Abstract
We investigated the efficacy of the traditional herbal extract 6-bromoindirubin-3′-oxime (BIO) against lipopolysaccharide (LPS)-induced mastitis in mice and inflammatory signaling in mouse mammary epithelial cells (MMECs). In vivo, breast inflammation scores and enzyme-linked immunosorbent assay (ELISA) detection of pro-inflammatory factor expression were used to assess the effect of BIO against mastitis. In vitro, the effects of BIO on LPS-induced changes in the expression levels of pro-inflammatory factors, anti-inflammatory cytokines, and signaling factors of the toll-like receptor 4 (TLR4)/nuclear factor-κB (NF-κB) and TLR4/mitogen-activated protein kinase (MAPK) pathways were examined by qRT-PCR and ELISA. In LPS-injected mice, BIO pretreatment downregulated the expression of the pro-inflammatory factors interleukin (IL)-1β, IL-6, tumor necrosis factor (TNF)-α, and myeloperoxidase (MPO) in mammary glands and reduced inflammatory lesions in breast tissue. In MMECs, BIO pretreatment downregulated the LPS-induced expression of IL-1β, IL-6, and TNF-α. Further, BIO inhibited both the expression and phosphorylation of TLR4/NF-κB and TLR4/MAPK signaling factors. Thus, BIO downregulates IL-6, IL-1β, TNF-α, and MPO expression, upregulates IL-10 expression, and suppresses LPS-induced inflammation by inhibiting the TLR4/NF-κB and TLR4/MAPK pathways. BIO may be a potential treatment agent for mastitis and other inflammatory diseases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Bovine mastitis is the most frequent and prevalent production-limiting disease in dairy herds [1, 2]. It develops most commonly in response to intramammary bacterial infections, and many microorganisms can cause mastitis, of which the Gram-negative bacterium Escherichia coli and Gram-positive bacterium Staphylococcus aureus are the most frequent causative pathogens [3]. Mastitis can be divided into clinical mastitis, subclinical mastitis, and recessive mastitis. S. aureus always causes subclinical mastitis and long-term persistent infection, whereas E. coli usually causes clinical mastitis in high-yielding dairy cows during the early lactation phase and when the number of somatic cells is low. Traditional antibiotic treatment is effective, but antibiotic residues are a potential hazard to human health.
Lipopolysaccharide (LPS) is a component of the outer membrane of E. coli that is widely used to induce mastitis in animal models. LPS is recognized by toll-like receptor 4 (TLR4), which is a major inducer of the immune response in acute mastitis caused by E. coli. Binding of LPS to TLR4 results in the activation of transcription factors, mainly nuclear factor-κB (NF-κB) and mitogen-activated protein kinases (MAPKs). The activation of transcription factors leads to the production of many pro-inflammatory cytokines and chemokines such as tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), and interleukin-6 (IL-6).
The indigo extract 6-bromoindirubin-3′-oxime (BIO) is an active ingredient of Danggui Longhui Wan, the first traditional Chinese medicine shown to be effective for the treatment of chronic myelogenous leukemia [4]. BIO is a known glycogen synthase kinase 3 inhibitor. Our previous research revealed that indirubin can inhibit LPS-induced inflammation via TLR4 abrogation mediated by the NF-κB and MAPK signaling pathways [5]; therefore, we hypothesized that BIO may also inhibit LPS-induced inflammation. In this study, we examined the effects and mechanism of BIO on the inflammatory response in a mouse model of LPS-induced mastitis.
The scoring of histopathologic changes was performed as described previously in studies conducted in mouse mastitis models [6, 7], with minor modifications. The maximum score was 11, and the scoring system is shown in Table 1.
MATERIALS AND METHODS
Chemicals and Reagents
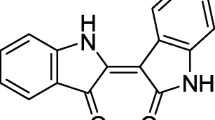
BIO (HPLC ≥ 98%; Fig. 1) was purchased from Shanghai Yuanye Biotechnology (Shanghai, China). Lipopolysaccharide, human epidermal growth factor (hEGF), insulin, and transferrin were obtained from Sigma (St. Louis, MO, USA), fetal bovine serum (FBS) and 0.25% trypsin-EDTA streptomycin from Gibco (Grand Island, NY, USA), and DMEM/F12 from Hyclone (Logan, UT, USA). Collagenase I and collagenase II (Collagenase I, II) were purchased from Solarbio, Trizol from Life Technologies (Carlsbad, CA, USA), and a BCA kit from Pik Wan. Company (Shanghai, China). An MTT Kit (A312-01/02), Hiscript® II RT Super Mix for PCR (R212-01), and AceQ qPCR SYBR Green Master Mix (Q111-02) were purchased from Nanjing Vazyme (Nanjing, China). Anti-β-Actin antibodies (BA2305) were purchased from Wuhan Boster Biological Engineering (Wuhan, Hubei, China). Anti-TLR4 antibodies were purchased from GeneTex (San Antonio, TX, USA). NF-κB pathway antibodies, MAPK family antibody, and phospho-MAPK family antibody kits were purchased from Cell Signaling Technology (Danvers, MA, USA). Goat anti-Mouse IgG antibody was purchased from Southern-Biotech (Wuhan, Hubei, China) and ECL Luminol from ThermoFisher (Beijing, China). Mouse IL-1β, IL-6, and TNF-α platinum ELISA kits with pre-coated plates were obtained from BioLegend (San Diego, CA, USA). Centrifuged tubes (15 mL and 50 mL), T175 vials, and culture plates (6-well, 12-well, and 96-well) were purchased from LabServ (Place). Cell strainers (40 μm and 70 μm) were purchased from FALCON (Corning, NY, USA). ABI fluorescence quantitative PCR plates (96A, 384-well) were purchased from Life Technologies.
