Abstract
NLRP3 inflammasome is a key multiprotein signaling platform that tightly controls inflammatory responses and coordinates antimicrobial host defenses by activating caspase-1 for the subsequent maturation of pro-inflammatory cytokines, IL-1β and IL-18, and induces pyroptosis. The assembly and activation of NLRP3 inflammasome are linked to the pathogenesis of several cardiovascular disease risk factors, such as hypertension and diabetes, and their major consequences—myocardial remodeling. The study of the NLRP3 inflammasome in these cardiovascular disease states may uncover important triggers and endogenous modulators of the disease, and lead to new treatment strategies. This review outlines current insights into NLRP3 inflammasome research associated with cardiovascular diseases and discusses the questions that remain in this field.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Inflammasomes are multiple protein complexes that serve as molecular signaling platforms to activate caspase-1 and regulate maturation of a potent pro-inflammatory cytokine IL-1 as well as inflammatory cell death, pyroptosis in response to pathogen-associated and danger/damage-associated molecular pattern molecules (PAMPs and DAMPs), reactive oxygen species (ROS), cholesterol crystals, and environmental irritants [8]. Although several types of inflammasomes have been identified so far, the best characterized is the NOD-like receptor family pyrin domain containing 3 (NLRP3) inflammasome that recognizes non-microbial danger signals and leads to sterile inflammatory responses in various disease conditions [50, 102]. NLRP3 inflammasome contains NLRP3 [an innate immune receptor, which is composed of three domains: C-terminal leucine-rich repeats (LRRs), a central nucleotide binding and oligomerization domain termed the NACHT domain, and an N-terminal pyrin domain (PYD) effector domain], ASC [an adaptor protein termed apoptosis-associated speck-like protein containing an N-terminal PYD and a C-terminal caspase recruitment domain (CARD), also known as Pycard] and cysteine protease precursor procaspase-1 (consists of a CARD and a caspase domain) [118]. Within this complex, NLRP3 initiates the formation of the inflammasome by interacting with ASC that recruits and activates procaspase-1 to generate the active caspase-1. Caspase-1 is an IL-1β-converting enzyme which cleaves preformed pro-IL-1β to its active form IL-1β [26].
IL-1β is a potent pro-inflammatory cytokine that is produced mainly by macrophages in tissue and primarily by monocytes in circulating blood [12, 83]. Mature IL-1β works as a significant mediator recruiting innate immune cells to the site of infection and modulating adaptive immune cells in many immune reactions [12]. Contrary to most other inflammatory cytokines, production of IL-1β requires a dual signal for its activation. First, intracellular pro-IL-1β is produced following stimulation of pattern-recognition receptors (PRRs), such as the toll-like receptors (TLRs) located on the cell surface or in endosomes and NOD-like receptors (NLRs) located in the cytoplasm. Second, to be functional IL-1β, pro-IL-1β requires proteolytic cleavage by caspase-1. In addition, IL-1β has many biological functions that are important in sterile inflammation, such as upregulation of those adhesion molecules on endothelial cells and induction of additional pro-inflammatory mediators [12, 31]. IL-1β is transcriptionally regulated when damage components are sensed by PRRs which can identify PAMPs and DAMPs [9].
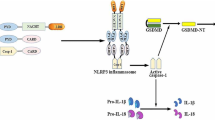
The activation of NLRP3 inflammasome by immune cells such as dendritic cells, monocytes, and macrophages is a two-step process including priming and triggering [24]. The priming is a process in which PAMPs, DAMPs or environmental stress are recognized by TLRs or cytokines such as tumor necrosis factor α (TNF-α) leading to the activation of nuclear factor-kappaB (NF-κB), which results in the expression and activation of NLRP3, pro-IL-1β and pro-IL-18, and transcriptional protein modifications such as NLRP3 deubiquitination and ASC phosphorylation [89, 118]. The triggering is characterized by promoting the oligomerization of inactive NLRP3, ASC and procaspase-1, resulting in proteolytic cleavage of caspase-1 and the maturation of IL-1β and leading to final inflammasome formation [27] (Fig. 1).
Overview of the activation of NLRP3 inflammasome. Activation of NLRP3 inflammasome requires a two-step process: priming and triggering. The priming is mediated by PAMPs/DAMPs recognized by TLRs or cytokines such as TNF-α, and is responsible for the upregulation of NLRP3 protein and pro-IL-1β levels in an NF-κB pathway. The triggering is a subsequent stimulus that activates the NLRP3 inflammasome by promoting the oligomerization of inactive NLRP3, ASC and procaspase-1, resulting in proteolytic cleavage of caspase-1 and the maturation of IL-1β. NLRP3 is composed of three domains: C-terminal LRRs, a central nucleotide binding and oligomerization domain termed the NACHT domain, and an N-terminal PYD effector domain. ASC contains an N-terminal PYD and a C-terminal CARD. Cysteine protease precursor procaspase-1 consists of a CARD and a caspase domain. DAMP danger/damage-associated molecular pattern molecule, PAMP pathogen-associated molecular pattern molecule, TLR toll-like receptor, TNFR tumor necrosis factor α receptor, NF-κB nuclear factor-kappaB, LRR leucine-rich repeats, ASC apoptosis-associated speck-like protein containing a caspase recruitment domain, PYD pyrin domain, CARD caspase recruitment domain
Although acute inflammation is a physiological attempt to eliminate destructive stimuli and combat pathogens, cellular damage and hazardous substances, chronic inflammation that occurs when noxious stimuli are not removed or attenuated can be detrimental to the host, and lead to tissue damage, aberrant collagen accumulation and fibrosis, and ultimately contribute to multiple chronic diseases. Recent studies have shown that the activation of NLRP3 inflammasome pathways is relevant to the pathogenesis of cardiovascular diseases such as hypertension, atherosclerosis, ischemic injury, cardiomyopathy and myocardial infarction [10, 39, 95, 101]. In this review, we highlight the current state of knowledge regarding the role of NLRP3 inflammasome in cardiovascular disease risk factors—hypertension and diabetes—and their major consequence—atherosclerosis and myocardial infarction—resulting in cardiac remodeling.
NLRP3 inflammasome in hypertension
Hypertension is a complex disease caused by multiple genetic and environmental factors. Hypertension and its complications, including ischemic heart disease, heart failure (HF), stroke, peripheral vascular disease, vision loss and chronic kidney disease, are responsible for substantial morbidity and mortality worldwide [74].
Inflammasome activity plays a key role in the progression of hypertension. Krishnan et al. observed elevated serum level of IL-1β in patients with high blood pressure and raised the question if high levels of serum IL-1β are a marker or inducer of systemic hypertension [49].
Salt-sensitive hypertension is characterized by chronic inflammation and elevated sympathetic outflow [60]. Oxidative stress is a major metabolic factor which leads to the onset of chronic diseases such as hypertension, metabolic syndrome, diabetes, and their complications. High-salt-induced inflammation and oxidative stress in the hypothalamic paraventricular nucleus contribute to the pathogenesis of salt-sensitive hypertension via sympathoexcitation [79]. NF-κB is an effective activator of NLRP3 and it induces inflammation which contributes to the pathophysiology of hypertension [114]. Blockade of NF-κB inhibits high-salt-induced hypertension by attenuating NLRP3 and caspase-1 activation, reducing pro-inflammatory cytokines and oxidative stress in the paraventricular nucleus of salt-sensitive hypertensive rats [75]. IL-1β is an important pro-inflammatory cytokine with pleiotropic effects and is mediated by NLRP3 inflammasome activation. Central IL-1β inhibition attenuates the renin-angiotensin system activation, decreases ROS generation in the paraventricular nucleus, and thereby delays hypertension-induced cardiovascular damage [30, 90]. In Dahl salt-sensitive hypertensive rats, infusion of IL-1β inhibitor gevokizumab into paraventricular nucleus suppresses sympathoexcitation and attenuates hypertensive responses by restoring the balance between pro- and anti-inflammatory cytokines and oxidative stress [76].
Angiotensin II (Ang II), a vasoconstrictive peptide generated as a result if activation of renin-angiotensin system, exerts physical effects through the activation of type 1 and type 2 receptors [105]. Ang II is locally formed in the kidney, and as an important mediator of hypertension plays a key role in regulating inflammatory processes associated with hypertension [54]. It has been shown that Ang II infusion (for 7 days) enhances the activation of NLRP3 inflammasome and the expression of IL-1β and other pro-inflammatory cytokines in the mouse heart [33]. Blockade of NLRP3 inflammasome activation markedly attenuates Ang II-induced cardiac fibrosis without affecting blood pressure, which indicates that NLRP3 inflammasome independently works to cardiac remodeling in Ang II-induced hypertensive mice [33].
