Abstract
Propofol is a widely used intravenous anesthetic agent with potential neuroprotective effect in diverse models of neuronal injury, including ischemic stroke and traumatic brain injury. However, few studies have been carried out to determine the effects and molecular mechanisms of propofol in classic microglial activation (M1 activation) related to neuronal injury. This study explored the anti-inflammatory effects of propofol in LPS-activated BV2 microglia. Propofol potently decreased the pro-inflammatory mediators, such as nitric oxide, TNF-α, and IL-6, at both the transcriptional and translational levels. Furthermore, propofol suppressed the expression of miR-155 in LPS-activated cells. Knockdown of miR-155 attenuated the anti-inflammatory effect of propofol in cells after LPS exposure. miR-155 was also confirmed as a negative regulator of SOCS1 expression. The inhibitory effect of propofol on LPS-induced inflammation involved the upregulation of SOCS1. Overall, these results suggest that propofol can suppress the neuroinflammatory response of microglia to LPS through the regulation of the miR-155/SOCS1 pathway.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Microglia, which comprise approximately 10–20% of the total glial cells of the brain, play a major role in host defense and tissue repair in the central nervous system [1]. Under physiologic conditions, microglial cells are typically found in a resting state. However, in response to injury, infection, or inflammation, microglia rapidly change into an activated state [2, 3]. Classic activated microglia are capable of producing various pro-inflammatory mediators, such as nitric oxide (NO), prostaglandin E2 (PGE2), reactive oxygen species (ROS), and pro-inflammatory cytokines, including interleukin (IL)-1β, IL-6, and tumor necrosis factor (TNF)-α, as well as potentially neurotoxic compounds [1, 4]. Although the activation of microglia may initially have protective effects for neurons, uncontrolled and overactivated microglia can trigger neurotoxicity. Indeed, microglia play a pivotal role in the inflammation-associated pathogenesis of brain injuries and various neurodegenerative disorders, including Alzheimer’s disease, Parkinson’s disease, and multiple sclerosis [5]. Thus, reducing microglial activation in the brain and inhibiting the release of pro-inflammatory mediators are considered to be an effective therapeutic approach to mitigate the progression of brain injuries and neurodegenerative diseases [6,7,8].
Propofol (2, 6-diisopropylphenol) is an intravenous hypnotic agent commonly used in anesthesia and intensive care. Propofol acts by potentiating the γ-aminobutyric acid type A (GABAA) receptor-mediated inhibitory tone in the central nervous system [9, 10]. Apart from its sedative effect, propofol has been shown to be neuroprotective in diverse models of neuronal injury, particularly in ischemic stroke and traumatic brain injury [11,12,13,14]. Propofol also has anti-inflammatory properties, decreasing the production of pro-inflammatory cytokines, altering the expression of nitric oxide, and inhibiting neutrophil function [15]. However, it is unclear whether the neuroprotection of propofol is associated with the inhibitory effect in classic microglial activation (M1 activation) related to neuronal injury. Moreover, the molecular mechanisms responsible for the anti-inflammatory activities of propofol in microglia remain to be elucidated.
MicroRNAs (miR) are a class of endogenous, noncoding RNA molecules that regulate nearly every biological process through inhibition of target messenger RNA expression. miR-155 has been found to be conserved across vertebrate species. Recent studies have suggested that altered expression or function of miR-155 is involved in various central nervous system pathologic conditions, including neuroinflammation [16], neurodegeneration [17], and autoimmune diseases [18]. Notably, targeting miR-155 has been found to be beneficial in animal models of amyotrophic lateral sclerosis and multiple sclerosis [19, 20].
In the current study, we hypothesized that the miR-155 signaling pathway plays a role in the protective effects of propofol in LPS-induced neuroinflammation. LPS activation of immortalized murine BV2 microglia has been widely used as an in vitro model to study the mechanisms underlying neuronal injury by various pro-inflammatory and neurotoxic factors released from activated microglia [21]. Our results showed that propofol could suppress the production of pro-inflammatory mediators, including NO, TNF-α, and IL-6, by downregulating the expression level of miR-155, which targeted SOCS1 signaling pathways.
