Abstract
Nobiletin (NOB), a citrus polymethoxy flavonoid, has been reported to exhibit anti-inflammatory, anti-cancer, and anti-insulin resistance activities. Although the anti-inflammatory activity of NOB already reported, its involvement in lung protection has not been reported. Thus, this study aimed to investigate the anti-inflammatory response of NOB in lipopolysaccharide (LPS)-stimulated A549 cells and LPS-induced acute lung injury (ALI) in mice. The animals were pre-treated with NOB (5, 10, and 20 mg/kg) or DEX (5 mg/kg) at 12 and 1 h before intranasal instillation of LPS. The severity of pulmonary injury was evaluated 6 h after LPS administration. Results suggested that treatment with NOB dramatically attenuated lung histopathological changes, wet-to-dry (W/D) ratio, myeloperoxidase (MPO) activity, the numbers of inflammatory cells, and TNF-α, IL-6, and NO in BALF induced by LPS. Furthermore, NOB also significantly inhibited the expression of iNOS and the phosphorylation of NF-κBp65 and IκBα. In vitro, NOB inhibited NF-κB activation and TNF-α, IL-6 production in LPS-stimulated A549 cells. Taken together, these results indicated that NOB exhibited a protective effect on ALI, and the possible mechanism is involved in inhibiting NF-κB activation, subsequently inhibiting LPS-induced inflammatory response.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Inflammation is a hallmark of many diseases and the continuance of this process may lead to various diseases related to acute or chronic inflammation, including acute lung injury (ALI), arthritis, sepsis [1], atherosclerosis, and even cancer [2]. ALI or acute respiratory distress syndrome (ARDS), which is the severest form of injury, is a common clinical problem associated with significant morbidity and mortality [3, 4]. Both of them are characterized by the disruption of endothelial and epithelial integrity, severe hypoxemia, the accumulation and recruitment of polymorphonuclear neutrophils, and the release of several pro-inflammatory cytokines and mediators. Despite the significant advances in anti-microbial therapy and supportive care made in the past several years, there are few effective measures or specific medicines recommend for ALI treatment [5]. Thus, it is critical to discover definitive and targeted drug therapies for ALI.
Lipopolysaccharide (LPS), a major constituent of the outer membrane of Gram-negative bacteria, has been reported to be an important risk for ALI and can induce a disturbance in immune and inflammatory responses [6]. Animal exposure to LPS has gained wide acceptance for induction of animal models of ALI for its similar characteristics of human ALI. Several previous studies have reported that LPS can activate numerous inflammatory cells and neutrophil infiltration and induce the release of pro-inflammatory mediators such as tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), and nitric oxide (NO). The up-regulation of these mediators may contribute to the pathogenesis of ALI [7]. Nuclear factor-kappa B (NF-κB) is a transcription factor and plays a key role in modulating the transcription of multiple inflammatory factors and cytokines. In resting cells, heterodimers of NF-κB components, mainly p50/p65, remain in the cytosol in inactive form by interacting with a family of regulatory proteins, a cytoplasmic inhibitor of NF-κB, IκB. Abundant researches have confirmed that NF-κB is activated in response to various inflammatory stimuli including LPS [8, 9]. The activated NF-κB is then translocated into the nucleus and induces the transcription of specific target genes, including cytokines and iNOS [10]. Thus, the suppression of NF-κB and inflammatory mediators may potentially have therapeutic effects in ALI.
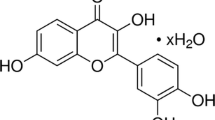
Nobiletin (NOB, Fig. 1), one of the major components of polymethoxyflavone family in citrus fruits, has been reported to have anti-inflammatory [11, 12], anti-cancer [13, 14], and anti-diabetes activities [15]. Several studies have showed that NOB can interfere with the production of prostaglandin E2 (PGE2) in human synovial fibroblasts by selectively inhibiting cyclooxygenase-2 (COX-2) activity [11] and also can down-regulate the gene expression of some cytokines like IL-6 and TNF-α in mouse macrophages [12]. Furthermore, NOB was found to have a protective effect on experimental colitis by reducing inflammation and restoring impaired intestinal barrier function [16]. However, there are few reports about the protective effects of NOB on LPS-induced ALI. In this study, we sought to assess the preventive effects and potential mechanism of NOB on LPS-induced ALI in mice.
