Abstract
The exact etiology and pathogenesis of chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS) are still unknown, as a result, available therapeutic options for patients are far from satisfactory. Therefore, there is a need to develop a valid therapeutic approach that can ameliorate the manifestations of CP/CPPS. Fifty male C57BL/6 mice were randomly divided into five groups of ten mice each. All groups except naïve were subcutaneously injected with 0.2 ml of T2 plus complete Freund adjuvant (CFA) on day 0 and 14 to generate valid CP/CPPS model. After successful CP/CPPS induction, model group was injected with 0.2 ml of normal saline while PLGA, PLGA-OVA, and PLGA-T2 groups were administered intravenously with 0.2 ml mixture of PLGA, PLGA-OVA, and PLGA-T2, respectively. Voiding behavior, pain threshold, and hematoxylin and eosin staining were used to assess micturition habits, pain intensity as well as prostate inflammation. Additionally, TNF-α, CRP, and IL-10 levels in plasma were measured by using ELISA kits. Mice administered with PLGA-T2 showed higher pain threshold, lower urine frequencies, mild edema, and inflammation in prostate tissue in comparison to other groups. Moreover, the expression of TNF-α and CRP levels was markedly decreased while IL-10 expression was increased in the PLGA-T2 treatment group as compared to the other groups. Our results showed that nanoparticles conjugated with autoantigen novel peptide T2 could successfully alleviate or even heal CP/CPPS to some extent in mice. This study provides an easy, useful, and economic tool for ameliorating the manifestations of CP/CPPS that will improve the therapeutic approaches.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Prostatitis is the most frequently diagnosed outpatient condition in urologic surgery. Chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS) accounts for about 90 to 95% of the incidence of prostatitis, affecting 10 to 14% of men and being the most common urological disease in men younger than 50 years [1,2,3]. According to the National Institutes of Health (NIH) category, this condition is also known as prostatitis type IIIa and type IIIb [4]. Type IIIA is characterized by the presence of white blood cells (WBC) in semen, after a prostate massage urine specimen (VB3) or expressed prostatic secretion (EPS); however, patients suffering from Type IIIB have pelvic pain with no evidence of inflammation on semen, VB3, or EPS. CP/CPPS is mainly characterized by chronic pelvic pain symptoms including pain and discomfort in the perineum, rectum, prostate, penis, testicles, and lower abdomen. The life quality of patients with CP/CPPS is as low as that of patients with congestive heart failure, diabetes, and Crohn’s disease [5,6,7]. Despite many years of clinical and basic research, CP/CPPS mechanism is still not well-established. Thus, available therapies for patients are very few and the effects are also far from satisfactory [8]. The current standard medical therapy for CP/CPPS is principally a multimodal approach using a combination of agents such as nonsteroidal anti-inflammatories, α-blockers, antimicrobial therapy, and 5a-reductase inhibitors [9,10,11].
A large body of evidence suggests that the predominant autoimmune theory is the main factor in the pathogenesis of CP/CPPS [12], and the autoimmune nature of CP/CPPS has also been validated by experimental models of prostatitis [13, 14]. Penna et al. have shown that NOD mice are susceptible to the induction of experimental autoimmune prostatitis (EAP) by injecting prostate extract, i.e., purified prostatic steroid-binding protein (PSBP) [15]. Keetch et al. also reported one of the first mouse models of antigen-induced prostate inflammation. They showed that all C57BL/6 male mice immunized once with prostate tissue extract developed autoimmune prostatitis on day 30 [16]. These EAP models were characterized by the presence of macrophages, dendritic cells, T lymphocytes, and B lymphocytes infiltrating into the prostate interstitium.
For the treatment of complex and refractory autoimmune diseases, researchers have found that the nanoparticle technology platform brings a new technological revolution. Intravenous administration of biodegradable poly lactic-co-glycolic acid (PLGA) nanoparticles combined with autoantigens using ethylene carbodiimide (ECDI) has been demonstrated to be a prospective method for treating autoimmune disease. This method could rebuild peripheral autoimmune tolerance in multiple autoimmune disease models by removing excess circulating inflammatory mediators, and reduce pathological damage [17]. Numerous researchers have already utilized tolerance-based therapies to modulate human autoimmune diseases including multiple sclerosis and type 1 diabetes mellitus [18, 19]. Here in this study, we showed that administration of novel autoantigen peptide T2 from the prostate coupled with PLGA induces immune tolerance and ameliorates the disease symptoms in CP/CPPS mice model.
