Abstract
Blunt chest (thoracic) trauma (TxT) and hemorrhagic shock (HS)-induced local and systemic inflammation with increased neutrophil activity often result in an impaired organ function. Next to increasing the trauma risk, binge drinking causes anti-inflammatory effects due to immunomodulatory properties of alcohol (ethanol, EtOH). The impact of clinically relevant acute binge drinking scenario on local and systemic inflammatory changes, notably regarding the activity and longevity of leukocytes, has been analyzed in a combinatory trauma model of TxT + H/R. Twenty-four female Lewis rats (190–240 g) received alcohol (5 g/kg, 30%) or saline gavage. Two hours after alcohol gavage, TxT with subsequent HS (60 min) and resuscitation (TxT + H/R) were induced. Sham-operated animals underwent surgical procedures. Bronchoalveolar lavage fluid (BAL), lung tissue, and blood were harvested 2 h after resuscitation. Pulmonary infiltration with PMN, IL-6 gene expression, systemic PMN activation, neutrophil and monocyte apoptosis (caspase-3/7), and pyroptosis/inflammasome activation (caspase-1) were evaluated. Lung damage was evaluated by hematoxylin-eosin (H/E) staining and determination of the total protein content in BAL (ANOVA, p < 0.05 was significant). TxT + H/R-induced increases in IL-6, PMN infiltration and BAL-protein concentration were significantly reduced by EtOH; however, histological morphology changes after trauma remained unaltered by EtOH. TxT + H/R-induced systemic leukocyte activation (increased CD11b and CD31, reduced CD62L expression) as well as inflammasome activation in monocytes were significantly diminished by EtOH. Apoptosis was prolonged only in PMN after TxT + H/R and was further prolonged by EtOH, an effect that was observed in sham animals as a trend as well. Acute EtOH exposure inhibits the activation of circulating leukocytes after trauma compared to controls. These EtOH-driven systemic changes may be associated with reduced infiltration with PMN after trauma as well as reduced local tissue inflammation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Particularly in young patients, trauma causes significant mortality rates worldwide, with approximately 5 million people dying upon traumatic injury [1]. Notably, patients suffering from traumatic brain injuries (47%) and/or chest trauma (46%) as well as hemorrhagic shock, which is the most preventable cause of death, contribute to patients’ mortality [2, 3]. Combinatory trauma consisting of a blunt chest (thoracic) trauma (TxT) and blood loss frequently results in a strong systemic and local inflammatory response to injury, which causes severe tissue damage, and subsequently may lead to multiple organ failure (MOF) or death [4].
The pathogenesis of pulmonary complications is not completely understood. Post-injury lung damage arises from multiple processes as response to microcirculatory disturbances and to the release of pathogen-associated molecular pattern (PAMP), e.g., endotoxins (LPS) and/or damage-associated molecular pattern (DAMP) after tissue damage [5]. This implies an inflammatory process, which is characterized by an excessive release of proinflammatory cytokines such as interleukin (IL)-6; activation of leukocytes (polymorphonuclear leukocytes, PMN, and monocytes), and pulmonary tissue infiltration with neutrophils, which collectively result in the injury of epithelial cells and the disruption of alveolar-capillary barrier [6, 7]. Activation, localization, and further extravasation of neutrophils from the circulation to injury site involve the modulation of cell adhesion molecules on their cell surface, such as CD62L and CD31 and CD11b/CD18 [8,9,10]. Although they are required for the clearance of invading PAMP and/or DAMP, not only the presence of activated neutrophils but their function as well contributes to pulmonary damage and dysfunction [6, 11]. As such, neutrophil apoptosis can be delayed after trauma [12], suggesting that their prolonged lifespan indicates a prolonged presence of activated neutrophils in the lungs inducing lung damage [13].
Next to increasing the risk for traumatic injury, alcohol as an immunomodulatory drug is associated with altered inflammatory response upon trauma in both animal studies as well as in trauma patients [14, 15]. As such, alcohol-reduced chemotactic activity of neutrophils, as well as their infiltration into the lung or site of infection, and an impaired expression of neutrophilic adhesion molecules have been observed [16]. At clinically relevant doses, alcohol blunts the ability of formyl-methionyl-leucyl-phenylalanine to upregulate CD11b on neutrophils [17]. Since alcohol consumption is associated with a serious percentage of all hospital admissions and tissue damage as well, in the present study, we characterized the systemic neutrophil biology and lung injury following blunt chest trauma and hemorrhagic shock and determined whether an acute alcohol intoxication prior trauma has any influence on those. We hypothesized that acute alcohol consumption will inhibit the increased systemic activation of both neutrophils and monocytes and decrease local pulmonary inflammation and lung damage after blunt chest trauma and hemorrhagic shock.
MATERIAL AND METHODS
Animals and Experimental Model
The study was approved by the Veterinary Department of the Regional Council in Darmstadt, Germany. The design and reporting of the study are in accordance with the ARRIVE guidelines to optimize the reproducibility of animal studies [18]. Twenty-four female Lewis rats (190–240 g, Janvier Labs, France) were used after an acclimation phase for at least 7 days. Prior experimentation, the animals received a single dose of alcohol (5 g/kg, 30% ethanol, EtOH) to mimic the clinical “drink and drive” scenario or control sodium chloride solution (NaCl 0.9%, vehicle) via oral gavage as described before [14, 19].
1.5 h later, the animals received buprenorphine. Mask anesthesia with isoflurane oxygen mixture was applied. The abdomen, chest, right inguinal region, and neck region were shaved; the incision sites were infiltrated with carbostesin; and the right femoral artery was cannulated with polyethylene tubing for monitoring of the blood pressure (BP analyzer, Siemens AG). These procedures lasted around 30 min. Then, the experimentation began.
Bilateral blunt chest (thoracic) trauma (TxT) was induced as described before [19, 20]. Briefly, after placing the animals in a supine position, a cylinder separated by a Mylar polyester film (0.190 mm, DuPont Teijin Films Luxembourg) was located above the ventral thorax. By applying compressed air, the polyester membrane perforated, and a standardized blast wave was provided. After a short stabilization phase of 5 min, the left jugular vein and the right carotid artery were cannulated with polyethylene tubing.