Collectively, these observations suggest that NLRP3 inflammasome/IL-1β nexus may be a novel therapeutic target in hypertensive heart diseases. Further studies on the assembly and activation of NLRP3 inflammasome are required to address more particularly on its functional role during hypertension progression.
NLRP3 inflammasome in diabetes
Diabetes is a group of chronic metabolic diseases characterized by high blood sugar levels over a prolonged period, which results from either the pancreas not producing enough insulin or the cells of the body not responding appropriately to the insulin produced [5, 14, 108]. Type 2 diabetes (T2D) is the most common type of diabetes, which is characterized by pancreatic islet β cell damage, insulin resistance, reduced insulin secretion, hyperglycemia, and permanent inflammatory response [84]. Diabetes can result in many complications that include myocardial ischemia, stroke, chronic kidney failure, foot ulcers, retinopathy and early death. The prevalence of T2D worldwide was estimated to be 2.8% in 2000, which increased to 8.3% in 2014, and is expected to affect approximately 592 million individuals world-wide by 2035 [104, 110]. It is estimated by the World Health Organization (WHO) that 422 million people had T2D worldwide in 2016 [109]. It is urgent to prevent, control and treat T2D by clarifying the steps leading to its pathogenesis and thereby developing effective prevention and treatment strategies.
Previous studies revealed a closer link between onset of T2D and inflammatory reaction mediated by innate immune system. As an important component of innate immune system, NLRP3 inflammasome plays an important role in the pathogenesis of T2D, and IL-1β is a key mediator. Menu and Vince summarized the role of abnormal activation of NLRP3 inflammasome and the overexpression of IL-1β in the development of diabetes and in its complications [65]. Liu et al. reviewed the critical role of NLRP3 in the pathogenesis of T2D [55]. It has been demonstrated that IL-1β is elevated in T2D patients, suggesting that IL-1β may be linked with the development of T2D [61]. Kim et al. reported that inhibition of NLRP3 inflammasome by γ-tocotrienol can ameliorate and delay the progression of T2D [46]. Coll et al. showed that suppression of NLRP3 inflammasome with MCC950 could be used as a potential therapy for inflammatory disorders such as T2D and atherosclerosis [17]. Thus, it seems that IL-1β is not only a primary inflammatory cytokine produced by NLRP3 inflammasome activation, but also is a key factor in the pathogenesis of T2D [82]. IL-1β can directly injure pancreatic islet β-cells and decrease the function of insulin and induce insulin resistance [88, 104]. IL-1β and other pro-inflammatory cytokines such as TNF-α can cooperatively interfere with glucose metabolism [87]. Elevated levels of pro-inflammatory cytokines lead to insulin resistance by antagonizing insulin signaling, which can inhibit the absorption of glucose, resulting in abnormal glucose tolerance, and development of T2D. Of note, IL-1β levels are elevated in circulation and in pancreatic islets during the progression from obesity to T2D. These observations suggest that IL-1β may be implicated as an important driver of disease [61].
Hyperglycemia can activate thioredoxin-interacting protein (TXNIP) expression in a variety of cell types including skeletal myocytes, pancreatic islet β cells, endothelial cells and adipocytes, and induce the production of IL-1β in endothelial cells, monocytes, and pancreatic islet β cells by direct interaction with NLRP3 inflammasome [25, 48]. TXNIP as an endogenous inhibitor of the ROS scavenging protein TRX (antioxidant thioredoxin), which can counter oxidative stress and maintain the physiologic state of cells [37]. TXNIP has been linked to glucose metabolism and T2D [13, 72]. It is down-regulated by insulin in β-cells, and its levels are elevated in subjects with T2D [70]. TXNIP released from the TXNIP-TRX complex, specifically binds with NLRP3 inflammasome to activate it to release capase-1 and IL-1β [1]. In addition, TXNIP mutations are associated with hypertriglyceridemia and hypertension in T2D patients [98]. Furthermore, reducing TXNIP gene expression has been shown to induce a significant decrease in intracellular pro-IL-1β and bioactive IL-1 production and to prevent endoplasmic reticulum stress-induced β-cell death [48]. Caspase-1 protein levels are markedly elevated in human adipose tissue exposed to high glucose. The absence of caspase-1 and TXNIP can improve insulin sensitivity and result in lower plasma glucose levels [40]. These studies highlight the role of TXNIP as a functional link between endoplasmic reticulum stress, NLRP3 inflammasome activation and inflammation in T2D patients [3].
The expression of IL-1β antagonists in pancreatic islets is decreased in T2D patients. High glucose-induced pancreatic islet β-cell function enhances IL-1β levels, and leads to impaired insulin secretion, decreases proliferation, and increases apoptosis [51]. The function of pancreatic islet β-cell is improved when IL-1β is low or deficient [62]. The use of IL-1β receptor antagonist (IL-1RA) can also improve the function of pancreatic islet β-cells [19]. Pancreatic islet β-cells can secrete IL-1β in T2D patients which would damage islet function, leading to insulin resistance [113]. Importantly, deletion of the NLRP3 gene leads to a decrease in IL-1β secretion, improvement of insulin signal transduction, and the protection of pancreatic islet β-cells [113]. In fact, islet cells are particularly susceptible to IL-1β because the expression level of IL-1β receptor in β-cells is higher than that in any other cells in the body [7].
Obesity, an important precursor of diabetes, is a state of chronic inflammation characterized by atherogenic lipid profiles, infiltration of immune cells in adipose tissue, and the secretion of inflammatory cytokines such as IL-1β and TNF-α [48]. IL-1β and TNF-α are also considered to be key contributors to the obesity-induced inflammation and subsequent insulin resistance, pancreatic islet β-cell dysfunction and apoptosis, and the onset of T2D [20, 22]. NLRP3 inflammasome is closely associated with obesity-induced inflammation and insulin resistance, and targeted interruption of NLRP3 can improve glucose tolerance in obese mice [52, 103].
These observations suggest that careful targeting of NLRP3 inflammatory signaling pathways may be beneficial in the treatment of T2D. However, the most challenging task is how to translate these preclinical findings into clinical studies that detect the effect of inflammation-regulating therapies on T2D in humans.
NLRP3 inflammasome in atherosclerosis
Atherosclerosis is characterized by lipid deposition in the arterial wall and inflammatory cells infiltration. This chronic pathologic state is a fundamental cause of many cardiovascular diseases and a leading cause of death and loss of productive life years [32]. It has been proposed that there is a link between lipid metabolism and inflammation not only because atherosclerotic plaque constituents such as crystalline cholesterol and oxidized low-density lipoprotein activate NLRP3 inflammasome, but also they share common important events including oxidative stress, mitochondrial dysfunction, endoplasmic reticulum stress, and lysosome rupture [39]. Atherosclerotic plaque is considered an inflammatory lesion with involvement of the innate immune system, and chronic inflammation plays an important role in its progression [15, 36].
Pathways mediating pro-IL-1β release have been strongly linked with the progression of atherosclerotic lesions [58]. Yajima et al. using ASC−/− mice for the first time showed that NLRP3 inflammasome contributed to atherogenesis. ASC deficiency reduced the expression of IL-1β and IL-18 in the neointimal lesion and attenuated neointimal formation after vascular injury [111]. This seminal work identified NLRP3 inflammasome to be responsible for triggering the maturity of IL-1β in atherosclerotic plaques. Zheng et al. reported that NLRP3 inflammasome was involved in atherogenesis and its silence led to the stabilization of atherosclerotic plaque [116]. Peng et al. demonstrated that P2X7R was involved in the progression of atherosclerosis through promoting NLRP3 inflammasome activation [71]. Tumurkhuu et al. showed that 8-oxoguanine glycosylase (OGG1) deficiency caused increased IL-1β production and accelerated atherogenesis through activating NLRP3 inflammasome [97]. Duewell et al. reported the seminal study showing that NLRP3 inflammasome is required for atherogenesis and could be activated by cholesterol crystals [23]. Using a combination of laser reflection and fluorescence confocal microscopy, they showed cholesterol crystals in the aortic sinus together with the appearance of immune cells in the sub-endothelial space in low density lipoprotein receptor (LDLr)−/− mice fed a high-fat diet-induced atherosclerosis. The most exciting data related to the LDLr−/− mice that received NLRP3-, ASC-, or IL-1α/β-deficient bone marrow followed by high-lipid diet had significantly reduced the early formation of atherosclerosis and inflammation. Abderrazak et al. used NLRP3 inflammasome inhibitor arglabin to inhibit cholesterol crystal-induced NLRP3 inflammasome activation and observed a marked reduce of atherosclerotic lesions in apolipoprotein E2.Ki mice fed a high-fat diet [4]. Together, these findings indicate that the NLRP3 inflammasome-mediated signaling regulates cholesterol crystal-induced inflammation in atherosclerosis. Freigang et al. showed that cholesterol crystals could activate the NLRP3 inflammasome via an Nrf2 (NF-E2-related factor 2)-dependent mechanism in macrophages, and Nrf2-deficient apolipoprotein E (ApoE)−/− mice were highly protected against diet-induced atherogenesis [29].