In Vivo Study
Experiment Design
Thirty-six Kunming pregnant mice were purchased from the Center for Experimental Animal or ABSL-3 Laboratory of Wuhan University (Hubei, China) and randomly divided into six groups. Mice were anesthetized with intraperitoneal (i.p.) injection of 10 mg pentobarbital sodium salt per 20 g body weight dissolved in phosphate buffered saline (PBS). Mice then received saline, vehicle, or LPS perfusion into the 4th and 5th pairs of mammary ducts with or without i.p. injection of BIO approximately one hour before and 12 hours after LPS. The treatment groups were as follows: (1) control group, PBS 50 μL; (2) dimethyl sulfoxide (DMSO) group (vehicle control), 0.1% DMSO 50 μL; (3) LPS group (positive control), 50 μL of 0.2 mg/mL LPS; (4) 5 μM BIO + LPS group, 50 μL of 0.2 mg/mL LPS plus 5 μM BIO; (5) 25 μM BIO + LPS group, 50 μL of 0.2 mg/mL LPS plus 25 μM BIO; (6) 50 μM BIO + LPS group, 50 μL of 0.2 mg/mL LPS plus BIO 50 μM; (7) LPS + dexamethasone (DEX), 50 μL of 0.2 mg/mL LPS plus DEX. At 24 hours following the LPS challenge, mice were euthanized by CO2, and then mammary gland tissues harvested and stored at − 80 °C in a tube freezer.
Histopathologic Evaluation of Mammary Gland Tissue
The tissue samples were fixed in 4% paraformaldehyde for 48–72 hours, dehydrated in a graded alcohol series, embedded in paraffin, section at 4 μm, and then stained with hematoxylin and eosin (HE). The scoring of histopathologic changes was done as previously described in studies conducted in mouse mastitis models [6, 7], with minor modifications. The maximum score was 11, and the scoring system was shown in Table 1.
Detection of Inflammatory Cytokine Levels
Each breast tissue sample was weighed and homogenized in phosphate buffer (w/v, 1:9) using a tissue homogenizer. All procedures were performed on ice. Tissue samples were centrifuged at 2000g for 40 min at 4 °C. The supernatant was collected and recentrifuged at 2000g for 20 min at 4 °C to remove any remaining lipid. Supernatants were used to detect tissue levels of IL-1β, IL-6, and TNF-α by ELISA according to the kit manufacturer’s instructions.
In Vitro Study
Experimental Design
Primary MMECs were isolated and cultured following a previously described method [5]. Cells were treated as follows: (1) control group (Con), PBS for 24 hours; (2) DMSO group, 0.1% DMSO for 24 hours as a vehicle control; (3) LPS group (LPS), LPS (1 μg/mL) for 24 hours; (4) BIO + LPS groups, BIO at 5, 25, or 50 nM for 1 hours followed by LPS (1 μg/mL) for 24 hours.
MTT Assay of Cell Viability
Cells were seeded into 96-well plates at 104/well and incubated overnight at 37 °C under 5% CO2 and then treated as indicated: control group (no drugs), 1 μg/mL LPS group, 0.1% DMSO group, 5, 25, and 50 nM BIO treatment groups, and BIO (5, 25, 50 nM) + LPS co-stimulation groups, with five replicate wells per group. A cell-free group was also included. Plates were incubated under 5% CO2 at 37 °C for 24 hours. Then, 10 μL of MTT was added to each well, and the supernatant was aspirated four hours later. Wells were carefully rinsed two or three times with PBS and then DMSO (150 μL) was added to each well. The OD of each well was measured at 490 nm and 570 nm after 10 minutes. Cell viability was calculated as follows: cell viability = (dosage group cell OD value − no cell group OD value) / (control group cell OD value − no cell group OD value). Each experiment was repeated three times with independently treated cultures.
