Abstract
Ferulic acid (FA), derived from fruits and vegetables, is well-known as a potent antioxidant of scavenging free radicals. However, the role and underlying mechanism of FA on kidney ischemia reperfusion (I/R) injury are limited. Here, we explored the effects of FA on kidney I/R injury. The kidney I/R injury models were carried out by clamping bilateral pedicles for 35 min followed by reperfusion for 24 h. Mice were orally pretreated with different doses of FA for three times 24 h before I/R. The renal function was assessed by serum creatine (Scr) and blood urea nitrogen (BUN). Kidney histology was examined by hematoxylin and eosin (HE) staining and terminal deoxynucleotidly transferased UTP nick-end labeling (TUNEL) assay. Proinflammatory cytokines, caspase-3 activity, adenosine generation, adenosine signaling molecules, and hypoxia inducible factor-1 alpha (HIF-1α) were also detected, respectively. The siHIF-1α adenovirus vectors were in vivo used to inhibit the expression of HIF-1α. The results showed that FA significantly attenuated kidney damage in renal I/R-operated mice as indicated by reducing levels of Scr and BUN, ameliorating renal pathological structural changes, and tubular cells apoptosis. Moreover, FA pretreatment inhibited I/R-induced renal proinflammatory cytokines and neutrophils recruitment. Interestingly, the levels of HIF-α, CD39, and CD73 mRNA and protein as well as adenosine production were all significantly increased after FA pretreatment in the kidney of I/R-performed mice, and inhibiting HIF-α expression using siRNA abolished this protection of FA on I/R-induced acute kidney injury as evidenced by more severe renal damage and reduced adenosine production. Our findings indicated that FA protected against kidney I/R injury by reducing apoptosis, alleviating inflammation, increasing adenosine generation, and upregulating CD39 and CD73 expression, which might be mediated by HIF-1α.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Acute kidney injury (AKI) is a common complication resulting from sepsis, transplantation, nephrotoxin, and I/R injury, and is strongly associated with high morbidity and mortality, poor outcome, and high financial costs among hospital patients [3, 20, 24, 38]. Although dramatic advances have been made in diagnosis and medical technologies, the incidence of AKI is growing over the past decades and there is still no specific effective therapy to treat AKI [18, 33, 38]. Kidney I/R injury is known as the leading cause of AKI; it is characterized by early moderate proximal tubule epithelium cells apoptosis, subsequent neutrophils infiltration, profound inflammatory and immune responses, and massive proximal tubule necrosis [11, 19, 30]. Therefore, new tools or drugs that prevent kidney I/R-induced tubule epithelium apoptosis and inflammatory responses are urgently needed.
Adenosine is an endogenous nucleoside and exists widely among all mammalians [31]. CD39 and CD73 are two vital enzymes to generate adenosine [1]. There are two steps in adenosine generation. The first step is the enzymatic hydrolysis of adenosine triphosphate (ATP) to adenosine monophosphate (AMP) by CD39, and then the AMP was converted to adenosine via CD73 [43]. Under physiological condition, the level of adenosine is relatively low in extracellular milieu, and adenosine is involved in regulating renin release, glomerular filtration rate, and vascular tone and tubular glomerular feedback [42]. However, adenosine increases rapidly under acute stress like hypoxia or ischemia, contributing to adapting the cells to anoxic environment and attenuating inflammation [2, 31]. Accumulating evidence revealed that adenosine plays a vital protective role on kidney I/R injury by attenuating tubule epithelium cellular apoptosis and inhibiting inflammatory responses [34, 42].
Ferulic acid (FA) belongs to a member of phenolic acids existing abundantly in various fruits and vegetables [27]. FA is known as an antioxidant for the ability of scavenging free radicals by its structure of phenoxy radical [45]. Since chemically synthesized for the first time in 1925, FA has been intensively investigated for nearly a century and confirmed to be effective in treating many diseases, like cancer, neurodegenerative diseases, and skin diseases [4, 12, 25, 37]. A recent study showed that FA exhibited antithrombotic activities via cyclic nucleotide signaling [17]. Previous studies have supported that FA could effectively attenuate I/R injury in the brain, liver, heart, and retina [5, 6, 21]. However, the role and underlying mechanism of FA on kidney I/R injury remain unclear.
In this study, we firstly explored the effect of FA on kidney I/R-induced kidney dysfunction, proximal tubule epithelium cell apoptosis and necrosis, and kidney inflammatory responses. Furthermore, we evaluated whether the protective effect of FA on kidney I/R injury is associated with adenosine generation. Our results indicated that FA exerted an effective protection on kidney I/R injury through inhibiting tubule epithelium cells apoptosis, attenuating inflammatory responses, and promoting adenosine generation, which might be associated with the up-regulation of CD39 or CD73 via HIF-1α.