Shortly after the role of NLRP3 inflammasome was identified in the murine model, another group reported that human macrophages also perceive cholesterol crystals via the NLRP3 inflammasome and the phagocytic cells engulf abundant amount of cholesterol crystals resulting in the activation of the NLRP3 inflammasome with subsequent activation of caspase-1 and IL-1β family cytokines, which strongly implicate cholesterol crystals as a potential source of inflammation in atherosclerotic lesions [77]. NLRP3 inflammasome is also highly expressed in the aorta of patients with atherosclerosis. This correlates with the severity of coronary artery disease and the atherosclerotic risk factors [117]. Paramel et al. recently reported that NLRP3 inflammasome-related genes were highly expressed in lipopolysaccharide-primed human atherosclerotic plaques and were sensitive to activation when exposed to adenosine triphosphate (ATP) and cholesterol crystals [69].
However, the role of the NLRP3 inflammasome in atherogenesis is not entirely consistent, and is confounded by inter-model modifiability. Menu et al. reported that atherogenesis in ApoE−/− mice could progress independently of the NLRP3 inflammasome activation [66]. They used the ApoE−/− mice model crossed with NLRP3−/−, ASC−/−, or caspase-1−/− mice and placed on high-fat diet, but could not identify any difference in progression of atherosclerosis, infiltration of plaques by macrophages, or plaque stability across genotypes. The divergence between these studies may due to the different mouse models used. Thus, the role of IL-1 in atherosclerosis development in the ApoE−/− mouse model is not fully established yet [44].
With respect to ROS playing a strong role in the link between oxidative stress and progression of atherosclerotic plaques, excessive generation of ROS induces expression of lectin-like oxidized low-density lipoprotein receptor-1 (LOX-1), which is a potent regulator of the immune system. LOX-1 activation in turn induces release of ROS, forming a positive feedback loop and playing a major role in the development of atherosclerosis [57, 73]. We recently showed that exposure of cultured human THP-1 macrophages to lipopolysaccharide markedly induced the expression of LOX-1 and ROS generation and initiated mitochondrial DNA (mtDNA) damage, subsequently caused accumulation of the damaged mtDNA that led to cell autophagy followed by NLRP3 inflammasome activation (Fig. 2). LOX-1 inhibition with a binding antibody or siRNA inhibited the subsequent series of events. ROS inhibitors and an autophagy inducer both reduced NLRP3 inflammasome expression. Inversely, autophagy inhibitor enhanced the expression of NLRP3 inflammasome. Furthermore, experiment with DNase II siRNA transfection confirmed the hypothesis that LOX-1-mediated autophagy and mtDNA damage play an essential role in NLRP3 inflammasome activation in a variety of cardiovascular diseases, including atherosclerosis, hypertension, and myocardial ischemia [21].
Activation of NLRP3 inflammasome accelerates atherosclerosis. In atherosclerotic plaque, cholesterol crystals, ATP and ROS activate the NLRP3 inflammasome, leading to inflammation and cell infiltration. Excessive generation of ROS induces the expression of scavenger receptors such as LOX-1, which would mediate autophagy and mtDNA damage. Damaged mitochondria that escape autophagy result in NLRP3 inflammasome activation. This amplified inflammatory cascade leads to the accumulation of extracellular lipids, resulting in cell injury and/or death, plaque formation and rupture, and finally accelerating atherosclerosis progression. ATP adenosine triphosphate, Nrf2 NF-E2-related factor 2, ROS reactive oxygen species, mtDNA mitochondrial DNA, LOX-1 lectin-like oxidized low-density lipoprotein receptor-1
These findings provide new insights into the important role of NLRP3 inflammasome in accelerating atherosclerosis, and point to new potential molecular targets for the therapy of atherosclerosis (Fig. 2). However, further studies are needed to acquire regulatory signaling pathways of NLRP3 inflammasome in atherosclerosis.
NLRP3 inflammasome in myocardial infarction
Cardiovascular diseases, particularly acute myocardial infarction (MI) and stroke, are the leading causes of death and disability worldwide [86]. Myocardial infarction is defined as myocardial cell death due to severe and prolonged ischemia. Myocardial infarction not only results in progressive deterioration of pump function in the heart (heart failure), but also to electrical instability and fatal arrhythmias [92]. The primary underlying cause of myocardial infarction is narrowing of the coronary arteries by atherosclerotic plaque and instability and disruption of the plaque. Myocardial infarction triggers an intense and complex inflammatory response that is responsible for cardiac repair and remodeling [27, 28, 93].
Inflammation plays not only a role in the pathophysiology of myocardial infarction in the acute stage, but also an important role in the determination of the extent of tissue injury and repair after myocardial infarction. Interventions targeted at inflammatory signals have been shown to reduce infarct size [2, 28, 43, 78, 112]. Inhibition of IL-1β activity using neutralizing antibodies and IL-1 receptor antagonist anakinra markedly reduced cardiac hypertrophy and myocardial dysfunction after acute myocardial infarction in mice [2]. Infusion of IL-10, an anti-inflammatory cytokine, limited cardiac fibrosis, improved cardiac remodeling and facilitated cardiac wound healing in the post-myocardial infarction mice via activating M2 macrophage polarization [43].
NLRP3 inflammasome recognizes various danger signals and induces sterile inflammatory responses and is regarded as a highly plausible candidate for pattern-recognition receptor in myocardial ischemia [12, 18, 94]. Mezzaroma et al. reported that inhibition of NLRP3 and the purinergic P2X7 receptor by siRNA or a pharmacological inhibitor prevented inflammasome activation and reduced cardiac cell death, resulting in alleviating cardiac remodeling after coronary artery ligation in wild-type mice [67]. VanHout et al. observed that NLRP3-inflammasome inhibitor MCC950 reduced infarct size and preserved cardiac function in a pig model of myocardial infarction induced by transluminal balloon occlusion, and indicated that NLRP3-inflammasome inhibition may have therapeutic potential in acute myocardial infarction patients [99].
Although successful reperfusion procedure improves the extent of myocardial injury, the reperfusion may also exacerbate injury to the myocardium, the so-called myocardial ischemia–reperfusion injury. Marchetti et al. reported that inhibition of the activity of NLRP3 reduced infarct size in a rat model of myocardial ischemia–reperfusion injury [64]. Liu et al. observed up-regulated expression of NLRP3, enhanced capase-1 activity and increased IL-1β and IL-18 in the heart in C57BL/6J mice subjected to myocardial ischemia–reperfusion [56]. In their studies, intramyocardial injection of NLRP3 siRNA or an intraperitoneal injection of inflammasome inhibitor BAY-11-7028 attenuated macrophage and neutrophil infiltration and decreased myocardial ischemia–reperfusion injury in the mouse hearts. Kawaguchi et al. established myocardial ischemia–reperfusion injury model induced by transient occlusion of the left anterior descending artery [45]. In this model, ASC expression was detected primarily in the infiltrating macrophages and neutrophils, and to some extent in vascular endothelial cells and cardiac resident fibroblasts. Compared with wild mice type, ASC−/− or caspase-1−/−mice exhibited a significant decrease in inflammatory cell infiltration and cytokine/chemokine expression. These mice also showed a significant reduction of infarct size, myocardial fibrosis and left ventricular dysfunction after myocardial infarction. These findings allow us to suggest that NLRP3 inflammasome may play a crucial role in the pathophysiology of myocardial ischemia–reperfusion injury as well as the extent of infarct following permanent coronary ligation. Of note, inflammatory responses occur more aggressively in the myocardial ischemia–reperfusion injury models than in permanent coronary ligation models [112].