Total RNA Extraction and qRT-PCR
Trizol-treated cell samples frozen at − 80 °C were removed and freeze-thawed. Chloroform (200 μL) was added to each treated sample. The samples were vortexed for 15 seconds on a vortexing shaker, allowed to stand for three minutes, and centrifuged at 12,000g for 15 minutes at 4 °C. The supernatants (approximately 500 μL per sample) were transferred to new RNase-free EP tubes. Chloroform (500 μL) was added to each 500 μL supernatant sample and the mixture vortexed for 15 s, allowed to stand for 3 minutes, and centrifuged at 12,000g for 15 minutes at 4 °C. The supernatant was then mixed with 500 μL of isopropanol, inverted gently, left for 10 min at room temperature, and centrifuged at 12,000g for 10 min at 4 °C. The top layer of supernatant was carefully removed and 1 mL of 75% ethanol in RNA-free water added to the precipitate, followed by gentle inversion. The pellet with 75% ethanol diluted in RNase-free water was centrifuged at 12,000g for 4 min at 4 °C, the supernatant carefully removed, and the pellet dried at room temperature for 10 min. An appropriate volume of 0.1% diethylpyrocarbonate (DEPC)-treated water was added to the EP tube and the pellet solubilized in a 55–60 °C water bath for 10–15 min. RNA concentration was measured using an ultra-micro spectrophotometer. The sample was then subjected to reverse transcription and qRT-PCR experiments following the kit manufacturer’s instructions.
The primers used are listed in Table 2. The PCR cycling conditions were two minutes at 50 °C followed by two minutes at 95 °C and then 40 cycles of 15 seconds at 95 °C, 30 seconds at 58 °C, and 30 s at 72 °C. Each reaction mixture contained 1 μL of cDNA, 5 μL of the SYBR Green Super Mix, and sense and anti-sense primers. Each sample was run three times in triplicate; the data were averaged. Melting curves were constructed to assess PCR accuracy. The 2−ΔΔCt method was used to measure the expression levels of calibrator genes. β-Actin served as an internal control. We calculated ΔCt values as follows: ΔCt = Ct (target gene) − Ct (housekeeping gene) and also the following: ΔΔCt = ΔCt (treatment) − ΔCt (control). Amplitude variation served as a surrogate measure of gene expression.
Enzyme-Linked Immunosorbent Assays
Pro-inflammatory cytokines were measured in cell-free supernatants using mouse ELISA kits according to the manufacturer’s instructions.
Western Blotting
Cells treated as indicated were washed three times with pre-cooled PBS and suspended in pre-cooled RIPA lysate buffer. Suspensions were then transferred to pre-chilled 1.5 mL EP tubes and lysed on ice for one hour at 4 °C, followed by centrifugation at 12,000g for 10 minutes. Supernatants were transferred to new 1.5 mL EP tubes and a 20-μL portion removed for total protein concentration measurement using a BCA kit according to the manufacturer’s instructions. Lysate samples were mixed with 2× sample buffer, boiled for 10 minutes, and then loaded onto polyacrylamide gels at 20 μg per lane for SDS-PAGE. Separated protein bands were wet transferred to PVDF membranes. The membrane was blocked in BSA (5%) at room temperature for two–three hours and then incubated overnight in primary antibody (1:500) at 4 °C. Blotted membranes were then incubated in goat anti-rabbit secondary antibody and goat anti-mouse secondary antibody (1:5000) at 37 °C for two hours, and labeling revealed by ECL luminescence assay.
Statistical Analysis
qRT-PCR results were analyzed with Prism 7.0 software and western blot grayscale analysis was performed with ImageJ software. Statistical analysis was performed using Prism 7.0 software. Group means were compared by analysis of variance (ANOVA) with post hoc X tests for pair-wise comparisons. A p < 0.05 was accepted as significant for all tests.
RESULTS
BIO Protects Mammary Gland Tissue Against LPS-Induced Damage
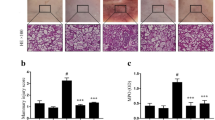
To evaluate the possible anti-inflammatory effects of BIO in mouse mastitis, we first compared the degree of tissue injury among control, LPS-treated, and BIO + LPS co-treated mammary duct tissues by macroscopic pathology and histological analysis (Fig. 2). In untreated control and DMSO (vehicle control) groups, no substantial pathological changes were observed and few inflammatory cells were present (Fig. 2a, b, h, i). In the LPS group, mammary gland tissue had evident edema, inflammatory hyperemia, milk stasis, and local tissue necrosis (Fig. 2c). In tissue, mammary alveoli were hyperemic and thicker than in other treatment groups, and neutrophil infiltration was evident in the alveolar lumen (Fig. 2j). Treatment with BIO or Dex significantly reversed these LPS-induced macroscopic changes (Fig. 2d–g). Fewer neutrophils and macrophages were observed in the alveolar lumen, the mammary alveoli were thinner, and hyperemia and edema were attenuated in a dose-dependent manner (Fig. 2k–n). Tissue in the LPS group had the highest histological injury score, while scores were reduced by co-treatment with BIO, especially at a BIO dose of 100 μM (Fig. 2o).