Subsequently, hemorrhagic shock was induced as described before [19]. Briefly, hemorrhage was initiated by withdrawing blood from the right artery into a heparinized syringe until a mean arterial blood pressure (MABP) was reduced to 35 ± 3 mmHg. The MABP was kept constant by further withdrawal or injection of small blood volumes for 60 min. Immediately after hemorrhagic shock, resuscitation followed by transfusing 60% of the shed blood, plus 50% volume of the maximal shed blood volume as lactated Ringer’s solution via the left jugular vein over 30 min. The catheters were removed, the vessels were occluded, and the wounds were closed. Two hours later, sacrifice was performed. At this time point, highly relevant inflammatory and pathophysiological changes have been observed after hemorrhagic shock and in the underlying model of blunt chest trauma and hemorrhagic shock in rats [14, 19, 21, 22]. Figure 1 shows the experimental time line.
Schematic timeline of the experimental design. EtOH ethanol (alcohol) application, HS hemorrhagic shock, MABP mean arterial blood pressure, R resuscitation, TxT blunt chest (thoracic) trauma. The groups received either EtOH or vehicle 2 h before experimentation. Then, HS followed by R was induced, and 2 h later, sacrifice was performed.
Group Allocation
Animals were randomly subdivided into four groups (n = 6 each). The sham groups received either EtOH or vehicle before the beginning of the experiments and underwent all surgical procedures without induction of TxT or H/R. TxT + H/R groups received TxT followed by H/R and either EtOH or vehicle prior trauma.
Examination of Lung Injury
After obtaining blood samples from the aorta in pyrogen-free heparinized tubes for cytometric flow analyses, bronchoalveolar lavage fluid (BAL) was obtained from the lungs at 2 h after resuscitation. BAL was obtained by instilling 8 ml saline into the lungs via a tracheal cannula and subsequently withdrawing exactly 6 ml BAL. The BAL was centrifuged at 2000×g for 15 min at 4 °C, and the cell-free supernatant was frozen at -80°C for subsequent total protein determination.
Then, the right middle lung lobe was ligated and removed for RNA isolation. The left lung lobe was used for histology. Histological damage of hematoxylin-eosin–stained lung sections (3 μm) has been evaluated by an independent examiner, who evaluated alveolar disruption with alveolar wall thickening and cellular infiltrates, in a blinded manner.
Quantification of Polymorphonuclear Leukocytes in the Lungs
Analysis of lung infiltration with PMN has been performed by a chloroacetate esterase staining (CAE, 4% pararosanilin, 4% sodium nitrite and naphthol solution) for 30 min at room temperature (RT) as described before [23]. All sections were counterstained with hematoxylin. The total number of infiltrating PMN was quantified by counting CAE-positive cells in a total of 20 high-power (400×) fields per section per animal in a blinded manner. Data from each tissue slide were pooled to determine means.
Ribonucleic Acid (RNA) Isolation, Semi-Quantitative Reverse-Transcription Polymerase Chain Reaction (qRT-PCR)
Snap-frozen lung tissue was used for RNA isolation (RNeasy-system, Qiagen, Hilden, Germany) according to the manufacturer’s instructions. RNase-Free DNase Set was applied to remove DNA (Qiagen, Hilden, Germany). Both quality and amount of the isolated RNA were determined photometrically by using the NanoDrop ND-1000 device (NanoDrop Technologies, Wilmington, DE, USA). One hundred nanograms of total lung RNA was reversely transcribed using the Affinity script QPCR-cDNA synthesis kit (Stratagene, La Jolla, CA, USA) according to manufacturer’s instructions and as reported before [24]. IL-6 gene expression was determined using a Stratagene MX3005p QPCR system (Stratagene) and a gene-specific primer for rat interleukin 6 (NM_012589.2, UniGene#: Rn.9873, Cat#: PPR06483B, SABiosciences, SuperArray, Frederick, MD, USA). mRNA expression of gapdh was determined as reference gene expression (rat Gapdh, NM_017008, UniGene#: Rn.91450, Cat#: PPR06557A, SABiosciences). PCR reaction was set up with 1× RT2 SYBR Green/Rox qPCR Master mix (SABiosciences) in a 25-μl volume. A two-step amplification protocol consisting of initial denaturation at 95°C for 10 min followed by 40 cycles with 15 s denaturation at 95 °C and 60 s annealing/extension at 60°C was used. Relative expression of the target gene mRNA was calculated by using the comparative threshold-cycle (CT) method (2−ΔΔCT method). The amount of target mRNA in each sample has been normalized to gapdh to provide ΔCT and then to a calibrator consisting of samples obtained from the vehicle sham group. Relative IL-6 mRNA expression is presented as percentage calculated in relation to 100% of the sham group.
Measurement of Cell Surface Receptor Expression by Flow Cytometry
Blood samples were collected via the aorta in pyrogen-free heparinized tubes at 2 h after resuscitation. Fifty microliters was transferred into polystyrene FACS tubes (BD Pharmingen™) and incubated with FITC mouse anti-rat CD11b (Clone WT.5, BD Bioscience, San Jose, CA), anti-rat CD31 (PECAM-1) PE-Cyanine7 (Clone TLD-3A12, eBioscience, San Diego, CA), and APC mouse anti-rat CD62L (Clone OX-85, BioLegend, San Diego, CA) antibodies. Control stainings with the corresponding isotype antibodies were applied for the settings. After 30 min at RT, 1 ml of the FACS Lysing Solution (BD Pharmingen™) was added for additional 10 min (RT). Then, samples were centrifuged at 400g for 7 min and washed twice with 2 ml phosphate buffered saline (PBS) supplemented with 0.5% bovine serum albumin (FACS buffer). After removal of supernatants, cells were diluted in 400 μl FACS buffer. Each cell population was defined by gating the corresponding forward and side scatter scan. From each sample, a minimum of 3.0 × 104 cells was measured, which were subsequently analyzed. The mean fluorescence units were assessed by flow cytometric analyses using a BD FACS Canto 2™ and FACD DIVA™ software (BD).
Caspase-1 and Caspase-3 Activation Assay
Active caspases were quantified by using a FAM-YVAD-FMK 660 caspase-1 detection kit and FAM-DEVD-FLICA caspase-3/7 kit (ImmunoChemistry Technologies) according to the manufacturer’s guidelines. Mean fluorescence units (MFU) of the stained cells were quantified by flow cytometry (using unstained cells to set the gate) by BD FACS Canto 2™ and FACD DIVA™ software.
Statistical Analysis
Normality distribution was assessed by Kolmogorov-Smirnov test with Dallal-Wilkinson-Lilliefor p value. Non-parametric Kruskal-Wallis test was applied to study the differences between the groups. For post hoc correction, Dunn’s multiple comparison test was applied. Data are given as mean ± standard error of the mean (s.e.m.). A p value below 0.05 was considered statistically significant. All statistical analyses were performed using GraphPad Prism 6 (Graphpad Software, Inc., San Diego, CA).