Interesting questions have arisen during the period of the investigations on the NLRP3 inflammasome in different models of myocardial injury. Sandanger et al. reported that NLRP3 inflammasome was predominantly up-regulated in the cardiac fibroblasts in the ischemic myocardium hearts subjected to ligation of coronary artery [80]. These authors also described that myocardial dysfunction and injury in response to ischemia–reperfusion were improved in NLRP3−/− mice, but not in ASC−/− mice, suggesting that NLRP3 and ASC may have different roles in myocardial damage after ischemia–reperfusion. Inoue et al. also observed that hepatic ischemia–reperfusion injury was ameliorated in NLRP3−/− mice, but not in ASC−/− mice [41]. These findings collectively indicate that the inflammasome components, such as NLRP3 and ASC can also function independently and that NLRP3 and ASC may have cell-intrinsic roles in different cell types such as cardiac fibroblasts, cardiomyocytes and leukocytes. Thus, the contribution of NLRP3 and ASC should be determined in genetically modified animals in a tissue-specific manner. These findings will lead to a better understanding of disease progression and aid in designing new therapeutic strategies for the treatment of myocardial infarction.
NLRP3 inflammasome in cardiac remodeling
Cardiac remodeling is a process characterized by a series of changes (gradual cardiac enlargement and dysfunction associated with molecular alterations) in the structure and function of the myocardium that involves several cell types, such as cardiomyocytes, fibroblasts and endothelial cells, as well as extracellular matrix, capillary microcirculation and neuronal networks [34, 68]. It is also associated with changes in genome expression [16, 38]. Left ventricular remodeling has been considered an adaptive response to pressure overload (aortic stenosis and hypertension), volume overload (valvular regurgitation, ischemia) and other pathologies, such as myocardial infarction, myocarditis, dilated cardiomyopathy and valvular heart diseases, which gradually lead to progressive decompensation [91]. Li et al. demonstrated that caspase activation and recruitment domain 3 (CARD3) serve as a positive modulator of left ventricular remodeling and dysfunction after myocardial infarction via the regulation of the NF-κB and p38MAPK signaling, and CARD3 plays a key role in regulating caspase-1 apoptotic activity in several cell lines [53]. Hence there may be an association between cardiac remodeling and NLRP3 inflammasome because both CARD3 and caspase-1 are important components in the activation of NLRP3 inflammasome.
It has been estimated that despite successful reperfusion, appropriate pharmacotherapy and lifestyle modification, cardiac remodeling is very common, ultimately leading to heart failure and death [81]. Myocardial necrosis triggers the production of inflammatory cytokines, such as TNF-α, IL-1, IL-6, IL-18, IL-33, and C-reactive protein, that in a complex fashion modulate myocardial remodeling, decreased contractility and fibrosis [42]. An intense sterile inflammatory response occurring during myocardial remodeling is believed to be mediated at least in part through NLRP3 inflammasome [11, 107] (Fig. 3).
NLR3 inflammasome in cardiac remodeling. Reactive oxygen species (ROS), ATP and other dangerous signals including bacteria, viruses, crystals, environmental irritants, etc. are recognized by toll-like receptors (TLRs) or NOD-like receptors (NLRs), followed by the activation of NLRP3 inflammasome through lysosomal destabilization and rupture, potassium (K+) efflux, calcium (Ca2+) influx, macrophage endocytosis, endoplasmic reticulum (ER) stress and mitochondrial ROS (mtROS) generation. Activation of NLRP3 inflammasome induces bioactive IL-1β release from cardiac fibroblasts and pyroptosis in cardiomyocytes, resulting in cardiac inflammation and remodeling
NLRP3 inflammasome predominantly exists in cardiac fibroblasts which are one of the most abundant cell types within the human heart. An inappropriate activation of NLRP3 in cardiac fibroblasts can lead to myocardial dysfunction. Exogenous carbon monoxide (CO) derived from CO-releasing molecule-3 improved myocardial function in mice with sepsis by inhibiting NLRP3 inflammasome activation in cardiac fibroblasts [115]. Heme oxygenase-1 (HO-1) is responsible for the catalysis of heme degrade to CO which negatively regulates NLRP3 inflammation activation [35]. HO-1 deficiency leads to late left ventricular remodeling due to overactive and prolonged post-ischemic inflammatory response in mice [96]. It is well known that inducible nitric oxide (NO) synthase (iNOS) regulates the production of bioactive NO which is one of the critical negative regulators of NLRP3 inflammasome activation [63]. Kingery et al. showed that iNOS is highly expressed in both macrophages and cardiomyocytes in the failing human heart. Further they observed that iNOS−/−c HF mice displayed significant improvement in survival, left ventricular function, hypertrophy, fibrosis, and inflammatory activation [47]. They conclude that iNOS is responsible for systemic inflammatory activation and cardiac remodeling in ischemic HF. Mitochondria are involved in a variety of cellular life activities such as energy, signaling, cell proliferation, differentiation and apoptosis. MtDNA released by mitochondrial damage can bind NLRP3 to activate NLRP3 inflammasome via NF-κB pathway [85]. MtDNA causes mitochondrial dysfunction and death in cardiomyocytes via activating NF-κB which is dependent on toll-like receptor 9 [6].
Studies in the transverse abdominal aortic constriction (TAC)-induced hypertension-complicated left ventricular remodeling mouse model, pirfenidone, an antifibrotic small-molecular-size drug with anti-inflammatory properties, reduced protein levels of ROS and transforming growth factor-β1 (TGF-β1), suppressed NLRP3 inflammasome formation and attenuated the expression of IL-1β and IL-1β-induced inflammatory and profibrotic responses [106]. In the high fat diet and low dose streptozotocin-induced T2D rats, which develop dilated cardiomyopathy, NLRP3 gene silencing therapy ameliorate cardiac inflammation, pyroptosis, fibrosis and cardiac dysfunction [59]. NLRP3 inflammasome signaling intervention has also been shown to reduce infarct size and preserve cardiac function post-myocardial infarction in several experimental animal models [100].
Summary
In conclusion, NLRP3 inflammasome plays an important role in the pathogenesis of cardiovascular disease risk factors, hypertension and diabetes, as well as the development of atherosclerosis and myocardial infarction following coronary occlusion. NLRP3 inflammasome and IL-1β activity seem to regulate PAMPs and DAMPs during infection, injury, inflammation or stress. Excess IL-1β activity contributes to a number of inflammatory disorders and its inhibition seems to reduce cardiovascular pathology (Fig. 4). Understanding the molecular mechanisms of NLRP3 inflammasome assembly and activation may lead to novel therapeutic targets to formulate safe therapies in the treatment of cardiovascular diseases.
