Abstract
Renal ischemic reperfusion (IR) injury is one of the major source of mortality and morbidity associated with acute kidney injury (AKI). Several flavonoids have shown to be renal protective against many nephrotoxic agents causing AKI. Fisetin, a promising flavonoid, is effective in the management of septic AKI, expected to ameliorate renal IR injury. The present study aimed to generate evidence for fisetin-mediated renal protection against IR injury. Male Wistar rats of 200–250 g were subjected to IR protocol by performing bilateral clamping for 45 min and reperfusion for 24 h. Fisetin was administrated 30 min (20 mg/kg b.wt, ip) before the surgery. Renal injury was evaluated by measuring the biomarkers in plasma, examining the ultra-structure of the kidney, and analyzing the apoptotic changes. Oxidative stress, antioxidant levels, and mitochondrial function were analyzed in the renal tissue. Fisetin administration significantly reduced the renal damages associated with IR by improving estimated glomerular filtration rate (eGFR: IR-0.35 ml/min, F_IR-9.03 ml/min), reducing plasma creatinine level (IR-2.2 mg/dl, F_IR-0.92 mg/dl), and lowering urinary albumin/creatinine ratio (IR-6.09 F_IR-2.16), caspase activity, decreased DNA fragmentation and reduced tubular injury score (IR- 11 F_IR-6.5). At the cellular level, fisetin significantly reduced renal oxidative stress and augmented the antioxidant levels. Fisetin was found to preserve mitochondrial electron transport chain activities and improved the ATP producing capacity in the renal tissue upon IR injury. Fisetin pretreatment attenuates renal IR injury by improving renal function, reducing the renal injury mediated by apoptosis, reducing free radical release, and augmenting mitochondrial function.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Renal insufficiency due to ischemia–reperfusion (IR) is one of the major concerns of clinicians in the management of acute kidney diseases where IR injury can compromise normal regulation of fluid, electrolyte, and acid–base homeostasis (Basile et al. 2012; Bienholz et al. 2015). This inevitable injury may occur after infarction, sepsis, and organ transplantation, leading to acute kidney injury by exacerbating tissue damage by triggering inflammatory cascade, reactive oxygen species, and inactivation of mitochondrial function (Bienholz et al. 2015). Long-term and short-term complications of AKI are associated with IR (Dong et al. 2019). The search for a suitable therapeutic agent to attenuate the clinically challenged IR is continuing.
Evidence from the literature testifies the beneficial effects of different agents in combating IR injury. The major groups of molecules include anti-apoptosis/necrosis agents, free radical scavengers, antisepsis, growth factors, vasodilators, and anti-inflammatory agents (Jo et al. 2007). Although these compounds showed beneficial effects in pre-clinical settings, clinical translation of its impact is questionable as the compounds look for specific targets, and it is difficult to rule out other overlapping or similar effects that could have been partly responsible for causing renal injury and dysfunction (Jo et al. 2007). This may be addressed by increasing the ability of the renal tissue to withstand IR or searching for a multi-targeted agent to tackle multifaceted IR pathology.
Natural products like flavonoids are drawing increasing attention for the treatment of renal diseases, primarily due to their ability to trigger multiple targets apart from their antioxidant potential (Vargas et al. 2018). Flavonoids are reported to have antihypertensive, antidiabetic, anti-carcinogenic, and anti-inflammatory effects (Panche et al. 2016). Many of them exert reno-protective actions in diseases such as glomerulonephritis, diabetic nephropathy, and chemically induced kidney insufficiency (Arab et al. 2016; Vargas et al. 2018). Flavonoids are known to prevent renal injury associated with arterial hypertension, both by decreasing blood pressure and by acting directly on the renal parenchymal cells (Bai et al. 2013; Vargas et al. 2018). These outcomes result from their interaction with multiple signaling pathways known to produce renal injury.
Fisetin (3,7,3,4-tetrahydroxyflavone) is a flavonoid widely present in many fruits and vegetables with the highest concentrations in strawberries (Khan et al. 2013). Fisetin is already reported to have several pharmacological benefits, including anti-inflammatory, anti-apoptotic, antioxidant, anti-tumorigenic, and anti-angiogenic effects (Antika and Dewi 2021). Physicochemical analysis of fisetin indicated that the compound and its metabolites had a high concentration in mouse kidneys compared to other organs, suggesting its potential in treating renal diseases (Touil et al. 2011). The reno-protective effects of fisetin have been confirmed in animal models of diabetic nephropathy, cisplatin-induced nephrotoxicity, and LPS-induced septic AKI (Sahu et al. 2014, Ge et al. 2019). Fisetin’s ability to ameliorate IR injury has been proven in organs such as the heart, liver, and brain (Shanmugam et al. 2018; Li et al. 2021; Zhang and Cui 2021). Fisetin has been reported to exert IR protective activity in the heart via the PI3K/Akt/GSK3β pathway (Shanmugam et al. 2018) and by reducing oxidative stress and inflammation in the liver and brain (Li et al. 2021; Zhang and Cui 2021). IR is a bioenergetic-related disorder rooted in parenchymal or stromal cells, where these cells varied in different organs and thus have different impacts and involve distinct mediators (Kisseleva and Brenner 2008). However, limited information is available about its IR protective role in renal ischemia–reperfusion.
The present study aims at unraveling the reno-protective effects of fisetin and underlying mechanisms in a rat model of renal ischemia–reperfusion injury.
Material methods
Animals
Male Wistar rats (230–250 g) were obtained from Central Animal facility, SASTRA deemed to be University. Animals were kept for at least one week prior to the experiments in the animal unit under standardized conditions of temperature (22 ± 1 °C), humidity (55 ± 5%), and 12-h/12 h-light/dark cycles with free access to food and water.
Anesthesia, analgesia, and surgical procedure
All the animal experiments were carried out to comply with the guidelines of the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA) (552/SASTRA/IAEC/RPP). Anesthesia with isoflurane and analgesia with meloxicam were used for the surgical procedure. The vascular pedicle of each kidney was mobilized. In animals undergoing clamping, both renal pedicles were occluded using atraumatic mini-bulldogs. After the ischemic period, the reperfusion is attained by removing the clamps and perfusing the kidney. Animals were sacrificed by a cardiac incision under deep isoflurane anesthesia at the end of the experiment.
