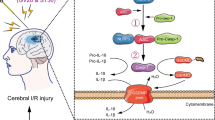
Abstract
Electroacupuncture (EA) pretreatment, electrical stimulation using metal needle at specific acupoints in advance, possesses the potential to prevent cerebral ischemia-reperfusion injury (CIRI). Transient receptor potential vanilloid 1 (TRPV-1) has been indicated to take part in cerebral protection of EA; however, the detailed mechanisms remain unclear. The aim of this study was to investigate whether neuroprotection of EA pretreatment against CIRI is associated with TRPV-1 and explore the underlying mechanisms. Middle cerebral artery occlusion (MCAO) was performed to induce CIRI after EA pretreatment at Baihui (GV20), bilateral Shenshu (BL23), and Sanyinjiao (SP6) acupoints in rats. Neurological deficit scores, infarct volumes, oxidative stress damage, inflammatory cytokine production, MAPK signaling activation, and the expression of TRPV-1 were assessed. EA pretreatment lowered neurological deficit scores, reduced infarct volumes, impeded oxidative stress injury, inhibited inflammatory cytokine production, curbed P38 phosphorylation, and suppressed TRPV-1 expression in MCAO rats. Attributing to inhibition of TRPV-1 expression, AMG-517 (TRPV-1 antagonist) showed the synergistic effect with EA pretreatment on the neuroprotection against ischemia-reperfusion injury. However, TRPV-1 agonists capsaicin significantly abrogated the neuroprotective effects of EA pretreatment in MCAO rats accompanying enhancement of TRPV-1 expression. These findings indicated EA pretreatment exerted neuroprotection in rats with cerebral ischemia-reperfusion injury, which at least partially were associated with TRPV1-mediated anti-oxidant stress and anti-inflammation via inhibiting P38 MAPK activation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Stroke, an acute focal injury of the central nervous system, is generally classified into two categories: hemorrhagic stroke and ischemic stroke [1]. Ischemia stroke makes up a majority of stroke cases in origin and often occurs in cerebral stroke patients, resulting in a high morbidity, disability, and mortality [2]. At present, alternative treatments for acute ischemic stroke are limited to tissue-type plasminogen activator (tPA) and mechanical thrombectomy [3, 4]. Therefore, new strategies that protect against CIRI or stroke are attracting more and more attention.
As one of the important components of traditional Chinese medicine, acupuncture has been widely used in China and Western countries, due to its simple, safe, convenient, and effective intervention compared with other conventional therapies [5]. Electroacupuncture (EA) has also been increasingly applied in clinical practice via the combination between traditional acupuncture and modern electrical stimulation [6]. On one hand, EA has a good repeatability and stability through the standardization of frequency, intensity, and duration. On the other hand, continuous low frequency electrical stimulation, with metal needle at specific acupoints in the body, can enhance the afferent impulses and improve acupuncture outcome associated with the local and systemic regulation [7].
Increasing evidence shows that EA pretreatment exerts neuroprotective effect in ischemic stroke, including attenuation of glutamate excitotoxicity [8], reduction of oxidative stress [9], inhibition of neuroinflammation [10], and suppression of cell apoptosis [11]. However, a majority of them are descriptive studies, lacking direct evaluation for action of EA pretreatment, and the detailed mechanisms still remain elusive.
Herein, we investigated the neuroprotective effect of EA pretreatment at Baihui (GV20), bilateral Shenshu (BL23), and Sanyinjiao (SP6) acupoints on ischemic stroke, which were associated with TRPV-1-mediated anti-oxidant stress and anti-inflammation via inhibiting P38 signaling activation. Our findings suggest that EA pretreatment is an alternative and promising candidate for the prevention of cerebral ischemia-reperfusion injury or stroke.
MATERIALS AND METHODS
Animals
Male Sprague-Dawley rats (6 weeks old, 220 ± 20 g) were provided by Hubei Research Center of Laboratory Animals (Wuhan, China). All the experimental procedures were approved by the Animal Care and Use Committee of Hubei University of Chinese Medicine (No. SYXK2017-0067), and all procedures were performed according to the National Institutes of Health Guidelines for Animal Research.