Macroscopic pathology and histological analysis of mammary gland tissue. Different concentrations of BIO (5, 25, and 50 μM) were given by i.p. injection 1 h before and 12 hours after LPS administration. Mouse mammary glands (n = 3–5) from each experimental group were processed for macroscopic pathological and histological evaluation at 24 hours after LPS challenge. Representative histological changes of the mammary gland from different groups: a and h are control group, b and i are DMSO group, c and j are LPS group, d and k are BIO (5 μM) and LPS group, e and l are BIO (25 μM) and LPS group, and f and m are BIO 50 (μM) and LPS group, (25 μM) and LPS group, and g and n are Dex and LPS group. o is inflammatory score (hematoxylin and eosin staining, magnification × 200). Red circle means mammary gland, and red arrow means neutrophil infiltration or mammary gland structure damage.
BIO Reduces Inflammatory Cell Infiltration in LPS-Treated Mammary Glands
Myeloperoxidase (MPO) activity was determined to assess neutrophil accumulation within the mammary gland tissue, as MPO activity is directly proportional to the number of polymorphonuclear cells (Fig. 3). Activity was significantly increased by LPS treatment compared to the control group, while co-treatment with 25 μM BIO reduced MPO activity compared to the LPS group. Moreover, MPO activity decreased as the BIO dose increased. MPO activity was significantly lower in the Dex-treated group than in the LPS and other treated groups.
BIO inhibits LPS-induced expression of MPO in serum and tissue homogenates. Effect of BIO on MPO in the serum and mammary gland in LPS-stimulated mastitis. Serum (a) and tissue homogenates (b) were used to evaluate MPO with ELISA. The values are presented as the means ± SEM of three independent experiments. *p < 0.05, **p < 0.01, and ***p < 0.001, versus LPS group.
BIO Reduces Inflammatory Cytokines in Mammary Gland Tissue
We used ELISA to measure the expression levels of the inflammatory cytokines IL-1β, IL-6, and TNF-α in serum (Fig. 4a–c) and mammary gland tissue homogenates (Fig. 4d–f). Compared to the control group, LPS challenge significantly increased IL-6, IL-1β, and TNF-α, while BIO co-treatment dose-dependently reduced the expression of all three inflammatory cytokines in LPS-induced mouse mastitis. Expression was significantly lower than the LPS group but still higher than the Dex-treated group.
BIO inhibits LPS-induced expression of pro-inflammatory cytokines in serum and tissue homogenates. Serum and tissue homogenates were used to evaluate IL-6 (a, d), IL-1β (b, e), and TNF-α (c, f) with ELISA, respectively. The values are presented as the means ± SEM of three independent experiments. *p < 0.05, **p < 0.01, and ***p < 0.001, versus LPS group.
Role of BIO in Cell Viability
The effects of BIO (5, 25, and 50 nM) and LPS on cell viability were examined by MTT assay (Fig. 5). Cell viability of DMSO group MMECs was slightly higher than the untreated controls but the difference did not show significance. The results suggest that DMSO and LPS have no effect on the growth of MMECs. In addition, neither BIO alone nor BIO and LPS co-stimulation had any inhibitory effect on cell growth. These findings suggest that results presented here were not influenced by changes in cell viability.
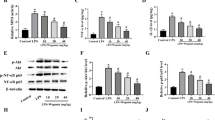
BIO Reduces Inflammatory Cytokine Expression and Enhances Anti-inflammatory Cytokine Expression in Cultured MMECs
The effects of BIO on IL-1β, IL-6, and TNF-α expression levels in LPS-treated MMECs were examined by ELISA and qRT-PCR (Fig. 6). The expression levels of all three pro-inflammatory cytokines in LPS-treated MMECs were significantly higher than in the control and vehicle control groups. BIO co-treatment significantly reduced IL-1β, IL-6, and TNF-α expression levels compared to LPS alone. Conversely, BIO dose-dependently enhanced expression of the anti-inflammatory cytokine IL-10 compared to the LPS group (Fig. 6d, h).
Assay of inflammatory cytokines and anti-inflammatory cytokines in MMEC. The mRNA expressions for IL-6 (a), IL-1β (b), TNF-α (c), and IL-10 (d) were measured by qRT-PCR. The concentration of IL-6 (e), IL-1β (f), TNF-α (g), and IL-10 (h) was measured by ELISA. The values are presented as the means ± SEM of three independent experiments. *p < 0.05, **p < 0.01, and ***p < 0.001, versus LPS group.
BIO Reduces MMCE TLR4 Expression and Activity
To examine the signaling pathways underlying the effects of BIO, we first examined the expression levels and activity of TLR4 in MMECs, the main LPS receptor, using RT-PCR and the TAK-242 inhibition assay (Fig. 7). Challenge with LPS markedly upregulated TLR4 protein expression, an effect completely reversed by 25 and 50 nM BIO (Fig. 7a, b). Similarly, BIO decreased TLR4 mRNA expression in MMECs (Fig. 7c). In cells pre-incubated with 10 nM Tak-242 for 1 h prior to LPS or BIO + LPS treatment, BIO no longer dose-dependently reduced TLR4 mRNA expression (Fig. 7d), suggesting that TLR4 is the major site for BIO-mediated effects.