In this study, rats were randomized into 6 groups consisting of 6 rats in each group: (I) sham (S): all procedures remained similar except the occlusion of renal arteries was not carried out; (II) fisetin sham (F_S): surgical procedures were similar to sham. Fisetin (20 mg/kg) administered intraperitoneally 15 min before initiating the procedure; (III) ischemia–reperfusion (IR): bilateral clamping was done to begin ischemia and maintained for 45 min after that the kidneys were subjected to 24 h of reperfusion; (IV) fisetin IR (F_IR): similar to IR procedure. Fisetin (20 mg/kg based on preliminary data in supplementary Fig. 1) was administered intraperitoneally 15 min before initiating the procedure; (V) wortmannin IR (W_IR): similar to IR procedure. wortmannin (0.015 mg/kg) administered intraperitoneally 30 min before initiating the procedure; and (VI) wortmannin fisetin IR (W_F_IR): wortmannin injected 15 min before fisetin administration and proceeded with IR procedure.
Biochemical parameters
Blood samples from all groups were collected before and after the surgery. Plasma was separated by centrifuging the tubes at 3000 rpm for 10 min and stored at − 80 °C for further analysis. Plasma creatinine and BUN were estimated using the respective diagnostic kits purchased from Agappe Diagnostics Ltd. (India).
Animals used for urine collection were housed in metabolic cages. The animals had free access to water during the entire experiment, which could last up to 24 h post-surgery. The urine was collected 24 h after the surgery, and the total urine volume was determined. Creatinine and blood urea nitrogen (BUN) were measured in plasma and urine using respective diagnostic kits from Agappe Diagnostics Ltd. (India). Creatinine, protein, and blood urea nitrogen (BUN) were measured in the urine using diagnostic kits (Agappe, India).
Glomerular filtration rate
eGFR calculation was done using the equation by Pestel et al. (2007):
-
Creatinine clearance = (1000 ⁎ urine volume ⁎ concentration of creatinine in urine)/concentration of creatinine in serum
-
BUN clearance = (urine volume ⁎ concentration of BUN in urine)/concentration of BUN in serum
-
eGFR = mean of (creatinine clearance, BUN clearance)
Histopathology
Immediate laparotomy was performed to collect both the kidneys after the reperfusion period. Isolated kidneys were cleaned off the extraneous tissue, weighed, and rinsed with ice-cold normal saline. A section from both kidneys was fixed with 10% formalin (v/v), embedded in paraffin, sectioned at 5 μm, and stained with hematoxylin and eosin for histopathological examination under a light microscope. Three kidney tissues per group were analyzed for histological changes.
Apoptosis
DNA was isolated using phenol–chloroform-isoamyl alcohol mixture as per standard protocol, and sample (1 μg/well) was run on an agarose gel (1.8%) for 2 h. Laddering/smearing patterns representing DNA breaks due to apoptosis were evaluated along with the standard DNA ladder, and images were taken using the Bio-Rad Chemi Doc XRS system.
Additionally, the renal tissue homogenate was prepared in Tris–HCl buffer (pH-8) with EDTA (25 mM) and NaCl (400 mM). The activity of active caspase-3 was estimated spectrophotometrically using caspase-3 fluorogenic substrate (Sigma Aldrich, USA). Briefly, the tissue homogenate was added to the buffer with the substrate (HEPES 100 mM, EDTA 0.5 mM, DTT 5 mM, glycerol 20%, CHAPS 0.1%, and substrate 25 μM), and the activity was monitored at ex/em 365/465 nm for 1 h at 37 °C.
Isolation of mitochondria
The differential centrifugation technique was used to isolate rat kidney mitochondria according to the method described by Palmer et al. (1977). Isolated mitochondrial function was analyzed as mentioned below.
Antioxidant enzymes and lipid peroxidation
The concentration of thiobarbituric acid reactive substances (TBARS) and glutathione (GSH) and activities of glutathione peroxidase (GPx), glutathione reductase (GR), superoxide dismutase (SOD), and catalase enzymes were measured in renal tissue and mitochondrial samples as per the procedures described previously (Ansari et al. 2019).
Mitochondria analysis
-
i)
Activity of electron transport chain enzymes, namely rotenone-sensitive NADH-oxidoreductase (NQR), succinate decylubiquinone DCPIP reductase (SQR), ubiquinol cytochrome c reductase (QCR), and cytochrome c oxidase (COX) analyzed spectrophotometrically as per the procedure described previously (Priante et al. 2019).
-
ii)
ATP level and ATP producing capacity: The ATPlite (Perkin Elmer) system was used to measure ATP levels in the isolated mitochondria of all the groups based on the luminescence produced by the reaction of ATP with the substrates luciferase and α-luciferin. The ATP producing ability was evaluated in the presence of glutamate/malate (5/2.5 mM) and succinate (2.5 mM) energized medium and compared with the ATP level in the non-energized conditions.
-
iii)
Mitochondrial permeability: Ca2 + -induced swelling was used to assess the opening of the mitochondrial permeability transition pore. Mitochondria were incubated at 30 °C in a swelling buffer containing 125 mM sucrose, 50 mM KCl, 5 mM HEPES, 2 mM KH2PO4, and 1 mM MgCl2 to assess non-energized swelling. The absorbance was recorded at 540 nm for 10 min. The swelling activity was reported as light scattering/mg protein at 540 nm.
-
iv)
Rhodamine 123 dye mediated transmembrane analysis: Mitochondria aliquots were centrifuged at 9000 g for 10 min. Pellets were then suspended in fresh incubation buffer (125 mM sucrose, 50 mM KCl, 5 mM HEPES, 2 mM KH2PO4, and 1 mM MgCl2) devoid of EDTA. 1.0 μM of Rhodamine 123 was added and incubated at 37° in a thermostatic bath for 15 min with gentle shaking. Mitochondrial pellets were separated by centrifugation at 9000 g for 10 min, and the concentration of Rhodamine 123 was measured in the pellet as well as in supernatant at an excitation wavelength of 508 nm and emission wavelength of 528 nm (Sigma). Membrane potentials (negative inside) were calculated by the Nernst equation: Δψm = 59 * log [(Rh 123)in/(Rh 123)out].
