heat sags [53]. Furthermore, studies have shown that intracellular mRNA levels of the NLRP3 inflammasome, ASC, and caspase-1 increases, when β-glycerophosphate induces the primary rat aorta VSMCs to get crystallize on calcium. In the same period, the mRNA levels were significantly upregulated in the calcified tissue of the human artery; also, caspase-1 activity was enhanced [54].
The melanoma-associated pattern recognition receptor (AIM2) can stimulate caspase-1 via the inflammatory pathway of the NLRP3 inflammasome and then initiates the inflammatory response by slicing GSDMD [55]. VSMCs also release IL-1β, IL-18, and other inflammatory factors under the action of the NLRP3 inflammasome, which exacerbate the inflammation, decrease the production of collagen, extracellular matrix, and destabilize the fibrous cap. Increased plaque instability thus results in plaque degradation and breach.
NLRP3 Inflammasome and Endoplasmic Reticulum Stress
Endoplasmic reticulum (ER) stress contribution to atherosclerosis development is also supported by increasing evidence. In this phase, the preserved homeostasis mediator in the unfolded protein reaction inositol-requiring enzyme 1(IRE1) shows a major contribution. In macrophages, proatherogenic genes are regulated by the enzyme IRE1 and result in NLRP3 inflammasome activation. In line with this concept, the use of the IRE1 inhibitor in an Apoe/mouse model showed a reduction in the size of atherosclerotic plaques by minimizing the accumulation and activation of macrophages [56]. Bioactive lipid palmitoleate avoids lipid-induced activation of the inflammasome in macrophages through its role in ER membrane remodeling, resulting in a decrease in the atherosclerotic plaque size of ER pressure in vitro in an Apoe/mouse model [57].
THERAPIES TAKING ADVANTAGE OF NLRP3 INFLAMMASOME INHIBITION
Since the NLRP3 inflammasome inflammatory response plays a significant role in atherosclerosis production, the NLRP3 inflammasome as the therapeutic target has become a hot topic in atherosclerotic drug research.
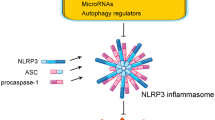
A prospective therapeutic target for atherosclerosis is the negative regulation of NLRP3 inflammasome. Recent studies have shown that autophagy, an intracellular degradation mechanism for cellular homeostasis, negatively regulated activation of NLRP3 inflammasome through several mechanisms. Because mitochondrial ROS is essential for the activation of NLRP3 inflammasome in response to many stimuli, autophagy inhibits its activation by clearing damaged mitochondria.
Another research showed that autophagy was capable of capturing and degrading the assembled NLRP3 inflammasome inflammatory complex through its ubiquitination and modulating its activity. Indeed, autophagy-defective Atg 5−/− mice developed accelerated atherosclerosis, together with increased NLRP3 inflammasome activation. Moreover, since lysosome is an important organelle for the degradation of a particulate, activation of lysosome biogenesis by overexpression of the transcription factor EB in macrophages has been shown to inhibit cholesterol-induced NLRP3 inflammasome activation and mitigate the development of atherosclerosis. Furthermore, protein kinase A (PKA) phosphorylation has been recorded as a negative regulator of NLRP3 inflammasome. Transmembrane G protein-coupled reception Tor 5 (TGR5), bile acid receptor-induced PKA activation leads to NLRP3 ubiquitination, which is associated with the PKA-induced NLRP3 phosphorylation (serine 291). Although agonists of TGR5 avoid atherosclerosis progression through enhanced cholesterol efflux and reduced inflammatory reactions, anti-inflammatory mechanisms are not fully elucidated. Further studies in atherosclerosis are therefore needed to clarify the accurate processes of NLRP3 inflammasome regulation [58, 59].
CONCLUDING REMARKS
The critical role played by inflammasomes in health and disease is inevitable. The pathophysiology of CVDs and other diseases is comprehendible after the discovery of the NLRP3 inflammasomes. Inflammasomes are needed for the maturation and release of IL-1β, IL-18, elimination of malignant or damaged cells along with other cellular contents, and host defense from infectious agents. Although multiple studies have been published that explore the mechanism of NLRP3 inflammasome activation, still some fundamental questions are needed to be addressed such as the mechanisms involved in regulating the sensing of DAMPs and/or PAMPs by cytoplasmic sensors.