RESULTS
Total Protein Concentration in the Bronchoalveolar Lavage Fluid
TxT + H/R induced a significant increase in total protein concentration to 1.26 ± 0.26 μg/μl at 2 h after resuscitation in the vehicle-treated group as compared to sham-operated controls (0.14 ± 0.02 μg/μl, p < 0.05, Fig. 2a). At 2 h after resuscitation, in the TxT + H/R EtOH group, total protein concentration in the BAL was significantly decreased to 0.69 ± 0.14 μg/μl compared to the TxT + H/R vehicle group (p < 0.05, Fig. 2a).
Lung damage after blunt chest trauma (TxT) and hemorrhagic shock followed by resuscitation (H/R). Total protein concentration in the bronchoalveolar lavage fluid (BAL, a) and lung tissue damage by hematoxylin-eosin staining (b–e) at 2 h after resuscitation was determined. Sham-operated animals underwent surgical procedures. Vehicle-treated animals received sodium chloride (NaCl, 0.9%) as control, and ethanol (EtOH, alcohol)-treated animals received 5 g/kg, 30% EtOH. Data are given as mean ± standard error of the mean, *p < 0.05, n = 6.
Histopathological Changes in Lung Tissue
Lung sections from the TxT + H/R vehicle group revealed alveolar disruption with alveolar wall thickening and cellular infiltrates compared to lung morphology of the sham groups (Fig. 2b–d). TxT + H/R-induced changes in the lung morphology were not markedly altered in the TxT-H/R EtOH group (Fig. 2d).
Lung Neutrophil Accumulation
Local infiltration of the lungs with PMN increased significantly to 48.82 ± 2.82 cells per high-power field at 2 h after resuscitation in the TxT + H/R vehicle group as compared to the sham vehicle group (26.68 ± 1.80 cells per high-power field, p < 0.05, Fig. 3a). EtOH markedly diminished lung PMN infiltration after TxT + H/R as compared to the vehicle group after TxT + H/R (35.79 ± 3.18 vs. 48.82 ± 2.82 cells per high-power field, p < 0.05, Fig. 3a).
Lung inflammation after blunt chest trauma (TxT) and hemorrhagic shock followed by resuscitation (H/R). Infiltration with polymorphonuclear leukocytes (PMN, a) and systemic interleukin (IL)-6 gene expression (b) at 2 h after resuscitation. Sham-operated animals underwent surgical procedures. Vehicle-treated animals received sodium chloride (NaCl, 0.9%) as control, and ethanol (EtOH, alcohol)-treated animals received 5 g/kg, 30% EtOH. Data are given as mean ± standard error of the mean, *p < 0.05, n = 6.
Interleukin-6 Gene Expression
The local immune response induced by TxT-H/R has been detected at 2 h after resuscitation by significantly increased IL-6 gene expression levels in the TxT-H/R vehicle group compared to the sham vehicle group (2393.00 ± 389.50 vs. 78.17 ± 18.39, p < 0.05, Fig. 3b). IL-6 gene expression was significantly reduced by EtOH after TxT + H/R compared to the TxT-H/R vehicle group (p < 0.05, Fig. 3b).
Cell Surface Expression of Intercellular Adhesion Molecules on Granulocytes
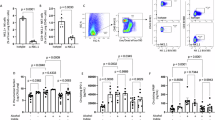
To analyze if and how EtOH modulates neutrophil migration after TxT + H/R, expression levels of CD11b, CD62L, and CD31 on granulocytes at 2 h after resuscitation were assessed. TxT + H/R increased significantly CD11b expression on granulocytes in vehicle animals compared to the corresponding sham vehicle group (p < 0.05, Fig. 4a). Though TxT-H/R induced an increase in CD11b expression in the EtOH group as well, this increase was not significant compared to the sham control groups (Fig. 4a). There was a tendency of decreasing CD11b expression in EtOH-gavaged animals of the sham group compared to sham vehicle group; however, this was not significant.
Flow cytometric analysis of cellular adhesion molecules CD11b (a), CD62 (b), and CD31 (c) on circulating neutrophils after blunt chest trauma (TxT) and hemorrhagic shock followed by resuscitation (H/R). Flow cytometric analysis was performed 2 h after resuscitation. Sham-operated animals underwent surgical procedures. Vehicle-treated animals received sodium chloride (NaCl, 0.9%) as control, and ethanol (EtOH, alcohol)-treated animals received 5 g/kg, 30% EtOH. Data are given as mean ± standard error of the mean, *p < 0.05, n = 6.
Regarding CD62L expression, there was no notable difference between the two sham groups. CD62L expression significantly decreased after TxT + H/R in the vehicle group compared to the sham vehicle control (p < 0.05, Fig. 4b). CD62L expression was significantly increased after TxT + H/R in the EtOH group as compared to the TxT + H/R vehicle group (p < 0.05, Fig. 4b).
TxT + H/R induced a significant increase of CD31 expression in both vehicle and EtOH group as compared to the sham vehicle control (p < 0.05, Fig. 4c). Gavage with EtOH further enhanced the CD31 expression both after sham as well as after TxT + H/R compared to either sham or TxT + H/R vehicle group; however, this difference was not significant (p < 0.05, Fig. 4c).
Monocyte and Granulocyte Apoptosis and Pyroptosis/Inflammasome Activation
To measure monocyte and granulocyte apoptosis and pyroptosis, the activities of caspase-3/7 and caspase-1, respectively, at 2 h after resuscitation have been assessed. Following TxT + H/R, caspase-3/7 activation in the vehicle group was significantly reduced when compared to the corresponding vehicle-treated sham group. Furthermore, EtOH treatment significantly lowered caspase-3/7 expression after TxT + H/R compared with the vehicle group after TxT + H/R (Fig. 5a). In sham groups, there was a clear tendency to decrease caspase-3/7 expression in EtOH-treated compared to vehicle-treated animals; however, this difference was not significant (Fig. 5a).
Caspase-3/7 (a and b) and caspase-1 (c and d) activation after blunt chest trauma (TxT) and hemorrhagic shock followed by resuscitation (H/R) in granulocytes (a and c) or monocytes (b and d). Granulocytes or monocytes were gated as described in material and methods. Sham-operated animals underwent surgical procedures. Vehicle-treated animals received sodium chloride (NaCl, 0.9%) as control, and ethanol (EtOH, alcohol)-treated animals received 5 g/kg, 30% EtOH. Data are given as mean ± standard error of the mean, *p < 0.05, n = 6.