MATERIALS AND METHODS
Animals
Fifty male C57BL/6 mice weighing 18–22 g and age 6 to 8 weeks were purchased from the Qinglong Mountain Animal Breeding Ground, China. They all were housed in an animal room under standard temperature (22 ± 2 °C) and humidity (50–60%) keeping a 12/12 h light-dark cycle with free access to food and water. All animal experiments were approved by and conducted in accordance with the guidelines of the Committee for Animal Care and Use of the China Pharmaceutical University.
Reagents
T2 peptide with an amino acid sequence of CSEEM RHRFR QLDTK LNDLKG is an antigenic epitope of transient receptor potential cation channel subfamily M member 8 (TRPM8). It was synthesized and purified by Wuhan Buyers Biotechnology Co Ltd., Wuhan, China. OVA was purchased from Beijing Solarbio Science & Technology Co Ltd., Beijing, China.
The 500 nm PLGA nanoparticles with surface carboxylate groups were purchased from Phosphorex, Inc., Hopkinton, Massachusetts, USA. They were resuspended to a concentration of 50 mg/mL in a sterile phosphate-buffered saline (PBS).
Particle Characterization
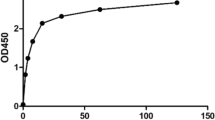
About 10 μ of 25 mg/mL PLGA nanoparticle suspension was diluted with 990 μL ultrapure water. Size and ζ-potential were measured by the Zetasizer Nano ZS from Malvern Instruments, Inc., MA, USA. Particle size was also confirmed by transmission electron microscopy. The efficiency of peptide coupling was determined by using the bicinchoninic acid (BCA) protein quantification kit (determinate supernatant for calculation of the amount of bound biomolecule).
Immunization
All mice were randomly divided into five groups each having 10 mice (n = 10) including the naive group, model group, PLGA group, PLGA-OVA group, and PLGA-T2 group. All groups except naïve were subcutaneously injected with 0.2 ml of T2 plus complete Freund adjuvant (CFA) on days 0 and 14 to generate a valid CP/CPPS model as described previously [20, 21].
T2 was prepared to 1 mg/ml of reserves for a combination of equal volume of CFA (St. Louis, MO, USA) before immunization. After generating a valid model of CP/CPPS on the 28th day based on previous methodology [20, 21], the model group was injected with 0.2 ml of normal saline while all other groups except naïve group were given subcutaneous injection of 200 μl or 0.2 ml of the individual mixture. The final concentration of T2 for mice was 225 μg/ml.
Tolerance Induction with Antigen-Coupled PLGA Nanoparticles
Antigenic peptide T2 was coupled to the PLGA nanoparticles using ECDI (Macklin Biochemical Co., Ltd., Shanghai) according to the manufacturer’s instructions (0.08 mg of peptide in the presence of 0.32 mg ECDI/mg of PLGA nanoparticles). Animals received intravenous injections of approximately 0.2–0.3 mg nanoparticles comprising 10–15 μg of the peptide on day 28. All mice were sacrificed by anesthesia on the 35 days after the final injection.
Pain Threshold Assessment
To evaluate mechanical allodynia, the animals were acclimatized for at least 30 min to the test environment, namely a plexiglass box on a metal grid. The up-down method was applied using a standard set of graded von Frey filaments (IITC Life Science Inc., Woodland Hill, CA, USA) on days 7, 14, 21, and 28 for CPPS induction evaluation and 35th day after treatment with nanoparticles [22]. We used the following filaments: 1.65 g, 2.36 g, 2.44 g, 2.83 g, 3.22 g, 3.61 g, 3.84 g, 4.08 g, 4.17 g, 4.31 g, 4.56 g, and 4.74 g. The probe was situated so that the filament was vertically oriented and applied to either the left or right side of the scrotum until a slight bend was observed in the filament. The filament was held in place for 10 s or until the animal showed a positive response. Sharp retraction of the abdomen, immediate licking and scratching, and jumping of mice were considered as a positive response to filament stimulation.
Voiding Behavior Analysis
We used metabolic cages to analyze the urination in immunized and naive mice. Mice were individually placed in the metabolic cages and allowed to acclimate for 1 h. Urination was monitored over the next hour by placing filter paper under each metabolic cage and recorded the number of urination. Water was withheld at the beginning of the acclimation period to the entire experiment. The placement of mice in the cages and removal from cages were performed calmly to avoid startling and causing reflex urination. The collected papers were imaged under UV light to visualize urine stains. Finally, urine spots were evaluated by using the Fiji version of ImageJ software. The number of urine spots represents urine frequency. Spot areas < 6.6 mm2 were excluded as they may be caused by claw or tooth marks. Data was collected by the blind observers.