Moreover, further studies are necessary to help in the understanding of inflammasome biology, so that better treatment strategy can be developed for NLRP3 inflammasome-associated diseases, and other essential inquiries will be solved. In addition, the mechanism of NLRP3 inflammasome inhibition is equally important and significant for providing novel drug therapy for the treatment of disease, e.g., CVDs including atherosclerosis.
References
Medzhitov, R. 2008. Origin and physiological roles of inflammation. Nature. 454: 428–435.
Lamkanfi, M., and V.M. Dixit. 2014. Mechanisms and functions of inflammasomes. Cell. 157: 1013–1022.
Amin, J., D. Boche, and S. Rakic. 2017. What do we know about the inflammasome in humans? Brain Pathol 27: 192–204.
Schroder, K., R. Zhou, and J. Tschopp. 2010. The NLRP3 inflammasome: A sensor for metabolic danger? Science. 327: 296–300.
Libby. Interleukin-1 beta as a target for atherosclerosis therapy: Jour of the Amer Colle of Cardio. 2017: 18; 2278–2289.
Broz, P., and V.M. Dixit. 2016. Inflammasomes: Mechanism of assembly, regulation and signaling. Nat Rev Immunol 16: 407–420.
Vanaja, S.K., V.A. Rathinam, and K.A. Fitzgerald. 2015. Mechanisms of inflammasome activation: Recent advances and novel insights. Trends Cell Biol 25: 308–315.
Bauernfeind, F.G., et al. 2009. Cutting edge: NF-κB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J Immunol 183: 787–791.
Lemmers, B., L. Salmena, N. Bidère, H. Su, E. Matysiak-Zablocki, K. Murakami, P.S. Ohashi, A. Jurisicova, M. Lenardo, R. Hakem, and A. Hakem. 2007. Essential role for caspase-8 in toll-like receptors and NFκB signaling. J Biol Chem 282: 7416–7423.
Gurung, P., P.K. Anand, R.K.S. Malireddi, L. Vande Walle, N. van Opdenbosch, C.P. Dillon, R. Weinlich, D.R. Green, M. Lamkanfi, and T.D. Kanneganti. 2014. FADD and caspase-8 mediate priming and activation of the canonical and noncanonical Nlrp3 inflammasomes. J Immunol 192: 1835–1846.
Py, B.F., et al. 2013. Deubiquitination of NLRP3 by BRCC3 critically regulates inflammasomes activity. Mol Cell 49: 331–338.
Bauernfeind, F., A. Rieger, F.A. Schildberg, P.A. Knolle, J.L. Schmid-Burgk, and V. Hornung. 2012. NLRP3 inflammasome activity is negatively controlled by miR-223. J Immunol 189: 4175–4181.
Haneklaus, M., J.D. O’Neil, A.R. Clark, S.L. Masters, and L.A.J. O’Neill. 2017. The RNA-binding protein tristetraprolin (TTP) is a critical negative regulator of the NLRP3 inflammasome. J Biol Chem 292: 6869–6881.
Stutz, A., C.C. Kolbe, R. Stahl, G.L. Horvath, B.S. Franklin, O. van Ray, R. Brinkschulte, M. Geyer, F. Meissner, and E. Latz. 2017. NLRP3 inflammasome assembly is regulated by phosphorylation of the pyrin domain. J Exp Med 214: 1725–1736.
Hernandez-Cuellar, E., K. Tsuchiya, H. Hara, R. Fang, S. Sakai, I. Kawamura, S. Akira, and M. Mitsuyama. 2012. Nitric oxide inhibits the NLRP3 inflammasome. J Immunol 189: 5113–5117.
Muñoz-Planillo, R., P. Kuffa, G. Martínez-Colón, B.L. Smith, T.M. Rajendiran, and G. Núñez. 2013. K+ efflux is the common trigger of NLRP3 inflammasome activation by bacterial toxins and particulate matter. Immunity. 38: 1142–1153.
He, Y., M. Zeng, D. Yang, B. Motro, and G. Núñez. 2016. NEK7 is an essential mediator of NLRP3 activation downstream of potassium efflux. Nature. 530: 354–357.
Karmakar, M., M.A. Katsnelson, G.R. Dubyak, and E. Pearlman. 2016. Neutrophil P2X7 receptors mediate NLRP3 inflammasome-dependent IL-1β secretion in response to ATP. Nat Commun 7: 10555.