There were no differences in monocyte apoptosis after TxT + H/R compared to shams or depending on the gavage fluid (Fig. 5b).
Regarding the pyroptosis, in granulocytes, caspase-1 activity trended to decrease after TxT + H/R and vehicle treatment compared with sham vehicle. Similar data were observed for the EtOH groups (Fig. 5c). EtOH markedly reduced caspase-1 activity in the sham group compared to vehicle sham control, while this difference was significant between the EtOH and vehicle groups after TxT + H/R in granulocytes (Fig. 5c).
In monocytes, caspase-1 activity was significantly increased after TxT + H/R and vehicle treatment compared to both sham groups (p < 0.05, Fig. 5d). After TxT + H/R, EtOH significantly decreased the TxT + H/R-induced caspase-1 increase in the vehicle group to the levels which were comparable to those of both sham groups (p < 0.05 Fig. 5d).
DISCUSSION
Several studies have described a dose and frequency-depending influence of alcohol on the immune system. While on the one hand chronic alcohol consumption has been associated with a twofold increased risk of complications, particularly pneumonia and other infections [25], reduced mortality, inflammatory response, and hepatic injury after isolated hemorrhage and resuscitation were found after acute alcohol consumption on the other hand [14]. Thoracic injuries as well as hemorrhagic shock account for significant causes of trauma-related death [2, 4]. Especially lung contusions after blunt chest trauma are related with post-traumatic, inflammation-triggered complications including acute respiratory distress syndrome (ARDS) and multiple organ dysfunction syndrome (MODS) [26]. In a clinically relevant double-hit model of blunt chest trauma and hemorrhagic shock, we examined the modulation of post-traumatic pulmonary as well as systemic inflammatory response by acute alcohol consumption.
Comparable to the observations from small and large in vivo models including blunt chest trauma and hemorrhagic shock, in the present study, we found that TxT + H/R induced a profound damage of lung tissue, which has been associated with enhanced local and systemic inflammation [27,28,29]. Chest trauma itself, as it was observed in porcine as well as in mouse or rat models, led to a mechanic cell damage and severe hemorrhage in lung tissue, observations which were partly found in our model as well, showing edema, wall thickness, and diffuse alveolar damage after trauma [27, 30]. Our results indicated that blunt chest trauma and hemorrhagic shock induced a local cellular histopathological lung damage, findings that were supported by increased pulmonary epithelial leakage, which was monitored by increased protein content in the bronchoalveolar lavage fluid after trauma/hemorrhage. Additionally, the abovementioned studies have described a significant IL-6 and IL-8 increase, of which the first was found in the lung tissue in our model after trauma as well [27, 30]. Inflammatory modulations after trauma/hemorrhage are important components of tissue pathophysiology. Increase of pro-inflammatory mediators has been associated with the infiltration of tissues with neutrophils after trauma/hemorrhage [14, 31]. In vitro findings have confirmed IL-6-induced increase in neutrophil adhesion rates to human lung epithelial cells [32]. Interestingly, treatment of human lung epithelial cells with alcohol significantly reduced the IL-6-induced inflammatory response as well as neutrophil adhesion rates to those cells [32]. These findings have been confirmed in vivo showing that hemorrhage-induced systemic and local IL-6 increase as well as local tissue infiltration with neutrophils could be diminished by acute exposure to alcohol [14]. Stimulating human lung epithelial cells with either IL-1β or sera from traumatized patients induced a strong release of IL-6 but also an increased adhesion of neutrophils to lung epithelial cells [33]. Similar to the above discussed studies, a consecutive acute exposure to alcohol decreased both effects [33]. The possible molecular mechanism is based on the inflammation-induced activation of the transcription factor NF-ĸB [33]. Acute exposure to alcohol reduced significantly p50 activation, which is representing the canonical NF-ĸB signaling [33]. Interestingly, alcohol did not reduce the activation of p52, which is representing the non-canonical pathway of NF-ĸB signaling [33]. Summarized, the anti-inflammatory effects of the acute exposure to alcohol were mediated via decreasing nuclear levels of activated p50 subunit involving the canonical NF-ĸB signaling pathway [33]. NF-ĸB is triggering the systemic and local propagation of inflammation [34, 35]. Although we did not evaluate the NF-ĸB signaling in this study, we have observed significant anti-inflammatory potential of alcohol, which is indicating at its key role in mediating the anti-inflammatory potential of alcohol. This apparently inflammation-suppressing influence of an acute alcohol consumption has been confirmed in the underlying study as well. Although the previously observed tissue-protective and anti-inflammatory effects of alcohol under acute inflammatory conditions were not obvious according to the lung morphology, the reduced protein concentration in the bronchoalveolar lavage fluid does imply beneficial inflammation-suppressive effects in our model. However, further studies with special regard to organ function and underlying mechanisms are clearly required.
Neutrophils play a pivotal role in the initiation and propagation of the inflammatory response to trauma [36]. Traumatic injury leads to marked alterations in the neutrophil phenotype, their function, and life span. Comparable to our data, the cell surface expression of CD62L, a receptor that facilitates tethering of neutrophils to the endothelium, has been shown to be significantly reduced on systemically circulating neutrophils after trauma [37, 38]. However, this was observed 6 h, 9 h, and 24 h after trauma. Interestingly, in traumatized patients, CD62L has also been reported to increase within a short observational period of 2.5 h (mean sample time = 1.2 h, median = 1 h) after trauma [39]. During neutrophil activation and migration, CD62L is shed from the cell into the circulation as soluble L-selectin [40]. It remains to be elucidated in further studies, when CD62L is shed in mice after blunt chest trauma and hemorrhagic shock and if alcohol suppresses or only postpones this process.
Interestingly, in line with previously reported data, our findings indicate as well that the surface expression of CD31 and CD11b, which forms part of the heterodimeric integrin Mac-1, that is responsible for mediating the firm adhesion to the endothelium is significantly increased after trauma [41]. Together, according to Hazeldine et al., the combined data for CD62L decrease and the CD11b increase suggest that systemic activation of the circulating neutrophil occurs following trauma [36]. Coherent with the systemic neutrophil activation, local inflammation as shown by, e.g., increased IL-6, may explain the significant rise in PMNL migration to lung tissue after blunt chest trauma and hemorrhagic shock, which has been observed in the underlying study. Additionally, as CD62L shedding is seen as a protective mechanism against excessive inflammation [42], the decreased CD62L shedding as a consequence of an acute alcohol consumption may indicate at a reduced systemic activation of neutrophils and subsequently their lower infiltration rates in the lungs. Together, these processes may end up in an improved barrier function as shown by less protein leakage into the bronchoalveolar lavage fluid upon alcohol consumption and blunt chest trauma followed by hemorrhagic shock. Nonetheless, these hypotheses remain to be elucidated in further studies.