MATERIALS AND METHODS
Drug and Reagents
FA (C10H10O4, FW = 194.18, purity > 99%) was obtained from Aladdin (Shanghai, China). Kits for serum creatine (Scr), blood urea nitrogen (BUN), myeloperoxidase (MPO), and caspase-3 assays were purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). The terminal deoxynucleotidly transferased UTP nick-end labeling (TUNEL) assay kit was obtained from Roche Applied Science (Basel, Switzerland). Enzyme-linked immunosorbent assay (ELISA) kits for tumor necrosis factor alpha (TNF-α) and interleukin-1beta (IL-1β) were purchased from Bender MedSystems (Vienna, Austria). The rat anti-mouse Ly6G-labbeled PE was purchased from BD Biosciences (Heidelberg, Germany). RNAiso reagent was obtained from Takara (Dalian, China). SYBR Green Polymerase Chain Reaction (PCR) kit was supplied by Roche (Basel, Switzerland). Primary antibodies of anti-CD39, anti-CD73, anti-HIF-1α, and HRP-conjugated secondary antibodies were purchased from Abcam (Cambridge, MA, UK), and anti-GAPDH was purchased from Santa Cruz (Texas, USA). were all purchased from Abcam (Cambridge, MA, UK).
Animals and Treatments
Male C57/BL6 mice (weighing 18~22 g) were supplied by Experimental Animal Center of Chongqing Medical University (Chongqing, China). All mice were kept in standard laboratory condition with a controlled temperature (25 ± 0.2 °C), humidity (50~60%), a 12-h light/dark cycle, and free access to standard laboratory chow diet. Mice were randomly assigned to four groups with six animals per group: Control group (sham), FA group (100 mg/kg FA alone in sham), I/R group, I/R + FA group. In I/R + FA group, FA was dissolved in 0.5% PBS and administrated by gavage for three times 24 h before I/R operation at the dose of 10, 30, and 100 mg/kg, respectively. For HIF-1α slicing, the adenovirus vectors were administrated via intravenous injection into both kidney for 72 h before the operation as reported previously [39].
Protocol of Kidney I/R Model
The animal model of kidney I/R was performed as recently reported [28]. In brief, mice were anesthetized by pentobarbital sodium (75 mg/kg) through intraperitoneal injection and placed on a pad with temperature controlled at approximately 37 °C. The flank incisions were performed and both kidney pedicels were bluntly dissected and clamped by atraumatic clamps for 35 min with color change. Then, the clamps were removed, both kidney were perfused for 24 h with both incisions closed. To prevent dehydration, all mice received 1 ml normal saline through intraperitoneal injection. Mice in sham group were subjected to the same surgical procedures without clamping renal pedicles. Mice were sacrificed 24 h after reperfusion, and the blood sample and kidney tissues were harvested for analyses. To evaluate survival rate, both kidneys were subjected to clamp for 50 min, and animals were observed for 7 days.
Assessment of Kidney Function
The blood sample was centrifuged at 3000 rpm at 4 °C for 5 min. The supernatant was collected and stored at − 80 °C. Scr and BUN were measured with commercial available kits in accordance with the manufacturer’s instructions.
Histology Examination
The histology of kidney was examined by H&E staining. Briefly, tissues were fixed with formalin, dehydrated, and embedded with paraffin. After cut in 5-μm sections, the kidney tissues were stained with H&E. The extent of damage was counted and scored in 10 randomly chosen fields (200×) as follows: 0, none; 1, < 10%; 2, 10~25%; 3, 26~45%; 46~75%; and 5, > 75%.
TUNEL Staining
The 5-μm section of kidney tissue was deparaffinized and dehydrated. The apoptosis in liver tissues was then detected using the commercial TUNEL kit according to the manufacturer’s protocol. TUNEL-positive cells were counted in 10 captured fields (200×) per slide.
Assay for Caspase-3 Activity
The caspase-3 activity was assayed with the corresponding kit according to the manufacturer’s protocols.
Cytokines Measurement by ELISA
The TNF-α and IL-1β were all detected by ELISA using commercial kits respectively according to the manufacturer’s protocols.
Flow Cytometry for Neutrophils
After perfusion of kidney with cold normal saline, kidney tissues were minced into pieces and then digested with collagenase (Worthington) and DNase at 37 °C. Kidney fragments were then passed serially through a 200-μm mesh. Single-cell suspensions from the kidney tissues were collected and then centrifuged at 50g for 5 min, and the supernatant was further centrifuged at 300g for 15 min. The pellet was treated with an RBC lysis solution, washed with PBS, and then incubated with anti-CD45-FITC antibody and anti-Ly6G-PE antibody for flow cytometry analysis.
MPO Activity Assay
Kidney tissues were thawed and homogenized in phosphate buffer containing 0.5% hexadecyltrimethylammonium bromide. MPO activity was measured using the commercial kit according to the manufacturer’s instructions.