Experimental Protocols
Experiment I
Rats were randomly divided into four groups (n = 8 for each group): Control (Control), sham-operation group (Sham), middle cerebral artery occlusion model (MCAO), and EA pretreated-MCAO group (EA + MCAO). As shown in Fig. 1a, rats were pretreated with EA stimulation at GV20, bilateral BL23, and SP6 acupoints for 1 h. Then, the rats were yield to unilateral right middle cerebral artery occlusion for 2 h, and then reperfused for 3 h, 6 h, 12 h, and 24 h, separately. Lastly, rats were sacrificed to collect the samples.
Effect of EA pretreatment in MCAO rats. a Schematic diagram of the experiment I as described in experimental protocols. b Neurological deficit scores at 3, 6, 12, and 24 h after reperfusion in MCAO group and EA + MCAO group were analyzed. c Representative TTC staining showed noninfarct (red) and infarct (white) regions in the experimental groups at 6 h after reperfusion. d Quantification of infarct volumes among the four groups at 6 h after reperfusion was statistically analyzed. **P < 0.01 and ***P < 0.001 vs. MCAO group.
Experiment II
Rats were randomly divided into four groups (n = 8 for each group): Middle cerebral artery occlusion model (MCAO), EA pretreated-MCAO group (EA + MCAO), AMG-517 administration before EA pretreatment in MCAO model (AMG-517 + EA + MCAO), and capsaicin administration before EA pretreatment in MCAO model (capsaicin + EA + MCAO). Based on other reports [12, 13], AMG-517 (TRPV-1 antagonists, 0.3 mg/kg, once a day for continuous 3 days, days 1–3) (S7115, Selleckchem, Houston, TX, USA) was used via i.p. before EA pretreatment (day 4), and capsaicin (TRPV-1 agonists, 0.2 mg/kg) was used via s.c. 3 h before EA pretreatment (day 4) (S1990, Selleckchem) in experiment II. As shown in Fig. 6a, AMG-517 or capsaicin was administered in advance. Then, the rats were pretreated with EA at GV20, bilateral BL23, and SP6 acupoints for 1 h followed by unilateral right middle cerebral artery occlusion for 2 h. After reperfusion for 6 h, rats were sacrificed to collect the samples.
Middle Cerebral Artery Occlusion (MCAO) Model
MCAO model was performed to represent CIRI. Briefly, rats were anesthetized with 5% chloral hydrate (0.6 mL/100 g via i.p.). Then, a monofilament nylon suture (3.0 cm in length) with a rounded tip coated with silicone rubber was inserted into the internal carotid artery 18–20 mm to block blood flow. After a 2-h occlusion, the middle cerebral artery was reperfused by withdrawing the nylon monofilament.
EA Pretreatment
EA stimulation was applied to Baihui (GV20, located at the intersection of sagittal midline and the line linking two rat ears), bilateral Shenshu (BL23, located adjacent to the second lumbar vertebra), and Sanyinjiao (SP6, located 10 mm above the prominence of the lateral malleolus of the hind limb) acupoints (HANS-200A, Beijing Huayun Science and Technology Co., Ltd., Beijing, China). The needles (diameter 0.2 mm and length 3.0 cm) (Suzhou Medical Apparatus Co., Ltd., Suzhou, China) were inserted perpendicularly to the skin. Then, a continuous wave at 2/100 Hz and 1 mA with electrical stimulation was used for 10 min, and kept the needles alone without electrical stimulation for 5 min. The process was continually repeated four times for 1 h in total as EA pretreatment.
Neurologic Deficit Scores
Based on Longa neurological deficit scores [14], neurologic deficit scores of all rats at 3, 6, 12, and 24 h after reperfusion were evaluated and determined according to the following criterion: 0, normal neurologic behavior; 1, flexion in the right forelimb; 2, failure to extend the right forelimb completely and strength to resist lateral push declined obviously; 3, forelimb flexion, rotation, and crawling toward the right side; and 4, unable or difficult to ambulate spontaneously. The mean value of three scores was recorded at each timepoint, and higher scores meant more severe neurological deficit.