A summary of NLRP3 inflammasome and cardiovascular diseases. The increase in ROS, cholesterol crystals, PAMPs, and DAMPs cause the activation of NLRP3 inflammasome, which further contribute to the pathogenesis of risk factors, such as hypertension and diabetes, and their consequences—atherosclerosis and myocardial infarction. The genesis of hypertension, diabetes, atherosclerosis and myocardial infarction further exacerbates NLRP3 inflammasome activation. Finally, the excess activation of NLRP3 inflammasome results in inflammation and cardiovascular pathology, including cell injury/death, hypertrophy and fibrosis. The inhibition of NLRP3 inflammasome activation seems to modify cardiovascular pathology. ROS reactive oxygen species, PAMPs pathogen-associated molecular pattern molecules, DAMPs danger/damage-associated molecular pattern molecules
References
Abais JM, Xia M, Zhang Y, Boini KM, Li PL (2015) Redox regulation of NLRP3 inflammasomes: ROS as trigger or effector? Antioxid Redox Signal 22:1111–1129. https://doi.org/10.1089/ars.2014.5994
Abbate A, Van Tassell BW, Seropian IM, Toldo S, Robati R, Varma A, Salloum FN, Smithson L, Dinarello CA (2010) Interleukin-1beta modulation using a genetically engineered antibody prevents adverse cardiac remodelling following acute myocardial infarction in the mouse. Eur J Heart Fail 12:319–322. https://doi.org/10.1093/eurjhf/hfq017
Abderrazak A, Syrovets T, Couchie D, El HK, Friguet B, Simmet T, Rouis M (2015) NLRP3 inflammasome: from a danger signal sensor to a regulatory node of oxidative stress and inflammatory diseases. Redox Biol 4:296–307. https://doi.org/10.1016/j.redox.2015.01.008
Abderrazak A, Couchie D, Mahmood DF, Elhage R, Vindis C, Laffargue M, Mateo V, Buchele B, Ayala MR, El GM, Syrovets T, Slimane MN, Friguet B, Fulop T, Simmet T, El HK, Rouis M (2015) Anti-inflammatory and antiatherogenic effects of the NLRP3 inflammasome inhibitor arglabin in ApoE2.Ki mice fed a high-fat diet. Circulation 131:1061–1070. https://doi.org/10.1161/CIRCULATIONAHA.114.013730
Barry E, Roberts S, Oke J, Vijayaraghavan S, Normansell R, Greenhalgh T (2017) Efficacy and effectiveness of screen and treat policies in prevention of type 2 diabetes: systematic review and meta-analysis of screening tests and interventions. BMJ 356:i6538. https://doi.org/10.1136/bmj.i6538
Bliksoen M, Mariero LH, Torp MK, Baysa A, Ytrehus K, Haugen F, Seljeflot I, Vaage J, Valen G, Stenslokken KO (2016) Extracellular mtDNA activates NF-kappaB via toll-like receptor 9 and induces cell death in cardiomyocytes. Basic Res Cardiol 111:42. https://doi.org/10.1007/s00395-016-0553-6
Boni-Schnetzler M, Thorne J, Parnaud G, Marselli L, Ehses JA, Kerr-Conte J, Pattou F, Halban PA, Weir GC, Donath MY (2008) Increased interleukin (IL)-1beta messenger ribonucleic acid expression in beta -cells of individuals with type 2 diabetes and regulation of IL-1beta in human islets by glucose and autostimulation. J Clin Endocrinol Metab 93:4065–4074. https://doi.org/10.1210/jc.2008-0396
Broz P, Dixit VM (2016) Inflammasomes: mechanism of assembly, regulation and signalling. Nat Rev Immunol 16:407–420. https://doi.org/10.1038/nri.2016.58
Bruder-Nascimento T, Ferreira NS, Zanotto CZ, Ramalho F, Pequeno IO, Olivon VC, Neves KB, Alves-Lopes R, Campos E, Silva CA, Fazan R, Carlos D, Mestriner FL, Prado D, Pereira FV, Braga T, Luiz JP, Cau SB, Elias PC, Moreira AC, Camara NO, Zamboni DS, Alves-Filho JC, Tostes RC (2016) NLRP3 inflammasome mediates aldosterone-induced vascular damage. Circulation 134:1866–1880. https://doi.org/10.1161/CIRCULATIONAHA.116.024369
Bullon P, Cano-Garcia FJ, Alcocer-Gomez E, Varela-Lopez A, Roman-Malo L, Ruiz-Salmeron RJ, Quiles JL, Navarro-Pando JM, Battino M, Ruiz-Cabello J, Jimenez-Borreguero LJ, Cordero MD (2017) Could NLRP3-inflammasome be a cardiovascular risk biomarker in acute myocardial infarction patients? Antioxid Redox Signal. https://doi.org/10.1089/ars.2016.6970
Cassel SL, Sutterwala FS (2010) Sterile inflammatory responses mediated by the NLRP3 inflammasome. Eur J Immunol 40:607–611. https://doi.org/10.1002/eji.200940207
Chen GY, Nunez G (2010) Sterile inflammation: sensing and reacting to damage. Nat Rev Immunol 10:826–837. https://doi.org/10.1038/nri2873
Chen W, Zhao M, Zhao S, Lu Q, Ni L, Zou C, Lu L, Xu X, Guan H, Zheng Z, Qiu Q (2016) Activation of the TXNIP/NLRP3 inflammasome pathway contributes to inflammation in diabetic retinopathy: a novel inhibitory effect of minocycline. Inflamm Res 66:157–166. https://doi.org/10.1007/s00011-016-1002-6
Clark A, Jones LC, de Koning E, Hansen BC, Matthews DR (2001) Decreased insulin secretion in type 2 diabetes: a problem of cellular mass or function? Diabetes 50(Suppl 1):S169–S171
Cochain C, Zernecke A (2016) Protective and pathogenic roles of CD8+ T cells in atherosclerosis. Basic Res Cardiol 111:71. https://doi.org/10.1007/s00395-016-0589-7
Cohn JN, Ferrari R, Sharpe N (2000) Cardiac remodeling-concepts and clinical implications: a consensus paper from an international forum on cardiac remodeling. Behalf of an International Forum on Cardiac Remodeling. J Am Coll Cardiol 35:569–582. https://doi.org/10.1016/s0735-1097(99)00630-0
Coll RC, Robertson AA, Chae JJ, Higgins SC, Munoz-Planillo R, Inserra MC, Vetter I, Dungan LS, Monks BG, Stutz A, Croker DE, Butler MS, Haneklaus M, Sutton CE, Nunez G, Latz E, Kastner DL, Mills KH, Masters SL, Schroder K, Cooper MA, O’Neill LA (2015) A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases. Nat Med 21:248–255. https://doi.org/10.1038/nm.3806
Davis BK, Wen H, Ting JP (2011) The inflammasome NLRs in immunity, inflammation, and associated diseases. Annu Rev Immunol 29:707–735. https://doi.org/10.1146/annurev-immunol-031210-101405
Dinarello CA, van der Meer JW (2013) Treating inflammation by blocking interleukin-1 in humans. Semin Immunol 25:469–484. https://doi.org/10.1016/j.smim.2013.10.008
Dinarello CA, Donath MY, Mandrup-Poulsen T (2010) Role of IL-1beta in type 2 diabetes. Curr Opin Endocrinol Diabetes Obes 17:314–321. https://doi.org/10.1097/MED.0b013e32833bf6dc
Ding Z, Liu S, Wang X, Dai Y, Khaidakov M, Deng X, Fan Y, Xiang D, Mehta JL (2014) LOX-1, mtDNA damage, and NLRP3 inflammasome activation in macrophages: implications in atherogenesis. Cardiovasc Res 103:619–628. https://doi.org/10.1093/cvr/cvu114
Donath MY, Shoelson SE (2011) Type 2 diabetes as an inflammatory disease. Nat Rev Immunol 11:98–107. https://doi.org/10.1038/nri2925
Duewell P, Kono H, Rayner KJ, Sirois CM, Vladimer G, Bauernfeind FG, Abela GS, Franchi L, Nunez G, Schnurr M, Espevik T, Lien E, Fitzgerald KA, Rock KL, Moore KJ, Wright SD, Hornung V, Latz E (2010) NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature 464:1357–1361. https://doi.org/10.1038/nature08938
Elliott EI, Sutterwala FS (2015) Initiation and perpetuation of NLRP3 inflammasome activation and assembly. Immunol Rev 265:35–52. https://doi.org/10.1111/imr.12286
Feng H, Gu J, Gou F, Huang W, Gao C, Chen G, Long Y, Zhou X, Yang M, Liu S, Lu S, Luo Q, Xu Y (2016) High glucose and lipopolysaccharide prime NLRP3 inflammasome via ROS/TXNIP pathway in mesangial cells. J Diabetes Res 2016:6973175. https://doi.org/10.1155/2016/6973175
Franchi L, Nunez G (2012) Immunology. Orchestrating inflammasomes. Science 337:1299–1300. https://doi.org/10.1126/science.1229010