Next to their phenotype alterations, trauma induces functional modulations of neutrophils such as of their increased life-span, which is caused by lowered apoptosis rates [41]. After blunt chest trauma followed by hemorrhagic shock, we observed a significant reduction in neutrophil apoptosis, demonstrated by decreased caspase-3/7 activation. Interestingly, Shults et al. have shown that excessive pulmonary inflammation, which was paralleled by decreased lung function, is mediated in part by alveolar macrophages [43]. In their burn trauma model, they have postulated that the restoration of lung tissue homeostasis was dependent on the eradication of neutrophils and removal of apoptotic cells, which are major functions of alveolar macrophages [43]. They uncovered that the loss of alveolar macrophages paralleled a strong increase in lung cell death and found that alcohol consumption delayed the resolution of the necessary inflammation [43]. Our study seems to substantiate their postulation, as we see a significantly lowered caspase-3/7 activity after EtOH treatment in the TxT + H/R groups and a likewise clear tendency in the sham groups. However, besides a small trend of decreased caspase-3/7 activity after EtOH treatment in the sham groups, there were no significant alterations in monocyte apoptosis after TxT + H/R or acute alcohol consumption. Next to apoptotic modulations, inflammation-induced apoptosis, so-called pyroptosis or inflammasome activation, is recently gaining more interest. As immunosuppression is a major complication of alcoholism, the caspase-1 activation indicating inflammasome activity has been evaluated [44]. Inflammasomes are cytosolic multi-protein complexes, which are of central importance for inflammatory processes by promoting the maturation of certain pro-inflammatory cytokines notably IL-1β or IL-18 [45]. A typical multimeric inflammasome consists of an adaptor protein apoptosis-associated speck-like protein containing a caspase activation and recruitment domain (CARD), abbreviated ASC, the zymogen pro-caspase-1, and a cytosolic pattern recognition receptor (PRR), which is capable of detecting intracellular PAMP related to infectious pathogens and/or DAMP related to cell stress [46]. Upon PRR stimulation, inflammasome assembly is mediated by the PYD/PYD interaction between ASC and a cytosolic PRR and a CARD/CARD interaction between ASC and pro-caspase-1 resulting into ASC-formed cytosolic specks [47]. This so-called speck formation promotes the auto-activation of inactive pro-caspase-1 into its active form caspase-1 [47, 48]. Then, active caspase-1 proteolytically cleaves the precursor of, e.g., IL-1β into its mature, secreted active form [47, 49]. Caspase-1 is the leading enzyme which mediates pyroptosis, a highly inflammatory form of programmed cell death [46, 50]. Though TxT + H/R did not modulate the inflammasome activation, alcohol reduced strongly the pro-inflammatory activity of neutrophils. Apparently, this is a general effect on inflammasome activation in neutrophils, since comparable data have been observed in both sham and trauma animals. Recently, Hoyt et al. have shown that in a mouse macrophage cell line, mouse bone marrow-derived dendritic cells, mouse neutrophils, and human peripheral blood mononuclear cells, ethanol and other short-chain alcohols inhibited inflammasome activation [51]. Upon TxT + H/R, increased inflammasome activation has been shown in monocytes, and in line with the above reports, alcohol intoxication reduced markedly the caspase-1 activity.
Other neutrophilic functions are modified by alcohol as well. It has been shown that the phagocytic and oxidative burst capacity of polymorphonuclear cells was suppressed in alcohol-intoxicated rodents after hemorrhagic shock [52]. Similarly, the ability of neutrophils to phagocytose virulent K2 Klebsiella pneumoniae was suppressed by ethanol at high concentrations [53]. This finding may account for the higher prevalence of pneumonia or other extrapulmonary infection in people with acute alcohol intoxication. If possible alterations in phagocytic and oxidative burst capacity of neutrophils are suppressed by alcohol in this model remains to be elucidated in further studies. However, the above described activation of neutrophils after blunt chest trauma followed by hemorrhagic shock, which was reduced by acute alcohol consumption, may indicate at a reduced systemic activation of neutrophils and subsequently less infiltration of the lungs with these cells. Similar data were reported by Oh and Diamond, who studied the effects of alcohol at physiological concentrations on neutrophil membrane tether pulling, adhesion lifetime, rolling, and firm arrest behavior in parallel-plate flow chamber assays with P-selectin-coated beads, P-selectin-coated surfaces, or IL-1-stimulated human endothelium [54]. They found that alcohol reduced rolling velocity and rolling flux on P-selectin surfaces compared with untreated neutrophils. On IL-1-stimulated endothelium, rolling velocity was unchanged by alcohol treatment, but the fraction of cells converting to firm arrest was reduced [54]. Overall, this data indicate that alcohol directly influences neutrophil adhesion rates as demonstrated in our study as well.
Taken together, we hypothesize that increased systemic activation of neutrophils and monocytes after blunt chest trauma and hemorrhagic shock is associated with enhanced local inflammation and reduced neutrophil apoptosis, which are associated with tissue damage. Acute alcohol consumption inhibits systemic activation of neutrophils, possibly via NF-ĸB signaling, thereby reducing neutrophil infiltration rates into the lungs, an effect that subsequently contributes to decreased local pulmonary inflammation and improved barrier function after blunt chest trauma and hemorrhagic shock.
There are several limitations of our study. The control group was not fed with isocaloric diet but instead received a sodium chloride solution, which is well-accepted as control solution; however, an isocaloric diet should be included in future studies. We have applied only one dose of ethanol, which may not allow for any dose dependency analyses, which are apparently indicated in the clinical scenario. Similarly, the impact of ethanol’s toxic metabolites was not studied and remains to be elaborated in further studies. In conclusion, the severity of the tissue damage may be dependent on the timing of alcohol intoxication, but also in the trauma severity itself. Additionally, we have investigated only one point in time and did not perform kinetics. Also, the impact of ethanol on apoptosis of leukocytes, NF-ĸB signaling pathway, and its relevance for tissue damage remain to be further evaluated in future studies.