Measurement of Infarct Volume
After neurobehavioral assessment, rats were sacrificed and the brains were collected. Infarct volume was determined according to previous description [15]. In brief, the brain tissue was sectioned into five coronal blocks in brain matrix with an approximate thickness of 2 mm. Then, the sections were stained with 2,3,5-triphenyltetrazolium chloride (TTC) (Sigma-Aldrich, St. Louis, MO, USA) and immersed in 4% paraformaldehyde overnight. Normal area was red and infarct area kept white. The percentage of infarct volumes were calculated following the formula:
- Vc:
-
volumes of normal gray matter in the control hemisphere
- VL:
-
volumes of normal gray matter in the s lesioned hemisphere.
Enzyme-Linked Immunosorbent Assay (ELISA)
Rat hippocampus were isolated and mechanically homogenized. Then, the supernatants were collected for ELISA analysis, such as interleukin-1β (IL-1β) (BGK5BKB0), tumor necrosis factor-α (TNF-α) (BGK16599) (PeproTech, Rocky Hill, NJ, USA), malondialdehyde (MDA) (S0131), glutathione (GSH) (S0053), and superoxide dismutase (SOD) (S0101) (Beyotime, Shanghai, China).
Immunohistochemical Analysis
Brain tissues were collected, fixed with 4% paraformaldehyde, and embedded in paraffin. Then, the tissues were serially sectioned at 5 μm in thickness. For immunohistochemical analysis, the sections were deparaffinized, blocked with 5% BSA, and treated with rabbit anti-rat TNF-α (ab6671) and rabbit anti-rat cytochrome C (ab13575) (Abcam, Cambridge, Massachusetts, USA) at 37 °C for 2 h, separately. The sections were incubated with goat anti-rabbit IgG for 30 min, and slides were observed with Olympus BX60 (Olympus Optical Co Ltd., Japan). Under × 400 magnification, the morphometric examination was performed in a blinded manner by two independent investigators. For each section, five visual fields were chosen at random and the mean number of the positive cells were represented.
Western Blotting
Rat hippocampus were removed and extracted using ice-cold RIPA lysis buffer (P0013B, Beyotime, Shang, China). Protein level was determined with the BCA Protein Assay Kit (Pierce, Rockford, IL, USA). Protein samples (30 μg) were separated by 10% polyacrylamide gel electrophoresis, and transferred to PVDF membranes. Subsequently, the membranes were blocked with 5% skim milk. After 2 h of treatment at room temperature, the membranes were incubated overnight at 4 °C with primary rabbit anti-rat antibodies against ERK, JNK, P38 (MAPK Family Antibody Sampler Kit #9926), p-P38, p-JNK, p-ERK (Phospho-MAPK Family Antibody Sampler Kit #9910), GAPDH (#5174) (Cell Signaling Technology Inc., Beverly, Massachusetts, USA), and TRPV-1 (PA5–77317) (Invitrogen, Carlsbad, CA, USA). Then, the membranes were held with HRP-conjugated goat anti-rabbit antibody (#7074) (Cell Signaling Technology Inc.). Changes in the density of the protein bands were detected by HP Scanjet 7400C (Hewlett-Packard Co., Palo Alto, CA, USA) and quantified with ImageJ software (National Institutes of Health, Bethesda, MD, USA).
Statistical Analysis
Results were expressed as the mean ± SEM. Data were analyzed with SPSS 19.0 software (SPSS Inc., Chicago, IL, USA). One-way analysis of variance (ANOVA) was used for comparisons of multiple samples. A Student’s t test was used for comparisons between two groups. A value of P < 0.05 indicated statistically significant.
RESULTS
EA Pretreatment Elicits Neuroprotection Against Cerebral Ischemia-Reperfusion Injury
According to the experimental progress described in Fig. 1a, we firstly observed the effect of EA stimulation in advance in MCAO rats. Compared with MCAO group, neurological deficit scores at 6 h after reperfusion in EA + MCAO group were significantly decreased (Fig. 1b), but not at 3 h, 12 h, or 24 h. Simultaneously, the percentage of cerebral infarct areas was obviously lowered in EA + MCAO group than those in MCAO model (Fig. 2c, d).