Frangogiannis NG (2014) The inflammatory response in myocardial injury, repair, and remodelling. Nat Rev Cardiol 11:255–265. https://doi.org/10.1038/nrcardio.2014.28
Frangogiannis NG, Smith CW, Entman ML (2002) The inflammatory response in myocardial infarction. Cardiovasc Res 53:31–47. https://doi.org/10.1016/S0008-6363(01)00434-5
Freigang S, Ampenberger F, Spohn G, Heer S, Shamshiev AT, Kisielow J, Hersberger M, Yamamoto M, Bachmann MF, Kopf M (2011) Nrf2 is essential for cholesterol crystal-induced inflammasome activation and exacerbation of atherosclerosis. Eur J Immunol 41:2040–2051. https://doi.org/10.1002/eji.201041316
Fujita T (2014) Mechanism of salt-sensitive hypertension: focus on adrenal and sympathetic nervous systems. J Am Soc Nephrol 25:1148–1155. https://doi.org/10.1681/ASN.2013121258
Gabay C, Lamacchia C, Palmer G (2010) IL-1 pathways in inflammation and human diseases. Nat Rev Rheumatol 6:232–241. https://doi.org/10.1038/nrrheum.2010.4
Galkina E, Ley K (2009) Immune and inflammatory mechanisms of atherosclerosis. Annu Rev Immunol 27:165–197. https://doi.org/10.1146/annurev.immunol.021908.132620
Gan W, Ren J, Li T, Lv S, Chenghe L, Liu Z, Yang M (2017) The SGK1 inhibitor EMD638683, prevents Angiotensin II-induced cardiac inflammation and fibrosis by blocking NLRP3 inflammasome activation. Biochim Biophys Acta 1864:1–10. https://doi.org/10.1016/j.bbadis.2017.10.001
Gjesdal O, Bluemke DA, Lima JA (2011) Cardiac remodeling at the population level—risk factors, screening, and outcomes. Nat Rev Cardiol 8:673–685. https://doi.org/10.1038/nrcardio.2011.154
Gomperts E, Belcher JD, Otterbein LE, Coates TD, Wood J, Skolnick BE, Levy H, Vercellotti GM (2017) The role of carbon monoxide and heme oxygenase in the prevention of sickle cell disease vaso-occlusive crises. Am J Hematol 92:569–582. https://doi.org/10.1002/ajh.24750
Hansson GK, Hermansson A (2011) The immune system in atherosclerosis. Nat Immunol 12:204–212. https://doi.org/10.1038/ni.2001
Heid ME, Keyel PA, Kamga C, Shiva S, Watkins SC, Salter RD (2013) Mitochondrial reactive oxygen species induces NLRP3-dependent lysosomal damage and inflammasome activation. J Immunol 191:5230–5238. https://doi.org/10.4049/jimmunol.1301490
Heusch G, Libby P, Gersh B, Yellon D, Bohm M, Lopaschuk G, Opie L (2014) Cardiovascular remodelling in coronary artery disease and heart failure. Lancet 383:1933–1943. https://doi.org/10.1016/S0140-6736(14)60107-0
Hoseini Z, Sepahvand F, Rashidi B, Sahebkar A, Masoudifar A, Mirzaei H (2017) NLRP3 inflammasome: its regulation and involvement in atherosclerosis. J Cell Physiol. https://doi.org/10.1002/jcp.25930
Hui ST, Andres AM, Miller AK, Spann NJ, Potter DW, Post NM, Chen AZ, Sachithanantham S, Jung DY, Kim JK, Davis RA (2008) Txnip balances metabolic and growth signaling via PTEN disulfide reduction. Proc Natl Acad Sci USA 105:3921–3926. https://doi.org/10.1073/pnas.0800293105
Inoue Y, Yasuda Y, Takahashi M (2013) Role of the inflammasome in inflammatory responses and subsequent injury after hepatic ischemia-reperfusion injury. Hepatology 58:2212. https://doi.org/10.1002/hep.26480
Jahng JW, Song E, Sweeney G (2016) Crosstalk between the heart and peripheral organs in heart failure. Exp Mol Med 48:e217. https://doi.org/10.1038/emm.2016.20
Jung M, Ma Y, Iyer RP, DeLeon-Pennell KY, Yabluchanskiy A, Garrett MR, Lindsey ML (2017) IL-10 improves cardiac remodeling after myocardial infarction by stimulating M2 macrophage polarization and fibroblast activation. Basic Res Cardiol 112:33. https://doi.org/10.1007/s00395-017-0622-5
Kamari Y, Shaish A, Shemesh S, Vax E, Grosskopf I, Dotan S, White M, Voronov E, Dinarello CA, Apte RN, Harats D (2011) Reduced atherosclerosis and inflammatory cytokines in apolipoprotein-E-deficient mice lacking bone marrow-derived interleukin-1alpha. Biochem Biophys Res Commun 405:197–203. https://doi.org/10.1016/j.bbrc.2011.01.008
Kawaguchi M, Takahashi M, Hata T, Kashima Y, Usui F, Morimoto H, Izawa A, Takahashi Y, Masumoto J, Koyama J, Hongo M, Noda T, Nakayama J, Sagara J, Taniguchi S, Ikeda U (2011) Inflammasome activation of cardiac fibroblasts is essential for myocardial ischemia/reperfusion injury. Circulation 123:594–604. https://doi.org/10.1161/CIRCULATIONAHA.110.982777
Kim Y, Wang W, Okla M, Kang I, Moreau R, Chung S (2016) Suppression of NLRP3 inflammasome by gamma-tocotrienol ameliorates type 2 diabetes. J Lipid Res 57:66–76. https://doi.org/10.1194/jlr.M062828
Kingery JR, Hamid T, Lewis RK, Ismahil MA, Bansal SS, Rokosh G, Townes TM, Ildstad ST, Jones SP, Prabhu SD (2017) Leukocyte iNOS is required for inflammation and pathological remodeling in ischemic heart failure. Basic Res Cardiol 112:19. https://doi.org/10.1007/s00395-017-0609-2
Koenen TB, Stienstra R, van Tits LJ, de Graaf J, Stalenhoef AF, Joosten LA, Tack CJ, Netea MG (2011) Hyperglycemia activates caspase-1 and TXNIP-mediated IL-1beta transcription in human adipose tissue. Diabetes 60:517–524. https://doi.org/10.2337/db10-0266
Krishnan SM, Sobey CG, Latz E, Mansell A, Drummond GR (2014) IL-1beta and IL-18: inflammatory markers or mediators of hypertension? Br J Pharmacol 171:5589–5602. https://doi.org/10.1111/bph.12876
Lamkanfi M, Dixit VM (2014) Mechanisms and functions of inflammasomes. Cell 157:1013–1022. https://doi.org/10.1016/j.cell.2014.04.007
Larsen CM, Faulenbach M, Vaag A, Volund A, Ehses JA, Seifert B, Mandrup-Poulsen T, Donath MY (2007) Interleukin-1-receptor antagonist in type 2 diabetes mellitus. N Engl J Med 356:1517–1526. https://doi.org/10.1056/NEJMoa065213
Lee BC, Lee J (2014) Cellular and molecular players in adipose tissue inflammation in the development of obesity-induced insulin resistance. Biochim Biophys Acta 1842:446–462. https://doi.org/10.1016/j.bbadis.2013.05.017
Li L, Wang X, Chen W, Qi H, Jiang DS, Huang L, Huang F, Wang L, Li H, Chen X (2015) Regulatory role of CARD3 in left ventricular remodelling and dysfunction after myocardial infarction. Basic Res Cardiol 110:56. https://doi.org/10.1007/s00395-015-0515-4
Lin L, Phillips WE, Manning RD (2009) Intrarenal Angiotensin ii is associated with inflammation, renal damage and dysfunction in dahl salt-sensitive hypertension. J Am Soc Hypertens 3:306–314. https://doi.org/10.1016/j.jash.2009.08.002
Liu SS, Ding Y, Lou JQ (2014) NLRP3, a potential therapeutic target for type 2 diabetes? Cardiovasc Drugs Ther 28:391–392. https://doi.org/10.1007/s10557-014-6531-z
Liu Y, Lian K, Zhang L, Wang R, Yi F, Gao C, Xin C, Zhu D, Li Y, Yan W, Xiong L, Gao E, Wang H, Tao L (2014) TXNIP mediates NLRP3 inflammasome activation in cardiac microvascular endothelial cells as a novel mechanism in myocardial ischemia/reperfusion injury. Basic Res Cardiol 109:415. https://doi.org/10.1007/s00395-014-0415-z
Lu J, Mitra S, Wang X, Khaidakov M, Mehta JL (2011) Oxidative stress and lectin-like ox-LDL-receptor LOX-1 in atherogenesis and tumorigenesis. Antioxid Redox Signal 15:2301–2333. https://doi.org/10.1089/ars.2010.3792
Lu X, Kakkar V (2014) Inflammasome and atherogenesis. Curr Pharm Des 20:108–124. https://doi.org/10.2174/13816128113199990586
Luo B, Li B, Wang W, Liu X, Xia Y, Zhang C, Zhang M, Zhang Y, An F (2014) NLRP3 gene silencing ameliorates diabetic cardiomyopathy in a type 2 diabetes rat model. PLoS One 9:e104771. https://doi.org/10.1371/journal.pone.0104771
Luzardo L, Noboa O, Boggia J (2015) Mechanisms of salt-sensitive hypertension. Curr Hypertens Rev 11:14–21. https://doi.org/10.2174/1573402111666150530204136
