Abstract
Chronic prostatitis/chronic pelvic pain syndromes (CP/CPPS) is a clinical tricky problem due to its enigmatic etiology, low cure rate, and high recurrence rate. The research on its pathogenesis has never stopped. In this experimental autoimmune prostatitis (EAP) model, male C57BL/6 mice were subcutaneously immunized with prostate extracts in an adequate adjuvant. For mice in the antibody intervention group, anti-T2 polyclonal antibodies were intraperitoneally injected during the induction of EAP. Animals were periodically monitored for pelvic pain. Hematoxylin and eosin staining was used to assess prostate inflammation. Tumor necrosis factor-α (TNF-α) levels in serum were measured by ELISA kits. The immunized animals developed prostatitis as a consequence of the immune response against prostate antigens. Pelvic pain thresholds were gradually decreased and TNF-α expression significantly increased. T2 plays an important role in the disease since polyclonal antibodies to T2 greatly ameliorated symptoms in animals induced for EAP. T2 peptide may represent the major autoantigen epitope in EAP, which could serve for a better understanding of the etiology of CP/CPPS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
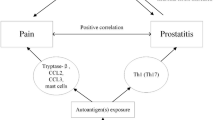
CP/CPPS constitutes approximately 90% of all chronic prostatitis cases [1]. The prevalence varies from 2 to 16% worldwide [2]. Apart from the local damage and inflammation of pelvic region including prostate, longitudinal clinical changes in CP/CPPS patients may also include painful bladder filling and/or painful urinary urgency [3], irritable bowel syndrome [4], fibromyalgia [5], chronic fatigue syndrome [6], structural and functional brain changes [7], depression, and panic disorders [8]. Management of CP/CPPS patients has always been a formidable task in clinical practice. The unclear etiology slows the development of therapies.
Various theories have been suggested to explain CP/CPPS. The autoimmune origin has emerged as an important area [9, 10]. The identification of target antigens in autoimmune diseases is always an important step towards understanding the etiology. We have previously examined the ability of T2 peptide to induce EAP in rats [11] and mice [12, 13] and concluded that when incorporated into an adequate adjuvant, T2 elicits cellular and humoral autoimmune responses and lesions in prostate. In this study, intraperitoneal injections of anti-T2 polyclonal antibodies ameliorated disease in the EAP model induced by prostate extracts, which preliminarily proved that the epitope contained within the sequence T2 represented the main autoantigen epitope of EAP.
METHODS
Antibody
The amino acid sequence of T2 peptide is CSEEMRHRFRQLDTKLNDLKG. Polyclonal antibodies to T2 were prepared by injecting New Zealand rabbits with purified T2 peptide-keyhole limpet hemocyanin (KLH) conjugates (200 μg per injection) subcutaneously in complete Freund’s adjuvant followed by subsequent boosts in incomplete Freund’s. The reactivity of polyclonal antibodies with T2 was confirmed using ELISA.
Experimental Autoimmune Prostatitis
Preparation of Prostate Extracts
Prostate extracts were prepared from adult C57BL/6 mice prostate glands. Anterior and ventral lobes of prostate from 35 mice were pooled and homogenized in 0.5% Triton X-100 at 4 °C. The homogenate was centrifuged at 10,000 rpm for 30 min, and the supernatant was used as antigens. Protein concentration was determined by the Bicinchoninic Acid assay (Beyotime biotechnology, China). The supernatant was diluted in phosphate-buffered saline (0.01 M, pH 7.2), and final concentration was adjusted to 10 mg/ml.
Immunization and Grouping
Twenty adult male C57BL/6 mice (18–22 g) were injected with 0.2-ml CFA containing 5 mg/ml prostate extracts subcutaneously on days 0 and 14 to induce EAP. 10 age-matched naive animals remained uninduced. The day of first injection was designated day 0. On days 3, 17, 19, and 21, ten animals in the antibody group were injected with T2 polyclonal antibodies (0.5 ml/injection per mouse, i.p.), and the model group was given a vehicle control (0.01 M phosphate-buffered saline, i.p.). The choice of antibody administration schedule was mainly based on two research articles [14, 15]. Animals were periodically monitored for pelvic pain. On day 28, animals were killed, and prostates were prepared for histopathological examination. All animal studies were approved by and conducted in accordance with the guidelines of the Committee for Animal Care and Use of the China Pharmaceutical University.