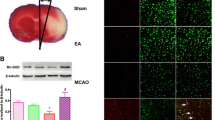
Effect of EA pretreatment on oxidative stress. a–c Oxidative injury at 6 h after reperfusion, including MDA, GSH, and SOD, were assessed by ELISA and statistically analyzed. d, e Representative immunohistochemical staining (× 400) showed cytochrome C expression in rat hippocampus at 6 h after reperfusion, and statistical analysis of cytochrome C-positive cells in the different groups was shown. **P < 0.01 vs. MCAO group.
EA Pretreatment Inhibits Oxidative Stress and Inflammation
As shown in Fig. 2a–c, the levels of MDA in EA + MCAO group were visibly downregulated, but the activities of GSH and SOD were markedly upregulated compared with those in MCAO group. EA pretreatment evidently decreased cytochrome C-positive cells in hippocampal neurons after CIRI (Fig. 3d, e). Additionally, EA pretreatment significantly reduced the release of TNF-α and IL-1β compared with model group (Fig. 3a, b). Immunohistochemical staining also indicated TNF-α-positive cells apparently were decreasing in MCAO rats with EA pretreatment (Fig. 3c, d).
Effect of EA pretreatment on inflammatory responses. a, b Inflammatory cytokine production at 6 h after reperfusion, such as TNF-α and IL-1β, was assessed by ELISA and statistically analyzed. c, d Representative immunohistochemical staining (× 400) showed TNF-α expression in rat hippocampus at 6 h after reperfusion, and statistical analysis of TNF-α-positive cells in the different groups was shown. *P < 0.05 and **P < 0.01 vs. MCAO group.
EA Pretreatment Curbs P38 MAPK Activation
Next, we investigated the underlying mechanisms related to the neuroprotective effects of EA pretreatment on ischemia stroke. Representative Western blotting showed EA pretreatment effectively prevented P38 MAPK activation, but not phosphorylation of ERK and JNK (Fig. 4a, b). The result suggested that inhibiting P38 MAPK activation might be associated with cerebral protection of EA pretreatment against CIRI.
Effect of EA pretreatment on MAPK signaling in MCAO rats. a MAPK signaling (including ERK, JNK, and P38) and protein phosphorylation were detected by Western blot. The representative results were displayed. b Relative protein expression of p-ERK, p-JNK, and p-P38 was statistically analyzed, respectively. *P < 0.05 vs. MCAO group.
EA Pretreatment Suppresses TRPV-1 Expression
Considering TRPV-1 channels play a pivotal role in ischemia-reperfusion injury, we further observed the effect of EA stimulation on TRPV-1. Compared with that in MCAO group, EA pretreatment obviously discouraged the expression of TRPV-1 protein (Fig. 5a, b), suggesting the relation between TRPV-1 and the neuroprotection of EA pretreatment in MCAO rats.
Involvement of TRPV-1 in EA Pretreatment-Evoked Neuroprotection
Following schematic diagram of the experiment II, we further assessed the neuroprotective effects of EA pretreatment utilizing AMG-517 and capsaicin, which is specific TRPV-1 antagonists and agonists, respectively (Fig. 6a). AMG-517 showed the synergistic effect with EA pretreatment on the inhibitive expression of TRPV-1 in MCAO rats, but capsaicin clearly enhanced TRPV-1 expression (Fig. 6b, c). Importantly, capsaicin significantly abrogated the neuroprotection of EA pretreatment in MCAO rats, including the increment of neurological deficit scores and the augmentation of cerebral infarct areas (Fig. 6d–f).
Effects of TRPV-1 antagonists and agonists in MCAO rats with EA pretreatment. a Schematic diagram of the experiment II as described in experimental protocols. b, c The protein expression of TRPV-1 was measured and analyzed. A representative Western blot was displayed. d Neurological deficit scores in the different groups were analyzed. e Representative TTC staining showed noninfarct (red) and infarct (white) regions. f Quantitative analysis of infarct volumes in different groups was shown. **P < 0.01 and ***P < 0.001 vs. MCAO group; #P < 0.05 and ##P < 0.01 vs. EA + MCAO group.