MATERIALS AND METHODS
Reagents
LPS (Escherichia coli serotype O55:B5) was obtained from Sigma (St. Louis, MO). NOB was purchased from Xi’an Xiaocao Botanical Development Co., Ltd. (Xi’an, China) and identified by the Pharmacognosy Laboratory, School of Pharmacy, Xi’ an Jiaotong University (Xi’an, China). Dexamethasone (DEX, as a positive control) was supplied by Xi’an Lijun Pharmaceutical Company Limited (Xi’an, China). TNF-α and IL-6 ELISA kits for mouse/human were purchased from R&D Systems (Minneapolis, MN, USA). The kit for biochemical analysis of myeloperoxidase (MPO) was provided by Jiancheng Bioengineering Institute (Nanjing, China). Griess reagent was purchased from Sigma (St. Louis, MO, USA). All antibodies were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). Other reagents were of commercially available analytical grade.
In Vivo Study
Animals
Healthy male Kunming mice, weighing 18–22 g, were purchased from the Experimental Animal Center, Xi’an Jiaotong University (Xi’an, China). All animals were kept in a room maintained at 23 ± 2 °C with 50 ± 10% humidity and a 12-h light/dark cycle. All animals were allowed free access to standard laboratory chow and water, and they were acclimated in rooms for 1 week before the initiation of the experiment. All experimental producers were performed in accordance with the Guide for the Care and Use of Laboratory Animals, published by the US National Institutes of Health.
Mouse Model of LPS-Induced ALI
All mice were randomly divided into six groups (n = 12/group): control group; LPS (30 mg/kg) group; LPS + NOB (5, 10, and 20 mg/kg) groups; LPS + DEX (5 mg/kg) group. The chosen doses of these drugs were based on our previous studies and preliminary experiments. Mice were orally administrated with NOB (5, 10, or 20 mg/kg) or equivalent volume of DEX (5 mg/kg) at 12 and 1 h, prior to the induction of ALI by intranasal administration of LPS. The control and LPS groups were pre-treated with an equal volume of 0.5% CMC-Na. Six hours after LPS administration, bronchoalveolar lavage fluid (BALF) and lung tissue were harvested for further study.
BALF Collection and Cell Count
For the BALF collection, tracheostomy was performed and a plastic cannula inserted into the trachea. The lungs were lavaged with 500 μL of sterile PBS (pH 7.2) three times (total volume 1.5 mL); the recovery ratio of the fluid was about 90%. BALF samples were centrifuged (4 °C, 1360 ×g, 10 min) to pellet the cells, and the supernatants were harvested for cytokine assay. The cell pellets were re-suspended in PBS for the total cell number using a standard hemacytometer, and differences in cell numbers were examined by counting on a smear prepared by Wright-Giemsa staining.
Lung Wet-to-Dry Weight Ratio
At 6 h after LPS challenge, mice were euthanized, the lungs were excised (the lung lobes were not lavaged) and weighed to obtain the “wet” weight. Then, the lung tissue was placed in an oven at 80 °C for 48 h to obtain the “dry” weight. The ratio of wet lung to dry lung was calculated to assess tissue edema.
Cytokine Assay
The levels of TNF-α and IL-6 in BALF were evaluated by mouse ELISA kits according to the manufacturer’s directions. The absorbance was read at 450 nm and the samples were detected three times. Accumulation in BALF and lung homogenate of extracellular nitrites (NO2−), an indicator of NO synthase activity, was measured by the Griess reaction. The absorbance was measured at 550 nm, and results were expressed as μmol/L.
Myeloperoxidase Activity in the Lung Tissues
MPO activity was determined by MPO activity kit. Briefly, lung tissues of 100 mg were homogenized and fluidized in extraction buffer to obtain 5% homogenate. The sample including 0.9 mL of homogenate and 0.1 mL of reaction buffer was heated to 37 °C in water for 15 min. The enzymatic activity was determined by measuring the absorbance at 460 nm using spectrophotometer, and results were expressed as U/mg protein.
Pulmonary Histopathology
Histopathologic examination was performed in mice which were not subjected to BALF collection. Lung tissues were fixed overnight in 10% buffered formalin, embedded in paraffin, sliced, and then stained with hematoxylin and eosin (H&E). After staining, pathological changes in the lung tissues were observed with a light microscope. The sections were assessed by an investigator who was blind to the group arrangement.
Western Blot
Proteins of lung tissues were extracted with lysis buffer (RIPA with protease and phosphatase inhibitor) on ice for 10 min. Extractions of nuclear and cytoplasmic proteins from the lungs were performed with nuclear and cytoplasmic proteins extractions reagent kits (Beyotime Institute of Biotechnology). Protein concentrations were determined using a BCA protein assay kit. Samples were separated on 10% SDS-PAGE and transferred to polyvinylidene difluoride membranes. After blocked with 5% nonfat milk, the membranes were incubated with primary antibody (p-NF-κBp65, p-IκBα, NF-κBp65, IκBα, and iNOS) and then incubated with the conjugated secondary antibodies. Immunodetection was performed using an enhanced chemiluminescence detection kit. The GAPDH was performed as an internal control of protein loading. All Western blots were repeated three times from three different experiments.
