Abstract
In our previous work, we showed that during inflammation-induced epithelial-to-mesenchymal transition (EMT), mesenteric mesothelial cells express ED1 (pan-macrophage marker), indicating that they are transformed into macrophage-like cells. In this paper, we provide additional evidences about this transition by following the phagocytic activity and the TNFα production of mesenteric mesothelial cells during inflammation. Upon injection of India ink particles or fluorescent-labeled bioparticles (pHrodo) into the peritoneal cavity of rats pretreated with Freund‘s adjuvant, we found that mesothelial cells efficiently engulfed these particles. A similar increase of internalization could be observed by mesothelial cells in GM-CSF pretreated primary mesenteric culture. Since macrophages are the major producers of tumor necrosis factor, TNFα, we investigated expression level of TNFα during inflammation-induced EMT and found that TNFα was indeed expressed in these cells, reaching the highest level at the 5th day of inflammation. Since TNFα is one of the target genes of early growth response (EGR1) transcription factor, playing important role in monocyte-macrophage differentiation, expression of EGR1 in mesothelial cells was also investigated by Western blot and immunocytochemistry. While mesothelial cells did not express EGR1, a marked increase was observed in mesothelial cells by the time of inflammation. Parallel to this, nuclear translocation of EGR1 was shown by immunocytochemistry at the day 5 of inflammation. Caveolin-1 level was high and ERK1/2 became phosphorylated as the inflammation proceeded showing a slight decrease when the regeneration started. Our present data support the idea that under special stimuli, mesenteric mesothelial cells are able to transdifferentiate into macrophages, and this transition is regulated by the caveolin-1/ERK1/2/EGR1 signaling pathway.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Monocytes and macrophages are professional phagocytic cells and are the major differentiated mediators of immune responses [1,2,3]. These cells are widely distributed in many tissues and organs. Under normal (steady state) conditions, large numbers of these phagocytic cells (called resident macrophages) reside in the peritoneal cavity [3] and in the “milky spots” of the mesentery [4, 5]. Since peritoneal macrophages can be easily isolated, they are widely used as excellent in vivo model to study various biological processes. As a result of inflammatory stimulus (intraperitoneal Freund’s adjuvant injection), these resident peritoneal macrophages become activated, and their number and endocytic activity are significantly increased [6]. The origin and plasticity of these chronic, heterogeneous inflammatory macrophages are not entirely known. Tissue-resident macrophages as well as infiltrating monocyte-derived macrophages play a distinct role in the progression of inflammation [3,4,5, 7], but the large number of these inflammatory macrophages suggests that cells originating from other, non-hematopoietic sources can also contribute to a subset of macrophages. Our previous results showed that during inflammation-induced epithelial-to-mesenchymal transformation (EMT) and on the action of granulocyte-macrophage colony-stimulating factor (GM-CSF), mesenteric mesothelial cells undergo morphological and biochemical changes. They lose their cell polarity, start to migrate, and express macrophage markers [8,9,10]. Although mesothelial cells are phenotypically epithelial cells, originating from mesoderm, they still have some mesenchymal characteristics. Mesothelial cells express nestin [8], a well-known multi-lineage progenitor cell’s marker [11], indicating that they are not terminally differentiated cells; instead, they can be taken as multipotent cells. [8]. These data raise the question whether these plastic, not terminally differentiated cells can transdifferentiate into macrophages and contribute to the large increase of macrophages during inflammation.
The aims of the present study were to answer this question and to provide evidence for a possible transdifferentiation. Using India ink particles and fluorescent-labeled bioparticles (pHrodo), we followed the changes of the phagocytic (endocytic) activity of mesenteric mesothelial cells. Besides being professional phagocytic cells, macrophages are the major producers of TNFα, the main regulator of inflammatory cytokine production [12]. The early growth response transcription factor, EGR1, is the main regulator of macrophage differentiation [13,14,15,16,17,18,19]. When EGR1 is activated, it increases the target genes’s (TNFα and CD115) activity [20, 21]. Our next question was whether mesothelial cells expressed TNFα and EGR1 during inflammation-induced EMT.