Histopathology
The anterior prostate (AP) and ventral prostate (VP) lobes were harvested and fixed in neutral buffered 10% formalin for 48 h, dehydrated in ethanol, embedded in paraffin, and serially sectioned (5 μm) for hematoxylin-eosin staining. The severity of prostatitis was analyzed by using a four-level grading scale. Two independent observers scored prostate sections in a randomized order. Standardized scoring categories were as follows: grade 0, normal architecture; grade 1, a portion of acini showed epithelial cell exfoliation, and minor focal inflammatory infiltrate; grade 2, most of the acini showed epithelial cell exfoliation and minor inflammatory infiltrate; and grade 3, atrophy in epithelia of most acini, hyperemia, and marked inflammatory infiltration. The slides were observed under a light microscope.
ELISA Assays
The concentrations of TNF-α, C-reactive protein (CRP), and IL-10 in plasma were determined by commercially available ELISA kits. The blood obtained from the carotid artery was put into the heparin for 30 min. It was then centrifuged at 2500 rpm for 20 min. Then the supernatant was collected and stored at − 80 °C for further use. The contents of TNF-α, CRP, and IL-10 were quantified by mouse TNF-α ELISA kit (Eecell Bio, Co., Ltd., China), mouse CRP ELISA kit (Cusabio biotech co., Ltd., China), and IL-10 ELISA kit (Proteintech Group, Rosemont, IL, USA) following the manufacturer’s instructions.
Statistical Analysis
Group data are represented as means ± standard error of the mean (SEM). Macroscopic and microscopic damage scores were measured using the Mann-Whitney U test or one-way analysis of variance (ANOVA). Cytokine ELISAs were analyzed by a one-way ANOVA. Figures were compiled using GraphPad Prism v 6.00 software. The P values (P < 0.05, P < 0.01, P < 0.001) were considered significant in all analyses.
RESULTS
PLGA Nanoparticle Characterization
PLGA nanoparticles were characterized by transmission electron microscopy and dynamic light scattering, both of which indicated a mean diameter between 400 and 500 nm (Fig. 1a &b). Population analyses by dynamic light scattering established that the z-average diameter of the PLGA particles was 485.7 ± 6.17 nm. And the ζ-potential of the PLGA nanoparticles was found to be − 47.1 ± 6.13 mV (Fig. 1c).
Behavioral Test
Pain Behavior
Pain threshold was recorded for all groups on day 28 of CP/CPPS induction and post-treatment with nanoparticles. As shown in Fig. 2, the model group, PLGA, PLGA-OVA, and PLGA-T2 groups showed a significant difference on day 28 as compared to the naïve group indicating that the EAP model has been induced in these groups.
After successful induction of the CP/CPPS model, all the mice were treated with different mixtures in different groups for 7 days and the resultant pain threshold on day 35 (post) was compared relative to day 28 (pre) pain threshold. The model group, PLGA group, and PLGA-OVA group showed no significant difference on day 35 as compared to the day 28; however, the PLGA-T2-treated group showed a significant increase in the withdrawal threshold indicating that PLGA-T2 nanoparticles successfully attenuated the pain on day 35 in comparison to day 28 (Fig. 2).
Voiding Behavior
This analysis was based on the Voided Stain On Paper (VSOP) method [23]. Urine stains on the paper were analyzed to determine voiding behavior. As shown in Fig. 3, the number of urine spots in the model group, PLGA, and PLGA-OVA groups was more significant in comparison to the naïve group. PLGA and PLGA-OVA treatment groups did not show any difference from the model group; however, when we compared PLGA-T2 group with the model group, we found a significant decrease in the number of urine spots with almost the same results as in the naive group (Fig. 3).
Voiding behavior analysis. a Analysis of urine spot using ImageJ software. The arrows indicate the excluded urine spot < 6.6 mm2. b Numbers of urine spots. *P < 0.05 indicating the comparison of naïve and PLGA-T2 groups with the model group. #P < 0.05 indicating the comparison of treated groups with the naive group.