High-Performance Liquid Chromatography (HPLC) for Adenosine Measurement
The whole kidney tissues were snapped in liquid nitrogen, and the frozen tissues were sonicated with 0.6 N ice-cold perchloric acid followed by neutralization with 0.6 M potassium phosphate tribasic. The supernatant was collected and detected by HPLC to measure adenosine level.
Western Blot Analysis
The kidney tissue was collected and homogenized with RIPA buffer supplemented with protease and phosphatase inhibitors (leupeptin, aprotinin, and pepstatin). Protein in lysate was then separated on 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred to a polyvinylidene fluoride membrane. The membranes were blocked in TBS containing 5% skimmed milk powder and 0.1%Tween-20 for 1 h, and then membranes were incubated overnight at 4 °C with primary antibodies as follows: rabbit anti-GAPDH (1:1000, sc32233), rabbit anti-CD39 (1:1000, ab108248), rabbit anti-CD73 (1:1000, ab175396), and HIF-1α (1:500, ab8366). After washing three times with TBST, the membranes were incubated again with secondary HRP-conjugated goat-anti-rabbit antibody (1,5000, ab136817) for 1 h. The protein was detected by enhanced chemiluminescence (ECL) system (Bio-Rad, USA) and handled with Image Lab software.
RNA Extraction and Quantitative Reverse Transcription (RT)-PCR
The mRNA was extracted from frozen kidney tissues using RNAiso reagent. Then, the complement DNA (cDNA) was synthesized by the reverse transcription kit (Takara, China). Quantitative PCR was performed with SYBR Green PCR kit according to the manufacturer’s protocol. The gene expression of CD39, CD73, and HIF-1α was normalized to GAPDH expression, and results were analyzed with 2−ΔΔ method. Primers used in this study were as follows: GAPDH, 5′-AGGTCGGTGTGAACGGATTTG-3′ (sense), 5′-TGTAGACCATGTAGTTGAGGTCA-3′ (antisense); CD73, 5′-GGACAT TTGACCTCGTCCAAT-3′ (sense), 5′-GGGCACTCGACACTTGGTG-3′ (antisense); CD39, 5′-AAGGTGAAGAGATTTTGCTCCAA-3′ (sense), 5′-TTTGTTCTGGGTC AGTCCCAC-3′ (antisense); and HIF-1α, 5′-ACCTTCATCGGAAACTCCAAAG-3′ (sense), 5′-CTGTTAGGCTGGGAAAAGTTAGG-3′ (antisense).
Statistical Analysis
Experimental data were presented as means ± SD. The two-tailed Student’s t test and ANOVA were used to analyze the comparison between different groups, and P < 0.05 was set to indicate a significant difference.
RESULT
FA Improved Kidney Function in Renal I/R Mice
To determine the effect of FA in I/R, mice were pretreated with different doses of FA and then subjected to 35 min of bilateral renal ischemia followed by 24 h reperfusion. As shown in Fig. 1b, c, there were no significant differences in levels of Scr and BUN between control group and FA alone group. But, Scr and BUN were significantly elevated in I/R group when compared with control group, and FA pretreatment showed a dose-dependent lower in Scr and BUN levels in comparison with I/R. To further evaluate the protective effect of FA, mice received vehicle or different concentrations of FA pretreatment and were subjected to 50 min kidney I/R. FA-pretreated mice survived longer than vehicle-pretreated animals in renal I/R, and 80% mice of FA (100 mg/kg) + I/R group completely survived, whereas 80% I/R-operated animals died within 5 days (Fig. 1d).
FA pretreatment improved kidney function in renal I/R injury. Mice were pretreated with FA (100 mg/kg) subjected to renal I/R operation. a The chemical structure of FA. b Scr levels. c BUN levels. d Survival rates of mice. All data were expressed as mean ± SD. ##P < 0.01 compared with control group; *P < 0.05; **P < 0.01 compared with renal I/R model (n = 6 per group).
FA Attenuated Kidney Tubular Damage and Apoptosis in Renal I/R Mice
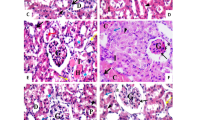
In parallel with the kidney functional results, histologic analysis from kidney sections by HE staining showed that compared with control sham group, there were no difference of kidney histological structural changes in FA alone group. However, the histologic score of tubular damage in renal I/R mice was 3.4 ± 1.0, which declined to 1.6 ± 0.8 (P < 0.01) in FA + I/R group (Fig. 2a, b). Additionally, TUNEL staining and caspase activity assays indicated that renal I/R elicited dramatically renal tubular cell apoptosis with higher TUNEL-positive cells and caspase 3 activity, which were significantly decreased in mice receiving FA pretreatment followed by I/R (Fig. 2c, d).
FA pretreatment ameliorated renal structural damage and tubular cell apoptosis after I/R. Mice were pretreated with FA (100 mg/kg) subjected to renal I/R operation. a Representative images of HE-stained kidney sections, original magnification ×200. b Semiquantitative evaluation of kidney damage scores. c The apoptosis of tubular cells was evaluated by TUNEL staining. d The caspase 3 activity on kidney tissues. All data were expressed as mean ± SD. ##P < 0.01 compared with control group; **P < 0.01 compared with renal I/R model (n = 6 per group).