We found that upon inflammation as well as GM-CSF-treatment, mesothelial cells efficiently phagocytosed both India ink and fluorescent bioparticles. The engulfed particles were present in large multivesicular body-like structures in the cytoplasm. By the time of inflammation, mesothelial cells express an increasing amount of TNFα and EGR1 reaching the maximum level on day 5 of inflammation. In our system, we found an increased ERK1/2 phosphorylation with a maximum level on day 3 of inflammation and caveolin-1 expression also changed during inflammation indicating that mesothelial cells to macrophage transdifferentiation (MMT) are regulated by caveolin-1/ERK1/2/EGR1 signaling pathway.
MATERIAL AND METHODS
In vivo Experiments
To induce peritonitis, 1-ml complete Freund’s adjuvant (Sigma, Saint Louis, Missouri) was injected into the peritoneal cavity of 70–90-day-old male Sprague-Dawley rats (200–250 g). After 3, 5, 8, and 11 days, the mesentery was isolated from control and treated animals (10 animals per group).
For in vitro experiments, mesentery was cut out from control animals and maintained in Dulbecco’s Modified Eagle Medium (Nutrient Mixture F-12 (DMEM/F12, Life Technologies, Paisley, UK) in humid condition at 37 °C with 5% CO2. To stimulate MMT, this primary mesentery culture was treated with 1 ng/ml GM-CSF (Sigma, Saint Louis, Missouri) diluted with the culture medium, for 24 h.
To study the phagocytic activity of transforming mesothelial cells in vivo, we injected India ink (1:2 India ink/PBS; Rotring-Werke Riepe KG, Hamburg) and fluorescent-labeled bioparticles (2 mg/3 ml/animal, Green Bioparticle pHrodo; ThermoFisher Scientific, Molecular Probe, Paisley, UK) into the peritoneal cavity of the control and Freund’s adjuvant-treated (days 3 and 5) rats. For in vitro experiments, pHrodo (0.5 mg/ml/Petri dish) was added to control and GM-CSF-treated (24 h) primary mesentery culture. After 24-h incubation with India ink or pHrodo, both in vivo and in vitro samples were fixed either in a mixture of 1% glutaraldehyde (GA) and 1% OsO4 in 0.1 M cacodylate buffer, pH 7.4 (1 h, on ice) or 4% paraformaldehyde (PFA) in 0.1 M phosphate-buffered saline (PBS), pH: 7.4 (1 h at room temperature). The GA and OsO4-fixed samples were proceeded for conventional transmission electron microscopy (EM), while PFA-fixed samples were used for light- (confocal) microscopic observation and immunocytochemistry.
IMMUNOCYTOCHEMISTRY
The PFA-fixed samples were stored in 1% PFA at 4 °C until further processing. For immunolabeling on frozen semithin sections, we applied a modified Tokuyashu technique [22]. The fixed samples were washed with 0.05 M glycine in PBS, infiltrated with 10% gelatin at 37 °C for 30 min. The gelatin (containing the mesentery) was solidified on ice and cut into small blocks. For cryoprotection, blocks were infiltrated with 2.3 M sucrose at 4 °C, mounted onto aluminum pins, and frozen in liquid nitrogen. The 0.6-μm-thick frozen sections were cut by Leica Ultracut S ultramicrotome (Vienna, Austria). The sections were mounted on microscope slides, and pHrodo-labeled mesentery sections were examined with confocal microscope. For immunocytochemistry, the sections were washed with 0.02 M glycine in PBS three times for 10 min and blocked with 1% BSA-PBS. Anti-TNFα (1:100; ABCAM, Cambridge, UK) and anti-EGR1 (1:100; Thermo Fisher Scientific, Waltham, Massachusetts, USA) were used as primary antibodies; anti-mouse IgG Alexa Fluor 488 and anti-rabbit IgG Alexa Fluor 555 (1:200, Molecular Probes, Leiden, The Netherlands) were applied as secondary antibodies. The nuclei were stained with DAPI (Vector Laboratories Inc. Burlingame, California). The samples were observed with Zeiss LSM 780 confocal microscope. Images were performed by Photoshop Elements 15 Editor.