Histopathology
H&E-stained slides were scored for severity of inflammation as described in the “MATERIALS AND METHODS” section. Naive animals showed normal prostatic histology with minimal inflammatory infiltrate, vascular changes, or tissue damage. However, significant inflammation and infiltration of inflammatory cells were observed even after the treatment with respective nanoparticles in the model group, PLGA group, and PLGA-OVA group. In contrast, PLGA-T2-treated group showed reduced pathology and no infiltration of inflammatory cells. Thus PLGA-T2 successfully reduced inflammatory signs and inflammatory cell infiltration (Fig. 4a).
a Histopathologic observations of the prostate tissue from C57BL/6 mice (magnification: × 400). The naive group displayed no inflammation and infiltration of the inflammatory cells. The model group, PLGA group, and PLGA-OVA group showed extensive inflammatory cell infiltration and inflammatory lesion as compared to the naïve group while PLGA-T2 group displayed no inflammatory cell infiltration after treatment. b Mean inflammatory scores of different groups. Inflammation scores of the model, PLGA, and PLGA-OVA groups showed a significant difference (###P < 0.001) as compared to the naïve group; PLG-T2-treated group exhibited (***P < 0.001) minimal prostate inflammation in comparison to the model group. ###P < 0.001 indicating significant difference of treated groups from the naïve group; ***P < 0.001 indicating a significant difference of naive and PLGA-T2 groups from the model group.
As expected, the inflammation scores of the model, PLGA, and PLGA-OVA groups were much higher than the naïve group indicating that inflammation of prostate gland was not reduced even after treatment with respective nanoparticles in these groups. Interestingly, mice treated with PLG-T2 showed minimal inflammation score demonstrating that these mice have minimal prostate inflammation as compared to the model group (Fig. 4b).
Plasma Levels of TNF-α, CRP, and IL-10
Elisa was used to measure the plasma levels of TNF-α, CRP, and IL-10 in each group. As shown in Table 1 and Fig. 5a, the plasma levels of TNF-α, and CRP in the model group (#P < 0.05) were significantly different as compared to the naïve group. The PLGA and PLGA-OVA groups showed no significant difference in TNF-α and CRP serum levels when compared to the naïve group. The plasma levels of TNF-α and CRP in PLGA-T2 group were also not significantly different from the naive group. When compared to the model group, no significant difference was seen in PLGA and PLGA-OVA groups while the PLGA-T2-treated group showed significant difference (*P < 0.05) and showed minimal TNF-α and CRP levels as compared to the model group. As expected, the plasma level of IL-10 in PLGA-T2 group was significantly higher (#*P < 0.05) than the naïve, model, and other treatment groups. PLGA and PLGA-OVA treatment groups showed no significant difference in comparison to the naïve and model groups.
Comparison of the expression levels of TNF-α, CRP, and IL-10 in plasma. Each value was represented as means ± SEM. a Quantification of TNF-α and CRP in all groups. The model group exhibited enhanced serum levels of TNF-α (##P < 0.01), and CRP (#P < 0.05), while PLGA, PLGA-OVA, and PLGA-T2 groups displayed no significant serum levels of TNF-α and CRP in comparison to the naïve group. b Quantification of IL-10 level in all groups. PLGA-T2 treatment group revealed higher expression of anti-inflammatory IL-10 (#*P < 0.05) as compared to naïve and model groups. #Significant difference (P < 0.05) from the naïve group; ##significant difference (P < 0.01) from the naïve group *significant difference (P < 0.05) from the model group; **significant difference (P < 0.05) from the model group.
DISCUSSION
Chronic prostatitis/chronic pelvic pain syndrome is one of the most complex, bothersome, and enigmatic diseases with unknown etiology and pathogenesis. While the exact cause of chronic prostatitis remains unknown, there is substantiating evidence to support the role of the immune system in its pathogenesis [24,25,26]. Previously, numerous animal models were established by immune methods to explore drugs for treating CP/CPPS. Recently, our lab also developed EAP animal model via multipoint subcutaneous injection of autoantigen T2 peptide from the prostate, which induced marked inflammatory cell infiltration into the prostate with increased expression of TNF-α, IL-1β, and CRP [20, 21]. The common treatments for autoimmune disease include the use of a wide range of immunosuppressive drugs and usually require long-term administration, which may lead to reactivation of latent pathogens, tumor development, and opportunistic infections [27].