Pelvic Pain Threshold Assessment
On days -6, 7, 14, 21 and 27, von Frey filaments were used to measure the mechanical pain threshold in the pelvic region of mice. According to the up and down method [16], eight filaments with force ranging from 0.008 to 4 were used to stimulate the vicinity of the mouse scrotum vertically until the filament was “S”-shaped and held for 6–8 s, or stopped when the animal showed a positive response. The interval between two stimulations is 7 s. Sharp contraction of the abdomen, immediate licking and biting of the stimulated area, or jumping of mice can all be considered as positive reactions to the filament stimulation.
Histopathological Evaluation
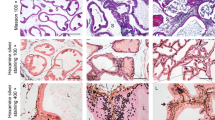
After mice’s death, the anterior and ventral lobes of prostate were collected, fixed with 10% formalin for 24 h, then dehydrated, embedded in paraffin, and sectioned (6 μm). Hematoxylin and eosin staining was performed. Morphological changes and inflammatory cells infiltration were assessed in a double-blind manner. The scoring criteria are as follows: 0, no inflammation; 1, slight inflammatory cells in the stroma, perivascular and periglandular regions; 2, moderate infiltration accompanied by degeneration of epithelial cells; 3, severe epithelial acini atrophy, inflammatory cells infiltration and congestion.
Cytokine Determination
Blood obtained from heart, 60 min for natural coagulation in ambient temperature, was centrifuged at 3000 rpm for 10 min. The supernatant was collected carefully and stored at − 80 °C. TNF-α levels were determined by ELISA kits (Nanjing Jiancheng Bioengineering Institute, China).
Statistical Analysis
All statistical analyses were performed using SPSS v 21.0 software. The data were shown as the mean ± standard deviation. One-way analysis of variance (ANOVA) was used to determine significance among groups. Figures were compiled using GraphPad Prism v 5.01 software. P < 0.05 was considered to indicate a statistically significant difference.
RESULTS
Production of Anti-T2 Polyclonal Antibodies
To determine whether T2 plays a key role in the induction of EAP, anti-T2 polyclonal antibodies were purified from the serum of T2-immunized rabbits. The concentration of the affinity-purified antibody measured by NanoDrop Spectrophotometer A280nm was 0.569 mg/ml, and the titer determined by ELISA was greater than 1/512,000 (Fig. 1), suggesting that anti-T2 polyclonal antibodies were able to effectively bind to its molecular target.
T2 Is Involved in the Development of EAP
Pain and inflammation without having the infection are hallmarks of CP/CPPS [17]. And prostate histopathology is the core indicator to judge the success of EAP models [18]. Pelvic pain threshold assessment for this study was shown in Fig. 2. The model group had a lower pain threshold, which was lowest on day 14. Mice treated with anti-T2 antibodies showed a small fluctuation in the pain threshold. The pain threshold decreased during 14–21 days and then rose to the original threshold, which cannot exclude the influence caused by intraperitoneal injections of antibodies on days 17 and 19.
Prostate histopathological scores were shown in Fig. 3. Compared with naive mice, the inflammation scores of the model group were significantly increased (P < 0.01), while mice treated with the antibody had no significant difference. Figure 4 showed the representative histopathological changes in these animals on day 28. Some epithelial acini atrophy, gland reduction and inflammatory cells infiltration were observed in the model group. However, in the naive and antibody groups, the glands had a uniform distribution, no obvious atrophy was found, and minimal inflammatory cells infiltrated in the interstitium. The detection of TNF-α in mouse serum further confirmed the role of T2 in the development of EAP. Compared with the naive group, TNF-α levels in the model group significantly increased (P < 0.05), while it decreased after antibody intervention (Fig. 5).
DISCUSSION
EAP models have long been used for studying CP/CPPS. Prostate extracts immunization is one of the typical induction of EAP [19, 20]. The histologic pattern of prostate inflammation, a prominent infiltration of inflammatory cells into the interstitium primarily surrounding vessels and acini, is strikingly similar to that of CP/CPPS patients. In addition, immunization of murine strains with other antigens, such as prostatic steroid-binding protein (PSBP) [21], prostatic acid phosphatase (PAP) [22] and T2 [13], can also induce autoantigen-specific T cell and antibody responses associated with histological evidence of prostate inflammation. Prostate extracts contained a variety of autoantigens. In this study, the antibody group used anti-T2 polyclonal antibodies to intervene the EAP induction after primary immunization with prostate extracts. During the 28-day induction period, the mechanical pain threshold of pelvic region had no significant change compared with the naive group. The inflammation of prostate was significantly reduced compared with the model group (P < 0.01), and the serum TNF-α level also showed a downward trend. These results indicated, at least in part, that T2 may represent the major autoantigen epitope in prostate extracts. Autoantigen exposure and subsequent recognition by the immune system are key starting points for autoimmune diseases. After intraperitoneal injections of anti-T2 polyclonal antibodies, the antibody binds to the T2 epitope of autoantigens, similar to the knockdown of T2, thus preventing the recruitment, further activation and proliferation of immune cells, and achieving the purpose of symptom relief and etiological treatment.