Katsnelson, M.A., L.G. Rucker, H.M. Russo, and G.R. Dubyak. 2015. K+ efflux agonists induce NLRP3 inflammasome activation independently of Ca2+ signaling. J Immunol 194: 3937–3952.
Murakami, T., J. Ockinger, J. Yu, V. Byles, A. McColl, A.M. Hofer, and T. Horng. 2012. Critical role for calcium mobilization in activation of the NLRP3 inflammasome. Proc Natl Acad Sci 109: 11282–11287.
Csordás, G., and G. Hajnóczky. 1787. SR/ER–mitochondrial local communication: Calcium and ROS. Biochim Biophys Acta Bioenerg 2009: 1352–1362.
Schorn, C., B. Frey, K. Lauber, C. Janko, M. Strysio, H. Keppeler, U.S. Gaipl, R.E. Voll, E. Springer, L.E. Munoz, G. Schett, and M. Herrmann. 2011. Sodium overload and water influx activate the NALP3 inflammasome. J Biol Chem 286: 35–41.
Verhoef, P.A., S.B. Kertesy, K. Lundberg, J.M. Kahlenberg, and G.R. Dubyak. 2005. Inhibitory effects of chloride on the activation of caspase-1, IL-1β secretion, and cytolysis by the P2X7 receptor. J Immunol 175: 7623–7634.
Compan, V., A. Baroja-Mazo, G. López-Castejón, A.I. Gomez, C.M. Martínez, D. Angosto, M.T. Montero, A.S. Herranz, E. Bazán, D. Reimers, V. Mulero, and P. Pelegrín. 2012. Cell volume regulation modulates NLRP3 inflammasome activation. Immunity. 37: 487–500.
Tang, T., X. Lang, C. Xu, X. Wang, T. Gong, Y. Yang, J. Cui, L. Bai, J. Wang, W. Jiang, and R. Zhou. 2017. CLICs-dependent chloride efflux is an essential and proximal upstream event for NLRP3 inflammasome activation. Nat Commun 8: 202.
Shimada, K., T.R. Crother, J. Karlin, J. Dagvadorj, N. Chiba, S. Chen, V.K. Ramanujan, A.J. Wolf, L. Vergnes, D.M. Ojcius, A. Rentsendorj, M. Vargas, C. Guerrero, Y. Wang, K.A. Fitzgerald, D.M. Underhill, T. Town, and M. Arditi. 2012. Oxidized mitochondrial DNA activates the NLRP3 inflammasome during apoptosis. Immunity. 36: 401–414.
Shi, H., Y. Wang, X. Li, X. Zhan, M. Tang, M. Fina, L. Su, D. Pratt, C.H. Bu, S. Hildebrand, S. Lyon, L. Scott, J. Quan, Q. Sun, J. Russell, S. Arnett, P. Jurek, D. Chen, V.V. Kravchenko, J.C. Mathison, E.M.Y. Moresco, N.L. Monson, R.J. Ulevitch, and B. Beutler. 2016. NLRP3 activation and mitosis are mutually exclusive events coordinated by NEK7, a new inflammasome component. Nat Immunol 17: 250–258.
Groß, C.J., R. Mishra, K.S. Schneider, G. Médard, J. Wettmarshausen, D.C. Dittlein, H. Shi, O. Gorka, P.A. Koenig, S. Fromm, G. Magnani, T. Ćiković, L. Hartjes, J. Smollich, A.A.B. Robertson, M.A. Cooper, M. Schmidt-Supprian, M. Schuster, K. Schroder, P. Broz, C. Traidl-Hoffmann, B. Beutler, B. Kuster, J. Ruland, S. Schneider, F. Perocchi, and O. Groß. 2016. K+efflux-independent NLRP3 inflammasome activation by small molecules targeting mitochondria. Immunity. 45: 761–773.
Halle, A., V. Hornung, G.C. Petzold, C.R. Stewart, B.G. Monks, T. Reinheckel, K.A. Fitzgerald, E. Latz, K.J. Moore, and D.T. Golenbock. 2008. The NALP3 inflammasome is involved in the innate immune response to amyloid-β. Nat Immunol 9: 857–865.