In Vitro Study
Cell Culture
A549 cells were purchased from Institute of radiation and radiation research in Military Medical Science Academy of the PLA (Beijing, China) and cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with antibiotics (100-U/ml penicillin and 100-U/ml streptomycin) and 10% fetal bovine serum, at 37 °C in a humidified incubator containing 5% CO2 and 95% air.
Cell Viability Assay
Cell viability was measured by MTT assay. Briefly, A549 cells were seeded onto 96-well plates at a density of 1 × 105 cells/mL. Twenty-four hours after plating, the cells were incubated for 24 h with different concentrations of NOB (10−4, 10−3, 10−2 mg/mL), followed by stimulation with 10-μg/mL LPS for 12 h. Then, 200-μL MTT (0.5 mg/mL) was added to each well. After 4-h incubation, the supernatants were removed, and the formation of formazan was resolved with 150 μL/well of DMSO. The optical density (OD) was measured at a wavelength of 490 nm using a Spectramax 250 microplate reader.
Cytokine Assays In Vitro (TNF-α, IL-6)
To investigate the effects of NOB on cytokines responses to LPS stimulation, A549 cells (1 × 105 cells/mL) were plated onto 24-well plates and incubated for 24 h. Then, cells were pre-treated with different concentrations of NOB (10−4, 10−3, 10−2 mg/mL) for 4 h before stimulation with LPS (10 μg/mL). The control group was added equal volume of medium. Cell-free supernatants were collected 12 h after LPS stimulation and cytokines were assayed by human ELISA kit according to the manufacturer’s instructions.
NF-κB Protein Expression in LPS-Induced A549 Cells
A549 cells were seeded into 6-well plates at concentrations of 1 × 106 cells/mL. After 24-h incubation, the cells were incubated for 4 h with NOB and then stimulated with 10-μg/mL LPS for 12 h. Next, cells were washed three times with cold PBS then lysed in cold lysis buffer (50-mM Tris–HCl, pH 7.5, 150-mM NaCl, 1-mM EDTA, 20-mM NaF, 0.5% NP-40, and 1% Triton X-100) containing a protease inhibitor and phosphatase inhibitor cocktail for 40 min. The medium was centrifuged at 12,000 rpm for 10 min 4 °C, and then supernatant was harvested for p-IκBα, p-NF-κBp65, IκBα, and NF-κBp65 analysis using Western blot as shown in “Western Blot.”
Statistical Analysis
Results were presented as mean ± SEM (standard error of mean). Statistical analysis was performed using the GraphPad Software (CA, USA). Differences between groups were analyzed by one-way ANOVA with Tukey multiple comparison test, with p < 0.05 were considered to be significant.
RESULTS
Effects of NOB on LPS-Induced Pulmonary Edema
LPS challenge produced a significant increase in capillary leakage and result in pulmonary edema. In this study, the severity of edema induced by LPS was reflected by wet-to-dry weight (W/D) ratio. As shown in Fig. 2, the lung W/D ratios were significantly higher than those in control group. In contrast, pre-treatment with NOB (10 and 20 mg/kg) and DEX (5 mg/kg) efficiently reduced the W/D ratios in LPS-induced ALI model. In addition, in low-dose group of NOB, there was no marked difference in lung W/D ratios from LPS group.
Effects of NOB on the lung W/D ratio in the LPS-induced ALI mice. Mice were sacrificed 6 h after LPS instillation. The lungs were excised and lung W/D ratio was examined. The results are expressed as mean ± SEM. ##p < 0.01 compared with control group; **p < 0.01 and *p < 0.05 compared with the LPS group.
Effects of NOB on Inflammatory Cell Count in BALF
Six hours after LPS administration, the numbers of total cells, neutrophils, and macrophages in BALF were detected. Compared with control group, total cells, neutrophils, and macrophages were significantly increased in mice challenged with LPS alone. Meanwhile, pre-treatment with NOB and DEX induced a significant decrease of total cells (Fig. 3a), neutrophils (Fig. 3b), and macrophages (Fig. 3c) in BALF of mice.