The effect of BIO on TLR4 activity. MMECs were cultured with different concentrations of BIO (5, 25, and 50 nM) for one hour and then stimulated with LPS (1 μg/mL) for 24 hours. Proteins were analyzed by western blotting (a). Densitometric analysis of the effects of BIO on TLR4 expression (b). Proteins were analyzed by western blotting; β-actin served as an internal control. TLR4 gene was analyzed by qRT-PCR (c). MMECs were treated with TAK-242(10 nM) for one hour, then treated with LPS for 1 h, finally treated with BIO for 24 hours. The TLR4 (d) gene was analyzed by qRT-PCR. The values are presented as the means ± SEM (n = 3). *p < 0.05, **p < 0.01, and ***p < 0.001, versus LPS group.
BIO Suppresses p65 and MAPK Activity
Finally, we examined the downstream signaling pathways mediating the anti-inflammatory effects of BIO by measuring the activities of p65 and MAPK by western blotting (Figs. 8 and 9). LPS enhanced p65 mRNA expression, an effect reversed by BIO pretreatment. The phosphorylation of p65 was also increased in LPS-stimulated MMECs, and this effect was reversed dose-dependently by BIO (except at 5 nM). The levels of MAPK proteins JNK, ERK, and p38 were significantly upregulated by LPS, and all were decreased dose-dependently by BIO pretreatment. Finally, BIO dose-dependently reduced JNK, ERK, and p38 phosphorylation in MMECs.
The effect of BIO on p65 activity. MMECs were pretreated with different concentrations of BIO (5, 25, and 50 nM) for 1 h and then stimulated with LPS (1 μg/mL) for 24 hours; cell lysates were then harvested. Proteins on NF-κB signal pathway were analyzed by western blotting (a). Densitometric analysis of the effects of BIO on phosphorylation of related proteins (b). The values are presented as the means ± SEM (n = 3). *p < 0.05, **p < 0.01, and ***p < 0.001, versus LPS group.
The effect of BIO on MAPK activity. MMECs were pretreated with different concentrations of BIO (5, 25, and 50 nM) for 1 h and then stimulated with LPS (1 μg/mL) for 24 hours; cell lysates were then harvested. Proteins on MAPK signal pathway were analyzed by western blotting (a). Densitometric analysis of the effects of BIO on phosphorylation of related proteins (b). The values are presented as the means ± SEM (n = 3). *p < 0.05 m, **p < 0.01, and ***p < 0.001, versus LPS group.
DISCUSSION
Mastitis is a major economic burden on the cattle industry. The disease is mainly caused by the adhesion of pathogenic microorganisms (bacteria, fungi, mycoplasma, etc.) to breast tissue, and antibiotic therapy is the main treatment for the disease. However, this increases the antibiotic resistance of mastitis-causing pathogens, and antibiotic residues can seriously threaten human health. Traditional Chinese medicines have low toxicity and few side effects. Moreover, active ingredients include numerous antimicrobials with promise for the clinical treatment of mastitis. 6-Bromoindirubin-3′-indole (BIO, also known as 6BIO) is a derivative of indirubin and a bioactive ingredient of Danggui Longhui Wan (Chinese Angelica, Gentian, and Aloe Pill). To study the possible efficacy of BIO for the treatment of mastitis, we established both in vitro and in vivo mouse models using LPS as an inflammation inducer. Indeed, BIO reduced inflammatory lesions in mice mammary tissue and suppressed the expression of pro-inflammatory cytokines and other inflammatory mediators in mouse mammary epithelial cells, possibly by inhibiting the TLR4/NF-κB and TLR4/MAPK pathways.
Lipopolysaccharide, the main component of the E. coli cell membrane, is widely used for in vivo and in vitro inflammation models. LPS can enter the blood and activates neutrophils, which then release the pro-inflammatory cytokines TNF-α and IL-1α [8]. These cytokines are potent inducers of other inflammatory cytokines by other immune and somatic cells. The genes encoding these pro-inflammatory factors are regulated by nuclear factor-κB (NF-κB), a key transcription factor in inflammation. Uterine injection of 2.5 mg/mL LPS can cause uterine injury, and pathological observation revealed a larger number of neutrophils in the uterus as evidenced by enhanced MPO and nitric oxide synthase (NOS) activity [9]. In vitro, LPS stimulates the RAW264.7 murine macrophage line to mimic endotoxin-induced inflammation during sepsis [10]. In this study, the mouse mastitis model was established by injection of 1 mg/mL LPS. The results showed that this concentration can induce mammary glandular swelling and hyperemia, and inflammatory reactions such as increased expression of MPO and pro-inflammatory cytokines. At 1 μg/mL, LPS upregulated expression of pro-inflammatory cytokines IL-6, IL-1β, and TNF-α in MMECs, and activated both TLR4/NF-κB and TLR4/MAPKs pathways. These results indicate that a mastitis model was successful established both in vivo and in vitro.