The emergence of nanoparticle-based therapies for inducing antigen-specific immune tolerance provides considerable promise for the future of immunotherapy [28, 29]. Five-hundred-nanometer NPs with negative zeta potential promote immune tolerance in autoimmune disease models. NPs from FDA-approved biocompatible and biodegradable polymers such as PLGA have shown enormous potential in immune modulatory therapies [28]. Hunter et al. reported that PLGA nanoparticles combined with PLP139–151 giving subcutaneously or intravenously into the experimental autoimmune encephalomyelitis (EAE) mice can prevent and treat recurrent EAE, reducing central nervous system pathogenic Th1, Th17 cells, and macrophage infiltration [30]. Daniel et al. also demonstrated that intravenous administration of either polystyrene or PLGA-bearing encephalitogenic peptides can prevent the onset of EAE and modify the course of EAE [31]. In light of these reports, we also demonstrated for the first time that intravenous administration of nanoparticles coupled to T2 peptide could successfully ameliorate CP/CPPS in experimental mice model. We have previously determined that T2 peptide is the dominant antigenic epitope of TRPM8 and plays a critical role in inducing CP/CPPS [20, 32]. T2 is a special peptide sequence isolated from a TRPM8 protein that is encoded by a prostate-specific gene [33]. It is an autoantigen peptide having amino acid sequence of CSEEM RHRFR QLDTK LNDLKG [18]. Coupling of this disease-relevant immunogenic T2 peptide to biodegradable PLGA nanoparticle resulted in the reversal of disease symptoms in our study. Here in this study, we first established EAP model by subcutaneously injecting a mixture of immunogenic novel peptide T2 and complete Freund’s Adjuvant (CFA) into all groups except naïve group to induce CP/CPPS as described in the literature. CP/CPPS was characterized by increased inflammatory cell infiltration such as T cell, macrophages, granulocytes, and proinflammatory cytokines into the prostate interstitium and epithelium [34,35,36]. After successful induction of CP/CPPS, the model group was injected with normal saline while PLGA, PLGA-OVA, and PLGA-T2 groups were administered intravenously with a mixture of PLGA, PLGA-OVA, and PLGA-T2. We observed a significant decrease of pelvic region tactile hyperalgesia in the PLGA-T2 group in comparison to the pretreated group and a significant difference in the urinary frequency of PLGA-T2 group in comparison to the model group. Also, a less number of inflammatory cell infiltration such as T cell, macrophages, and granulocytes into the prostate interstitium and epithelium were observed in the PLGA-T2 group as compared to the model group. Furthermore, less expression of TNF-α and CRP while high expression of IL-10 in PLGA-T2 group were observed against the other groups. In other words, we can say that groups immunized with PLGA-T2 showed less inflammation than the other groups, which leads to the conclusion that nanoparticles conjugated with autoantigen novel peptide T2 could successfully alleviate CP/CPPS in mice.
Many researchers believe that autoimmunity is the key factor involved in the development of CP/CPPS. Immune disturbance in CP/CPPS leads to enhanced accumulation of T cells, macrophages, B cells, and granulocytes in the prostate of CP/CPPS patients without infection. The previous successful model of CP/CPPS was characterized by T cell and antibody responses to antigen origin from the prostate [37, 38]. In our study, there was obvious inflammation and infiltration of inflammatory cells in the model, PLGA, and PLGA-OVA groups. However, PLGA-T2 group was not significantly different from the naive group, although some of the tissues did exhibit a scattered mild inflammatory infiltrates.
Cytokines are important molecules in the immune response which allows inflammatory cells to communicate and regulate local and systemic events of the immune response. Recently, cytokines such as TNF-α, IL-1β, and IL-10 as a proximal mediator in the pathogenesis of CP/CPPS have attracted much attention. TNF-α is synthesized by monocytes and macrophages and has a prominent role in infection and inflammation. Alexander et al. demonstrated that the seminal plasma in patients with CPPS had significantly higher levels of the pro-inflammatory cytokines TNF-α and IL-1β [39]. In another study, it has been demonstrated that the level of TNF-α was elevated in EPS specimens suffering from CP and believes to be involved in the progression of CP [40, 41]. C-reactive protein (CRP) is a β globulin factor present in normal serum in trace amounts which may increase as much as 3000-folds in the acute phase of inflammation [42]. Serum CRP levels have been associated with benign prostatic hyperplasia (BPH)/lower urinary tract symptoms (LUTS) [43]. Disease progression can be assessed by evaluating serum CRP levels. On the other hand, IL-10 is an anti-inflammatory factor produced by Th2 cells and activated B cells. It plays a key role in down-regulating inflammatory response and antagonizing inflammatory mediators in various inflammatory reactions. Miller et al. showed that IL-10 plays a crucial role in the inflammatory immune response of chronic prostatitis. Examination of IL-10 levels can indirectly understand the local inflammatory response of tissues [44]. In light of these evidence, we can say that a patient suffering from CP/CPPS will have increased levels of pro-inflammatory cytokines such as TNF-α and IL-1β and elevated level of CRP protein while decreased level of IL-10. In our research, we observed that PLGA-T2 group expressed lower serum level of TNF-α in comparison to the model group. Likewise, we observed a significant difference in the plasma level of CRP for PLGA-T2 group in comparison to the model group. Furthermore, we also observed that the plasma level of IL-10 for the PLGA-T2 group was significantly higher than the naïve and model groups while no significant difference was observed in other groups as compared to the naïve and model groups.