EA Pretreatment Elicits Neuroprotection Associated with TRPV1-Mediated Anti-Oxidant Stress and Anti-Inflammation
Attributing to the inhibitive expression of TRPV-1, AMG-517 reinforced the anti-oxidant stress and anti-inflammation related to EA pretreatment, such as reducing the production of TNF-α, IL-1β, and MDA; increasing the release of GSH and SOD; and dampening the phosphorylation of P38 (Fig. 7). In contrast to AMG-517, capsaicin markedly reversed EA pretreatment-induced suppressive effects on inflammatory cytokine production and oxidant stress. It was worth noting that activation of P38 MAPK signaling was also inversed in response to capsaicin compared with that in EA + MCAO group (Fig. 7f, g).
Effects of TRPV-1 antagonists and agonists on the neuroprotection of EA pretreatment against CIRI. a, b Production of inflammatory cytokines (TNF-α, IL-1β) and c–e oxidative injury (MDA, GSH, and SOD) were detected by ELISA and statistically analyzed. f, g Activation of P38 was detected and analyzed. A representative Western blot was shown. **P < 0.01 and ***P < 0.001 vs. MCAO group; #P < 0.05 and ##P < 0.01 vs. EA + MCAO group.
DISCUSSION
As we know, MCAO treatment in rats and mice has been widely used as experimental model to study CIRI or ischemia stroke [16]. EA pretreatment at GV20 induced tolerance against CIRI in MCAO rats through inhibition of the autophagy pathway [17]. EA preconditioning at GV20 and Dazhui (GV14) acupoints could improve neural function after ischemic injury in MCAO mice [18]. Additionally, EA treatment at Chize (LU5), Hegu (LI4), SP6, and Zusanli (ST36) acupoints exerted neuroprotection against CIRI in JNK knockout mice [19]. Although there were different reasons for acupoint selection as described above, GV20, BL23, and SP6 acupoints were used together for 1 h of EA stimulation in this study. Then, ischemic rats were yielded to a 2-h occlusion followed by reperfusion for 6 h (Fig. 1a). EA pretreatment elicited neuroprotective effects in MCAO rats (Fig. 1b–d), which might be due to the difference in the time referred to occlusion and reperfusion, acupoint selection, combination of multi-acupoints, EA pretreatment time, and frequency and amplitude of electrical stimulation.
Following CIRI, neurons injury and damage were triggered by a cascade of events, such as oxidative stress, neuroinflammation, glutamate excitotoxicity, and apoptosis [20]. In mitochondria, cytochrome C shows peroxidase activity to promote cellular oxidation [21]. Due to an imbalance between harmful reactive oxygen species (ROS) and endogenous anti-oxidant protection, ROS-induced MDA is significantly accumulated; and anti-oxidant enzymes, such as SOD and GSH, correspondingly decreased [22]. In this study, EA pretreatment presented the anti-oxidant potential via upregulating MDA and cytochrome C as well as downregulating SOD and GSH (Fig. 2). During oxidative stress related to neurons injury, inflammatory cascade reaction, the amplification of cytokines and chemokine production, leads to an exacerbated ischemic brain injury [23]. Our data showed that the release of inflammatory cytokines, including TNF-α and IL-1β, were significantly reduced with EA pretreatment (Fig. 3). The result was consistent with previous reports that EA preconditioning ameliorated CIRI in MCAO rats via discouraging the production of IL-1β, IL-6, and TNF-α [11, 12]. These findings suggest the anti-oxidant stress and anti-inflammation of EA pretreatment in MCAO rats.
Mitogen-activated protein kinase (MAPK) family includes extracellular signal-regulated kinase 1/2 (ERK1/2), c-Jun N terminal kinases (JNK), and p38, which are activated through phosphorylation, leading to the expression of target genes [24]. Previous studies indicated that the involvement of P38 MAPK-mediated anti-apoptotic signaling in the neuroprotective activity of EA preconditioning in MCAO rats [11, 25]. Even though we also observed that EA pretreatment effectively curbed P38 MAPK activation (Fig. 4), further studies were needed to illuminate the concerns if other signaling pathway (such as PI3/Akt, Wnt/β-catenin, and Notch) or transcription factor (NF-κB, HIF-1α, and CREB) participated in the neuroprotection of EA pretreatment against ischemic stroke.