Effects of NOB on TNF-α, IL-6, and NO Production
To evaluate the inflammatory cytokine levels in response to LPS stimulation, TNF-α and IL-6 in BALF were measured using corresponding kits. As shown in Fig. 4, LPS administration significantly increased TNF-α (p < 0.001) and IL-6 (p < 0.01) production in comparison to control group. In contrast, NOB intervention prevented the release of these cytokines in a dose-dependent manner; similar results were observed in DEX group. In the same way, pre-treatment with NOB significantly reduced the production of NO in BALF and lung homogenate.
Effects of NOB on MPO Activity in Lung Tissues of LPS-Challenged Mice
MPO, a marker of neutrophil infiltration, was measured in present study. As shown in Fig. 5, mice exposed to LPS alone showed a significant increase of MPO activity (p < 0.001). However, this increase in LPS-induced MPO activity was found to be significantly inhibited in the NOB and DEX group (p < 0.05 or p < 0.01).
Effects of NOB on Lung Histopathologic Changes
Lung histological changes were detected in this study. As illustrated in Fig. 6a, no evident histological alteration was observed in lung specimens of normal mice. In contrast, the lung specimens from LPS group showed marked pathologic changes, such as infiltration of inflammatory cells, alveolar wall thickening, and interstitial edema. Conversely, these pathological changes were improved by pre-treatment with NOB (Fig. 6d–f) and DEX (Fig. 6c).
Effects of NOB on histopathological changes in lung tissues in LPS-induced ALI mice. Lungs from each experimental group were processed for histological evaluation at 6 h after LPS challenge. a The lung section from the control group. b The lung section from LPS-induced ALI model group. c The lung section from the mice exposed to LPS and treated with DEX (5 mg/kg). d, e, and f The lung section from the mice exposed to LPS and treated with 5, 10, and 20 mg/kg of NOB, respectively.
Effects of NOB on NF-κB Activation and iNOS Expression in ALI Model
In the inflammation response, the activation of NF-κB signaling pathway is particularly important in the regulation of inflammatory cascade. To explore the potential mechanism of NOB on ALI, we investigated the effect of NOB on NF-κB activation in lung tissues. As shown in Fig. 7, in LPS group, the phosphorylation levels of NF-κB were increased significantly. However, the increased phosphorylations of NF-κBp65, IκBα were dramatically blocked by NOB or DEX pre-treatment. iNOS, as a downstream protein of NF-κB signaling pathway, was also increased in LPS group. In contrast, pre-treatment with NOB significantly reduced iNOS expression and the positive group showed the similar effects.
Effects of NOB on activity of NF-κB and iNOS in ALI model. Similar results were obtained in three independent experiments and one of the three representative experiments is shown. The values presented are the means ± SEM. ###p < 0.001 and ##p < 0.01 when compared with the control group; **p < 0.01 and *p < 0.05 when compared with the LPS group.
Effects of NOB on Cell Viability
The MTT assay was used to evaluate changes in cell viability after treatment with NOB and LPS. As shown in Fig. 8, with NOB treated alone or in the presence of LPS (10 μg/mL), NOB (10−4, 10−3, 10−2 mg/mL) did not display any cellular toxicity against A549 cells over 24-h incubation. Thus, the effects of NOB on A549 cells were not attributable to cytotoxic effects.
Effects of NOB on TNF-α and IL-6 Production in LPS-Stimulated A549 Cells
To investigate the effects of NOB on inflammatory cytokines secretion in vitro, levels of TNF-α and IL-6 in LPS-stimulated A549 cells were measured using ELISA kits. As demonstrated in Fig. 9, LPS treatment markedly increased TNF-α and IL-6 levels compared with non-stimulated cells. However, NOB treatment significantly inhibited the production of TNF-α and IL-6 (p < 0.05 or p < 0.01).
Effects of NOB on the secretion TNF-α (a) and IL-6 (b) in LPS-stimulated A549 cells. The cells were pre-treated with different concentrations (10−4, 10−3, 10−2 mg/mL) of NOB for 4 h prior to stimulation with 10-μg/mL LPS for 12 h. The values presented are the mean ± SEM. ###p < 0.001 and ##p < 0.01 when compared with the control group; **p < 0.01 and *p < 0.05 when compared with the LPS group.
Effects of NOB on NF-κB Pathway in LPS-Stimulated A549 Cells
To further investigate the potential effect of NOB in vitro, the expression of IκBα and NF-κBp65 protein in A549 cells was determined by Western blot. As shown in Fig. 10, NF-κB pathway was activated in LPS-induced A549 cells and more importantly, NOB pre-treatment dramatically inhibited the phosphorylation of IκBα and NF-κBp65 (p < 0.05 or p < 0.01).