The precise mechanism underpinning immune tolerance is unclear in this study. However, we speculate that PLGA-T2 might enhance the level of regulatory/immunosuppressive cytokine, IL-10 in the blood. This cytokine is critical for tolerance induction, as previously published studies indicated that IL-10-deficient mice or mice treated with anti-IL-10 cannot be tolerated [45]. IL-10 was initially defined as a T cell cytokine, secreted from macrophages and regulatory B cells as well as T cells [46]. Production of IL-10 as a result of nanoparticle-coupled-T2 peptide finally induced antigen-specific immune tolerance, suppressed inflammation, antigen-presentation [47] and monocyte-macrophage function, and down-regulated TNF-α [40] that eventually led to the suppression of the disease. In light of these findings, we can speculate that PLGA-T2 could not only inhibit the production of pro-inflammatory factor TNF-α and C-reactive protein (CRP) in the plasma but also increase the level of anti-inflammatory factor IL-10, that simultaneously improves the inflammatory response of the prostate tissue. Though some progress has been made, considerable work still remains to be done to warrant further research. The future investigation of our study should include but not limit to explore the role of antigen-specific Treg cells that plays a critical role in maintaining long-term tolerance induction that will help in better understanding of the underlying mechanism.
CONCLUSION
In summary, this study demonstrated that PLGA nanoparticles conjugated to T2 peptide could successfully mitigate or even heal CP/CPPS to some extent. This study provides an easy, useful, and economic tool for ameliorating the manifestations of CP/CPPS that will improve the therapeutic approaches.
References
Krieger, J.N., D.E. Riley, P.Y. Cheah, M.L. Liong, and K.H. Yuen. 2003. Epidemiology of prostatitis: new evidence for a world-wide problem. World Journal of Urology 21 (2): 70–74.
Nickel, J.C., J. Downey, D. Hunter, and J. Clark. 2001. Prevalence of prostatitis-like symptoms in a population based study using the National Institutes of Health chronic prostatitis symptom index. The Journal of Urology 165 (3): 165(3):842–5.
Khan, F.U., A.U. Ihsan, H.U. Khan, R. Jana, J. Wazir, P. Khongorzul, M. Waqar, and X. Zhou. 2017. Comprehensive overview of prostatitis. Biomedicine & Pharmacotherapy 94: 1064–1076.
Vasdev N and Thorpe AC. Chronic prostatitis / chronic pelvic pain syndrome. Clinical Management of Complicated Urinary Tract Infection. 2011.
Wagenlehner, F.M.E., J.W.O. van Till, V. Magri, G. Perletti, J.G.A. Houbiers, W. Weidner, and J.C. Nickel. 2013. National Institutes of Health Chronic Prostatitis Symptom Index (NIH-CPSI) symptom evaluation in multinational cohorts of patients with chronic prostatitis/chronic pelvic pain syndrome. European Urology 63 (5): 953–959.
McNaughton Collins, M., M.A. Pontari, and M.P. O'Leary. 2001. Quality of life is impaired in men with chronic prostatitis: the Chronic Prostatitis Collaborative Research Network. Journal of General Internal Medicine 16 (10): 656–662.
Wenninger, K., J.R. Heiman, I. Rothman, J.P. Berghuis, and R.E. Berger. 1996. Sickness impact of chronic nonbacterial prostatitis and its correlates. The Journal of Urology 155 (3): 965–968.
Yang, C.C., et al. 2003. Pain sensitization in male chronic pelvic pain syndrome: why are symptoms so difficult to treat? The Journal of Urology 170 (3): 823–826 discussion 826-7.
Polackwich, A.S., and D.A. Shoskes. 2016. Chronic prostatitis/chronic pelvic pain syndrome: a review of evaluation and therapy. Prostate Cancer and Prostatic Diseases 19 (2): 132–138.
Rees, J., M. Abrahams, A. Doble, A. Cooper, and the Prostatitis Expert Reference Group (PERG). 2015. Diagnosis and treatment of chronic bacterial prostatitis and chronic prostatitis/chronic pelvic pain syndrome: a consensus guideline. BJU International 116 (4): 509–525.
Herati, A.S., and R.M. Moldwin. 2013. Alternative therapies in the management of chronic prostatitis/chronic pelvic pain syndrome. World Journal of Urology 31 (4): 761–766.