As a non-selective cation channel, TRPV-1 is primarily expressed in dorsal root ganglions sensory neurons and brain, which functions as a polymodal nociceptor activated by heat, acidic conditions, and capsaicin [26]. A previous study reported that brain TRPV-1 channels were activated by ischemic stroke, in which the neurological and motor deficits, and infarct volumes in TRPV-1-KO mice were lower than those of WT mice after brain ischemia [27]. Other study also indicated that MCAO-induced cerebral ischemia was accompanied by overexpression of N-methyl-D-aspartate receptor subtype 1 (NR1) and TRPV-1 receptors, and EA at GV20 acupoint reversed the deficit of behavior in vascular dementia via reversal of NR1 and TRPV-1-mediated neurotoxicity [28]. Similar with these results, TRPV-1 expression was increased in MCAO rats compared with that in EA + MCAO group (Fig. 5). Following intervention of capsaicin (TRPV-1 agonist), but not AMG-517 (TRPV-1 antagonist), markedly reversed the neuroprotection of EA pretreatment (Figs. 6 and 7).
Lastly, two limitations of this study should be mentioned. Firstly, TRPV-1 antagonists and agonists could not completely mimic the blocking of TRPV-1 in vivo. MCAO model with TRPV-1 knockout would be more convincing and provide more support for our conclusions. Secondly, the sham-EA group (EA at non-acupoint points) was not presented in the present study. Our previous studies confirmed that EA treatment at non-acupoint points had little effects to regulate the local and systemic reactions, such as delayed-type hypersensitivity and allergic contact dermatitis [29, 30]. In addition, preliminary data revealed that EA pretreatment at non-acupoint points showed no obvious neuroprotection in MCAO rats (data not shown).
In summary, EA pretreatment inhibited the ischemia-reperfusion injury-induced mitochondrial damage and P38 MAPK activation via suppressing TRPV-1. As a result, oxidative injury and inflammatory cytokine production were controlled, which at least partially contributed to the cerebral protection of EA pretreatment on ischemia stroke (Fig. 8). These findings highlight its preventive potential of EA pretreatment for patients with high risk of ischemic stroke.
Proposed mechanisms for cerebral protection of EA pretreatment against CIRI. EA pretreatment at GV20, bilateral BL23, and SP6 acupoints reduced TRPV-1 expression in rat hippocampus, resulting in the suppression of CIRI-induced mitochondrial damage and P38 MAPK phosphorylation. On one hand, EA pretreatment obviously inhibited oxidative injury, such as the reduced cytochrome C and regulated release of MDA, GSH, and SOD. On the other hand, EA pretreatment also significantly curbed the production of inflammatory cytokines, including TNF-α and IL-1β. These findings suggest the involvement of TRPV1-mediated anti-oxidant stress and anti-inflammation in neuroprotection of EA pretreatment against CIRI.
References
Grysiewicz, R.A., K. Thomas, and D.K. Pandey. 2008. Epidemiology of ischemic and hemorrhagic stroke: incidence, prevalence, mortality, and risk factors. Neurologic Clinics 26: 871–895.
Pan, J., A.A. Konstas, B. Bateman, G.A. Ortolano, and J. Pile-Spellman. 2007. Reperfusion injury following cerebral ischemia: pathophysiology, MR imaging, and potential therapies. Neuroradiology 49: 93–102.
Adibhatla, R.M., and J.F. Hatcher. 2008. Tissue plasminogen activator (tPA) and matrix metalloproteinases in the pathogenesis of stroke: therapeutic strategies. CNS & Neurological Disorders Drug Targets 7: 243–253.
Campbell, B.C.V., G.A. Donnan, P.J. Mitchell, and S.M. Davis. 2016. Endovascular thrombectomy for stroke: current best practice and future goals. Stroke and Vascular Neurology 1: 16–22.