FA Alleviated Kidney Inflammatory Responses in Renal I/R Mice
I/R-induced local inflammatory responses in the kidney were determined by renal pro-inflammatory cytokines and neutrophils. Data in Fig. 3a, b showed that proinflammatory cytokines IL-1β and TNF-α in kidney tissues were significantly elevated in I/R group when compared with control group (P < 0.01). However, FA pretreatment markedly inhibited I/R-induced production of these cytokines. Similar to pro-inflammatory cytokines, the results of flow cytometry analysis and MPO activity assay indicated that neutrophils with CD45+Ly6G+ and high MPO activity infiltration in the kidney were prevalent in renal I/R mice, however, FA pretreatment alleviated I/R-primed recruitment of neutrophils in the kidney (Fig. 3c, d).
FA pretreatment attenuated I/R-induced kidney inflammation. Mice were pretreated with FA (100 mg/kg) subjected to renal I/R operation. a Kidney TNF-α and b IL-1β levels were measured by ELISA. c Kidney neutrophil infiltration was analyzed by flow cytometry. d Kidney MPO activity. All data were expressed as mean ± SD. ##P < 0.01 compared with control group; **P < 0.01 compared with renal I/R model (n = 6 per group).
FA Promoted Kidney Adenosine Generation, CD39, CD73, and HIF-1α Expression in Renal I/R Mice
To examine whether the protective effect of FA is related to adenosine generation on I/R-induced kidney injury, the adenosine and its key enzymes CD39 and CD73 were determined by HPLC, Western blot, and RT-PCR, respectively. As expected, I/R induced a slight increase in adenosine generation, which was drastically enhanced by FA pretreatment (Fig. 4a). Moreover, compared with control group, the expression levels of CD39 and CD73 mRNA and protein were mildly upregulated by I/R operation, and FA pretreatment markedly enhanced this upregulation of CD39 and CD73 induced by I/R (Fig. 4b, c). Notably, Western blot and RT-PCR analysis showed that HIF-1α mRNA and protein were slightly induced by renal I/R, and this inductive response was profoundly increased in FA + IR group (Fig. 4b, c).
FA pretreatment promoted kidney adenosine generation and upregulated CD39 and CD73 and HIF-1α expression in renal I/R mice. Mice were pretreated with FA (100 mg/kg) subjected to renal I/R operation. a Adenosine production in kidney tissues was measured by HPLC. b The CD39 and CD73 and HIF-1α protein expression was determined by Western blot. c The CD39 and CD73 and HIF-1α mRNA expression was determined by quantitative RT-PCR. All data were expressed as mean ± SD. #P < 0.05 compared with control group; **P < 0.01 compared with renal I/R model (n = 6 per group).
HIF-1α Contributed to Adenosine Generation and Renoprotection of FA in Renal I/R Mice
To further explore whether HIF-1α participated in the protective effect of FA on renal I/R injury, we then used adenoviruses-mediated siRNA (Ad-siHIF-1α) to inhibit HIF-1α expression in different groups. As shown in Fig. 5a, b, the efficacy of siRNA at abrogating FA-induced kidney HIF-1α mRNA and protein expression in renal I/R was confirmed. Compared with Ad-RFP scrambled siRNA administration, transduction of Ad-siHIF-1α showed a lower CD 39 and CD73 expression in FA + I/R group (Fig. 5b). Along with lower protein expression, the effects of FA on adenosine generation (Fig. 5c), Scr and BUN (Fig. 5d, e), and HE pathological scores (Fig. 5f) on renal I/R injury were significantly abolished by Ad-siHIF-1α in comparison with Ad-RFP control vector in FA + I/R mice.
HIF-1α contributed to adenosine generation and renal protection of FA in renal I/R mice. Mice with Ad-HIF-1α siRNA or Ad-RFP were pretreated with FA (100 mg/kg) subjected to renal I/R operation. a HIF-1α mRNA was determined by quantitative RT-PCR. b The expression of HIF-1α, CD73, and CD39 protein was determined by Western blot. c Adenosine generation in the kidney was analyzed by HPLC. d Scr levels. e BUN levels. f Kidney damage scores. #P < 0.05 compared with Ad-RFP + I/R group; **P < 0.01 compared with Ad-RFP + I/R group; &&P < 0.01 compared with Ad-RFP + FA + I/R group (n = 6 per group).
DISCUSSION
The present study demonstrated that FA dose-dependently attenuated renal I/R injury as assessed by improved survival of mice and kidney function and alleviated I/R-induced tubular cell apoptosis and renal inflammatory responses. Interestingly, FA markedly up-regulated CD73 and CD39 expression, and increased adenosine generation in the kidney of I/R-performed mice. Moreover, FA induced a dramatical increase of HIF-1α mRNA and protein expression in the kidney of mice by I/R operation. Importantly, these beneficial effects of FA on I/R-induced renal injury, up-regulated CD73 and CD39 expression, and adenosine generation were abolished by HIF-1α siRNA.