DISCUSSION
In the present study, the anti-inflammatory activities of NOB on LPS-induced ALI model and LPS-stimulated A549 cells were examined. In vivo, NOB pre-treatment decreased inflammatory cell count and pro-inflammatory cytokine levels in BALF, neutrophil infiltration in lung, as well as the pulmonary histological changes. NOB pre-treatment also inhibited iNOS expression and NF-κB activation induced by LPS. In vitro, NOB inhibited NF-κB activation and inflammatory cytokines induced by LPS. Thus, NOB may be a potential agent for preventing LPS-induced ALI.
ALI and ARDS are common syndromes with several clinical disorders that affect both medical and surgical patients. LPS, the principle component of the outer cell wall of Gram-negative bacteria, has been referred to be an important risk factor of ALI [17, 18]. LPS challenge in mice can induce a clinically relevant model of ALI characterized by the widespread destruction of the capillary endothelium, the parenchymal infiltration of neutrophils and macrophages, the release of inflammatory cytokines, and other pulmonary inflammation [19,20,21]. NOB is a bioactive component extracted from natural plants citrus genus and possesses various pharmacological activities. In our previous study, we have proved that NOB exerted a protective effect on LPS-induced endotoxic shock model. Here, we assessed the protective effects of NOB against LPS-induced ALI. Mice treated with NOB at a dose of 20 mg/kg did not show any signs of acute toxicity, which suggests that it is a safe dose for in vivo acute experimental protocols. It is well known that edema is a typical feature of inflammation not only in systemic inflammation but also in local inflammation [22]. Considering the fact that edema is one of the major symptoms ALI, we firstly evaluated the lung W/D weight ratio to quantify the magnitude of pulmonary lung. Our result showed that the lung W/D ratio was evidently higher after LPS challenge when compared with the normal group. However, pre-treatment with NOB (10 and 20 mg/kg) obviously decreased the W/D ratio, which gave the first evidence for the protection of NOB in ALI.
In humans and animals, neutrophil influx and vascular leakage at the site of injury are hallmarks of ALI [23]. The total inflammatory cells include neutrophils and macrophages; both of them play a critical role during inflammatory reaction. Clinical studies have shown that neutrophilic granulocytes are important inflammatory cells and alveolar macrophages are one of the main sources of inflammatory cytokines. In this study, we found that prophylactic administration of NOB significantly lowered the LPS-induced increase in the number of total cells, neutrophils, and macrophages in BALF. Activated neutrophils contribute to the occurrence and development of lung injury induced by endotoxin or other stimuli. MPO, an enzyme located mostly in the primary granules of neutrophils, is proportional with the numbers of neutrophils in lung tissues [24]. Our present data illustrated increased MPO activity in the lung tissue in LPS model, while pre-treatment with NOB dramatically reduced MPO activity. In addition, histopathological analysis showed that NOB pre-treatment attenuated neutrophil infiltration and pathological changes in lung. These results suggest that the effects of NOB on LPS-induced ALI are associated with a decrease of inflammatory cells and neutrophil accumulation in lung tissues.
One of the major causes of ALI is over-production of pro-inflammatory cytokines with pleiotropic activities, such as TNF-α and IL-6, which lead to inflammation cascade followed by a series of pathologic responses [25]. Increased levels of TNF-α and IL-6 in BALF have been noted in patients with ALI and were related to the poor outcomes [26]. TNF-α, as a first-line cytokine, plays a fundamental role in inflammatory conditions through triggering leukocyte activation and accumulation. IL-6 is another crucial pro-inflammatory cytokine, and hyperproduction of IL-6 can promote the release of active vascular substances and neutrophils and cause a cascade effect of inflammation to increase tissue injury [27, 28]. On the other hand, the intense inflammatory response characterizing ALI is also associated with elevated iNOS expression and increased NO production [29]. NO, a short-lived small molecule, is involved in various important physiological processes. Under pathophysiological conditions, NO deprived from iNOS may have disadvantaging effects. Previous work has demonstrated TNF-α can stimulate the expression of iNOS [30], and NO production increased in response to LPS-induced lung injury via induction of iNOS [31]. These cytokines are important predictors in patients with ALI and contribute to the severity of lung injury. To investigate the protective and treatment mechanism of NOB, the possible changes of TNF-α, IL-6, and NO in the BALF and cell-free supernatants were detected. Our results showed that the production of these inflammatory mediators induced by LPS was obviously inhibited in NOB-treatment group both in vivo and vitro. These results suggest that the suppression of inflammation by NOB in lung is associated with its inhibition on inflammatory cytokine release.