ELECTRON MICROSCOPY
The GA and OsO4-fixed samples were washed in 0.1 M cacodylate buffer, dehydrated with ethanol, and stained with 1% uranyl acetate in 70% ethanol for 1 h (at room temperature) prior to araldite embedding. Semithin sections were stained with toluidine blue solution for light microscopy. Ultrathin sections were contrast-stained with uranyl acetate and lead citrate, and the samples were analyzed in a Hitachi H-7500 (Tokyo, Japan) transmission electron microscope.
IMMUNOBLOT ANALYSIS
The peritoneal cavity was washed with PBS to remove cells attached to the surface of mesentery. The isolated mesentery was incubated with 0.2% collagenase, type II (Sigma, Saint Louis, Missouri) in DMEM/F12 for 1 h in humid condition at 37 °C. The solid remnants (adipose and connective tissue) were removed; the samples were washed three times in PBS by centrifugation at 1000 rpm, for 10 min at 4 °C. Pellets were then placed into liquid nitrogen for 30 min and stored at − 80 °C until use for biochemical investigation. The isolated mesothelial cells were dissolved in lysis buffer containing 50 mM TRIS-HCl, pH:7.5, 150 mM NaCl, 2 mM EDTA, 200 mM Na3VO4, 1 mM NaF, 1% Nonidet P-40, and protease inhibitor mixture (Complete Mini, Roche, Mannheim, Germany), kept for 1 h on ice, followed by centrifugation at 12,000 rpm for 20 min at 4 °C to remove insoluble material. The supernatants were collected and the protein contents were determined by BCA method [23] and diluted to a concentration of 1 mg/ml. Afterwards, the samples were mixed with the same amount of reducing TRIS-SDS buffer (0.5 M TRIS pH:6.8, 10% glycerol, 2% SDS, 0.00125% bromophenol blue, 0.5% mercaptoethanol) and boiled at 100 °C for 4 min. Cell extracts were subjected to SDS-PAGE on 10% gels. Proteins were transferred to nitrocellulose blotting membrane (Amersham Hybond ECL, Germany) and probed with antibodies to TNFα (1:500), EGR1 (1:250) phosphoERK1/2 (1:250; Cell Signaling, Damers, Massachusetts, USA), and caveolin-1 (BD Transduction Laboratories, Lexington, Kentucky, USA). The signal was detected with species-specific peroxidase-conjugated secondary antibodies (Amersham, GE Healthcare Biosciences, Pittsburgh). The same membranes also were probed for β-tubulin (1:1000; Millipore, Temecula, California) as a loading control. Band densities of the transferred proteins were measured by the ImageJ software (U.S. National Institutional of Health, Bethesda, Maryland) and analyzed by the Microsoft Excel 2013 program.