Maedler K, Dharmadhikari G, Schumann DM, Storling J (2009) Interleukin-1 beta targeted therapy for type 2 diabetes. Expert Opin Biol Ther 9:1177–1188. https://doi.org/10.1517/14712590903136688
Maedler K, Schumann DM, Sauter N, Ellingsgaard H, Bosco D, Baertschiger R, Iwakura Y, Oberholzer J, Wollheim CB, Gauthier BR, Donath MY (2006) Low concentration of interleukin-1beta induces FLICE-inhibitory protein-mediated beta-cell proliferation in human pancreatic islets. Diabetes 55:2713–2722. https://doi.org/10.2337/db05-1430
Mao K, Chen S, Chen M, Ma Y, Wang Y, Huang B, He Z, Zeng Y, Hu Y, Sun S, Li J, Wu X, Wang X, Strober W, Chen C, Meng G, Sun B (2013) Nitric oxide suppresses NLRP3 inflammasome activation and protects against LPS-induced septic shock. Cell Res 23:201–212. https://doi.org/10.1038/cr.2013.6
Marchetti C, Chojnacki J, Toldo S, Mezzaroma E, Tranchida N, Rose SW, Federici M, Van Tassell BW, Zhang S, Abbate A (2014) A novel pharmacologic inhibitor of the NLRP3 inflammasome limits myocardial injury after ischemia-reperfusion in the mouse. J Cardiovasc Pharmacol 63:316–322. https://doi.org/10.1097/FJC.0000000000000053
Menu P, Vince JE (2011) The NLRP3 inflammasome in health and disease: the good, the bad and the ugly. Clin Exp Immunol 166:1–15. https://doi.org/10.1111/j.1365-2249.2011.04440.x
Menu P, Pellegrin M, Aubert JF, Bouzourene K, Tardivel A, Mazzolai L, Tschopp J (2011) Atherosclerosis in ApoE-deficient mice progresses independently of the NLRP3 inflammasome. Cell Death Dis 2:e137. https://doi.org/10.1038/cddis.2011.18
Mezzaroma E, Toldo S, Farkas D, Seropian IM, Van Tassell BW, Salloum FN, Kannan HR, Menna AC, Voelkel NF, Abbate A (2011) The inflammasome promotes adverse cardiac remodeling following acute myocardial infarction in the mouse. Proc Natl Acad Sci USA 108:19725–19730. https://doi.org/10.1073/pnas.1108586108
Nadruz W (2015) Myocardial remodeling in hypertension. J Hum Hypertens 29:1–6. https://doi.org/10.1038/jhh.2014.36
Paramel VG, Folkersen L, Strawbridge RJ, Halvorsen B, Yndestad A, Ranheim T, Krohg-Sorensen K, Skjelland M, Espevik T, Aukrust P, Lengquist M, Hedin U, Jansson JH, Fransen K, Hansson GK, Eriksson P, Sirsjo A (2016) NLRP3 inflammasome expression and activation in human atherosclerosis. J Am Heart Assoc. https://doi.org/10.1161/JAHA.115.003031
Parikh H, Carlsson E, Chutkow WA, Johansson LE, Storgaard H, Poulsen P, Saxena R, Ladd C, Schulze PC, Mazzini MJ, Jensen CB, Krook A, Bjornholm M, Tornqvist H, Zierath JR, Ridderstrale M, Altshuler D, Lee RT, Vaag A, Groop LC, Mootha VK (2007) TXNIP regulates peripheral glucose metabolism in humans. PLoS Med 4:e158. https://doi.org/10.1371/journal.pmed.0040158
Peng K, Liu L, Wei D, Lv Y, Wang G, Xiong W, Wang X, Altaf A, Wang L, He D, Wang H, Qu P (2015) P2X7R is involved in the progression of atherosclerosis by promoting NLRP3 inflammasome activation. Int J Mol Med 35:1179–1188. https://doi.org/10.3892/ijmm.2015.2129
Perrone L, Devi TS, Hosoya KI, Terasaki T, Singh LP (2010) Inhibition of TXNIP expression in vivo blocks early pathologies of diabetic retinopathy. Cell Death Dis 1:e65. https://doi.org/10.1038/cddis.2010.42
Pothineni N, Karathanasis SK, Ding Z, Arulandu A, Varughese KI, Mehta JL (2017) LOX-1 in atherosclerosis and myocardial ischemia: biology, genetics, and modulation. J Am Coll Cardiol 69:2759–2768. https://doi.org/10.1016/j.jacc.2017.04.010
Poulter NR, Prabhakaran D, Caulfield M (2015) Hypertension. Lancet 386:801–812. https://doi.org/10.1016/S0140-6736(14)61468-9
Qi J, Yu XJ, Shi XL, Gao HL, Yi QY, Tan H, Fan XY, Zhang Y, Song XA, Cui W, Liu JJ, Kang YM (2016) NF-kappaB blockade in hypothalamic paraventricular nucleus inhibits high-salt-induced hypertension through NLRP3 and caspase-1. Cardiovasc Toxicol 16:345–354. https://doi.org/10.1007/s12012-015-9344-9
Qi J, Zhao XF, Yu XJ, Yi QY, Shi XL, Tan H, Fan XY, Gao HL, Yue LY, Feng ZP, Kang YM (2016) Targeting interleukin-1 beta to suppress sympathoexcitation in hypothalamic paraventricular nucleus in Dahl salt-sensitive hypertensive rats. Cardiovasc Toxicol 16:298–306. https://doi.org/10.1007/s12012-015-9338-7
Rajamaki K, Lappalainen J, Oorni K, Valimaki E, Matikainen S, Kovanen PT, Eklund KK (2010) Cholesterol crystals activate the NLRP3 inflammasome in human macrophages: a novel link between cholesterol metabolism and inflammation. PLoS One 5:e11765. https://doi.org/10.1371/journal.pone.0011765
Rienks M, Carai P, Bitsch N, Schellings M, Vanhaverbeke M, Verjans J, Cuijpers I, Heymans S, Papageorgiou A (2017) Sema3A promotes the resolution of cardiac inflammation after myocardial infarction. Basic Res Cardiol 112:42. https://doi.org/10.1007/s00395-017-0630-5
Rust P, Ekmekcioglu C (2017) Impact of salt intake on the pathogenesis and treatment of hypertension. Adv Exp Med Biol 956:61–84. https://doi.org/10.1007/5584_2016_147
Sandanger O, Ranheim T, Vinge LE, Bliksoen M, Alfsnes K, Finsen AV, Dahl CP, Askevold ET, Florholmen G, Christensen G, Fitzgerald KA, Lien E, Valen G, Espevik T, Aukrust P, Yndestad A (2013) The NLRP3 inflammasome is up-regulated in cardiac fibroblasts and mediates myocardial ischaemia-reperfusion injury. Cardiovasc Res 99:164–174. https://doi.org/10.1093/cvr/cvt091
Savoye C, Equine O, Tricot O, Nugue O, Segrestin B, Sautiere K, Elkohen M, Pretorian EM, Taghipour K, Philias A, Aumegeat V, Decoulx E, Ennezat PV, Bauters C (2006) Left ventricular remodeling after anterior wall acute myocardial infarction in modern clinical practice (from the REmodelage VEntriculaire [REVE] study group). Am J Cardiol 98:1144–1149. https://doi.org/10.1016/j.amjcard.2006.06.011
Schroder K, Zhou R, Tschopp J (2010) The NLRP3 inflammasome: a sensor for metabolic danger? Science 327:296–300. https://doi.org/10.1126/science.1184003
Sharma AA, Jen R, Kan B, Sharma A, Marchant E, Tang A, Gadawski I, Senger C, Skoll A, Turvey SE, Sly LM, Cote HC, Lavoie PM (2015) Impaired NLRP3 inflammasome activity during fetal development regulates IL-1beta production in human monocytes. Eur J Immunol 45:238–249. https://doi.org/10.1002/eji.201444707
Shi Y, Hu FB (2014) The global implications of diabetes and cancer. Lancet 383:1947–1948. https://doi.org/10.1016/S0140-6736(14)60886-2
Shimada K, Crother TR, Karlin J, Dagvadorj J, Chiba N, Chen S, Ramanujan VK, Wolf AJ, Vergnes L, Ojcius DM, Rentsendorj A, Vargas M, Guerrero C, Wang Y, Fitzgerald KA, Underhill DM, Town T, Arditi M (2012) Oxidized mitochondrial DNA activates the NLRP3 inflammasome during apoptosis. Immunity 36:401–414. https://doi.org/10.1016/j.immuni.2012.01.009
Sidhu RK (2016) Association between acute myocardial infarction and periodontitis: a review of the literature. J Int Acad Periodontol 18:23–33
Stanley TL, Zanni MV, Johnsen S, Rasheed S, Makimura H, Lee H, Khor VK, Ahima RS, Grinspoon SK (2011) TNF-alpha antagonism with etanercept decreases glucose and increases the proportion of high molecular weight adiponectin in obese subjects with features of the metabolic syndrome. J Clin Endocrinol Metab 96:E146–E150. https://doi.org/10.1210/jc.2010-1170
Stienstra R, Joosten LA, Koenen T, van Tits B, van Diepen JA, van den Berg SA, Rensen PC, Voshol PJ, Fantuzzi G, Hijmans A, Kersten S, Muller M, van den Berg WB, van Rooijen N, Wabitsch M, Kullberg BJ, van der Meer JW, Kanneganti T, Tack CJ, Netea MG (2010) The inflammasome-mediated caspase-1 activation controls adipocyte differentiation and insulin sensitivity. Cell Metab 12:593–605. https://doi.org/10.1016/j.cmet.2010.11.011