-
v)
Gene expression: The expression for the mitochondrial encoded Nd 1, and nuclear-encoded β actin, Tfam, Polg, Pgc 1α, Fis 1, Mff, Dnm 1, Mfn 1, Mfn 2, Opa 1, Pink 1, Parkin, and Optn was analyzed using qPCR (ABI7500, Thermo Scientific, USA) in rat kidney samples. The primer details are presented in Table 1. Nd 1 expression was analyzed in both cellular DNA and cDNA, whereas other gene expressions were estimated in cDNA and normalized with β actin in respective samples. The DNA isolation is done using the phenol–chloroform-isoamyl alcohol method according to the manufacturer’s instructions (Himedia, Mumbai). The mRNA extraction was carried out using TRIzol reagent (15,596,026, Thermo Scientific, USA) as per the instructions, and gene expression was quantified using Sybr green chemistry (F415, Thermo Scientific, USA). The expression of genes was calculated as per the procedure of Livak and Schmittgen (Livak and Schmittgen 2001). The primer sequence of the genes is presented in Table 1.
Table 1 Primer details of mitochondrial quality control genes
Statistical analysis
All data are expressed as mean ± standard deviation of the mean (SD). Intergroup comparisons were performed by using a one-way analysis of variance, followed by a post hoc Dunnet’s test was employed to determine the difference among the groups, p < 0.05 was considered statistically significant.
Results
-
1.
The effects of fisetin on eGFR, plasma BUN, creatinine, and urinary albumin/creatinine ratio in rats subjected to renal ischemia–reperfusion injury
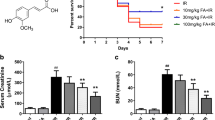
The effect of fisetin on the renal physiological function and corresponding renal markers were measured, and the results are given in Fig. 1. According to Fig. 1a, IR induced a fall in the eGFR from the sham group (S-15.91 ml/min, IR-0.35 ml/min). The plasma level of BUN (S-18.34 mg/dl, IR-111.83 mg/dl), creatinine (S-0.69 mg/dl, IR-2.20 mg/dl), and urinary albumin/creatinine ratio (Al/Cr-S-0.46, IR-6.09) were significantly increased from the normal range in IR group (Fig. 1b–d).
Fisetin pretreatment prior to IR protocol protected the kidney from damage and improved the glomerular function significantly (p < 0.05) from the IR (IR-0.35 ml/min, F_IR-9.03 ml/min). The injury markers exhibited a marked reduction in their level in fisetin pretreated renal tissues from the IR group (F-IR: Cr-0.92 mg/dl, BUN-81.03 mg/dl, Al/Cr-2.16).
-
2.
Effect of fisetin on renal injury in rats subjected to renal ischemia–reperfusion
Histopathological analysis of the renal tissue from different experimental groups reveals normal architecture in the sham group, which was significantly altered in the IR tissue (Table 2). Compared with the sham (Fig. 1e), the kidneys from the IR group rats (Fig. 1f) showed severe glomerular congestion, tubular dilatation, tubular degeneration with vacuolization in proximal tubules, and cast formation. Bowman’s capsule was found to be enlarged, and the gap between Bowman’s capsule and the glomerular capillaries convolute increased in the IR compared with sham (Fig. 1j). The pathological changes in the renal tissues from IR groups were minimal with prior treatment of fisetin (Fig. 1h), confirming the protective effect of fisetin. Accordingly, the characteristic histological changes observed in F_IR were a decrease in renal congestion and tubular dilatation, minimal tubular cast formation, and a decrease in Bowman’s capsule enlargement, indicating a reduced injury (Fig. 1i).
Table 2 Ultrastructural changes in the kidney after ischemia reperfusion (H&E): Histological changes were scored on a 4-point scale: (-) none, (+) mild, (++) moderate, and (+++) severe damage -
3.
Fisetin attenuates the IR-induced apoptosis in rats subjected to renal ischemia–reperfusion injury
Apoptotic renal injury was assessed by measuring the caspase 3 activities and visualizing the fragmented DNA in agarose gel (Fig. 1j and k). Caspase 3 activity was high in IR tissue by 27% from the sham tissue. Fragmented DNA was visible in the agarose gel electrophoresis of IR tissue which was absent in the sham group. However, compared with the IR, fisetin pretreated IR renal tissue exhibited significantly low apoptotic injury evident by the reduced fragmentation and decline in caspase 3 activity by 19% (Fig. 1k).
-
4.
Fisetin reduced the oxidative stress associated with renal ischemia–reperfusion injury
Figure 2 shows the renal lipid peroxidation level and antioxidant enzyme activities in the tissues of different experimental groups. Upon reperfusion, the renal tissues exhibited an increased concentration of TBARS (in nM MDA/mg tissue, S-2.00, IR-3.41) and correspondingly decreased level of GSH/GSSG (S-0.43, IR-0.27). The antioxidant enzymes like SOD (in U/mg protein, S-5.8, IR-3.56), catalase (mU/mg protein, S-12.8, IR-6.03), and GPx (μM GSH/mg protein, S-0.94, IR-0.65) were declined in their activities. Preconditioning the animal with fisetin showed a significant decline in the level of TBARS (IR-3.41, F_IR-2.74) and improved the endogenous antioxidant GSH/GSSG level (IR-0.27, F_IR-0.34) compared with the IR group. The antioxidant enzyme activities (SOD, catalase, GPx, and GR) were improved in F_IR (Fig. 2c–f) group than IR.
Figure 3 shows the lipid peroxidation and antioxidant status in the renal mitochondria. Lipid peroxidation measured in terms of TBARS was high in the IR group (11% higher than in the homogenate). The corresponding decline in reduced glutathione (GSH) was prominent in IR tissue (31% higher than in homogenate). Similar patterns of changes were observed in the antioxidant enzymes of mitochondrial fraction from IR tissue. Fisetin pretreatment significantly reduced TBARS (42%) and increased GSH/GSSG (18%) level in the mitochondria from the IR group animals. Activities of antioxidant enzymes like catalase (63%), SOD (35%), and GPx (20%) were also improved significantly from the IR group.