RESULTS
Phagocytosis
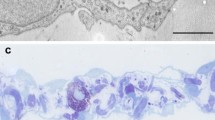
For studying the phagocytic activity of the transforming mesothelial cells, we injected India ink and fluorescent-labeled pHrodo bioparticles into the peritoneal cavity on days 3 and 5 of inflammation. On semithin sections, the engulfed ink particles could be identified as brownish dots in the cytoplasm of mesothelial cells. Control mesothelial cells without adjuvant treatment did not engulf India ink particles. On day 3 of inflammation, not all, but some mesothelial cells contained ink particles in the cytoplasm (Fig. 1a). Both the number of cells with phagocytosed ink particles and the number of particles/cells increased significantly by day 5 (Fig. 1b). Our electron microscopic results revealed that India ink particles were present in large multivesicular bodies and phagosomes in the cytoplasm of the transforming cells on days 3 (Fig. 1c) and 5 (Fig. 1d). Similarly, using fluorescent bioparticles (pHrodo), we could detect strong and increasing labeling in the cytoplasm of the mesothelial cells on days 3 and 5 of inflammation, respectively (Fig. 2b, c), while control mesothelial cells did not internalize pHrodo particles (Fig. 2a). Since fluorescence appears only in acidic environment, the strong fluorescence signal means that bioparticles must have been present in phagolysosomes, giving strong evidence of the phagocytic capability of transforming mesothelial cells. Engulfed pHrodo particles could also be detected in GM-CSF-treated cultured cells.
Phagocytosis of India ink particles in transforming mesothelial cells (in vivo experiments). a Engulfed India ink particles injected into the peritoneal cavity on day 3 of inflammation appeared in the cytoplasm of some mesothelial cells as brownish dots. b When the ink was injected into the peritoneal cavity of rats on day 5 of inflammation, the number of the cells containing ink particles and the number of the particles/cells significantly increased. c Our electron microscopic results showed that on day 3, ink particles were found in large vacuoles (arrows) in the cytoplasm. d–f On day 5, an increased number of multivesicular bodies containing engulfed ink particles was found in the cytoplasm. Bars: a, b 20 μm, c 0.5 μm; d 0.7 μm, e 0.3 μm, f 0.1 μm.
Phagocytosis of fluorescent-labeled bioparticles (in vivo and in vitro experiments). (a) Control mesenteric mesothelial cells do not internalized fluorescent-labeled bioparticles (pHrodo). A few bioparticles were engulfed by macrophages present in the mesenteric connective tissue. b, c On day 3 (b) and day 5 (c) of inflammation, increasing amount of bioparticles could be detected in the cytoplasm of transforming mesothelial cells. On day 5 (c), strong labeling was found in the cytoplasm at the perinuclear region indicating that bioparticles were not only engulfed but were transported into the acidic (lysosomal) compartments. d, e On electron microscopic pictures of GM-CSF-treated (for 24 h) primary mesenteric mesothelial cells, the bioparticles (arrows) were present in secondary lysosome-like structures (as small spherical particles) in the cytoplasm of the mesothelial cells. f Multivesicular body containing bioparticles with high magnification (red mesothelin, green Phrodo, blue DAPI). Bars: a–c 15 μm; d 0.4 μm; e 0.5 μm, f 0.3 μm.
TNFα Expression
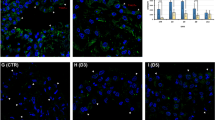
Control, mesothelial cells practically did not express TNFα (Fig. 3a). On day 3 of inflammation, mesothelial cells started to express this cytokine (Fig. 3b), and we could detect a strong cytoplasmic labeling on day 5 (Fig. 3c). Our Western blot results strongly support these morphological data (Fig. 3d): the TNFα expression reached the maximum level on day 5.
TNFα expression in mesothelial cells (in vivo experiments). a Our immunocytochemical results show that control, non-treated mesothelial cells practically do not express TNFα (a slight labeling—arrow—could be seen in a single mesothelial cell). b On day 3 of inflammation, mesothelial cells start to express TNFα, and a prominent labeling could be detected in the cytoplasm. c On day 5, we could detect strong cytoplasmic labeling in mesothelial cells. d Our Western blot results show, that by the time of inflammation, the TNFα expression significantly increased, reached the maximum level on day 5, and was still expressed on days 8 and 11 (green TNFα, blue DAPI). Bars: a–c 15 μm.