Breser, M.L., F.C. Salazar, V.E. Rivero, and R.D. Motrich. 2017. Immunological mechanisms underlying chronic pelvic pain and prostate inflammation in chronic pelvic pain syndrome. Frontiers in Immunology 8: 898.
Alexander, R.B., F. Brady, and S. Ponniah. 1997. Autoimmune prostatitis: evidence of T cell reactivity with normal prostatic proteins. Urology 50 (6): 893–899.
Rivero, V. 2002. Prostatein or steroid binding protein (PSBP) induces experimental autoimmune prostatitis (EAP) in NOD mice. Clinical Immunology 105 (2): 176–184.
Penna, G., S. Amuchastegui, C. Cossetti, F. Aquilano, R. Mariani, N. Giarratana, E. de Carli, B. Fibbi, and L. Adorini. 2007. Spontaneous and prostatic steroid binding protein peptide-induced autoimmune prostatitis in the nonobese diabetic mouse. The Journal of Immunology 179 (3): 1559–1567.
Keetch, D.W., P. Humphrey, and T.L. Ratliff. 1994. Development of a mouse model for nonbacterial prostatitis. The Journal of Urology 152 (1): 247–250.
Smith, D.M., J.K. Simon, and J.R. Baker Jr. 2013. Applications of nanotechnology for immunology. Nature Reviews. Immunology 13 (8): 592–605.
Miller, S.D., D.M. Turley, and J.R. Podojil. 2007. Antigen-specific tolerance strategies for the prevention and treatment of autoimmune disease. Nature Reviews. Immunology 7 (9): 665–677.
Luo, X., K.C. Herold, and S.D. Miller. 2010. Immunotherapy of type 1 diabetes: where are we and where should we be going? Immunity 32 (4): 488–499.
Khan, F.U., A.U. Ihsan, W. Nawaz, M.Z. Khan, M. Yang, G. Wang, X. Liao, L. Han, and X. Zhou. 2017. A novel mouse model of chronic prostatitis/chronic pelvic pain syndrome induced by immunization of special peptide fragment with aluminum hydroxide adjuvant. Immunology Letters 187: 61–67.
Zhang, L., et al. 2018. Establishment of experimental autoimmune prostatitis model by T2 peptide in aluminium hydroxide adjuvant. Andrologia 50 (3).
Dixon, W.J. 1980. Efficient analysis of experimental observations. Annual Review of Pharmacology and Toxicology 20: 441–462.
Sugino, Y., A. Kanematsu, Y. Hayashi, H. Haga, N. Yoshimura, K. Yoshimura, and O. Ogawa. 2008. Voided stain on paper method for analysis of mouse urination. Neurourology and Urodynamics 27 (6): 548–552.
Murphy, S.F., A.J. Schaeffer, and P. Thumbikat. 2014. Immune mediators of chronic pelvic pain syndrome. Nature Reviews. Urology 11 (5): 259–269.
Vykhovanets, E.V., M.I. Resnick, G.T. MacLennan, and S. Gupta. 2007. Experimental rodent models of prostatitis: limitations and potential. Prostate Cancer Prostatic Disease 10: 15–29.
Rivero, V.E., R.D. Motrich, M. Maccioni, and C.M. Riera. 2007. Autoimmune etiology in chronic prostatitis syndrome: an advance in the understanding of this pathology. Critical Reviews in Immunology 27 (1): 33–45.
Carbone, J., N. del Pozo, A. Gallego, and E. Sarmiento. 2011. Immunological risk factors for infection after immunosuppressive and biologic therapies. Expert Review of Anti-Infective Therapy 9 (4): 405–413.
Getts, D.R., L.D. Shea, S.D. Miller, and N.J.C. King. 2015. Harnessing nanoparticles for immune modulation. Trends in Immunology 36 (7): 419–427.
Serra, P., and P. Santamaria. 2018. Nanoparticle-based approaches to immune tolerance for the treatment of autoimmune diseases. European Journal of Immunology 48 (5): 751–756.
Hunter, Z., D.P. McCarthy, and W.T. Yap. 2014. A biodegradable nanoparticle platform for the induction of antigen-specific immune tolerance for treatment of autoimmune disease. ACS Nano 8 (3): 2148–2160.
Getts, D.R., A.J. Martin, D.P. McCarthy, R.L. Terry, Z.N. Hunter, W.T. Yap, M.T. Getts, M. Pleiss, X. Luo, N.J.C. King, L.D. Shea, and S.D. Miller. 2012. Microparticles bearing encephalitogenic peptides induce T-cell tolerance and ameliorate experimental autoimmune encephalomyelitis. Nature Biotechnology 30 (12): 1217–1224.