-
5.
Fisetin improved the mitochondrial bioenergetic functions in rats subjected to ischemia–reperfusion injury
The quality of mitochondria in the renal tissue is a critical factor that regulates renal function. The different mitochondria functions like bioenergetics (Fig. 4) and its regulatory events such as copy number (Fig. 5), mitophagy, mitofusion, and mitofission (Fig. 6) were evaluated to assess the renal mitochondrial quality.
The bioenergetic potential of renal mitochondria was measured via ETC enzyme activities like NQR, SQR, QCR, and COX, along with ATP level (Fig. 4). Animals subjected to IR exhibited low enzyme activities in NQR, SQR, QCR, and COX by 41%, 43%, 50%, and 82%, respectively, from the sham. ATP level in IR tissue was significantly low (18%) from the sham. However, fisetin treatment in tissue subjected to IR improved the mitochondrial ETC enzyme activities of NQR (25%), SQR (25%), QCR (39%), COX (71%), and ATP level (22%) from the IR control (Fig. 4a–d and 7a).
To assess if the mitochondrial copy number plays a significant role in the reduced functional activity, we evaluated the mitochondrial copy number in the rat renal tissues from all the experimental groups and noted a significant (p < 0.05) reduction in the copy number in IR-treated animal which was improved by the pretreatment of the animals with fisetin (Fig. 5a).
Further analysis of the expression level of PGC-1α (Fig. 5b), which plays a critical role in the regulation of mitochondrial biogenesis and respiratory function (bioenergetics), found to be downregulated 2.6-fold (p < 0.05) in IR rat kidney from the sham. Fisetin pretreatment upregulated the PGC-1α expression from the IR group (PGC-1α gene expression in folds: IR- 0.38, F_IR-1.03) confirming the mitochondrial biogenetic potential of the drug. However, fisetin was found to exert minimal impact on the mitochondrial transcription regulation factor A (Tfam) and mitochondrial DNA polymerase γ (POLG), which regulates the mitochondrial encoded gene expressions, where 13 mitochondrial encoded genes influenced the activity of bioenergetic enzymes (Fig. 5c–d).
-
6.
Fisetin improved renal mitochondrial dynamics and mitophagy in rats subjected to ischemia–reperfusion injury
The damage to mitochondria associated with IR injury can disrupt the balance of the dynamic processes controlling mitochondrial fusion and fission. In order to analyze the same, we estimated the gene expression of mitochondrial fission and fusion genes in different experimental groups, and the results are given in Fig. 6. We observed an augmented expression of mitochondrial fission genes Mff, Fis 1, and Dnm 1 by 1.66-, 1.17-, and 2.23-folds respectively in the IR-treated groups compared with sham (Fig. 6a–c). Treatment with fisetin prior to the IR reduced the mitochondrial fission (Dnm 1 expressed in fold change: IR-2.23 F_IR-1.21, Fis 1: IR-1.17 F_IR-0.35, Mff: IR-1.66 F_IR-1.14). Furthermore, the mRNA expression of the Mfn1 gene, which codes for the principal GTP-dependent membrane tethering protein for mitochondrial fusion, exhibited a significant (p < 0.05) increase in IR (S-1.00, IR-1.57) group animals from sham. However, mfn1 expression was further increased in the F_IR group (IR-1.57, F_IR-1.92).
To determine the effect of fisetin on mitophagy, a critical event that maintains the mitochondrial quality was evaluated by measuring the gene expression of the key regulatory molecules such as Pink, Parkin, and Optn (Fig. 6f–h). Pink and Parkin showed significant elevation (3.7-fold) in the gene expression in response to IR induction in the sham control among the three genes. On the other hand, compared to sham, all the three genes showed significantly higher expression in F_IR. Two out of three genes were significantly expressed in the F_IR group compared to the IR control group (Parkin: IR-1.25, F_IR-1.98, and Optn: IR-1.00, F_IR-1.28). In general, fisetin pretreatment prior to IR challenged improved the mitochondrial function and maintained the mitochondrial homeostasis and quality.
-
7.
Fisetin improved the mitochondrial ATP producing capacity and attenuated swelling and mPTP opening in rats subjected to ischemia–reperfusion injury
In order to assess if alterations in the electron transport chain affected the ATP machinery in renal tissue, we estimated the ATP producing capacity of isolated mitochondria in different energized conditions (glutamate-malate/succinate) and non-energized conditions. After incubating the mitochondria in the respective respiratory buffers for 10 min, ATP content was measured by using an ATPlite kit, and the results are presented in Fig. 7 (a–c). The IR significantly compromised the ATP producing capacity of the isolated renal mitochondria in both energized and non-energized conditions. However, isolated renal mitochondria from the fisetin pretreatment group retain the ATP producing capacity significantly. We also noted another interesting observation where the ATP level showed a striking increase in the fisetin control group than the sham control.
The corresponding mitochondrial membrane potential, which determines the oxidative phosphorylation, in different energized mediums, did not show significant changes across the experimental groups (Fig. 7d–f). However, mitochondrial swelling was high in the IR group in both energized and non-energized conditions, which was improved substantially in the fisetin-treated IR group (Fig. 7g–i).
-
8.