EGR1 Expression
With immunocytochemistry, we found that EGR1 was not expressed in control, non-treated mesothelial cells (Fig. 4a). By the time of inflammation, an increasing EGR1 labeling could be detected in the cytoplasm of transforming mesothelial cells (Fig. 4b, c). On day 5, EGR1 could also be detected in the nuclei of some transforming mesothelial cells (Fig. 4c insert), suggesting that this transcription factor was translocated into the nucleus. Our Western blot results (Fig. 4d) show that control, non-treated mesenteric mesothelial cells did not express EGR1. We could detect a strong EGR1 expression on day 5 of inflammation, and it was still expressed with a decreasing tendency on days 8 and 11.
EGR1 expression (in vivo experiments). a Control mesothelial cells do not express EGR1. b On day 3 of inflammation, a significant EGR1 labeling could already be detected in the cytoplasm of mesothelial cells. c We found strong labeling on day 5. The increased fluorescence is well seen in the cytoplasm of all the mesothelial cells, but in some cells, the labeling appeared in the nucleus as well (insert), indicating that the EGR1 is already translocated into the nucleus. d Our Western blot results also show that by the time of inflammation, the EGR1 expression increased significantly, reached the maximum level on day 5, but still was high on days 8 and 11 (green EGR1, blue DAPI). Bars: a–c 15 μm.
ERK1/2 Phosphorylation
To test whether ERK1/2 is involved in the regulation of EGR1 expression in our system, we followed the phosphorylation level of ERK1/2 during inflammation. Our Western blot results clearly show that in control cells, phosphorylated ERK1/2 was not present, but as the inflammation proceeded, the expression level of phosphorylated ERK1/2 was elevated. The highest phosphorylation level could be detected on day 3, but it was still high on day 5 (Fig. 5a).
Western blot: ERK1/2 phosphorylation and caveolin-1 expression. a In control cells, we could not detect ERK1/2 phosphorylation. The highest level of the phosphorylated ERK1/2 in mesothelial cells was found on day 3 of inflammation, which was followed by a slight decrease until day 11. b Two caveolin-1 isoforms are expressed in mesothelial cells during MMT and MET. High level of caveolin-1α was found in control cells and on day 3 of inflammation. On day 5, the amount of the β isoform started to increase, reached the maximum on day 8, but α isoform was still present. On day 11, the β was the only caveolin-1 isoform expressed in mesothelial cells.
Caveolin-1 Level
Our Western blot results show that two caveolin-1 isoforms (α and β) were expressed in transforming mesothelial cells. The expression level of these two isoforms changed oppositely during the inflammation period (Fig. 5b). The level of the α isoform was high in control cells and on day 3 of inflammation, decreased on day 5 and entirely disappeared on day 11. The expression level of the β isoform, however, increased on day 5 and reached its maximum on day 8. Although its level decreased, it remained the only caveolin-1 isoform on day 11.
DISCUSSION
Our previous data showed that under inflammatory stimuli, the number of peritoneal macrophages dramatically increased [6]. The origin of these chronic, heterogeneous inflammatory macrophages is not entirely known. The amount of blood-derived monocytes and activated resident macrophages should not be enough to provide such a large amount of inflammatory macrophages. We supposed that cells originating from non-hematopoietic sources should contribute to this large subset of macrophages. One candidate of these non-hematopoietic cells is the mesothelial cells. Mesothelial cells are present all over the peritoneal cavity, covering the internal organs of the abdomen. Their nestin expression [8] clearly indicates that they are not terminally differentiated cells, instead they are multipotent “young” cells with high regenerative capacity, and they can differentiate into various types of cells [11]. During inflammation, mesenteric mesothelial cells undergo morphological and biochemical transition [8, 9], and they start to express macrophage markers [8, 9], suggesting that they might be transformed into macrophage-like cells [10]. Our question was whether these multipotent cells can really transdifferentiate into macrophages. Since GM-CSF—a member of the hemopoietic cytokine family [24]—promotes the survival and activation of granulocytes, macrophages and dendritic cell differentiation in vivo, and it also stimulates proliferation of several non-hemopoietic cell types (osteoblasts, smooth muscle, endothelial and epithelial cells) [24], we tried to stimulate MTM by GM-CSF treatment. We found that GM-CSF-treated mesothelial cells also started to express macrophage markers [10].