The transient receptor potential melastatin 8 (TRPM8), which was initially identified as a prostate-specific antigen [23], has sparked our interest in the study of the possible relationship between TRPM8 and CP/CPPS due to its later discovery in human multiple organs and tissues [24] and its role as a cold receptor [25, 26], corresponding to the systemic symptoms [3], cold exacerbation symptoms, and cold-induced relapse [27] in CP/CPPS patients. T2 peptide envelops the epitope derived from TRPM8 (1075–1094 amino acid), which is specific to other transient receptor potential (TRP) channels. Previously, our laboratory has successfully induced CP/CPPS in rats [11] and mice [12, 13] using T2 peptide combined with adjuvants. Intravenous nanoparticle–coupled T2 [28, 29] and oral T2 peptide [30] can both induce immune tolerance and ameliorate CP/CPPS, indicating that T2 caused an autoimmune response in the body. And CD4+ T cells and macrophages play important pathological roles [18]. These data constituted additional proof for the role of T2 in the development of disease. TNF-α is produced chiefly by activated macrophages. Similar to CP/CPPS patients [17], serum TNF-α levels were also increased in EAP mice. Antibodies to T2 reduced the level of TNF-α, suggesting a weakened inflammatory response. Furthermore, because antibodies were given in only four injections (days 3, 17, 19, and 21) and would be predicted to be cleared from the animal after 3 days, it proposes that the immune response to T2 during 1 week after immunization is critical to disease development.
There are many deficiencies in this study. Antibodies to T2 ameliorated CP/CPPS, which may be attributed to the knockdown of the major antigen epitope, thus reducing T cell activation. On the other hand, T2 may be involved in the clonal amplification of activated T cells by directly inhibiting PD-1. These possible mechanisms need our further experiments to demonstrate. In addition, the histopathological and cytokine evaluations only performed on day 28. A time course study about these findings would provide more insights into the interactions between anti-T2 antibodies and the progression of EAP. Also, it seems reasonable to determine whether there is any correlation between the inflammatory and destructive elements of EAP and the appearance of T2 in blood or prostate.
The identification of the T2 autoantigen epitope has provided a further refinement and characterization of our EAP model, which could serve for a better understanding of the etiology, pathogenesis and pathophysiology of CP/CPPS, and lead to more definite diagnostic procedures and more useful therapeutic approaches.
CONCLUSION
In this study, the intervention of anti-T2 polyclonal antibodies during the induction of EAP can effectively improve the symptoms of CP/CPPS. T2 is proposed to represent the major autoantigen epitope in EAP. The specific mechanism and anti-T2 treatment regimen warrant further study.
References
Stein, A., T. May, and Y. Dekel. 2014. Chronic pelvic pain syndrome: a clinical enigma. Postgraduate Medicine 126 (4): 115–123. https://doi.org/10.3810/pgm.2014.07.2789.
Smith, Christopher P. 2016. Male chronic pelvic pain: an update. Indian journal of urology : IJU : journal of the Urological Society of India 32 (1): 34–39. https://doi.org/10.4103/0970-1591.173105.
Clemens, J.Q., C. Mullins, A.L. Ackerman, T. Bavendam, A. van Bokhoven, B.M. Ellingson, S.E. Harte, et al. 2019. Urologic chronic pelvic pain syndrome: insights from the MAPP research network. Nature Reviews Urology 16 (3): 187–200. https://doi.org/10.1038/s41585-018-0135-5.
Liao, Chun-Hou, Herng-Ching Lin, and Chao-Yuan Huang. 2016. Chronic prostatitis/chronic pelvic pain syndrome is associated with irritable bowel syndrome: a population-based study. Scientific Reports 6. doi:https://doi.org/10.1038/srep26939, 6.
Polackwich, A.S., and D.A. Shoskes. 2016. Chronic prostatitis/chronic pelvic pain syndrome: A review of evaluation and therapy. Prostate Cancer and Prostatic Diseases 19 (2): 132–138. https://doi.org/10.1038/pcan.2016.8.