Fisetin’s protective ability towards renal IR injury is independent of PI3K/Akt signaling pathway
Renal physiology and injury analysis of sham and fisetin-treated rats subjected to IR injury. Renal functional markers a eGFR, b plasma BUN, c plasma creatinine, and d urinary albumin/creatinine ratio. H&E-stained severe necrotic derangement images for e S, f IR, g F_S, and h F_IR (magnification: 10 ×) and images of H&E-stained Bowman’s capsule and capillaries for i S, j IR, k F_S, and l F_IR (magnification: 40 ×). m Renal injury score from H&E staining. Renal cell death analysis by n caspase activity and o DNA fragmentation. *p < 0.05 vs S, #p < 0.05 vs IR. The data are presented as mean ± SD (n = 6/group). S, sham; IR, ischemia–reperfusion; F_S, fisetin sham; F_IR, fisetin ischemia–reperfusion
Oxidative stress analysis and antioxidants levels in the renal homogenate of a sham- and fisetin-treated rats subjected to IR injury. a Shows lipid peroxidation measured by TBARS, b GSH/GSSG ratio, c SOD activity, d catalase activity, and e represents GPx activity. *p < 0.05 vs S, #p < 0.05 vs IR. The data are presented as mean ± SD (n = 6/group). S, sham; IR, ischemia–reperfusion; F_S, fisetin sham; F_IR, fisetin ischemia–reperfusion
Oxidative stress analysis and antioxidants levels in the renal mitochondrial fraction of sham- and fisetin-treated rats subjected to IR injury. a Shows lipid peroxidation measured by TBARS, b GSH/GSSG ratio, c SOD activity, d catalase activity, and e represents GPx activity. *p < 0.05 vs S, #p < 0.05 vs IR. The data are presented as mean ± SD (n = 6/group). S, sham; IR, ischemia–reperfusion; F_S, fisetin sham; F_IR, fisetin ischemia–reperfusion
Mitochondrial ETC enzymes in sham- and fisetin-treated rats subjected to IR injury. a NQR activity, b SQR activity, c QCR activity, and d COX activity. NQR activity was expressed as μmol NADH oxidized min−1 mg−1 protein; SQR activity was expressed in μmol DCPIP reduced min−1 mg−1 protein; QCR activity was expressed in μmol cytochrome C reduced min−1 mg−1 protein; COX activity was expressed in μmol cytochrome C oxidized min−1 mg−1 protein. *p < 0.05 vs S, #p < 0.05 vs IR. The data are presented as mean ± SD (n = 6/group). S, sham; IR, ischemia–reperfusion; F_S, fisetin sham; F_IR, fisetin ischemia–reperfusion
Gene expression analysis in sham and fisetin-treated rats subjected to IR injury. The graph represents a mitochondrial copy number and b Pgc-1α expression, c Tfam expression, and d Polg in renal tissues. *p < 0.05 vs S, #p < 0.05 vs IR. The data are presented as mean ± SD (n = 6/group). S, sham; IR, ischemia–reperfusion; F_S, fisetin sham; F_IR, fisetin ischemia–reperfusion
Expression analysis of genes encoding mitochondrial fission, fusion, and mitophagy in renal tissues from sham- and fisetin-treated rats subjected to IR injury. The graph represents the mitochondrial fission genes a Dnm 1, b Fis 1, and c Mff; mitochondrial fusion genes d Mfn 1 and e Mfn; mitophagic genes f Pink, g Parkin, and h Optn. *p < 0.05 vs S, #p < 0.05 vs IR. The data are presented as mean ± SD (n = 6/group). S, sham; IR, ischemia–reperfusion; F_S, fisetin sham; F_IR, fisetin ischemia–reperfusion
Analysis of changes in mitochondrial ATP production, membrane potential, and swelling in renal tissues from sham- and fisetin-treated rats subjected to IR injury. The graph represents measured ATP content using ATPlite luminescence kit, in a non-energized, b glutamate/malate energized, and c succinate energized medium. d, e, and f represent mitochondrial membrane potential (∆Ψm) measured by Rhodamine 123 fluorescent dye in non-energized, succinate energized, and glutamate/malamute energized medium respectively; g, h, and i represent calcium-induced mitochondrial swelling in non-energized, succinate energized, and glutamate/malamute energized medium respectively. *p < 0.05 vs S, #p < 0.05 vs IR. The data are presented as mean ± SD (n = 6/group). S, sham; IR, ischemia–reperfusion; F_S, fisetin sham; F_IR, fisetin ischemia–reperfusion
In order to assess the role of the PI3K/Akt pathway in renal protection mediated by fisetin, we used an inhibitor-based study. The study results presented in Fig. 8 shows that the IR induced renal dysfunction was found to have an elevated trend in the presence of wortmannin in all the physiological and injury parameters (IR: eGFR-0.35 ml/min, Cr-2.20 mg/dl, BUN-111.83 mg/dl, Al/Cr-6.09); W_IR: eGFR-0.30 ml/min, Cr-2.70 mg/dl, BUN-123.04 mg/dl, Al/Cr-6.87), but the significant difference was found only in creatinine. However, the improved protection of IR kidney by fisetin in the presence of wortmannin suggested its mode of action may be independent of PI3K/Akt signaling pathway. Immunoblot analysis and activity of GSK 3b showed an increased protein expression and its activities in the renal IR tissue which was successfully reverted by the fisetin treatment (Supplementary Fig. 2). Overall, the results suggest that the protective ability of fisetin was mediated by the inactivation of GSK3b, the downstream mediator of the PI3K signaling pathway.
Renal physiology and injury analysis of wortmannin treated rats subjected to IR injury. Renal functional markers a eGFR, b plasma creatinine, c plasma BUN, and d urinary albumin/creatinine ratio. H&E-stained severe necrotic derangement images for e S, f IR, g F_S, h F_IR, i W_IR, and j W_F_IR and k renal injury score from H and E staining. The images are presented at 10 × magnification. Renal cell death analysis by l caspase activity. *p < 0.05 vs S, #p < 0.05 vs IR. The data are presented as mean ± SD (n = 6/group). S, sham; IR, ischemia–reperfusion; F_S, fisetin sham; F_IR, fisetin ischemia–reperfusion
Discussion
Renal ischemia–reperfusion injury contributes significantly to the development of acute kidney injury that is continuously associated with high morbidity and mortality rate (Basile et al. 2012). Early reports suggest that ischemia–reperfusion injury in rat hearts can be effectively ameliorated by fisetin administration (Shanmugam et al. 2018). Pathophysiology of IR in the heart and kidney are different due to differences in the composition of cell types (Kisseleva and Brenner 2008). However, the therapeutic efficacy of fisetin in attenuating IR injury in renal tissue is yet to be explored. The important findings of the present study are as follows: (1) IR induced reduction in eGFR and increased plasma BUN, creatinine, and urinary Al/Cr ratio was reversed by fisetin and thereby preserved renal physiology from the impact of ischemia–reperfusion; (2) IR increased oxidative stress and mitochondrial dysfunction was attenuated by fisetin; (3) mitochondrial copy number reduction in the tissues challenged to ischemia–reperfusion were restored by fisetin; (4) IR-associated decline in the mitochondrial quality in renal tissues were protected by fisetin pretreatment via reversing the gene expression of Pgc 1α, reducing mitochondrial fission (Mff, Fis, and Dnm 1), increasing mitochondrial fusion (Mfn 1) and mitophagy (Parkin and Optn) (5); and the protective effect of fisetin against renal ischemia–reperfusion injury is independent of PI3K/Akt signaling pathway.