It is well-known that inflammatory response plays a critical role in ischemic AKI [11]. During renal inflammatory response, increased inflammatory cells infiltration and massive pro-inflammatory cytokines production are major hallmarks of I/R-induced kidney injury pathology [19]. Neutrophil trafficking from peripheral blood into ischemia/reperfusion tissues is a critical step to aggravate kidney damage, and blocking neutrophil recruitment prevents renal I/R injury [41]. Studies have shown that the production of IL-1β and TNF-α is an important component of renal I/R injury, TNF-α-deficient mice have alleviated kidney damage in response to I/R, or inhibiting IL-1β limits the damage caused by I/R in mice [9, 10, 32]. In this study, I/R-induced neutrophil infiltration as well as pro-inflammatory cytokines TNF-α and IL-1β production was significantly inhibited by FA pretreatment, suggesting that the protective effect of FA against kidney I/R injury was able to be through reducing inflammation.
Accumulating evidence suggests that CD39 or CD73-derived endogenous adenosine exerts an anti-inflammatory role in ischemic AKI [2]. It has been demonstrated that adenosine could mitigate renal tubular cell death including necrosis and apoptosis, relieve inflammatory response, and adapt renal tubular cells to ischemia/hypoxia. Previous investigations have indicated that overexpression of CD39 in transgenic mice exhibited renoprotection by reducing tubular apoptosis and necrosis with increased adenosine production [8]. Similarly, increased the expression of CD73 protected mice against kidney I/R injury [22]. Mice deficient in CD39 or CD73 abolished the renoprotection of ischemia preconditioning with less adenosine production [13, 14]. In the present study, we found that the protective effect of FA on kidney I/R injury was accompanied with enhanced CD39 and CD73 expression and adenosine generation in the kidney tissues. Hence, we speculated that the renoprotection of FA might be associated with increased adenosine production by CD39 and CD73.
Notably, apart from upregulation of CD39 and CD73, FA also exhibited a marked increase on the expression of HIF-1α mRNA and protein in renal I/R mice. In fact, as an oxygen-sensitive transcription factor, HIF-1α binds to its promoter containing hypoxia response sequences (HREs) and further modulates the transcription of target genes. HIF-1α has been reported to be involved in many biological processes, including cell proliferation and apoptosis, erythropoiesis, energy metabolism, and angiogenesis [15, 36, 40]. However, the role of HIF-1α in inflammation and immune is controversial. Using conditional knockouts of HIF-1α in mice, Thorsten Cramer and Randall Johnson et al. found that activation of HIF-1α is essential for myeloid cell infiltration and activation in vivo through regulating glycolytic capacity in myeloid cells, indicating that HIF-1α exhibits pro-inflammatory action [7]. In contrast, a previous study showed that HIF-1α-deficiency caused defects in proliferation of immature B cells that led to the development of autoimmunity [23]. Virtually, HIF-1α is widely expressed and detected in different cells, including immune cells and parenchymal cells [29]. Thereby, we deduced that the different roles of HIF-1α might be related to different cell types. Previous studies have found that enhanced HIF-1α activation or stabilization could effectively protect mice against renal I/R injury [16, 35]. Additionally, ischemia or xenon preconditioning protected mice from kidney I/R injury by enhancing HIF-1α activation, during which CD73 expression is also enhanced [26, 44]. Here, we proposed that the increased adenosine production as well as up-regulated CD39 and CD73 expression by FA pretreatment may be associated with HIF-1α. Interestingly, we found that FA significantly increased HIF-1α protein in the kidney of I/R-performed mice. Usually, HIF-1α is post-transcriptionally regulated through prolylhydroxylases (PHDs) to hydroxylate and ubiquitination. But in this study, we found that FA up-regulated HIF-1α mRNA at a transcriptional level. To further question whether the protective effect of FA on renal I/R injury is associated with upregulation of HIF-1α, we performed mice with Ad-HIF-1α siRNA to inhibit HIF-1α expression, as expected, the kidney damage was exacerbated, and CD73 and CD39 expression with the adenosine generation were markedly decreased in FA + I/R mice. All these results implied that HIF-1α was responsible for FA-increased adenosine generation, which might be via enhancing expression of CD39 and CD73. However, there are also limits in our present study. For instance, the upstream signal pathway to control HIF-1a expression and which adenosine receptor is involved in this protection of FA in renal I/R injury remains to be clarified.
CONCLUSION
In summary, our study clearly demonstrated that FA protected mice from kidney I/R injury. The underlying renoprotective mechanism may be associated by increasing adenosine generation via HIF-1α-induced expression of CD39 and CD73. Thus, FA may provide a novel therapy for AKI in the future.