Aaron, Leslie A., Richard Herrell, Suzanne Ashton, Megan Belcourt, Karen Schmaling, Jack Goldberg, and Dedra Buchwald. 2001. Comorbid clinical conditions in chronic fatigue. Journal of General Internal Medicine 16 (1): 24–31. https://doi.org/10.1111/j.1525-1497.2001.03419.x.
Farmer, Melissa A., Mona L. Chanda, Elle L. Parks, Marwan N. Baliki, A. Vania Apkarian, and Anthony J. Schaeffer. 2011. Brain functional and anatomical changes in chronic prostatitis/chronic pelvic pain syndrome. The Journal of Urology 186 (1): 117–124. https://doi.org/10.1016/j.juro.2011.03.027.
Clemens, J.Q., S.O. Brown, and E.A. Calhoun. 2008. Mental health diagnoses in patients with interstitial cystitis/painful bladder syndrome and chronic prostatitis/chronic pelvic pain syndrome: a case/control study. Journal of Urology 180 (4): 1378–1382. https://doi.org/10.1016/j.juro.2008.06.032.
Batstone, G.R., A. Doble, and J.S. Gaston. 2002. Autoimmune T cell responses to seminal plasma in chronic pelvic pain syndrome (CPPS). Clinical and Experimental Immunology 128 (2): 302–307.
Tomaskovic, I., B. Ruzic, D. Trnski, and O. Kraus. 2009. Chronic prostatitis/chronic pelvic pain syndrome in males may be an autoimmune disease, potentially responsive to corticosteroid therapy. Medical Hypotheses 72 (3): 261–262. https://doi.org/10.1016/j.mehy.2008.10.020.
Ihsan, Awais Ullah, Farhan Ullah Khan, Waqas Nawaz, Muhammad Zahid Khan, Mengqi Yang, and Xiaohui Zhou. 2017. Establishment of a rat model of chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS) induced by immunization with a novel peptide T2. Biomedicine & Pharmacotherapy 91: 687–692. https://doi.org/10.1016/j.biopha.2017.05.004.
Khan, Farhan Ullah, Awais Ullah Ihsan, Waqas Nawaz, Muhammad Zahid Khan, Mengqi Yang, Gang Wang, Xiaoqian Liao, Lei Han, and Xiaohui Zhou. 2017. A novel mouse model of chronic prostatitis/chronic pelvic pain syndrome induced by immunization of special peptide fragment with aluminum hydroxide adjuvant. Immunology Letters 187: 61–67. https://doi.org/10.1016/j.imlet.2017.05.008.
Zhang, L., A.U. Ihsan, Y. Cao, Y. Cheng, and X. Zhou. 2018. Establishment of experimental autoimmune prostatitis model by T-2 peptide in aluminium hydroxide adjuvant. Andrologia 50 (3). https://doi.org/10.1111/and.12922.
Barnes, D.A., J. Tse, M. Kaufhold, M. Owen, J. Hesselgesser, R. Strieter, R. Horuk, and H.D. Perez. 1998. Polyclonal antibody directed against human RANTES ameliorates disease in the Lewis rat adjuvant-induced arthritis model. Journal of Clinical Investigation 101 (12): 2910–2919. https://doi.org/10.1172/jci2172.
Wang, Xiaoqian, Zhang Yu, Zhiding Wang, Xiaoling Liu, Gaizhi Zhu, Gencheng Han, Guojiang Chen, et al. 2018. Anti-IL-39 (IL-23p19/Ebi3) polyclonal antibodies ameliorate autoimmune symptoms in lupus-like mice. Molecular Medicine Reports 17 (1): 1660–1666. https://doi.org/10.3892/mmr.2017.8048.
Chaplan, S.R., F.W. Bach, J.W. Pogrel, J.M. Chung, and T.L. Yaksh. 1994. Quantitative assessment of tactile allodynia in the rat paw. Journal of Neuroscience Methods 53 (1): 55–63. https://doi.org/10.1016/0165-0270(94)90144-9.
Liu, Yuqian, Reyaj Mikrani, Dianyou Xie, Junaid Wazir, Sajan Shrestha, Rahat Ullah, Mirza Muhammad Faran Ashraf Baig, et al. 2020. Chronic prostatitis/chronic pelvic pain syndrome and prostate cancer: study of immune cells and cytokines. Fundamental and Clinical Pharmacology 34 (2): 160–172. https://doi.org/10.1111/fcp.12517.
Wang, Wenlu, Muhammad Naveed, Fatima Majeed, Xingxing Cui, Awais Ullah Ihsan, Ziwei Liu, Hafiz Muhammad Zubair, Meng Tang, Muhammad Sohail, and Zhou Xiaohui. 2019. Morphological reseach on expression of inflammatory mediators in murine models of chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS) induced by T2 antigen. Andrologia 51 (11): e13435.