Inflammation is a hallmark of many diseases and the continuance of this process may lead to various diseases related to acute or chronic inflammation. There are numerous signaling pathways involved in inflammatory process; MAPKs and PI3K/Akt have been considered as two major signaling pathways that attenuate the translocation of NF-κB in inflammation [32]. So far in mammalian cells in LPS-stimulated inflammatory process, three subgroups of MAPKs have been identified, including p38 MAPK, JNK, and ERK 1/2. Once activated, MAPKs are phosphorylated at specific sites and induced a series of inflammatory mediators’ expression. In addition, activation of PI3K will result in the phosphorylation of its main downstream target, and Akt modulates various transcription factors including NF-κB which further regulate the production of inflammatory factors [33]. Accumulating evidence has indicated that activated NF-κB often facilitates transcription of numerous genes, including iNOS, TNF-α, and IL-6, resulting in inflammation [34].
In our study, we chose to investigate the effects of NOB on the activation of NF-κB signaling pathway. Under normal conditions, NF-κB normally localizes to the cytoplasm by its inhibitor IκB proteins. Once stimulated with LPS, the IκB protein is degraded. Then the freed NF-κB translocates into the nucleus, regulating gene transcription including the pro-inflammatory mediators such as iNOS and TNF-α [35, 36]. Activation of the NF-κB transcription factor is associated with nuclear translocation of the p65 component of the complex. Clinical research has revealed that the degree of NF-κB activation was increased in patients with sepsis or ALI [37]. Thus, NF-κB is regarded as a great potential for the molecule therapy for lung injury. In the present study, the phosphorylation of IκBα and NF-κBp65 was markedly increased in mice challenged with LPS. However, NOB pre-treatment significantly inhibited the NF-κB activation. On the other hand, pre-treatment with NOB can markedly decreased the levels of TNF-α, IL-6, and NO. So, we presume that the protective effect of NOB may be attributed to the suppression of NF-κB transcription factor that results in inhibition of iNOS and the production of pro-inflammatory cytokines (TNF-α, IL-6, and NO).
In summary, we have demonstrated the protective effects of NOB on lung injury induced by LPS. It is evidenced by descending pro-inflammatory cytokine expression and inflammatory cell numbers in BALF, improvement of pulmonary changes, abate edema, and neutrophil infiltration in LPS-challenged mice. Furthermore, NOB was shown to inhibit NF-κB pathway activation in vivo and in vitro. These findings suggest that NOB has a protective effect on LPS-induced ALI in mice.
References
Hu, J., Y. Wang, X. Wei, X. Wu, G. Chen, G. Cao, X. Shen, X. Zhang, Q. Tang, G. Liang, and X. Li. 2013. Synthesis and biological evaluation of novel thiazolidinone derivatives as potential anti-inflammatory agents. European Journal of Medicinal Chemistry 64 (6): 292–301.
Tilg, H., and A.R. Moschen. 2006. Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nature Reviews. Immunology 6 (10): 772–783.
Rubenfeld, G.D., and M.S. Herridge. 2007. Epidemiology and outcomes of acute lung injury. Chest 131 (2): 554–562.
Roch, A., S. Hraiech, E. Masson, D. Grisoli, J.M. Forel, M. Boucekine, P. Morera, C. Guervilly, M. Adda, S. Dizier, R. Toesca, F. Collart, and L. Papazian. 2014. Outcome of acute respiratory distress syndrome patients treated with extracorporeal membrane oxygenation and brought to a referral center. Intensive Care Medicine 40 (1): 74–83.
Reutershan, J., A. Basit, E.V. Galkina, and K. Ley. 2005. Sequential recruitment of neutrophils into lung and bronchoalveolar lavage fluid in LPS-induced acute lung injury. American Journal of Physiology. Lung Cellular and Molecular Physiology 289 (5): L807–L815.
Conti, G., S. Tambalo, G. Villetti, S. Catinella, C. Carnini, F. Bassani, N. Sonato, A. Sbarbati, and P. Marzola. 2010. Evaluation of lung inflammation induced by intratracheal administration of LPS in mice: comparison between MRI and histology. Magma 23 (2): 93–101.
Bhatia, M., and S. Moochhala. 2004. Role of inflammatory mediators in the pathophysiology of acute respiratory distress syndrome. The Journal of Pathology 202 (2): 145–156.
Baeuerle, P.A., and T. Henkel. 1994. Function and activation of NF-kappa B in the immune system. Annual Review of Immunology 33 (2): 141–179.