Zhuang, Y., J.J. Xing, J. Li, B.Y. Zeng, and F.R. Liang. 2013. History of acupuncture research. International Review of Neurobiology 111: 1–23.
Ulett, G.A., S. Han, and J.S. Han. 1998. Electroacupuncture: mechanisms and clinical application. Biological Psychiatry 44: 129–138.
Zhan, J., R. Pan, M. Zhou, F. Tan, Z. Huang, J. Dong, and Z. Wen. 2018. Electroacupuncture as an adjunctive therapy for motor dysfunction in acute stroke survivors: a systematic review and meta-analyses. BMJ Open 8: e017153.
Sun, N., X. Zou, J. Shi, X. Liu, L. Li, and L. Zhao. 2005. Electroacupuncture regulates NMDA receptor NR1 subunit expression via PI3-K pathway in a rat model of cerebral ischemia-reperfusion. Brain Research 1064: 98–107.
Siu, F.K., S.C. Lo, and M.C. Leung. 2004. Electroacupuncture reduces the extent of lipid peroxidation by increasing superoxide dismutase and glutathione peroxidase activities in ischemic-reperfused rat brains. Neuroscience Letters 354: 158–162.
Chen, Y., Y. Lei, L.Q. Mo, J. Li, M.H. Wang, J.C. Wei, and J. Zhou. 2016. Electroacupuncture pretreatment with different waveforms prevents brain injury in rats subjected to cecal ligation and puncture via inhibiting microglial activation, and attenuating inflammation, oxidative stress and apoptosis. Brain Research Bulletin 127: 248–259.
Cheng, C.Y., J.G. Lin, N.Y. Tang, S.T. Kao, and C.L. Hsieh. 2015. Electroacupuncture at different frequencies (5Hz and 25Hz) ameliorates cerebral ischemia-reperfusion injury in rats: possible involvement of p38 MAPK-mediated anti-apoptotic signaling pathways. BMC Complementary and Alternative Medicine 15: 241.
Bai, J., F. Liu, L.F. Wu, Y.F. Wang, and X.Q. Li. 2018. Attenuation of TRPV1 by AMG-517 after nerve injury promotes peripheral axonal regeneration in rats. Molecular Pain 14: 1744806918777614.
Pegorini, S., D. Braida, C. Verzoni, C. Guerini-Rocco, G.G. Consalez, L. Croci, and M. Sala. 2005. Capsaicin exhibits neuroprotective effects in a model of transient global cerebral ischemia in Mongolian gerbils. British Journal of Pharmacology 144: 727–735.
Longa, E.Z., P.R. Weinstein, S. Carlson, and R. Cummins. 1989. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke 20: 84–91.
Shen, M.H., C.B. Zhang, J.H. Zhang, and P.F. Li. 2016. Electroacupuncture attenuates cerebral ischemia and reperfusion injury in middle cerebral artery occlusion of rat via modulation of apoptosis, inflammation, oxidative stress, and excitotoxicity. Evidence-based Complementary and Alternative Medicine 2016: 9438650.
Kumar, A., Aakriti, and V. Gupta. 2016. A review on animal models of stroke: an update. Brain Research Bulletin 122: 35–44.
Wu, Z., Z. Zou, R. Zou, X. Zhou, and S. Cui. 2015. Electroacupuncture pretreatment induces tolerance against cerebral ischemia/reperfusion injury through inhibition of the autophagy pathway. Molecular Medicine Reports 11: 4438–4446.
Jung, Y.S., S.W. Lee, J.H. Park, H.B. Seo, B.T. Choi, and H.K. Shin. 2016. Electroacupuncture preconditioning reduces ROS generation with NOX4 down-regulation and ameliorates blood-brain barrier disruption after ischemic stroke. Journal of Biomedical Science 23: 32.
Wu, C.X., Y.H. Feng, L. Yang, Z.L. Zhan, X.H. Xu, X.Y. Hu, Z.H. Zhu, and G.P. Zhou. 2018. Electroacupuncture exerts neuroprotective effects on ischemia/reperfusion injury in JNK knockout mice: the underlying mechanism. Neural Regeneration Research 13: 1594–1601.