It was previously reported that 3 μM and 10 μM BIO inhibited the proliferation and invasion of the human cancer cell lines T24, HuH-7, and MDA-MB-231 via the JAK/STAT3 pathway [11]. However, there have been no reports on the effect of BIO on mouse mammary cells. In this study, BIO at concentrations up to 50 nM had no substantial effect on MMEC viability, but 5, 25, and 50 nM BIO reversed the LPS-induced increase in the transcript levels of IL-6, IL-1β, and TNF-α in a dose-dependent manner. Based on the effects of BIO on MMEC cell viability and the expression of IL-6, IL-1β, and TNF-α after LPS treatment, we selected BIO concentrations of 5, 25, and 50 nM for the in vitro experiments; BIO concentrations of 5, 25, and 50 μM were used for in vivo experiments (data not shown).
Overexpression of IL-1β, IL-6, and TNF-α can cause inflammatory damage to tissues. IL-1 is a strong inflammatory mediator secreted mainly in the form of IL-1β. Under normal circumstances, the secretion of IL-1β is extremely low, but it increases dramatically after stimulation by foreign antigens. In turn, IL-1β can induce the release of IL-3, IL-8, IL-13, and other pro-inflammatory cytokines [12]. IL-6 is a multifunctional cytokine secreted by macrophages, T cells, fibroblasts, and epithelial cells. IL-6 regulates immune responses and participates in acute phase reactions and hematopoiesis [13]. Significant upregulation of IL-6 may increase pain or hyperalgesia in a variety of pathological conditions [14]. TNF-α is a pro-inflammatory member of the TNF family produced in response to tissue damage, endotoxin stimulation, and infection. Studies have shown that TNF-α inhibits the activity of caspases in cells expressing RIPK3 and induces necroptosis. Therefore, TNF-induced inflammation in vivo is considered harmful to the body [15]. We used LPS to stimulate inflammation in mouse mammary glands and observed redness and congestion, infiltration of large numbers of inflammatory cells, and incomplete acinar structures. Alternatively, in vivo pretreatment with 5, 25, and 50 μM BIO suppressed these signs of pathogenic inflammation, as well as overexpression of IL-6, IL-1β, and TNF-α, suggesting that these lesions are caused by local cytokine overproduction.
The IL-10 anti-inflammatory factor family promotes the innate immune response in epithelial cells to limit the damage caused by viral and bacterial infections. These cytokines can also promote tissue healing from injuries caused by infection or inflammation [1]. Bruno (2015) found that IL-10-knockout mice exhibited poor recovery from sciatic nerve injury, indicating that IL-10 is indispensable for the recovery of tissue damage [16]. In this study, LPS stimulation inhibited the production of IL-10, whereas BIO promoted the secretion of IL-10, suggesting that BIO protects the mammary gland from inflammatory injury by promoting IL-10 expression.
Myeloperoxidase (MPO) is a heme peroxidase enzyme highly expressed in neutrophils as a lysosomal protein stored mainly in azurin particles. It is released outside the cell during threshing [17]. MPO has been shown to be an important mediator of tissue damage in various inflammatory diseases and so is likely an important target for the treatment of inflammation [18]. Neutrophils play an important role in preventing bacterial and fungal infections. In this study, a large amount of MPO was detected in serum and mammary tissue homogenates of the LPS-induced mastitis model compared to the control group (p < 0.001). This may be due to LPS damaging the mammary glands, resulting in chemotaxis of a large number of neutrophils and local release of MPO, aggravating the mammary gland inflammatory response. Intraperitoneal injection of BIO 1 h earlier reduced LPS-induced MPO activity to some extent, indicating that BIO can exert anti-inflammatory effects by targeting MPO.
Numerous studies have shown that the NF-κB and MAPK signaling pathways regulate the expression of TNF-α and IL-1β [19]. NF-κB is a nuclear transcription factor that exists in the cytoplasm until the cell is stimulated by stress, viruses, bacteria, inflammatory stimulants, cytokines, free radicals, carcinogens, tumor promoters, or endotoxins [20]. Upon activation, NF-κB translocates to the nucleus and regulates the expression of nearly 400 different genes, including pro-inflammatory or pro-oxidant enzymes (COX-2 and iNOS), cytokines (TNF, IL-1, IL-6, IL-8, and chemokines), adhesion molecules, cell cycle regulators, and angiogenic factors [21]. TLR4-mediated intracellular signaling pathways converge on and activate NF-κB, inducing the transcription of cytokines and chemokines that participate in the initiation or regulation of inflammatory responses [22]. This study revealed that BIO, even at 50 nM, can inhibit the activity of NF-κB by simultaneously inhibiting the expression of p65 transcription and phosphorylation.