Li, Y.C., C.H. Yeh, M.L. Yang, and Y.H. Kuan. 2012. Luteolin suppresses inflammatory mediator expression by blocking the Akt/NFkappaB pathway in acute lung injury induced by lipopolysaccharide in mice. Evidence-based Complementary and Alternative Medicine 2012: 383608.
Niu, X., H. Hu, W. Li, Y. Li, H. Huang, Q. Mu, H. Yao, and H. Li. 2014. Protective effect of total alkaloids on lipopolysaccharide-induced acute lung injury. The Journal of Surgical Research 189 (1): 126–134.
Lin, N., T. Sato, Y. Takayama, Y. Mimaki, Y. Sashida, M. Yano, and A. Ito. 2003. Novel anti-inflammatory actions of nobiletin, a citrus polymethoxy flavonoid, on human synovial fibroblasts and mouse macrophages. Biochemical Pharmacology 65 (12): 2065–2071.
Murakami, A., T. Shigemori, and H. Ohigashi. 2005. Zingiberaceous and citrus constituents, 1′-acetoxychavicol acetate, zerumbone, auraptene, and nobiletin, suppress lipopolysaccharide-induced cyclooxygenase-2 expression in RAW264.7 murine macrophages through different modes of action. The Journal of Nutrition 135 (12 Suppl): 2987S–2992S.
Kunimasa, K., M. Ikekita, M. Sato, T. Ohta, Y. Yamori, M. Ikeda, S. Kuranuki, and T. Oikawa. 2010. Nobiletin, a citrus polymethoxyflavonoid, suppresses multiple angiogenesis-related endothelial cell functions and angiogenesis in vivo. Cancer Science 101 (11): 2462–2469.
Li, S., M.-H. Pan, C.-Y. Lo, D. Tan, Y. Wang, F. Shahidi, and C.-T. Ho. 2009. Chemistry and health effects of polymethoxyflavones and hydroxylated polymethoxyflavones. Journal of Functional Foods 1 (1): 2–12.
Lee, Y.S., B.Y. Cha, K. Saito, H. Yamakawa, S.S. Choi, K. Yamaguchi, T. Yonezawa, T. Teruya, K. Nagai, and J.T. Woo. 2010. Nobiletin improves hyperglycemia and insulin resistance in obese diabetic ob/ob mice. Biochemical Pharmacology 79 (11): 1674–1683.
Xiong, Y., D. Chen, C. Yu, B. Lv, J. Peng, J. Wang, and Y. Lin. 2015. Citrus nobiletin ameliorates experimental colitis by reducing inflammation and restoring impaired intestinal barrier function. Molecular Nutrition & Food Research 59 (5): 829–842.
Shibayama, T., M. Tachibana, H. Tazaki, and K. Nakamura. 1991. Studies on anti-tumor activity of tumor necrosis factor alpha against human renal cell carcinoma cells heterotransplanted into nude mice. Nihon Hinyokika Gakkai Zasshi 82 (10): 1611–1619.
Rubenfeld, G.D., E. Caldwell, E. Peabody, J. Weaver, D.P. Martin, M. Neff, E.J. Stern, and L.D. Hudson. 2005. Incidence and outcomes of acute lung injury. The New England Journal of Medicine 353 (16): 1685–1693.
Hernandez, M.L., M. Herbst, J.C. Lay, N.E. Alexis, W.J. Brickey, J.P. Ting, H. Zhou, and D.B. Peden. 2012. Atopic asthmatic patients have reduced airway inflammatory cell recruitment after inhaled endotoxin challenge compared with healthy volunteers. The Journal of Allergy and Clinical Immunology 130 (4): 869–876.
Martin, T.R. 2000. Recognition of bacterial endotoxin in the lungs. American Journal of Respiratory Cell and Molecular Biology 23 (2): 128–132.
Suda, K., M. Tsuruta, J. Eom, C. Or, T. Mui, J.E. Jaw, Y. Li, N. Bai, J. Kim, J. Man, D. Ngan, J. Lee, S. Hansen, S.W. Lee, S. Tam, S.P. Man, S. Van Eeden, and D.D. Sin. 2011. Acute lung injury induces cardiovascular dysfunction: effects of IL-6 and budesonide/formoterol. American Journal of Respiratory Cell and Molecular Biology 45 (3): 510–516.
Soromou, L.W., N. Chen, L. Jiang, M. Huo, M. Wei, X. Chu, F.M. Millimouno, H. Feng, Y. Sidime, and X. Deng. 2012. Astragalin attenuates lipopolysaccharide-induced inflammatory responses by down-regulating NF-kappaB signaling pathway. Biochemical and Biophysical Research Communications 419 (2): 256–261.