Abbreviations
- APC:
-
Allophycocyanine
- ARDS:
-
Acute respiratory distress syndrome
- ASC:
-
Apoptosis-associated speck-like protein containing CARD
- BAL:
-
Bronchoalveolar lavage fluid
- CAE:
-
Chloroacetate esterase
- CARD:
-
Caspase activation and recruitment domain
- CD:
-
Cluster of differentiation
- CT:
-
Comparative threshold-cycle
- DAMP:
-
Damage-associated molecular pattern
- DFG:
-
Deutsche Forschungsgemeinschaft, German Research Foundation
- EtOH:
-
Ethanol
- Fig.:
-
Figure
- FITC:
-
Fluorescein isothiocyanate
- g :
-
Earth’s gravitational acceleration
- GAPDH:
-
Glyceraldehyde 3-phosphate dehydrogenase
- HE:
-
Hematoxylin-eosin
- Hg:
-
Mercury
- HS:
-
Hemorrhagic shock
- IL:
-
Interleukin
- LPS:
-
Lipopolysaccharide
- MABP:
-
Mean arterial blood pressure
- MFU:
-
Mean fluorescence units
- MOF:
-
Multiple organ failure
- MODS:
-
Multiple organ dysfunction syndrome
- NaCl:
-
Sodium chloride
- NF-ĸB:
-
Nuclear factor kappa-light-chain-enhancer of activated B cells
- p :
-
P value
- PAMP:
-
Pathogen-associated molecular pattern
- PRR:
-
Pattern recognition receptor
- PMN:
-
Polymorphonuclear leukocyte
- R:
-
Resuscitation
- RNA:
-
Ribonucleic acid
- RT:
-
Room temperature
- qRT-PCR:
-
Semi-quantitative real-time polymerase chain reaction
- s.e.m.:
-
Standard error of the mean
- TxT:
-
Blunt thoracic/chest trauma
- U:
-
Unit
References
Sakran, J.V., S.E. Greer, E. Werlin, and M. McCunn. 2012. Care of the injured worldwide: Trauma still the neglected disease of modern society. Scandinavian Journal of Trauma, Resuscitation and Emergency Medicine 20: 64.
Esmer, E., P. Derst, R. Lefering, M. Schulz, H. Siekmann, K.S. Delank, and das TraumaRegister DGU. 2017. Prehospital assessment of injury type and severity in severely injured patients by emergency physicians: An analysis of the TraumaRegister DGU(R). Der Unfallchirurg 120: 409–416.
Spahn, D.R., B. Bouillon, V. Cerny, T.J. Coats, J. Duranteau, E. Fernandez-Mondejar, D. Filipescu, B.J. Hunt, R. Komadina, G. Nardi, E. Neugebauer, Y. Ozier, L. Riddez, A. Schultz, J.L. Vincent, and R. Rossaint. 2013. Management of bleeding and coagulopathy following major trauma: An updated European guideline. Critical Care 17: R76.
Dutton, R.P., L.G. Stansbury, S. Leone, E. Kramer, J.R. Hess, and T.M. Scalea. 2010. Trauma mortality in mature trauma systems: Are we doing better? An analysis of trauma mortality patterns, 1997-2008. The Journal of Trauma 69: 620–626.
Raymond, S.L., D.C. Holden, J.C. Mira, J.A. Stortz, T.J. Loftus, A.M. Mohr, L.L. Moldawer, F.A. Moore, S.D. Larson, and P.A. Efron. 2017. Microbial recognition and danger signals in sepsis and trauma. Biochimica et Biophysica Acta 1863: 2564–2573.
Bruns, B., T. Honle, P. Kellermann, A. Ayala, and M. Perl. 2017. Divergent effects of neutrophils on Fas-induced pulmonary inflammation, apoptosis, and lung damage. Shock 47: 225–235.
Ware, L.B., and M.A. Matthay. 2000. The acute respiratory distress syndrome. The New England Journal of Medicine 342: 1334–1349.
Chian, C.F., C.H. Chiang, C. Yuan-Jung, C.H. Chuang, S.L. Liu, J. Yi-Han, H. Zhang, and J.H. Ryu. 2012. Apocynin attenuates lipopolysaccharide-induced lung injury in an isolated and perfused rat lung model. Shock 38: 196–202.
Relja, B., R. Taraki, M.P. Teuben, K. Mors, N. Wagner, S. Wutzler, F. Hildebrand, M. Perl, and I. Marzi. 2016. Sera from severe trauma patients with pneumonia and without infectious complications have differential effects on neutrophil biology. BMC Pulmonary Medicine 16: 171.
Wedepohl, S., F. Beceren-Braun, S. Riese, K. Buscher, S. Enders, G. Bernhard, K. Kilian, V. Blanchard, J. Dernedde, and R. Tauber. 2012. L-selectin--a dynamic regulator of leukocyte migration. European Journal of Cell Biology 91: 257–264.
Hoth, J.J., J.D. Wells, E.M. Hiltbold, C.E. McCall, and B.K. Yoza. 2011. Mechanism of neutrophil recruitment to the lung after pulmonary contusion. Shock 35: 604–609.
Matute-Bello, G., W.C. Liles, F. Radella 2nd, K.P. Steinberg, J.T. Ruzinski, L.D. Hudson, and T.R. Martin. 2000. Modulation of neutrophil apoptosis by granulocyte colony-stimulating factor and granulocyte/macrophage colony-stimulating factor during the course of acute respiratory distress syndrome. Critical Care Medicine 28: 1–7.
Weckbach, S., C. Hohmann, S. Braumueller, S. Denk, B. Klohs, P.F. Stahel, F. Gebhard, M.S. Huber-Lang, and M. Perl. 2013. Inflammatory and apoptotic alterations in serum and injured tissue after experimental polytrauma in mice: distinct early response compared with single trauma or “double-hit” injury. Journal of Trauma and Acute Care Surgery 74: 489–498.
Relja, B., C. Hohn, F. Bormann, K. Seyboth, D. Henrich, I. Marzi, and M. Lehnert. 2012. Acute alcohol intoxication reduces mortality, inflammatory responses and hepatic injury after haemorrhage and resuscitation in vivo. British Journal of Pharmacology 165: 1188–1199.
Relja, B., J. Menke, N. Wagner, B. Auner, M. Voth, C. Nau, and I. Marzi. 2016. Effects of positive blood alcohol concentration on outcome and systemic interleukin-6 in major trauma patients. Injury 47: 640–645.