MAPKs are a family of highly conserved serine/threonine protein kinases involved in the regulation of key cellular processes such as gene induction, cell survival/apoptosis, proliferation, differentiation, cellular stress, and inflammatory responses [23]. Each MAPK can be activated by many different upstream MKKs and MKKKs, each with unique regulatory mechanisms, substrate specificities, and activation kinetics [24]. In this study, we found that LPS could increase the phosphorylation level of each protein, suggesting that BIO can inhibit LPS-induced inflammatory cytokine production by suppressing multiple MAPK signaling pathways.
We confirmed our previous hypothesis by evaluating the activities of NF-κB and MAPKs. BIO may downregulate pro-inflammatory factors such as IL-6, IL-1β, and TNF-α by suppressing TLR4/NF-κB and TLR4/MAPK signaling. In this study, the activities of NF-κB and MAPKs were inhibited at BIO concentrations as low as 5 nM, with marked inhibitory effects similar to those of clinical anti-inflammatory dexamethasone at 50 nM. It is thus clear that BIO treatment of LPS-induced mastitis in a mouse model and its activity in MMECs acts via TLR4 and its two main Myd88-dependent pathways, NF-κB and MAPK signaling (Fig. 10). However, the anti-inflammatory effect of BIO in vitro differs somewhat from that in vivo. The conditions within an organism are not perfectly replicated in vitro, and therefore generalizing the results of in vitro experiments to the biology of complete organisms is challenging. Here, we examined how BIO exerts its anti-inflammatory effects, and whether BIO has the same effect in vivo. Further study is necessary to explore the effects of BIO on mammary tissue, and whether it can inhibit the TLR4/NF-κB and TLR4/MAPK pathways.
BIO suppressed LPS-induced inflammation via inhibition of TLR4/NF-κB and TLR4/MAPK signaling pathways. BIO inhibit TLR4 expression, and the phosphorylation of P38, ERK1/2, and JNK. BIO also has an inhibit effect on P-p65 and Iκ-B, thus preventing P-p65; the production of cytokines such as IL-6, IL-1β, TNF-α, and MPO was inhibited.
CONCLUSION
In conclusion, we demonstrated that BIO exerted anti-inflammatory effects on mice. BIO pretreatment attenuated mammary gland histopathological changes and reduced the expression levels of pro-inflammatory genes, including those encoding IL-1β, IL-6, and TNF-α, by inhibiting TLR4 expression and suppressing phosphorylation of IκBα, NF-κB P65, JNK, ERK, and P38. Thus, BIO might be a potential treatment of mastitis and other inflammatory diseases.
References
Ouyang, W., S. Rutz, N.K. Crellin, P.A. Valdez, and S.G. Hymowitz. 2011. Regulation and functions of the IL-10 family of cytokines in inflammation and disease. Annual Review of Immunology 29 (29): 71–109.
Kiku, Y., T.O. Zawa, H.T. Akahashi, et al. 2017. Effect of intramammary infusion of recombinant bovine GM-CSF and IL-8 on CMT score, somatic cell count, and milk mononuclear cell populations in Holstein cows with Staphylococcus aureus subclinical mastitis. Veterinary Research Communications: 1–8.
Gilbert, F.B., P.C. Unha, K.J. Ensen, et al. 2013. Differential response of bovine mammary epithelial cells to Staphylococcus aureus or Escherichia coli agonists of the innate immune system. Veterinary Research 44 (1): 40.
Wang, C., X. Bai, and C. Wang. 2014. Traditional Chinese medicine: a treasured natural resource of anticancer drug research and development. American Journal of Chinese Medicine 42 (3): 1450035.
Lai, J.L., Y.H. Liu, C. Liu, M.P. Qi, R.N. Liu, X.F. Zhu, Q.G. Zhou, Y.Y. Chen, A.Z. Guo, and C.M. Hu. 2017. Indirubin inhibits LPS-induced inflammation via TLR4 abrogation mediated by the NF-κB and MAPK signaling pathways. Inflammation. 40 (1): 1–12.
Engel, M.A., C.A. Kellermann, G. Burnat, E.G. Hahn, T. Rau, and P.C. Konturek. 2010. Mice lacking cannabinoid CB1-, CB2-receptors or both receptors show increased susceptibility to trinitrobenzene sulfonic acid (TNBS)-induced colitis. Journal of Physiology and Pharmacology 61 (1): 89–97.
Lai, J.L., Y.H. Liu, Y.C. Peng, P. Ge, C.F. He, C. Liu, Y.Y. Chen, A.Z. Guo, and C.M. Hu. 2017. Indirubin treatment of lipopolysaccharide-induced mastitis in a mouse model and activity in mouse mammary epithelial cells. Mediators of Inflammation: 3082805. https://doi.org/10.1155/2017/3082805.
Michlewska, S.M.C.F., I.I. Dransfield, and A. Rossi. 2009. Macrophage phagocytosis of apoptotic neutrophils is critically regulated by the opposing actions of pro-inflammatory and anti-inflammatory agents: key role for TNF-alpha. FASEB Journal 23 (3): 844–854.