Hornung, V., F. Bauernfeind, A. Halle, E.O. Samstad, H. Kono, K.L. Rock, K.A. Fitzgerald, and E. Latz. 2008. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat Immunol 9: 847–856.
Wang, Z., W. Hu, C. Lu, Z. Ma, S. Jiang, C. Gu, D. Acuña-Castroviejo, and Y. Yang. 2018. Targeting NLRP3 (nucleotide-binding domain, Leucine-rich–containing family, pyrin domain–Containing-3) inflammasome in cardiovascular disorders. Arterioscler Thromb Vasc Biol 38: 2765–2779.
Karasawa, T., and M. Takahashi. 2017. Role of NLRP3 inflammasomes in atherosclerosis. Review. J Atheroscler Thromb 24: 000–000.
Zheng, F., S. Xing, Z. Gong, and Q. Xing. 2013. NLRP3 inflammasomes show high expression in aorta of patients with atherosclerosis. Heart Lung Circ 22: 746–750.
Varghese, G.P., L. Folkersen, R.J. Strawbridge, et al. 2016. NLRP3 inflammasome expression and activation in human atherosclerosis. J Am Heart Assoc 5: 3031.
Shi, X., W.L. Xie, W.W. Kong, D. Chen, and P. Qu. 2015. Expression of the NLRP3 inflammasome in carotid atherosclerosis. J Stroke Cerebrovasc Dis 24: 2455–2466.
Duewell, P., H. Kono, K.J. Rayner, C.M. Sirois, G. Vladimer, F.G. Bauernfeind, G.S. Abela, L. Franchi, G. Nuñez, M. Schnurr, T. Espevik, E. Lien, K.A. Fitzgerald, K.L. Rock, K.J. Moore, S.D. Wright, V. Hornung, and E. Latz. 2010. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 464: 1357–1361.
Hendrikx, T., M.L.J. Jeurissen, P.J. Van Gorp, et al. 2015. Bone marrow-specific caspase-1/11 deficiency inhibits atherosclerosis development in Ldlr−/− mice. FEBS J 282: 2327–2338.
Wang, R., Y. Wang, and N. Muetal. 2017. Activation of NLRP3inflammasomes contributes to hyperhomocysteinemia-aggravated inflammation and atherosclerosis in apoE-deficient mice. Lab Investig 97: 922–934.
Ding, Z., S. Liu, X. Wang, Y. Dai, M. Khaidakov, X. Deng, Y. Fan, D. Xiang, and J.L. Mehta. 2014. LOX-1, mtDNA damage, and NLRP3 inflammasome activation in macrophages: Implications in atherogenesis. Cardiovasc Res 103: 619–628.
Mehta, J.L., N. Sanada, C.P. Hu, et al. 2007. Deletion of LOX-1 reduces atherogenesis in LDLR knockout mice fed high cholesterol diet. J Circ Res 100: 1634–1642.
Chen, L., Q. Yao, S. Xu, H. Wang, and P. Qu. 2018. Inhibition of the NLRP3 inflammasome attenuates foam cell formation of THP-1 macrophages by suppressing ox-LDL uptake and promoting cholesterol efflux. Biochem Biophys Res Commun 495: 382–387.
Tumurkhuu, G., K. Shimada, J. Dagvadorj, et al. 2016. Ogg1-dependent DNA repair regulates NLRP3 inflammasome and prevents atherosclerosis. Circ Res 119: 76–90.
Wang, Y., Z. Han, Y. Fan, J. Zhang, K. Chen, L. Gao, H. Zeng, J. Cao, and C. Wang. 2017. MicroRNA-9 inhibits NLRP3 inflammasome activation in human atherosclerosis inflammation cell models through the JAK1/STAT signaling pathway. Cell Physiol Biochem 41: 1555–1571.
Rajamaki, K., J. Lappalainen, K. Oorni, et al. 2010. Cholesterol crystals activate the NLRP3 inflammasome in human macrophages: a novel link between cholesterol metabolism and inflammation. PLoS One 7: e11765.
Tabas, I., and A.H. Lichtman. 2017. Monocyte-macrophages and T cells in atherosclerosis. Immun. 4: 621–634.
Hendrikx T, Jeurissen M.L.J, van Gorp P. J, et al. Bone marrow-specific caspase-1/11 deficiency inhibits atherosclerosis development inLdlr−/− mice. FEBS J. 2015;12:2327–2338.