Boe, D.M., S. Nelson, P. Zhang, L. Quinton, and G.J. Bagby. 2003. Alcohol-induced suppression of lung chemokine production and the host defense response to Streptococcus pneumoniae. Alcoholism, Clinical and Experimental Research 27: 1838–1845.
Patel, M., A. Keshavarzian, V. Kottapalli, B. Badie, D. Winship, and J.Z. Fields. 1996. Human neutrophil functions are inhibited in vitro by clinically relevant ethanol concentrations. Alcoholism, Clinical and Experimental Research 20: 275–283.
Kilkenny, C., W.J. Browne, I.C. Cuthill, M. Emerson, and D.G. Altman. 2010. Improving bioscience research reporting: The ARRIVE guidelines for reporting animal research. PLoS Biology 8: e1000412.
Wagner, N., N. Franz, S. Dieteren, M. Perl, K. Mors, I. Marzi, and B. Relja. 2017. Acute alcohol binge deteriorates metabolic and respiratory compensation capability after blunt chest trauma followed by hemorrhagic shock - a new research model. Alcoholism, Clinical and Experimental Research 41: 1559–1567.
Weckbach, S., M. Perl, T. Heiland, S. Braumuller, P.F. Stahel, M.A. Flierl, A. Ignatius, F. Gebhard, and M. Huber-Lang. 2012. A new experimental polytrauma model in rats: Molecular characterization of the early inflammatory response. Mediators of Inflammation 2012: 890816.
Wagner, N., S. Dieteren, N. Franz, K. Kohler, K. Mors, L. Nicin, J. Schmidt, M. Perl, I. Marzi, and B. Relja. 2018. Ethyl pyruvate ameliorates hepatic injury following blunt chest trauma and hemorrhagic shock by reducing local inflammation, NF-kappaB activation and HMGB1 release. PLoS One 13: e0192171.
Relja, B., N. Wagner, N. Franz, S. Dieteren, K. Mors, J. Schmidt, I. Marzi, and M. Perl. 2018. Ethyl pyruvate reduces acute lung damage following trauma and hemorrhagic shock via inhibition of NF-kappaB and HMGB1. Immunobiology 223: 310–318.
Relja, B., E. Tottel, L. Breig, D. Henrich, H. Schneider, I. Marzi, and M. Lehnert. 2011. Effects of green tea catechins on the pro-inflammatory response after haemorrhage/resuscitation in rats. The British Journal of Nutrition 105: 1791–1797.
Relja, B., J.P. Horstmann, K. Kontradowitz, K. Jurida, A. Schaible, C. Neunaber, E. Oppermann, and I. Marzi. 2015. Nlrp1 inflammasome is downregulated in trauma patients. Journal of Molecular Medicine (Berlin) 93: 1391–1400.
Bird, M.D., M.A. Choudhry, P.E. Molina, and E.J. Kovacs. 2009. Alcohol and trauma: A summary of the satellite symposium at the 30th annual meeting of the Shock Society. Alcohol 43: 247–252.
Veysi, V.T., V.S. Nikolaou, C. Paliobeis, N. Efstathopoulos, and P.V. Giannoudis. 2009. Prevalence of chest trauma, associated injuries and mortality: A level I trauma centre experience. International Orthopaedics 33: 1425–1433.
Horst, K., T.P. Simon, R. Pfeifer, M. Teuben, K. Almahmoud, Q. Zhi, S.A. Santos, C.C. Wembers, S. Leonhardt, N. Heussen, P. Stormann, B. Auner, B. Relja, I. Marzi, A.T. Haug, M. van Griensven, M. Kalbitz, M. Huber-Lang, R. Tolba, L.K. Reiss, S. Uhlig, G. Marx, H.C. Pape, and F. Hildebrand. 2016. Characterization of blunt chest trauma in a long-term porcine model of severe multiple trauma. Scientific Reports 6: 39659.
Seitz, D.H., M. Perl, U.C. Liener, B. Tauchmann, S.T. Braumuller, U.B. Bruckner, F. Gebhard, and M.W. Knoferl. 2011. Inflammatory alterations in a novel combination model of blunt chest trauma and hemorrhagic shock. The Journal of Trauma 70: 189–196.
Wu, X.J., H.M. Liu, X.M. Song, B. Zhao, Y. Leng, E.Y. Wang, L.Y. Zhan, Q.T. Meng, and Z.Y. Xia. 2018. Penehyclidine hydrochloride inhibits TLR4 signaling and inflammation, and attenuates blunt chest trauma and hemorrhagic shock-induced acute lung injury in rats. Molecular Medicine Reports 17: 6327–6336.
Knoferl, M.W., U.C. Liener, D.H. Seitz, M. Perl, U.B. Bruckner, L. Kinzl, and F. Gebhard. 2003. Cardiopulmonary, histological, and inflammatory alterations after lung contusion in a novel mouse model of blunt chest trauma. Shock 19: 519–525.
Denk, S., S. Weckbach, P. Eisele, C.K. Braun, R. Wiegner, J.J. Ohmann, L. Wrba, F.M. Hoenes, P. Kellermann, P. Radermacher, U. Wachter, S. Hafner, O. McCook, A. Schultze, A. Palmer, S. Braumuller, F. Gebhard, and M. Huber-Lang. 2018. Role of hemorrhagic shock in experimental polytrauma. Shock 49(2):154-163. https://doi.org/10.1097/SHK.0000000000000925.
Relja, B., N. Omid, A. Schaible, M. Perl, S. Meier, E. Oppermann, M. Lehnert, and I. Marzi. 2015. Pre- or post-treatment with ethanol and ethyl pyruvate results in distinct anti-inflammatory responses of human lung epithelial cells triggered by interleukin-6. Molecular Medicine Reports 12: 2991–2998.
Mors, K., J.A. Horauf, S. Kany, N. Wagner, R. Sturm, M. Woschek, M. Perl, I. Marzi, and B. Relja. 2017. Ethanol decreases inflammatory response in human lung epithelial cells by inhibiting the canonical NF-kB-pathway. Cellular Physiology and Biochemistry 43: 17–30.
Mandrekar, P., D. Catalano, and G. Szabo. 1997. Alcohol-induced regulation of nuclear regulatory factor-kappa beta in human monocytes. Alcoholism, Clinical and Experimental Research 21: 988–994.
Mandrekar, P., D. Catalano, B. White, and G. Szabo. 2006. Moderate alcohol intake in humans attenuates monocyte inflammatory responses: Inhibition of nuclear regulatory factor kappa B and induction of interleukin 10. Alcoholism, Clinical and Experimental Research 30: 135–139.