Khoshnam, S.E., W. Winlow, M. Farzaneh, Y. Farbood, and H.F. Moghaddam. 2017. Pathogenic mechanisms following ischemic stroke. Neurological Sciences 38: 1167–1186.
Hüttemann, M., P. Pecina, M. Rainbolt, T.H. Sanderson, V.E. Kagan, L. Samavati, J.W. Doan, and I. Lee. 2011. The multiple functions of cytochrome c and their regulation in life and death decisions of the mammalian cell: From respiration to apoptosis. Mitochondrion 11: 369–381.
Li, W., and S. Yang. 2016. Targeting oxidative stress for the treatment of ischemic stroke: Upstream and downstream therapeutic strategies. Brain Circulation 2: 153–163.
Amantea, D., C. Tassorelli, F. Petrelli, M. Certo, P. Bezzi, G. Micieli, M.T. Corasaniti, and G. Bagetta. 2014. Understanding the multifaceted role of inflammatory mediators in ischemic stroke. Current Medicinal Chemistry 21: 2098–2117.
Sun, J., and G. Nan. 2016. The mitogen-activated protein kinase (MAPK) signaling pathway as a discovery target in stroke. Journal of Molecular Neuroscience 59: 90–98.
Lan, X., X. Zhang, G.P. Zhou, C.X. Wu, C. Li, and X.H. Xu. 2017. Electroacupuncture reduces apoptotic index and inhibits p38 mitogen-activated protein kinase signaling pathway in the hippocampus of rats with cerebral ischemia/reperfusion injury. Neural Regeneration Research 12: 409–416.
Randhawa, P.K., and A.S. Jaggi. 2017. A review on potential involvement of TRPV1 channels in ischemia-reperfusion injury. Experimental Neurology 295: 66–76.
Miyanohara, J., H. Shirakawa, K. Sanpei, T. Nakagawa, and S. Kaneko. 2015. A pathophysiological role of TRPV1 in ischemic injury after transient focal cerebral ischemia in mice. Biochemical and Biophysical Research Communications 467: 478–483.
Lin, Y.W., and C.L. Hsieh. 2010. Electroacupuncture at Baihui acupoint (GV20) reverses behavior deficit and long-term potentiation through N-methyl-d-aspartate and transient receptor potential vanilloid subtype 1 receptors in middle cerebral artery occlusion rats. Journal of Integrative Neuroscience 9: 269–282.
Wang, Z., T. Chen, M. Long, L. Chen, L. Wang, N. Yin, and Z. Chen. 2017. Electro-acupuncture at Acupoint ST36 ameliorates inflammation and regulates Th1/Th2 balance in delayed-type hypersensitivity. Inflammation 40: 422–434.
Wang, Z., T. Yi, M. Long, Y. Gao, C. Cao, C. Huang, Q. Wang, N. Yin, and Z. Chen. 2017. Electro-acupuncture at Zusanli Acupoint (ST36) suppresses inflammation in allergic contact dermatitis via triggering local IL-10 production and inhibiting p38 MAPK activation. Inflammation 40: 1351–1364.
Funding
This work was supported by a grant from National Natural Science Foundation of China (No. 81574055).
Author information
Authors and Affiliations
Contributions
M.L., Z.G.W., N.N.Y., and Z.B.C. designed the study. M.L and Z.G.W. performed the experiments, analyzed the data, and wrote the manuscript. D.Z., W.T.T., and J.J.C contributed to the animal feeding and preparation of MCAO model. L.W. and N.N.Y. provided technical assistance of sample collection and results analysis for transmission electron microscope. N.N.Y. and Z.B.C revised the manuscript.
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Long, M., Wang, Z., Zheng, D. et al. Electroacupuncture Pretreatment Elicits Neuroprotection Against Cerebral Ischemia-Reperfusion Injury in Rats Associated with Transient Receptor Potential Vanilloid 1-Mediated Anti-Oxidant Stress and Anti-Inflammation. Inflammation 42, 1777–1787 (2019). https://doi.org/10.1007/s10753-019-01040-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-019-01040-y