Ischemia–reperfusion injury compromised renal function by altering renal hemodynamics and thereby promoting the development of AKI (Basile et al. 2012). In the present study, the IR-associated deterioration of renal function was assessed by measuring the efficiency of eGFR and the levels of plasma BUN, creatinine, and urinary albumin to creatinine ratio (Fig. 1a–d). The improvement of these parameters in fisetin-treated IR challenged kidney tissue confirmed the ability of the compound to render renal protection against IR. Renal cell death can contribute to deteriorated IR-associated renal insufficiency (Priante et al. 2019). Hence, we evaluated the ultra-structure of kidneys in all experimental groups. Accordingly, we found severe renal injury in the IR group and this injury was significantly reduced with fisetin pretreatment (Fig. 1e–i). Fisetin was reported to attenuate IR-associated apoptotic cell death in the heart, brain, and liver (Shanmugam et al. 2018; Li et al. 2021; Zhang and Cui 2021). In accordance with this finding, the present study demonstrated an evident decline in apoptotic injury in the F_IR group (Fig. 1j–k).
The renal proximal tubule is the primary target of ischemia–reperfusion injury and is involved in the subsequent progression of kidney diseases. This may be due to the presence of a large number of mitochondria in PCT (Chevalier 2016). The IR injury in any organ is developed when there is an imbalance between energy supply and demand, which is regulated by mitochondria (Kalogeris et al. 2012). In the present study, we noted deteriorated mitochondrial function in IR-challenged renal tissue. Notably, most of the IR-associated mitochondrial-specific changes were improved in renal tissue that was priorly treated with fisetin. The overall function of mitochondria can be assessed by measuring different mitochondrial events like bioenergetics, mitophagy, mitofusion, and mitochondrial copy number, collectively called mitochondrial quality control. Recent evidence emphasizes the significance of mitochondrial quality control as a molecular target in managing ischemia–reperfusion.
Previous studies reported the role of the master regulatory gene-Pgc 1α, in energy metabolism (Liang and Ward 2006). Pagel-Langenickel identified Pgc 1α as a bidirectional regulatory link integrating insulin-signaling and mitochondrial homeostasis in skeletal muscle (Pagel-Langenickel et al. 2008). In the present study, expression of Pgc 1α was significantly low in IR tissues (60% from sham) was recovered to near normal level by fisetin treatment (Fig. 5). The results are in corroboration with our previous results reported in the heart (Shanmugam et al. 2018). Consistent with this observation, we found the improvement of bioenergetic function in fisetin-treated renal tissue where NQR, SQR, QCR, and COX were significantly elevated from the IR control animal (Fig. 4). In addition, the ATP level in the tissue was augmented with fisetin treatment (Fig. 7).
Pgc 1α is not only a key regulator of energy metabolism but also controls mitochondrial biogenesis (Liang and Ward 2006). Flavonoids belonging to all classes have been found to stimulate mitochondrial biogenesis in both in vitro and in vivo by activating PGC 1α (Pagel-Langenickel et al. 2008; Kicinska and Jarmuszkiewicz 2020). In this direction, we found significant improvement in mitochondrial fusion genes like Mfn 1 in fisetin-treated renal tissue that may mitigate the stress in mitochondria by undergoing fusion with healthy mitochondria (Fig. 6). Previous studies have shown that Pgc 1α regulates the expression of mitochondrial antioxidant enzymes (Lu et al. 2010). In the present study, we measured the oxidative stress experienced by the mitochondria and tissue homogenate, and the results were complemented with Pgc 1α, where an improved antioxidant status in renal tissues from IR was observed in rats pretreated with fisetin (Fig. 2 and 3).
Literature suggests that when the mitochondria experience low membrane potential, it triggers mitochondrial events that favor fission that, in turn, enhances mitophagy to eliminate the dysfunctional mitochondria (Twig and Shirihai 2011). The mitochondrial fusion genes were significantly elevated in renal IR tissue with subsequent upregulation of mitophagic genes (Fig. 6). Fisetin pretreatment reduced the expression of mitochondrial fission genes and increased the expression of fusion and phagy genes. Similar effects were shown by quercetin, another dietary flavonoid that is structurally similar to fisetin, in vascular smooth muscle cells (Cui et al. 2017). The overall mitochondrial functional improvement by fisetin treatment was reflected in restoring mitochondrial DNA copy number (Fig. 5).
In order to reconfirm the fisetin-mediated mitochondrial protection, we isolated the mitochondria from different experimental groups and incubated the organelle in energized (GM/succinate) and non-energized medium before measuring the ATP producing capacity and related mitochondrial membrane potential and swelling behavior (Fig. 7). Accordingly, the results given in Fig. 7a–c suggest high ATP producing capacity wherein both fisetin-treated groups in all conditions. This observation was in line with the previous finding that suggests the activation of complex 1 by polyphenolic compound resveratrol (Gueguen et al. 2015). Structural similarities of fisetin with resveratrol predict the ability of fisetin to donate electrons to ETC, thereby promoting the generation of proton motive force (PMF) that may culminate in ATP production.
Fisetin was reported to render cardioprotection against IR via activating PI3K signaling pathway (Shanmugam et al. 2021). Contrary to this finding, the present study result did not support PI3K-mediated protection in renal IR, where the loss of function of PI3K by wortmannin did not show any significant decline in renal protection mediated by fisetin (Fig. 8). However, as the previous study suggested, fisetin probably retained its potential to bind with the GSK 3β in renal IR (Supplementary Fig. 2) (Shanmugam et al. 2018). And overall, the results suggested that in renal IR, the protective ability of fisetin was mediated by the inactivation of GSK3β, the downstream mediator of the PI3K pathway.