Keetch, D.W., P. Humphrey, and T.L. Ratliff. 1994. Development of a mouse model for nonbacterial prostatitis. Journal of Urology 152 (1): 247–250. https://doi.org/10.1016/s0022-5347(17)32871-9.
Qi, X., L. Han, X. Liu, J. Zhi, B. Zhao, D. Chen, F. Yu, and X. Zhou. 2012. Prostate extract with aluminum hydroxide injection as a novel animal model for chronic prostatitis/chronic pelvic pain syndrome. Urology 80 (6):1389.e1389-1315. doi:https://doi.org/10.1016/j.urology.2012.07.030.
Maccioni, M., V.E. Rivero, and C.M. Riera. 1998. Prostatein (or rat prostatic steroid binding protein) is a major autoantigen in experimental autoimmune prostatitis. Clinical and Experimental Immunology 112 (2): 159–165. https://doi.org/10.1046/j.1365-2249.1998.00588.x.
Penna, G., B. Fibbi, M. Maggi, and L. Adorini. 2009. Prostate autoimmunity: from experimental models to clinical counterparts. Expert Review of Clinical Immunology 5 (5): 577–586. https://doi.org/10.1586/eci.09.37.
Tsavaler, L., M.H. Shapero, S. Morkowski, and R. Laus. 2001. Trp-p8, a novel prostate-specific gene, is up-regulated in prostate cancer and other malignancies and shares high homology with transient receptor potential calcium channel proteins. Cancer Research 61 (9): 3760–3769.
Liu, Yuqian, Reyaj Mikrani, Yanjun He, Mirza Muhammad Faran Asraf Baig, Muhammad Abbas, Muhammad Naveed, Meng Tang, Qin Zhang, Cuican Li, and Xiaohui Zhou. 2020. TRPM8 channels: a review of distribution and clinical role. European Journal of Pharmacology: 173312. https://doi.org/10.1016/j.ejphar.2020.173312.
McKemy, D.D., W.M. Neuhausser, and D. Julius. 2002. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature 416 (6876): 52–58. https://doi.org/10.1038/nature719.
Stein, R.J., S. Santos, J. Nagatomi, Y. Hayashi, B.S. Minnery, M. Xavier, A.S. Patel, et al. 2004. Cool (TRPM8) and hot (TRPV1) receptors in the bladder and male genital tract. Journal of Urology 172 (3): 1175–1178. https://doi.org/10.1097/01.ju.0000134880.55119.cf.
Hedelin, Hans, and Karin Jonsson. 2007. Chronic abacterial prostatitis and cold exposure: an explorative study. Scandinavian Journal of Urology and Nephrology 41 (5): 430–435. https://doi.org/10.1080/00365590701365123.
Cao, Yanfang, Yijie Cheng, Awais Ullah Ihsan, Farhan Ullah Khan, Dianyou Xie, Xingxing Cui, Wenlu Wang, and Xiaohui Zhou. 2019. A nanoparticle-coupled T2 peptide induces immune tolerance and ameliorates chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS) in mice model. Fundamental and Clinical Pharmacology 33 (3): 267–276. https://doi.org/10.1111/fcp.12438.
Cheng, Yijie, Yanfang Cao, Awais Ullah Ihsan, Farhan Ullah Khan, Li Xue, Dianyou Xie, Xingxing Cui, Wenlu Wang, Ziwei Liu, and Cunyu Li. 2019. Novel treatment of experimental autoimmune prostatitis by nanoparticle-conjugated autoantigen peptide T2. Inflammation 42 (3): 1071–1081. https://doi.org/10.1007/s10753-019-00968-5.
Tang, Meng, Rahat Ullah, Junaid Wazir, Farhan Ullah Khan, Awais Ullah Ihsan, Xingxing Cui, Wenlu Wang, Min Hu, Yuqian Liu, and Xiaohui Zhou. 2019. Effect of oral T2 antigen on chronic prostatitis/chronic pelvic pain syndrome in mice model. Inflammation 42: 2086–2094. https://doi.org/10.1007/s10753-019-01072-4.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, Y., Tang, M., Zhang, Q. et al. T2 Peptide Represents a Major Autoantigen Epitope in Experimental Autoimmune Prostatitis. Inflammation 44, 243–248 (2021). https://doi.org/10.1007/s10753-020-01326-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-020-01326-6