Since macrophages are professional phagocytic cells and the major producers of TNFα, we thought that studying the phagocytic activity and the TNFα production of mesothelial cells during inflammation could provide persuading evidence about the MTM. As the mesothelial cells start to transform (during inflammation and GM-CSF treatment), their TNFα expression and phagocytic activity increased, they engulfed efficiently India ink particles and fluorescent-labeled bioparticles (pHrodo) both in vivo and in vitro.
The early growth response (EGR1) transcriptional factor is a member of early-immediate growth response gene family coupling the acute perturbation of the extracellular milieu to short-lived changes in target gene expression [19]. As a unique transcription factor—affecting only cells of monocyte/macrophage lineage, not other hemopoietic cells, [7, 13,14,15,16]—EGR1 plays essential role in macrophage differentiation [18]. Variety of extracellular signaling molecules—TGFβ [25] and other cytokines, like M-CSF, G-CSF, GM-CSF [18, 19], are able to induce EGR1 gene expression. In turn, EGR1 up-regulates TNFα, the “master regulator” of inflammatory cytokine production [12]. Since during Freund’s adjuvant-induced inflammation, TGFβ and GM-CSF were produced and secreted into the peritoneal cavity [10, 26], we were interested in whether EGR1 was also expressed in mesothelial cells. We found that by the time of inflammation, the EGR1 expression increased in transforming mesothelial cells, and in some cells, it was also translocated into the nucleus. ERK1/2 phosphorylation plays crucial role in the monocyte/macrophage differentiation, whereas the expression and the nuclear translocation of EGR1 are regulated by phosphorylation of ERK1/2 [20]. At the early time (day 3) of inflammation, when the transformation started, we could also detect high ERK1/2 phosphorylation level in mesothelial cells. ERK1/2 is known to localize in caveolae, and caveolin-1 (the major protein of caveolae) can regulate the ERK1/2 signaling pathway [27]. In mesothelial cells, caveolae—the small omega—or flask-shaped plasma membrane invaginations—and caveolin-1 are abundantly present, especially at the early time of inflammation when the macrophage marker’s expression starts, and prominent phagocytic activity can be detected (days 3 and 5). Caveolin-1 is expressed in two isoforms, α and β [28], and both isoforms have a slightly different role in caveolae formation [29]. There are caveolae composed of only (or mainly) β isoform as well as caveolae with both isoforms present [29, 30]. Although we could also detect two caveolin-1 isoforms in our system, and we found a shift in their expression level during MMT and regeneration (MET), it is not clear yet what the biological significance of this shift can be. It seems likely, however, that the α isoform plays important role in MMT signaling, while the β isoform can regulate the regeneration (MET).
Taking together all these data, we can conclude that under special stimuli (inflammation, GM-CSF treatment), the multipotent mesenteric mesothelial cells transdifferentiate into macrophages, and in addition to emigrating blood monocytes and resident macrophages, they could provide the third source of peritoneal macrophages during inflammation. Our results indicate that similarly to monocyte/macrophage differentiation, the MMT is also regulated by caveolin-1/ERK1/2/EGR1 signaling pathway.
References
van Furth, R., Z.A. Cohn, J.G. Hirsch, J.H. Humphry, W.G. Spector, and H.L. Lange-Woort. 1972. Mononuclear phagocytic system: New classification of macrophages, monocytes and their cell line. Bulletin of the World Health Organization 47: 651–658.
Van Furth, R. 1988. Phagocytic cells: Development and distribution of mononuclear phagocytes in normal steady state and inflammation. In Inflammation. Basic principles and clinical correlates, ed. J.I. Gallin, I.M. Goldstein, and R. Snyderman, 281–295. New York: Raven Press.