References
Antonioli, L., P. Pacher, E.S. Vizi, and G. Hasko. 2013. CD39 and CD73 in immunity and inflammation. Trends in Molecular Medicine 19 (6): 355–367. https://doi.org/10.1016/j.molmed.2013.03.005.
Bauerle, J.D., A. Grenz, J.H. Kim, H.T. Lee, and H.K. Eltzschig. 2011. Adenosine generation and signaling during acute kidney injury. Journal of the American Society of Nephrology 22 (1): 14–20. https://doi.org/10.1681/ASN.2009121217.
Bellomo, R., J.A. Kellum, and C. Ronco. 2012. Acute kidney injury. Lancet 380 (9843): 756–766. https://doi.org/10.1016/S0140-6736(11)61454-2.
Chang, C.J., J.H. Chiu, L.M. Tseng, C.H. Chang, T.M. Chien, C.W. Wu, and W.Y. Lui. 2006. Modulation of HER2 expression by ferulic acid on human breast cancer MCF7 cells. European Journal of Clinical Investigation 36 (8): 588–596. https://doi.org/10.1111/j.1365-2362.2006.01676.x.
Chao, H.M., D.E. Lin, Y. Chang, W.M. Hsu, S.M. Lee, F.L. Lee, C.W. Chi, W.H.T. Pan, T.Y. Liu, W.Y. Lui, L.T. Ho, C.D. Kuo, C.C. Chan, and F.P. Chao. 2008. Ferulic acid, but not tetramethylpyrazine, significantly attenuates retinal ischemia/reperfusion-induced alterations by acting as a hydroxyl radical scavenger. Journal of Ocular Pharmacology and Therapeutics 24 (5): 461–472. https://doi.org/10.1089/jop.2008.0005.
Cheng, C.Y., T.Y. Ho, E.J. Lee, S.Y. Su, N.Y. Tang, and C.L. Hsieh. 2008. Ferulic acid reduces cerebral infarct through its antioxidative and anti-inflammatory effects following transient focal cerebral ischemia in rats. American Journal of Chinese Medicine 36 (6): 1105–1119. https://doi.org/10.1142/s0192415x08006570.
Cramer, T., Y. Yamanishi, B.E. Clausen, I. Forster, R. Pawlinski, N. Mackman, V.H. Haase, et al. 2003. HIF-1alpha is essential for myeloid cell-mediated inflammation. Cell 112 (5): 645–657.
Crikis, S., B. Lu, L.M. Murray-Segal, C. Selan, S.C. Robson, A.J. D'Apice, H.H. Nandurkar, P.J. Cowan, and K.M. Dwyer. 2010. Transgenic overexpression of CD39 protects against renal ischemia-reperfusion and transplant vascular injury. American Journal of Transplantation 10 (12): 2586–2595. https://doi.org/10.1111/j.1600-6143.2010.03257.x.
Dinarello, C.A. 2011. A clinical perspective of IL-1beta as the gatekeeper of inflammation. European Journal of Immunology 41 (5): 1203–1217. https://doi.org/10.1002/eji.201141550.
Donnahoo, K.K., X. Meng, A. Ayala, M.P. Cain, A.H. Harken, and D.R. Meldrum. 1999. Early kidney TNF-alpha expression mediates neutrophil infiltration and injury after renal ischemia-reperfusion. The American Journal of Physiology 277 (3 Pt 2): R922–R929.
Eltzschig, H.K., and T. Eckle. 2011. Ischemia and reperfusion—from mechanism to translation. Nature Medicine 17 (11): 1391–1401. https://doi.org/10.1038/nm.2507.
Graf, E. 1992. Antioxidant potential of ferulic acid. Free Radical Biology and Medicine 13 (4): 435–448.
Grenz, A., H. Zhang, T. Eckle, M. Mittelbronn, M. Wehrmann, C. Kohle, D. Kloor, L.F. Thompson, H. Osswald, and H.K. Eltzschig. 2007. Protective role of ecto-5′-nucleotidase (CD73) in renal ischemia. Journal of the American Society of Nephrology 18 (3): 833–845. https://doi.org/10.1681/ASN.2006101141.
Grenz, A., H. Zhang, M. Hermes, T. Eckle, K. Klingel, D.Y. Huang, C.E. Muller, S.C. Robson, H. Osswald, and H.K. Eltzschig. 2007. Contribution of E-NTPDase1 (CD39) to renal protection from ischemia-reperfusion injury. The FASEB Journal 21 (11): 2863–2873. https://doi.org/10.1096/fj.06-7947com.
Haase, V.H. 2006. Hypoxia-inducible factors in the kidney. American Journal of Physiology: Renal Physiology 291 (2): F271–F281. https://doi.org/10.1152/ajprenal.00071.2006.