Ihsan, A.U., F.U. Khan, W. Nawaz, M.Z. Khan, M. Yang, and X. Zhou. 2017. Establishment of a rat model of chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS) induced by immunization with a novel peptide T2. Biomedicine & Pharmacotherapy 91: 687–692.
Du, S., et al. 2007. Differential expression profile of cold (TRPA1) and cool (TRPM8) receptors in human urogenital organs. Urology 72 (2): 450–455.
Jarrell, J., M.A. Giamberardino, and M. Robert. 2011. Bedside testing for chronic pelvic pain: discriminating visceral from somatic pain. Pain Research and Treatment 692102. https://doi.org/10.1155/2011/692102.
Jarrell, J. 2009. Demonstration of cutaneous allodynia in association with chronic pelvic pain. Journal of Visualized Experiments 28.
Schaeffer, J.A. 2006. Chronic prostatitis and the chronic pelvic pain syndrome. The New England Journal of Medicine 355 (16): 1690–1698.
Ludwig, M., C. Steltz, and P. Huwe. 2001. Immunocytological analysis of leukocyte subpopulations in urine specimens before and after prostatic massage. European Urology 39: 277–282.
Yang, Z., X. Wang, G. Zhu, Z. Zhou, Y. Wang, D. Chen, and Z. Meng. 2011. Effect of surgical castration on expression of TRPM8 in urogenital tract of male rats. Molecular Biology Reports 39 (4): 4797–4802.
Alexander, R.B., S. Ponniah, J. Hasday, and J.R. Hebel. 1998. Elevated levels of proinflammatory cytokines in the semen of patients with chronic prostatitis/chronic pelvic pain syndrome. Urology 52 (5): 744–749.
Pontari, M.A., and M.R. Ruggieri. 2004. Mechanisms in prostatitis/chronic pelvic pain syndrome. The Journal of Urology 172 (3): 839–845.
Nadler, R.B., et al. 2000. IL-1beta and TNF-alpha in prostatic secretions are indicators in the evaluation of men with chronic prostatitis. The Journal of Urology 164 (1): 214–218.
Girgis, S.M., E. Ekladios, R.M. Iskandar, S. El-Haggar, N. Moemen, and S.M. El-Kassem. 1983. C-reactive protein in semen and serum of men with chronic prostatitis. Andrologia 15 (2): 151–154.
Liao, C.H., S.D. Chung, and H.C. Kuo. 2011. Serum C-reactive protein levels are associated with residual urgency symptoms in patients with benign prostatic hyperplasia after medical treatment. Urology 78 (6): 1373–1378.
Miller, L.J., K.A. Fischer, S.J. Goralnick, M. Litt, J.A. Burleson, P. Albertsen, and D.L. Kreutzer. 2002. Interleukin-10 levels in seminal plasma: implications for chronic prostatitis-chronic pelvic pain syndrome. The Journal of Urology 167 (2 Pt 1): 753–756.
Getts, D.R., D.M. Turley, C.E. Smith, C.T. Harp, D. McCarthy, E.M. Feeney, M.T. Getts, A.J. Martin, X. Luo, R.L. Terry, N.J.C. King, and S.D. Miller. 2011. Tolerance induced by apoptotic antigen-coupled leukocytes is induced by PD-L1+ and IL-10-producing splenic macrophages and maintained by T regulatory cells. Journal of Immunology 187 (5): 2405–2417.
Zhang, Y., H.J. Kim, S. Yamamoto, X. Kang, and X. Ma. 2010. Regulation of Interleukin-10 gene expression in macrophages engulfing apoptotic cells. Journal of Interferon & Cytokine Research 30 (3): 113–122.
Ankit Saxena, A., et al. 2015. Interleukin-10 paradox: a potent immunoregulatory cytokine that has been difficult to harness for immunotherapy. Cytokine 74: 27–34.
Funding
This work was supported by the National Natural Science Foundation of China (grant numbers 30973003; 30901993); and the Administration of TCM of Jiangsu Province (grant number LZ11093).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
All authors declare no conflict of interest.
Submission Declaration and Verification
This manuscript has not been published previously, and it is not under consideration elsewhere. All authors approved to submit this manuscript to this journal.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cheng, Y., Cao, Y., Ihsan, A.U. et al. Novel Treatment of Experimental Autoimmune Prostatitis by Nanoparticle-Conjugated Autoantigen Peptide T2. Inflammation 42, 1071–1081 (2019). https://doi.org/10.1007/s10753-019-00968-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-019-00968-5