Conclusion
The present study shows that IR challenge compromised renal function (reduced eGFR and increased kidney injury markers), deteriorated mitochondrial function, along with low mitochondrial quality. These changes were successfully reverted by the fisetin treatment and protected the kidney from renal IR injury.
Data availability statement
The datasets generated and analyzed during the current study are submitted as supplementary material.
Abbreviations
- IR:
-
Ischemia-reperfusion
- AKI:
-
Acute kidney injury
- eGFR:
-
Estimated glomerular filtration rate
- ATP:
-
Adenosine triphosphate
- Al/Cr:
-
Urinary albumin/creatinine ratio
- PI3k:
-
Phosphoinositide 3-kinases
- Akt:
-
Protein kinase B
- GSK3 β:
-
Glycogen synthase kinase 3 beta
- BUN:
-
Blood urea nitrogen
- MDA:
-
Malondialdehyde
- GSH:
-
Reduced glutathione
- GSSG:
-
Oxidized glutathione
- GPx:
-
Glutathione peroxidase
- SOD:
-
Superoxide dismutase
- GR:
-
Glutathione reductase
- NQR:
-
NADH-oxidoreductase
- SQR:
-
Succinate-coenzyme Q reductase
- QCR:
-
QH2:cytochrome c oxidoreductase
- COX:
-
Cytochrome c oxidase
- Pgc 1α:
-
Peroxisome proliferator-activated receptor-gamma coactivator
- Tfam:
-
Transcription regulation factor A
- Polg:
-
Mitochondrial DNA polymerase γ
- Dnm 1:
-
Dynamin-1
- Fis 1:
-
Mitochondrial fission protein 1
- Mff:
-
Mitochondrial fission factor
- Mfn1:
-
Mitofusin 1
- Mfn 2:
-
Mitofusin 2
- Pink:
-
PTEN-induced kinase 1
- Optn:
-
Optineurin
References
Ansari M, Gopalakrishnan S, Kurian GA (2019) Streptozotocin-induced type II diabetic rat administered with nonobesogenic high-fat diet is highly susceptible to myocardial ischemia-reperfusion injury: an insight into the function of mitochondria. J Cell Physiol 234(4):4104–4114
Antika LD, Dewi RM (2021) Pharmacological aspects of fisetin. Asian Pac J Trop Biomed 11(1):1
Arab HH, Mohamed WR, Barakat BM (2016) Arafa el-SA: Tangeretin attenuates cisplatin-induced renal injury in rats: impact on the inflammatory cascade and oxidative perturbations. Chem Biol Interact 258:205–213. https://doi.org/10.1016/j.cbi.2016.09.008
Bai S, Huang ZG, Chen L, Wang JT, Ding BP (2013) Effects of felodipine combined with puerarin on ACE2-Ang (1–7)-Mas axis in renovascular hypertensive rat. Regul Pept 184:54–61. https://doi.org/10.1016/j.regpep.2013.03.005
Basile DP, Anderson MD, Sutton TA (2012) Pathophysiology of acute kidney injury. Compr Physiol 2(2):1303–1353. https://doi.org/10.1002/cphy.c110041
Bienholz A, Wilde B, Kribben A (2015) From the nephrologist’s point of view: diversity of causes and clinical features of acute kidney injury. Clin Kidney J 8(4):405–414. https://doi.org/10.1093/ckj/sfv043
Chevalier RL (2016) The proximal tubule is the primary target of injury and progression of kidney disease: role of the glomerulotubular junction. Am J Physiol Renal Physiol 311(1):F145–F161. https://doi.org/10.1152/ajprenal.00164.2016
Cui L, Li Z, Chang X, Cong G, Hao L (2017) Quercetin attenuates vascular calcification by inhibiting oxidative stress and mitochondrial fission. Vascul Pharmacol 88:21–29. https://doi.org/10.1016/j.vph.2016.11.006
Dong Y, Zhang Q, Wen J, Chen T, He L, Wang Y, Yin J, Wu R, Xue R, Li S, Fan Y, Wang N. Ischemic duration and frequency determines AKI-to-CKD progression monitored by dynamic changes of tubular biomarkers in IRI mice. Front Physiol. 10:153, 2019.. Published 2019 Feb 26. doi:https://doi.org/10.3389/fphys.2019.00153
Ge C, Xu M, Qin Y, Gu T, Lou D, Li Q, Hu L, Nie X, Wang M, Tan J (2019) Fisetin supplementation prevents high fat diet-induced diabetic nephropathy by repressing insulin resistance and RIP3-regulated inflammation. Food Funct 10(5):2970–2985. https://doi.org/10.1039/c8fo01653d
Gueguen N, Desquiret-Dumas V, Leman G, Chupin S, Baron S, Nivet-Antoine V, Vessières E, Ayer A, Henrion D, Lenaers G, Reynier P, Procaccio V: Resveratrol directly binds to mitochondrial complex I and increases oxidative stress in brain mitochondria of aged mice. PLoS One 18;10(12):e0144290, 2015 doi: https://doi.org/10.1371/journal.pone.0144290
Jo SK, Rosner MH, Okusa MD (2007) Pharmacologic treatment of acute kidney injury: why drugs haven’t worked and what is on the horizon. Clin J Am Soc Nephrol 2(2):356–365. https://doi.org/10.2215/CJN.03280906
Kalogeris T, Baines CP, Krenz M, Korthuis RJ (2012) Cell biology of ischemia/reperfusion injury. Int Rev Cell Mol Biol 298:229–317. https://doi.org/10.1016/B978-0-12-394309-5.00006-7
Khan N, Syed DN, Ahmad N, Mukhtar H (2013) Fisetin: a dietary antioxidant for health promotion. Antioxid Redox Signal 19(2):151–162. https://doi.org/10.1089/ars.2012.4901
Kicinska A, Jarmuszkiewicz W (2020) Flavonoids and mitochondria: activation of cytoprotective pathways? Molecules 25(13):3060. https://doi.org/10.3390/molecules25133060
Kisseleva T, Brenner DA (2008) Fibrogenesis of parenchymal organs. Proc Am Thorac Soc 5(3):338–342. https://doi.org/10.1513/pats.200711-168DR
Li Z, Wang Y, Zhang Y, Wang X, Gao B, Li Y, Li R, Wang J (2021) Protective effects of fisetin on hepatic ischemia-reperfusion injury through alleviation of apoptosis and oxidative stress. Arch Med Res 52(2):163–173, 202. doi: https://doi.org/10.1016/j.arcmed.2020.10.009.