Hill, P., D. Shukla, M.G. Tran, J. Aragones, H.T. Cook, P. Carmeliet, and P.H. Maxwell. 2008. Inhibition of hypoxia inducible factor hydroxylases protects against renal ischemia-reperfusion injury. Journal of the American Society of Nephrology 19 (1): 39–46. https://doi.org/10.1681/ASN.2006090998.
Hong, Q., Z.C. Ma, H. Huang, Y.G. Wang, H.L. Tan, C.R. Xiao, Q.D. Liang, H.T. Zhang, and Y. Gao. 2016. Antithrombotic activities of ferulic acid via intracellular cyclic nucleotide signaling. European Journal of Pharmacology 777: 1–8. https://doi.org/10.1016/j.ejphar.2016.01.005.
Hoste, E.A., S.M. Bagshaw, R. Bellomo, C.M. Cely, R. Colman, D.N. Cruz, K. Edipidis, et al. 2015. Epidemiology of acute kidney injury in critically ill patients: The multinational AKI-EPI study. Intensive Care Medicine 41 (8): 1411–1423. https://doi.org/10.1007/s00134-015-3934-7.
Jang, H.R., and H. Rabb. 2015. Immune cells in experimental acute kidney injury. Nature Reviews. Nephrology 11 (2): 88–101. https://doi.org/10.1038/nrneph.2014.180.
Jones, D.R., and H.T. Lee. 2008. Perioperative renal protection. Best Practice & Research: Clinical Anaesthesiology 22 (1): 193–208.
Kim, H.Y., and S.M. Lee. 2012. Ferulic acid attenuates ischemia/reperfusion-induced hepatocyte apoptosis via inhibition of JNK activation. European Journal of Pharmaceutical Sciences 45 (5): 708–715. https://doi.org/10.1016/j.ejps.2012.01.010.
Kim, M., A. Ham, J.Y. Kim, K.M. Brown, V.D. D'Agati, and H.T. Lee. 2013. The volatile anesthetic isoflurane induces ecto-5′-nucleotidase (CD73) to protect against renal ischemia and reperfusion injury. Kidney International 84 (1): 90–103. https://doi.org/10.1038/ki.2013.43.
Kojima, H., H. Gu, S. Nomura, C.C. Caldwell, T. Kobata, P. Carmeliet, G.L. Semenza, and M.V. Sitkovsky. 2002. Abnormal B lymphocyte development and autoimmunity in hypoxia-inducible factor 1alpha -deficient chimeric mice. Proceedings of the National Academy of Sciences of the United States of America 99 (4): 2170–2174. https://doi.org/10.1073/pnas.052706699.
Lameire, N.H., A. Bagga, D. Cruz, J. De Maeseneer, Z. Endre, J.A. Kellum, K.D. Liu, et al. 2013. Acute kidney injury: An increasing global concern. Lancet 382 (9887): 170–179. https://doi.org/10.1016/S0140-6736(13)60647-9.
Lin, F.H., J.Y. Lin, R.D. Gupta, J.A. Tournas, J.A. Burch, M.A. Selim, N.A. Monteiro-Riviere, J.M. Grichnik, J. Zielinski, and S.R. Pinnell. 2005. Ferulic acid stabilizes a solution of vitamins C and E and doubles its photoprotection of skin. Journal of Investigative Dermatology 125 (4): 826–832. https://doi.org/10.1111/j.0022-202X.2005.23768.x.
Mahfoudh-Boussaid, A., M.A. Zaouali, K. Hadj-Ayed, A.H. Miled, D. Saidane-Mosbahi, J. Rosello-Catafau, and H. Ben Abdennebi. 2012. Ischemic preconditioning reduces endoplasmic reticulum stress and upregulates hypoxia inducible factor-1alpha in ischemic kidney: The role of nitric oxide. Journal of Biomedical Science 19: 7. https://doi.org/10.1186/1423-0127-19-7.
Mancuso, C., and R. Santangelo. 2014. Ferulic acid: Pharmacological and toxicological aspects. Food and Chemical Toxicology 65: 185–195. https://doi.org/10.1016/j.fct.2013.12.024.
Noel, S., M.N. Martina, S. Bandapalle, L.C. Racusen, H.R. Potteti, A.R. Hamad, S.P. Reddy, and H. Rabb. 2015. T lymphocyte-specific activation of Nrf2 protects from AKI. Journal of the American Society of Nephrology 26: 2989–3000. https://doi.org/10.1681/ASN.2014100978.
Palazon, A., A.W. Goldrath, V. Nizet, and R.S. Johnson. 2014. HIF transcription factors, inflammation, and immunity. Immunity 41 (4): 518–528. https://doi.org/10.1016/j.immuni.2014.09.008.
Price, P.M., and R. Hodeify. 2012. A possible mechanism of renal cell death after ischemia/reperfusion. Kidney International 81 (8): 720–721. https://doi.org/10.1038/ki.2011.495.
Rabadi, M.M., and H.T. Lee. 2015. Adenosine receptors and renal ischaemia reperfusion injury. Acta Physiologica (Oxford, England) 213 (1): 222–231. https://doi.org/10.1111/apha.12402.