Yin, Y., X. Li, X. Sha, et al. 2015. Early hyperlipidemia promotes endothelial activation via a caspase-1-sirtuin 1 pathway. Arterio, Thromb, and Vascu Bio 35: 804–816.
Wu, X., H. Zhang, W. Qi, et al. 2018. Nicotine promotes atherosclerosis via ROS-NLRP3-mediated endothelial cell pyroptosis. Cell Death & Disease 2: 171.
Mestas, J., and K. Ley. 2008. Monocyte-endothelial cell interactions in the development of atherosclerosis. Trends Cardiovasc Med 6: 228–232.
Sheikine, Y., and A. Sirsjö. 2008. CXCL16/SR-PSOX-A friend or a foe in atherosclerosis? Atherosc 197: 487–495.
Kawaguchi, M., M. Takahashi, T. Hata, Y. Kashima, F. Usui, H. Morimoto, A. Izawa, Y. Takahashi, J. Masumoto, J. Koyama, M. Hongo, T. Noda, J. Nakayama, J. Sagara, S.’. Taniguchi, and U. Ikeda. 2011. Inflammasome activation of cardiac fibroblasts is essential for myocardial ischemia/reperfusion injury. Cir. 123: 594–604.
Fang, L., K.K. Wang, P.F. Zhang, T. Li, Z.L. Xiao, M. Yang, and Z.X. Yu. 2020. Nucleolin promotes Ang II-induced phenotypic transformation of vascular smooth muscle cells by regulating EGF and PDGF-BB. J Cell Mol Med 24: 1917–1933.
Bennett, M.R., S. Sinha, and G.K. Owens. 2016. Vascular smooth muscle cells in atherosclerosis. Circ Res 118: 692–702.
Wen, C., X. Yang, Z. Yan, et al. 2013. Nlrp3 inflammasome is activated and required for vascular smooth muscle cell calcification. Int J Cardiol 168: 2242–2247.
Man, S.M., Q. Zhu, L. Zhu, Z. Liu, R. Karki, A. Malik, D. Sharma, L. Li, R.K.S. Malireddi, P. Gurung, G. Neale, S.R. Olsen, R.A. Carter, D.J. McGoldrick, G. Wu, D. Finkelstein, P. Vogel, R.J. Gilbertson, and T.D. Kanneganti. 2015. Critical role for the DNA sensor AIM2 in stem cell proliferation and cancer. Cell. 162: 45–58.
Luchetti, F., R. Crinelli, E. Cesarini, B. Canonico, L. Guidi, C. Zerbinati, G. di Sario, L. Zamai, M. Magnani, S. Papa, and L. Iuliano. 2017. Endothelial cells, endoplasmic reticulum stress and oxysterols. Redox Biol 13: 581–587.
Çimen, I., B. Kocatürk, S. Koyuncu, et al. 2016. Prevention of atherosclerosis by bioactive palmitoleate through suppression of organelle stress and inflammasome activation. Sci Transl Med 8: 126.
Rsazani, B., C. Feng, T. Coleman, et al. 2012. Autophagy links inflammasomes to athero- sclerotic progression. Cell Metab 15: 534–544.
Pols, T.W., M. Nomura, T. Harach, et al. 2011. TGR5 activation inhibits atherosclerosis by reducing macrophage inflammation and lipid loading. Cell Metab 14: 747–757.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Cardiovascular diseases involving heart and vasculature disorders are a serious global health burden that is presently the world’s leading cause of death.
• Multiple danger signals such as cholesterol crystals, calcium phosphate crystals, and oxidized low-density lipoprotein triggered NLRP3 inflammasome activation in macrophages to initiate inflammatory reactions in atherosclerotic lesions.
• Understanding NLRP3 inflammasome activation mechanisms will allow its particular inhibitors to be developed to treat NLRP3-related illnesses
Electronic supplementary material
ESM 1
(DOCX 96.9 kb)
Rights and permissions
About this article
Cite this article
Liaqat, A., Asad, M., Shoukat, F. et al. A Spotlight on the Underlying Activation Mechanisms of the NLRP3 Inflammasome and its Role in Atherosclerosis: A Review. Inflammation 43, 2011–2020 (2020). https://doi.org/10.1007/s10753-020-01290-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-020-01290-1