Ware, L.B., and M.A. Matthay. 2000. The acute respiratory distress syndrome. The New England Journal of Medicine 342 (18): 1334–1349.
Niu, X., Y. Wang, W. Li, Q. Mu, H. Li, H. Yao, and H. Zhang. 2015. Protective effects of Isofraxidin against lipopolysaccharide-induced acute lung injury in mice. International Immunopharmacology 24 (2): 432–439.
Zhong, W.T., L.X. Jiang, J.Y. Wei, A.N. Qiao, M.M. Wei, L.W. Soromou, X.X. Xie, X. Zhou, X.X. Ci, and D.C. Wang. 2013. Protective effect of esculentoside A on lipopolysaccharide-induced acute lung injury in mice. The Journal of Surgical Research 185 (1): 364–372.
Pereira, M.M., T.P. Santos, R. Aras, R.D. Couto, M.L. Atta, and A.M. Atta. 2014. Serum levels of cytokines and chemokines associated with cardiovascular disease in Brazilian patients treated with statins for dyslipidemia. International Immunopharmacology 18 (1): 66–70.
Shin, N.R., I.S. Shin, H.H. Song, J.M. Hong, O.K. Kwon, C.M. Jeon, J.H. Kim, S.W. Lee, J.K. Lee, H. Jin, W.Y. Li, S.R. Oh, K.W. Hahn, and K.S. Ahn. 2015. Callicarpa Japonica Thunb. reduces inflammatory responses: a mouse model of lipopolysaccharide-induced acute lung injury. International Immunopharmacology 26 (1): 174–180.
Zhang, Y.M., S.K. Zhang, and N.Q. Cui. 2014. Intravenous infusion of mesenteric lymph from severe intraperitoneal infection rats causes lung injury in healthy rats. World Journal of Gastroenterology 20 (16): 4771–4777.
Kristof, A.S., P. Goldberg, V. Laubach, and S.N. Hussain. 1998. Role of inducible nitric oxide synthase in endotoxin-induced acute lung injury. American Journal of Respiratory and Critical Care Medicine 158 (6): 1883–1889.
Goldring, S. R., M. B. Goldring. 2004. The role of cytokines in cartilage matrix degeneration in osteoarthritis. Clin Orthop Relat Res 427 Suppl): S27-36.
Forstermann, U., and W.C. Sessa. 2012. Nitric oxide synthases: regulation and function. European Heart Journal 33 (7): 829–837.
Andy, S. N., C. K. Chan, H. A. Kadir. 2017. Deoxyelephantopin from Elephantopus scaber modulates neuroinflammatory response through MAPKs and PI3K/Akt-dependent NF-κB signaling pathways in LPS-stimulated BV-2 microglial cells. J Funct Foods 38 (Part A): 221-231.
Fukao, T., and S. Koyasu. 2003. PI3K and negative regulation of TLR signaling. Trends in Immunology 24 (7): 358–363.
Ma, S.F., D.N. Grigoryev, A.D. Taylor, S. Nonas, S. Sammani, S.Q. Ye, and J.G.N. Garcia. 2005. Bioinformatic identification of novel early stress response genes in rodent models of lung injury. American Journal of Physiology Lung Cellular & Molecular Physiology 289 (3): 468–477.
Surh, Y.J., K.S. Chun, H.H. Cha, S.S. Han, Y.S. Keum, K.K. Park, and S.S. Lee. 2001. Molecular mechanisms underlying chemopreventive activities of anti-inflammatory phytochemicals: down-regulation of COX-2 and iNOS through suppression of NF-kappa B activation. Mutation Research 480-481.
Lawrence, T., D.W. Gilroy, P.R. Colville-Nash, and D.A. Willoughby. 2001. Possible new role for NF-kappaB in the resolution of inflammation. Nature Medicine 7 (12): 1291–1297.
Schwartz, M.D., E.E. Moore, F.A. Moore, R. Shenkar, P. Moine, J.B. Haenel, and E. Abraham. 1996. Nuclear factor-kappa B is activated in alveolar macrophages from patients with acute respiratory distress syndrome. Critical Care Medicine 24 (8): 1285–1292.
Funding
This work was supported by a research grant (no.: 2013KW26-02) from the Natural Science Foundation of International Cooperation Projects (Shaanxi Province, PR China).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, W., Zhao, R., Wang, X. et al. Nobiletin-Ameliorated Lipopolysaccharide-Induced Inflammation in Acute Lung Injury by Suppression of NF-κB Pathway In Vivo and Vitro. Inflammation 41, 996–1007 (2018). https://doi.org/10.1007/s10753-018-0753-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-018-0753-3