Stutz A, Kolbe CC, Stahl R, Horvath GL, Franklin BS, van Ray O, Brinkschulte R, Geyer M, Meissner F, Latz E (2017) NLRP3 inflammasome assembly is regulated by phosphorylation of the pyrin domain. J Exp Med 214:1725–1736. https://doi.org/10.1084/jem.20160933
Su Q, Qin DN, Wang FX, Ren J, Li HB, Zhang M, Yang Q, Miao YW, Yu XJ, Qi J, Zhu Z, Zhu GQ, Kang YM (2014) Inhibition of reactive oxygen species in hypothalamic paraventricular nucleus attenuates the renin–angiotensin system and proinflammatory cytokines in hypertension. Toxicol Appl Pharmacol 276:115–120. https://doi.org/10.1016/j.taap.2014.02.002
Sutton MG, Sharpe N (2000) Left ventricular remodeling after myocardial infarction: pathophysiology and therapy. Circulation 101:2981–2988. https://doi.org/10.1161/01.CIR.101.25.2981
Takahashi M (2014) NLRP3 inflammasome as a novel player in myocardial infarction. Int Heart J 55:101–105. https://doi.org/10.1536/ihj.13-388
Takahashi M (2010) Role of the SDF-1/CXCR4 system in myocardial infarction. Circ J 74:418–423. https://doi.org/10.1253/circj.cj-09-1021
Takahashi M (2011) Role of the inflammasome in myocardial infarction. Trends Cardiovasc Med 21:37–41. https://doi.org/10.1016/j.tcm.2012.02.002
Toldo S, Mezzaroma E, Mauro AG, Salloum F, Van Tassell BW, Abbate A (2015) The inflammasome in myocardial injury and cardiac remodeling. Antioxid Redox Signal 22:1146–1161. https://doi.org/10.1089/ars.2014.5989
Tomczyk M, Kraszewska I, Szade K, Bukowska-Strakova K, Meloni M, Jozkowicz A, Dulak J, Jazwa A (2017) Splenic Ly6Chi monocytes contribute to adverse late post-ischemic left ventricular remodeling in heme oxygenase-1 deficient mice. Basic Res Cardiol 112:39. https://doi.org/10.1007/s00395-017-0629-y
Tumurkhuu G, Shimada K, Dagvadorj J, Crother TR, Zhang W, Luthringer D, Gottlieb RA, Chen S, Arditi M (2016) Ogg1-dependent DNA repair regulates NLRP3 inflammasome and prevents atherosclerosis. Circ Res 119:e76–e90. https://doi.org/10.1161/CIRCRESAHA.116.308362
van Greevenbroek MM, Vermeulen VM, Feskens EJ, Evelo CT, Kruijshoop M, Hoebee B, van der Kallen CJ, de Bruin TW (2007) Genetic variation in thioredoxin interacting protein (TXNIP) is associated with hypertriglyceridaemia and blood pressure in diabetes mellitus. Diabet Med 24:498–504. https://doi.org/10.1111/j.1464-5491.2007.02109.x
van Hout GP, Bosch L, Ellenbroek GH, de Haan JJ, van Solinge WW, Cooper MA, Arslan F, de Jager SC, Robertson AA, Pasterkamp G, Hoefer IE (2016) The selective NLRP3-inflammasome inhibitor MCC950 reduces infarct size and preserves cardiac function in a pig model of myocardial infarction. Eur Heart J. https://doi.org/10.1093/eurheartj/ehw247
van Hout GP, Arslan F, Pasterkamp G, Hoefer IE (2016) Targeting danger-associated molecular patterns after myocardial infarction. Expert Opin Ther Targets 20:223–239. https://doi.org/10.1517/14728222.2016.1088005
Van Tassell BW, Toldo S, Mezzaroma E, Abbate A (2013) Targeting interleukin-1 in heart disease. Circulation 128:1910–1923. https://doi.org/10.1161/CIRCULATIONAHA.113.003199
Vanaja SK, Rathinam VA, Fitzgerald KA (2015) Mechanisms of inflammasome activation: recent advances and novel insights. Trends Cell Biol 25:308–315. https://doi.org/10.1016/j.tcb.2014.12.009
Vandanmagsar B, Youm YH, Ravussin A, Galgani JE, Stadler K, Mynatt RL, Ravussin E, Stephens JM, Dixit VD (2011) The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat Med 17:179–188. https://doi.org/10.1038/nm.2279
Wada J, Makino H (2016) Innate immunity in diabetes and diabetic nephropathy. Nat Rev Nephrol 12:13–26. https://doi.org/10.1038/nrneph.2015.175
Wang X, Phillips MI, Mehta JL (2011) LOX-1 and angiotensin receptors, and their interplay. Cardiovasc Drugs Ther 25:401–417. https://doi.org/10.1007/s10557-011-6331-7
Wang Y, Wu Y, Chen J, Zhao S, Li H (2013) Pirfenidone attenuates cardiac fibrosis in a mouse model of TAC-induced left ventricular remodeling by suppressing NLRP3 inflammasome formation. Cardiology 126:1–11. https://doi.org/10.1159/000351179
Westman PC, Lipinski MJ, Luger D, Waksman R, Bonow RO, Wu E, Epstein SE (2016) Inflammation as a driver of adverse left ventricular remodeling after acute myocardial infarction. J Am Coll Cardiol 67:2050–2060. https://doi.org/10.1016/j.jacc.2016.01.073
WHO (2017) World Health Organization diabetes fact sheet. http://www.who.int/mediacentre/factsheets/fs312/en/. Accessed July 2017
WHO (2016) World Health Organization, global report on diabetes. Geneva. http://who.int/diabetes/global-report/en/. Accessed April 2016. ISBN: 978 92 4 156525 7 (NLM classification: WK 810)
Wild S, Roglic G, Green A, Sicree R, King H (2004) Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 27:1047–1053. https://doi.org/10.2337/diacare.27.10.2568
Yajima N, Takahashi M, Morimoto H, Shiba Y, Takahashi Y, Masumoto J, Ise H, Sagara J, Nakayama J, Taniguchi S, Ikeda U (2008) Critical role of bone marrow apoptosis-associated speck-like protein, an inflammasome adaptor molecule, in neointimal formation after vascular injury in mice. Circulation 117:3079–3087. https://doi.org/10.1161/CIRCULATIONAHA.107.746453
Yellon DM, Hausenloy DJ (2007) Myocardial reperfusion injury. N Engl J Med 357:1121–1135. https://doi.org/10.1056/NEJMra071667
Youm YH, Adijiang A, Vandanmagsar B, Burk D, Ravussin A, Dixit VD (2011) Elimination of the NLRP3-ASC inflammasome protects against chronic obesity-induced pancreatic damage. Endocrinology 152:4039–4045. https://doi.org/10.1210/en.2011-1326
Yu XJ, Zhang DM, Jia LL, Qi J, Song XA, Tan H, Cui W, Chen W, Zhu GQ, Qin DN, Kang YM (2015) Inhibition of NF-kappaB activity in the hypothalamic paraventricular nucleus attenuates hypertension and cardiac hypertrophy by modulating cytokines and attenuating oxidative stress. Toxicol Appl Pharmacol 284:315–322. https://doi.org/10.1016/j.taap.2015.02.023
Zhang W, Tao A, Lan T, Cepinskas G, Kao R, Martin CM, Rui T (2017) Carbon monoxide releasing molecule-3 improves myocardial function in mice with sepsis by inhibiting NLRP3 inflammasome activation in cardiac fibroblasts. Basic Res Cardiol 112:16. https://doi.org/10.1007/s00395-017-0603-8
Zheng F, Xing S, Gong Z, Mu W, Xing Q (2014) Silence of NLRP3 suppresses atherosclerosis and stabilizes plaques in apolipoprotein E-deficient mice. Mediat Inflamm 2014:507208. https://doi.org/10.1155/2014/507208
Zheng F, Xing S, Gong Z, Xing Q (2013) NLRP3 inflammasomes show high expression in aorta of patients with atherosclerosis. Heart Lung Circ 22:746–750. https://doi.org/10.1016/j.hlc.2013.01.012
Zhou R, Yazdi AS, Menu P, Tschopp J (2011) A role for mitochondria in NLRP3 inflammasome activation. Nature 469:221–225. https://doi.org/10.1038/nature09663
Acknowledgements
This work was supported by the National Natural Science Foundation of China (no. 81370428), research grant of Xinxiang Medical University (nos. 300/505186 and 300/505233), and the Department of Veterans Affairs, Washington, DC.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Liu, D., Zeng, X., Li, X. et al. Role of NLRP3 inflammasome in the pathogenesis of cardiovascular diseases. Basic Res Cardiol 113, 5 (2018). https://doi.org/10.1007/s00395-017-0663-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00395-017-0663-9