Lv, X., K. Fu, W. Li, Y. Wang, J. Wang, H. Li, W. Tian, and R. Cao. 2015. TIIA attenuates LPS-induced mouse endometritis by suppressing the NF-κB signaling pathway. Canadian Journal of Physiology and Pharmacology 93 (11): 967–971.
Fang, W.F., Y.M. Chen, C.Y. Lin, H.L. Huang, H. Yeh, Y.T. Chang, K.T. Huang, and M.C. Lin. 2018. Histone deacetylase 2 (HDAC2) attenuates lipopolysaccharide (LPS)-induced inflammation by regulating PAI-1 expression. Journal of Inflammation 15 (1): 3.
Braig, S., C.A. Kressirer, J. Liebl, F. Bischoff, S. Zahler, L. Meijer, and A.M. Vollmar. 2013. Indirubin derivative 6 BIO suppresses metastasis. Cancer Research 73 (19): 6004–6012.
Eljaafari, A., M. Robert, M. Chehimi, S. Chanon, C. Durand, G. Vial, N. Bendridi, A.M. Madec, E. Disse, M. Laville, J. Rieusset, E. Lefai, H. Vidal, and L. Pirola. 2015. Adipose tissue-derived stem cells from obese subjects contribute to inflammation and reduced insulin response in adipocytes through differential regulation of the Th1/Th17 balance and monocyte activation. Diabetes 64 (7): 2477–2488.
Banks, W.A., A.J. Kastin, and E.G. Gutierrez. 1994. Penetration of interleukin-6 across the murine blood-brain barrier. Neuroscience Letters 179 (1–2): 53–56.
Schaper, F., and S. Rose-John. 2015. Interleukin-6: Biology, signaling and strategies of blockade. Cytokine & Growth Factor Reviews 26 (5): 475–487.
Kearney, C.J., S.P. Cullen, G.A. Tynan, C.M. Henry, D. Clancy, E.C. Lavelle, and S.J. Martin. 2015. Necroptosis suppresses inflammation via termination of TNF- or LPS-induced cytokine and chemokine production. Cell Death and Differentiation 22 (8): 1313–1327.
Siqueira, M.B., A. Kroner, E.I. Girolami, et al. 2015. Role of IL-10 in resolution of inflammation and functional recovery after peripheral nerve injury. The Journal of Neuroscience: the Official Journal of the Society for Neuroscience 35 (50): 16431–16442.
Jr, J.C., and R. Medzhitov. 2002. Innate immune recognition. Annual Review of Immunology 20 (1): 197–216.
Aratani, Y. 2018. Myeloperoxidase: its role for host defense, inflammation, and neutrophil function. Archives of Biochemistry and Biophysics 640: 47–52.
Zhang, X., Y. Wang, C. Xiao, Z. Wei, J. Wang, Z. Yang, and Y. Fu. 2017. Resveratrol inhibits LPS-induced mice mastitis through attenuating the MAPK and NF-κB signaling pathway. Microbial Pathogenesis 107: 462–467.
May, M.J., and S. Ghosh. 1998. NF-kappaB and Rel proteins: evolutionarily conserved mediators of immune responses. Immunology Today 19.
Serasanambati, M., and S.R. Chilakapati. 2016. Function of nuclear factor kappa B (NF-κB) in human diseases—a review. South Indian Journal of Biological Sciences 2 (4): 368–387.
Liu, Y., H. Yin, M. Zhao, and Q. Lu. 2014. TLR2 and TLR4 in autoimmune diseases: a comprehensive review. Clinical Reviews in Allergy & Immunology 47 (2): 136–147.
Thalhamer, T., M.A. Mcgrath, and M.M. Harnett. 2008. MAPKs and their relevance to arthritis and inflammation. Rheumatology 47 (4): 409–414.
Kyriakis, J.M., and J. Avruch. 2012. Mammalian MAPK signal transduction pathways activated by stress and inflammation: a 10-year update. Physiological Reviews 92 (2): 689–737.
Acknowledgments
We would like to thank all our coworkers in the State Key Laboratory of Agricultural Microbiology (Wuhan, China) for useful discussions. This work was supported by the National Key Research and Development Plan (No. 2016YFD0500906), and the special fund for the China Agriculture Research System (Beef/Yak cattle) (No. CARS-38), and the Research Fund for National Distinguished Scholars in Agriculture Research and Technical Innovative Team.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
All co-authors implicated in this research approved this article to be published. The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, C., Tang, X., Zhang, W. et al. 6-Bromoindirubin-3′-Oxime Suppresses LPS-Induced Inflammation via Inhibition of the TLR4/NF-κB and TLR4/MAPK Signaling Pathways. Inflammation 42, 2192–2204 (2019). https://doi.org/10.1007/s10753-019-01083-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-019-01083-1