Ginsel, L.A., L.P. Rijfkogel, and W.T. Deams. 1985. A dual origin of macrophages? Review and hypothesis. In Macrophage biology, ed. S. Reichard and M. Kojima, 621–649. New York Press.
De Bakker, J.M., A.W. de Wit, H. Woelders, L.A. Ginsel, and W.T. Deams. 1985. On the origin of peritoneal resident macrophages. II. Recovery of the resident macrophage population in the peritoneal cavity and milky spots after peritoneal cell depletion. Journal of Submicroscopic Cytology 7: 141–151.
Papadimitriou, J.M., and R.B. Ashman. 1989. Macrophages: Current view on their differentiation, structure and function. Ultrastructural Pathology 13: 343–372.
Kiss, A.L., and A. Kittel. 1995. Early endocytotic steps in elicited macrophages: Omega-shaped plasma membrane vesicles at their cell surface. Cell Biology International 9: 527–538.
Geissmann, F., M.G. Manz, S. Jung, M.H. Sieweke, M. Merad, and K. Ley. 2010. Development of monocytes, macrophages and dendritic cells. Science 327: 656–661.
Katz, S., P. Balogh, and A.L. Kiss. 2011. Mesothelial cells can detach from the mesentery and differentiate into macrophage-like cells. Acta Pathologica, Microbiologica, et Immunologica Scandinavica 119: 782–793.
Katz, S., P. Balogh, N. Nagy, and A.L. Kiss. 2012. Epithelial-to-mesenchymal transition induced by Freund’s adjuvant treatment in rat mesothelial cells: A morphological and immunocytochemical study. Pathology Oncology Research 18: 641–649.
Katz, S., V. Zsiros, N. Doczi, A. Szabó, Á. Biczó, and A.L. Kiss. 2016. GM-CSF and GM-CSF receptor have regulatory role in transforming rat mesenteric mesothelial cells into macrophage-like cells. Inflammation Research 65: 827–836.
Wiese, C., A. Rolletschek, G. Kania, P. Blyszczuk, K.V. Tarasova, R.P. Wersto, K.R. Boheler, and A.M. Wobus. 2004. Nestin expression-a property of multi-lineage progenitor cells? Cellular and Molecular Life Sciences 61: 2510–2522.
Parameswaran, N., and S. Patial. 2010. Tumor necrosis factor-α signaling in macrophages. Critical Reviews in Eukaryotic Gene Expression 20 (2): 87–103.
Laslo, P., C.J. Spooner, A. Warmflash, D.W. Laneki, H.J. Lee, R. Sciammas, B.N. Gantner, A.R. Dinner, and H. Singh. 2006. Multilineage transcriptional priming and determination of alternate hematopoietic cell fates. Cell 126: 755–766.
Krishnaraju, K., H.Q. Nguyen, D.A. Liebermann, and B. Hoffman. 1995. The zinc finger transcription factor Egr-1 potentiates macrophage differentiation of hematopoietic cells. Molecular and Cellular Biology 15: 5499–5507.
Krishnaraju, K., B. Hoffman, and D.A. Liebermann. 1998. The zinc finger transcription factor EGR-1 activates macrophage differentiation in M1 myeloblastic leukemia cells. Blood 92: 1957–1966.
Krishnaraju, K., B. Hoffman, and D.A. Liebermann. 2001. Early growth response gene 1 stimulates development of hematopoietic progenitor cells along the macrophage lineage at the expense of the granulocyte and erythroid lineages. Blood 97: 1298–1305.
Nguyen, H.Q.B., B. Hoffman-Liebermann, and D.A. Liebermann. 1993. The zinc finger transcription factor EGR-1 is essential for and restricts differentiation along macrophage lineage. Cell 72: 197–209.