Liang H, Ward WF (2006) PGC-1alpha: a key regulator of energy metabolism. Adv Physiol Educ 30(4):145–151. https://doi.org/10.1152/advan.00052.2006
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods 1;25(4):402–8.
Lu Z, Xu X, Hu X, Fassett J, Zhu G, Tao Y, Li J, Huang Y, Zhang P, Zhao B, Chen Y (2010) PGC-1 alpha regulates expression of myocardial mitochondrial antioxidants and myocardial oxidative stress after chronic systolic overload. Antioxid Redox Signal 13(7):1011–1022. https://doi.org/10.1089/ars.2009.2940
Pagel-Langenickel I, Bao J, Joseph JJ, Schwartz DR, Mantell BS, Xu X, Raghavachari N, Sack MN (2008) PGC-1alpha integrates insulin signaling, mitochondrial regulation, and bioenergetic function in skeletal muscle. J Biol Chem 283(33):22464–22472. https://doi.org/10.1074/jbc.M800842200
Palmer JW, Tandler B, Hoppel CL (1977) Biochemical properties of subsarcolemmal and interfibrillar mitochondria isolated from rat cardiac muscle. J Biol Chem 252(23):8731–8739
Panche AN, Diwan AD, Chandra SR (2016) Flavonoids: an overview. J Nutr Sci 5:e47. https://doi.org/10.1017/jns.2016.41
Pestel S, Krzykalla V, Weckesser G (2007) Measurement of glomerular filtration rate in the conscious rat. J Pharmacol Toxicol Methods 56(3):277–289. https://doi.org/10.1016/j.vascn.2007.03.001
Prasath GS, Subramanian SP (2011) Modulatory effects of fisetin, a bioflavonoid, on hyperglycemia by attenuating the key enzymes of carbohydrate metabolism in hepatic and renal tissues in streptozotocin-induced diabetic rats. Eur J Pharmacol 668(3):492–496. https://doi.org/10.1016/j.ejphar.2011.07.021
Priante G, Gianesello L, Ceol M, Del Prete D, Anglani F: Cell death in the kidney. Int J Mol Sci 20(14):3598, 2019. Published 2019 Jul 23. doi:https://doi.org/10.3390/ijms20143598.
Sahu BD, Kalvala AK, Koneru M, Mahesh Kumar J, Kuncha M, Rachamalla SS, Sistla R (2014) Ameliorative effect of fisetin on cisplatin-induced nephrotoxicity in rats via modulation of NF-κB activation and antioxidant defence. PLoS ONE 9(9):e105070. https://doi.org/10.1371/journal.pone.0105070
Shanmugam K, Ravindran S, Kurian GA, Rajesh M (2018) Fisetin confers cardioprotection against myocardial ischemia reperfusion injury by suppressing mitochondrial oxidative stress and mitochondrial dysfunction and inhibiting glycogen synthase kinase 3β activity. Oxid Med Cell Longev 2018:9173436. https://doi.org/10.1155/2018/9173436
Shanmugam K, Boovarahan SR, Prem P, Sivakumar B, Kurian GA (2021) Fisetin attenuates myocardial ischemia-reperfusion injury by activating the reperfusion injury salvage kinase (RISK) signaling pathway. Front Pharmacol 12:566470. https://doi.org/10.3389/fphar.2021.566470
Touil YS, Auzeil N, Boulinguez F, Saighi H, Regazzetti A, Scherman D, Chabot GG (2011) Fisetin disposition and metabolism in mice: identification of geraldol as an active metabolite. Biochem Pharmacol 82(11):1731–1739. https://doi.org/10.1016/j.bcp.2011.07.097
Twig G, Shirihai OS (2011) The interplay between mitochondrial dynamics and mitophagy. Antioxid Redox Signal 14(10):1939–1951. https://doi.org/10.1089/ars.2010.3779
Vargas F, Romecín P, García-Guillén AI, Wangesteen R, Vargas-Tendero P, Paredes MD, Atucha NM, García-Estañ J (2018) Flavonoids in kidney health and disease. Front Physiol 9:394. https://doi.org/10.3389/fphys.2018.00394
Zhang P, Cui J: Neuroprotective effect of fisetin against the cerebral ischemia-reperfusion damage via suppression of oxidative stress and inflammatory parameters [published online ahead of print, 2021 Feb 22]. Inflammation https://doi.org/10.1007/s10753-021-01434-x, 2021. doi:https://doi.org/10.1007/s10753-021-01434-x
Acknowledgements
The authors thank Dr. David C Raj for his extensive help in carrying out the animal surgeries. We thank SASTRA Deemed University for providing all the needed infrastructure and facilities.
Funding
The authors sincerely thank the Department of Science and Technology, India, for supporting this research through grant-in-aid (EMR/2017/000669). Ms. Priyanka N P was supported by the Council of Scientific and Industrial Research, India (CSIR) fellowship grant (09/1095/(0040)/2018-EMR-1).
Author information
Authors and Affiliations
Contributions
P.N.P. has processed the experimental data, performed analysis, drafted the manuscript, designed figures, and tables, and compiled the literature sources. G.A.K. has contributed to the design and implementation of the research, to the interpretation of the results, and to the writing of the manuscript. The authors declare that all data were generated in-house and that no paper mill was used.
Corresponding author
Ethics declarations
Ethics approval
All the animal experiments carried out are in compliance with the guidelines of the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA) and approved by the Institutional animal ethical committee (552/SASTRA/IAEC/RPP).
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Prem, P.N., Kurian, G.A. Fisetin attenuates renal ischemia/reperfusion injury by improving mitochondrial quality, reducing apoptosis and oxidative stress. Naunyn-Schmiedeberg's Arch Pharmacol 395, 547–561 (2022). https://doi.org/10.1007/s00210-022-02204-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-022-02204-8