Hazeldine, J., P. Hampson, and J.M. Lord. 2014. The impact of trauma on neutrophil function. Injury 45: 1824–1833.
Groeneveld, K.M., F. Hietbrink, T.C. Hardcastle, B.L. Warren, L. Koenderman, and L.P. Leenen. 2014. Penetrating thorax injury leads to mild systemic activation of neutrophils without inflammatory complications. Injury 45: 522–527.
Visser, T., F. Hietbrink, K.M. Groeneveld, L. Koenderman, and L.P. Leenen. 2011. Isolated blunt chest injury leads to transient activation of circulating neutrophils. European Journal of Trauma and Emergency Surgery 37: 177–184.
Cocks, R.A., and T.Y. Chan. 1997. Alteration in leukocyte adhesion molecule expression following minor, moderate and major trauma. European Journal of Emergency Medicine 4: 193–195.
Jutila, M.A., L. Rott, E.L. Berg, and E.C. Butcher. 1989. Function and regulation of the neutrophil MEL-14 antigen in vivo: Comparison with LFA-1 and MAC-1. Journal of Immunology 143: 3318–3324.
Junger, W.G., S.G. Rhind, S.B. Rizoli, J. Cuschieri, A.J. Baker, P.N. Shek, D.B. Hoyt, and E.M. Bulger. 2013. Prehospital hypertonic saline resuscitation attenuates the activation and promotes apoptosis of neutrophils in patients with severe traumatic brain injury. Shock 40: 366–374.
Mommsen, P., T. Barkhausen, F. Hildebrand, C. Zeckey, C. Krettek, and M. van Griensven. 2011. Regulation of L-selectin expression by trauma-relevant cytokines. Pathology, Research and Practice 207: 142–147.
Shults, J.A., B.J. Curtis, D.M. Boe, L. Ramirez, and E.J. Kovacs. 2016. Ethanol intoxication prolongs post-burn pulmonary inflammation: Role of alveolar macrophages. Journal of Leukocyte Biology 100: 1037–1045.
O'Brien, M., D. Moehring, R. Munoz-Planillo, G. Nunez, J. Callaway, J. Ting, M. Scurria, T. Ugo, L. Bernad, J. Cali, and D. Lazar. 2017. A bioluminescent caspase-1 activity assay rapidly monitors inflammasome activation in cells. Journal of Immunological Methods 447: 1–13.
Martinon, F., K. Burns, and J. Tschopp. 2002. The inflammasome: A molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Molecular Cell 10: 417–426.
Awad, F., E. Assrawi, C. Louvrier, C. Jumeau, S. Georgin-Lavialle, G. Grateau, S. Amselem, I. Giurgea, and S.A. Karabina. 2018. Inflammasome biology, molecular pathology and therapeutic implications. Pharmacology & Therapeutics 187:133-149. https://doi.org/10.1016/j.pharmthera.2018.02.011.
Place, D.E., and T.D. Kanneganti. 2017. Recent advances in inflammasome biology. Current Opinion in Immunology 50: 32–38.
Elliott, J.M., L. Rouge, C. Wiesmann, and J.M. Scheer. 2009. Crystal structure of procaspase-1 zymogen domain reveals insight into inflammatory caspase autoactivation. The Journal of Biological Chemistry 284: 6546–6553.
Thornberry, N.A., H.G. Bull, J.R. Calaycay, K.T. Chapman, A.D. Howard, M.J. Kostura, D.K. Miller, S.M. Molineaux, J.R. Weidner, J. Aunins, K.O. Elliston, J.M. Ayala, F.J. Casano, J. Chin, G.J.F. Ding, L.A. Egger, E.P. Gaffney, G. Limjuco, O.C. Palyha, S.M. Raju, A.M. Rolando, J.P. Salley, T.T. Yamin, T.D. Lee, J.E. Shively, M. MacCross, R.A. Mumford, J.A. Schmidt, and M.J. Tocci. 1992. A novel heterodimeric cysteine protease is required for interleukin-1 beta processing in monocytes. Nature 356: 768–774.
Brennan, M.A., and B.T. Cookson. 2000. Salmonella induces macrophage death by caspase-1-dependent necrosis. Molecular Microbiology 38: 31–40.
Hoyt, L.R., J.L. Ather, M.J. Randall, D.P. DePuccio, C.C. Landry, M.D. Wewers, M.A. Gavrilin, and M.E. Poynter. 2016. Ethanol and other short-chain alcohols inhibit NLRP3 inflammasome activation through protein tyrosine phosphatase stimulation. Journal of Immunology 197: 1322–1334.
Molina, P.E., K.L. Zambell, K. Norenberg, J. Eason, H. Phelan, P. Zhang, C.V. Stouwe, J.W. Carnal, and C. Porreta. 2004. Consequences of alcohol-induced early dysregulation of responses to trauma/hemorrhage. Alcohol 33: 217–227.
Chiu, C.H., Y.C. Wang, K.M. Yeh, J.C. Lin, L.K. Siu, and F.Y. Chang. 2018. Influence of ethanol concentration in the phagocytic function of neutrophils against Klebsiella pneumoniae isolates in an experimental model. Journal of Microbiology, Immunology, and Infection 51: 64–69.
Oh, H., and S.L. Diamond. 2008. Ethanol enhances neutrophil membrane tether growth and slows rolling on P-selectin but reduces capture from flow and firm arrest on IL-1-treated endothelium. Journal of Immunology 181: 2472–2482.
Acknowledgements
We thank Katrin Jurida, Kerstin Kontradowitz, and Alexander Schaible for outstanding technical assistance.
Funding
This study was supported by grants from DFG RE 3304/5-1 and DFG PE 908/3-1.
Author information
Authors and Affiliations
Contributions
BR and MP designed the study and obtained the grant. NF, NW, and SD performed the experiments. NF, BR, and NW performed the statistical analysis and wrote the manuscript. KK evaluated the histology. KM, RS, and IM made important intellectual contributions to the study and revised the manuscript.
Corresponding author
Ethics declarations
Ethical Approval
Animal protocols were approved by the Veterinary Department of the Regional Council in Darmstadt, Germany.
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Franz, N., Dieteren, S., Köhler, K. et al. Alcohol Binge Reduces Systemic Leukocyte Activation and Pulmonary PMN Infiltration After Blunt Chest Trauma and Hemorrhagic Shock. Inflammation 42, 690–701 (2019). https://doi.org/10.1007/s10753-018-0927-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-018-0927-z