Carter, J.H., and W.G. Tourtellotte. 2007. Early response transcriptional regulators are dispensable for macrophage differentiation. Journal of Immunology 178: 3038–3047.
Baron, V., E.D. Adamson, A. Calogero, G.F. Ragona, and D. Mercola. 2006. The transcription factor Egr1 is a direct regulator of multiple tumor suppressors including TGFbeta1, PTEN, p53 and fibronectin. Cancer Gene Therapy 13: 115–124.
Fu, Y., X.-L. Moore, M.K.S. Lee, M.A. Fernandez-Rojo, M.-O. Parat, R.G. Parton, P.J. Meikle, D. Sviridov, and J.P. Chin-Dusting. 2012. Caveolin-1 plays a critical role in the differentiation of monocytes into macrophages. Arteriosclerosis, Thrombosis, and Vascular Biology 32: 117–125.
Hume, D.A., H. Ross, S.R. Himes, R.T. Sasmono, C.A. Well, and T. Ravasi. 2002. The mononuclear phagocyíte system revisited. Journal of Leukocyte Biology 72: 621–627.
Slot, J.W., and H.J. Geuze. 2007. Cryosectioning and immunolabeling. Nature Protocols 2: 2480–2491.
Olson, B.J., and J. Markwell. 2007. Assays for determination of protein concentration. Current Protocols in Protein Science. https://doi.org/10.1002/0471140864.ps0304s48.
Rasko, J.E.J., and M.M. Grough. 1994. Granulocyte macrophage-colony stimulating factor. In Cytokine handbook, ed. A.W. Thomson, 2nd ed., 342–369. New York: Academic Press.
Bhattacharyya, S., S.J. Chen, M. Wu, M. Warner-Blankenship, H. Ning, G. Lakos, Y. Mori, E. Chang, C. Nihijima, K. Takehara, C. Feghali-Bostwick, and J. Varga. 2008. Smad-independent transforming growth factor-beta regulation of early growth response-1 and sustained expression in fibrosis: Implications for scleroderma. The American Journal of Pathology 173: 1085–1099.
Balogh, P., A. Szabó, S. Katz, I. Likó, A. Patócs, and A.L. Kiss. 2013. Estrogen receptor alpha is expressed in mesenteric mesothelial cells and is internalized in caveolae upon Freund’s adjuvant treatment. PLoS One 8: 1–10.
Anderson, R.G. 1993. Caveolae: Where incoming and outgoing messengers meet. Proceedings of the National Academy of Sciences of the United States of America 90: 10909–10913.
Scherer, P.E., Z. Tang, M. Chun, M. Sargiacomo, H.E. Lodish, and M.P. Lisanti. 1995. Caveolin isoforms differ in their N-terminal protein sequence and subcellular distribution. Identification and epitope mapping of an isoform-specific monoclonal antibody probe. The Journal of Biological Chemistry 270: 16395–16401.
Fujimoto, T., H. Kogo, R. Nomura, and T. Une. 2000. Isoforms of caveolin-1 and caveolar structure. Journal of Cell Science 113: 3509–3517.
Nohe, A., E. Keating, C. Loh, T.M. Underhill, and N.O. Petersen. 2004. Caveolin-1 isoforms reorganization studied by image correlation spetroscopy. Faraday Discussions 126: 185–195.
Acknowledgements
We would like to express our thankfulness to Professor Pál Röhlich for the precious comments and accurate language correction of the manuscript. Special thanks are due to Katalin Lőcsey for her valuable technical help.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted.
Rights and permissions
About this article
Cite this article
Katz, S., Zsiros, V., Dóczi, N. et al. Inflammation-Induced Epithelial-to-Mesenchymal Transition and GM-CSF Treatment Stimulate Mesenteric Mesothelial Cells to Transdifferentiate into Macrophages. Inflammation 41, 1825–1834 (2018). https://doi.org/10.1007/s10753-018-0825-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-018-0825-4