Ramesh, G., and W.B. Reeves. 2002. TNF-alpha mediates chemokine and cytokine expression and renal injury in cisplatin nephrotoxicity. The Journal of Clinical Investigation 110 (6): 835–842. https://doi.org/10.1172/JCI15606.
Rewa, O., and S.M. Bagshaw. 2014. Acute kidney injury-epidemiology, outcomes and economics. Nature Reviews: Nephrology 10 (4): 193–207. https://doi.org/10.1038/nrneph.2013.282.
Roberts, V., B. Lu, K.M. Dwyer, and P.J. Cowan. 2014. Adenosine receptor expression in the development of renal fibrosis following ischemic injury. Transplantation Proceedings 46 (10): 3257–3261. https://doi.org/10.1016/j.transproceed.2014.09.151.
Schley, G., B. Klanke, J. Schodel, F. Forstreuter, D. Shukla, A. Kurtz, K. Amann, M.S. Wiesener, S. Rosen, K.U. Eckardt, P.H. Maxwell, and C. Willam. 2011. Hypoxia-inducible transcription factors stabilization in the thick ascending limb protects against ischemic acute kidney injury. Journal of the American Society of Nephrology 22 (11): 2004–2015. https://doi.org/10.1681/ASN.2010121249.
Schodel, J., S. Grampp, E.R. Maher, H. Moch, P.J. Ratcliffe, P. Russo, and D.R. Mole. 2016. Hypoxia, hypoxia-inducible transcription factors, and renal cancer. European Urology 69 (4): 646–657. https://doi.org/10.1016/j.eururo.2015.08.007.
Sgarbossa, A., D. Giacomazza, and M. di Carlo. 2015. Ferulic acid: A hope for Alzheimer’s disease therapy from plants. Nutrients 7 (7): 5764–5782. https://doi.org/10.3390/nu7075246.
Waikar, S.S., K.D. Liu, and G.M. Chertow. 2008. Diagnosis, epidemiology and outcomes of acute kidney injury. Clinical Journal of the American Society of Nephrology 3 (3): 844–861. https://doi.org/10.2215/CJN.05191107.
Wan, X., L.J. Hou, L.Y. Zhang, W.J. Huang, L. Liu, Q. Zhang, B. Hu, W. Chen, X. Chen, and C.C. Cao. 2015. IKKalpha is involved in kidney recovery and regeneration of acute ischemia/reperfusion injury in mice through IL10-producing regulatory T cells. Disease Models & Mechanisms 8 (7): 733–742. https://doi.org/10.1242/dmm.018200.
Wu, D., N. Potluri, J. Lu, Y. Kim, and F. Rastinejad. 2015. Structural integration in hypoxia-inducible factors. Nature 524 (7565): 303–308. https://doi.org/10.1038/nature14883.
Yago, T., B.G. Petrich, N. Zhang, Z. Liu, B. Shao, M.H. Ginsberg, and R.P. McEver. 2015. Blocking neutrophil integrin activation prevents ischemia-reperfusion injury. The Journal of Experimental Medicine 212 (8): 1267–1281. https://doi.org/10.1084/jem.20142358.
Yap, S.C., and H.T. Lee. 2012. Adenosine and protection from acute kidney injury. Current Opinion in Nephrology and Hypertension 21 (1): 24–32. https://doi.org/10.1097/MNH.0b013e32834d2ec9.
Yegutkin, G.G. 2008. Nucleotide- and nucleoside-converting ectoenzymes: Important modulators of purinergic signalling cascade. Biochimica et Biophysica Acta 1783 (5): 673–694. https://doi.org/10.1016/j.bbamcr.2008.01.024.
Zhao, H., A. Yoshida, W. Xiao, R. Ologunde, K.P. O'Dea, M. Takata, C. Tralau-Stewart, A.J. George, and D. Ma. 2013. Xenon treatment attenuates early renal allograft injury associated with prolonged hypothermic storage in rats. The FASEB Journal 27 (10): 4076–4088. https://doi.org/10.1096/fj.13-232173.
Zhao, Z., and M.H. Moghadasian. 2008. Chemistry, natural sources, dietary intake and pharmacokinetic properties of ferulic acid: A review. Food Chemistry 109 (4): 691–702. https://doi.org/10.1016/j.foodchem.2008.02.039.
Funding
This study was supported by the National Natural Science Foundation of China (No. 81200540).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Electronic Supplementary Material
ESM1
(DOCX 401 kb)
Rights and permissions
About this article
Cite this article
Zhou, Q., Gong, X., Kuang, G. et al. Ferulic Acid Protected from Kidney Ischemia Reperfusion Injury in Mice: Possible Mechanism Through Increasing Adenosine Generation via HIF-1α. Inflammation 41, 2068–2078 (2018). https://doi.org/10.1007/s10753-018-0850-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-018-0850-3