MATERIALS AND METHODS
BV2 Microglial Cell Culture
The murine BV2 cell line was obtained from China Center for Type Culture Collection (Wuhan, China) and was cultured as previously described [22]. Briefly, the cells were cultivated in Dulbecco’s Modified Eagle’s medium supplemented with 10% fetal bovine serum, 100 U/ml penicillin, and 100 mg/ml streptomycin at 37 °C in a humidified incubator with 5% CO2. For the experiments, confluent cultures were passaged by trypsinization and treated in culture medium overnight before treatments. In all experiments, the cells were treated with propofol and LPS at the indicated concentrations before sampling. Based on our previous observations and those in other laboratories, LPS at 10 ng/ml concentration can induce significant inflammatory responses in BV2 cells [13, 23].
Measurement of Nitrite Production
The concentrations of NO in the culture supernatants were determined by nitrite measurement with the use of Griess reagent (Invitrogen, USA). BV2 microglial cells (2 × 105 cells/well) were placed on 96-well plates and treated with different concentrations of propofol (0~100 μM) plus 10 ng/ml LPS (Sigma) for 24 h. The cell supernatants were collected and mixed with Griess reagent according to the manufacturer’s instruction, followed by incubation at room temperature for 30 min. The absorbance at 540 nm was measured on a microplate reader. The nitrite concentration was determined from a sodium nitrite standard curve.
Measurement of TNF-α and IL-6 Production
BV2 microglial cells were plated at a density of 2 × 105 cells/well on a 96-well plate. The cells were then treated with propofol and 10 ng/ml LPS for 24 h. The production of TNF-α and IL-6 in the cell-free supernatant was measured by using ELISA kits (R&D Systems, Inc., USA) according to the manufacturer’s instructions. The absorbance at 450 nm was determined by using a microplate reader.
Isolation of Total RNA and Reverse Transcription Polymerase Chain Reaction
BV2 microglia cells were treated with 10 ng/ml LPS and/or 50 μM propofol for 24 h. The total RNA was isolated by using TRIzol reagent (Ambion) and was obtained from cells by using the PrimeScript™ RT reagent kit with gDNA Eraser (Takara Bio Inc.) to produce cDNAs. The inducible nitric oxide synthase (iNOS), TNF-α, and IL-6 genes were amplified from the cDNA by PCR. The PCR primers used were: mouse iNOS (5′-ATCTTGAAGCCCCGCTACT-3′ and 5′-TCCTTTGAGCCCTTTGTGCT -3′), TNF-α (5′-CAAAAGATGGGGGGCTTC-3′ and 5′-TGTGGGTGAGGAG CACGTAGT-3′), IL-6 (5′-ATGAAGTTCCTCTCTGCAAGAGACT-3′ and 5′-CACT AGGTTTGCCGAGTAGATCTC-3′), and β-actin (5′-AAGGCGACAGCAGTTGG TT-3′ and 5′-ACCTGGGCCATTCAGAAATT-3′). After amplification, PCR reactions were separated electrophoretically on 1% agarose gel and visualized by ethidium bromide staining. β-Actin was used as an internal control. The relative mRNA expression was expressed as a percentage of the maximum LPS response.
Real-Time PCR
Quantitative real-time PCR was used to measure the expression of miR-155. The total RNA (10 ng) from BV2 cells was reverse transcribed by using the PrimeScript™ RT reagent kit with gDNA Eraser (Takara Bio Inc.) with miRNA-specific primers. Real-time PCR was done in a LightCycler® 480 system (Roche) by using SYBR Premix Ex Taq™ II (Takara Bio Inc.). The expression of miR-155 was normalized to that of endogenous RNU6B, which served as internal controls of the RNA amount and integrity. Each measurement was carried out in triplicate. The relative amount of transcripts was calculated by using the 2-ΔΔCt formula.
Western Blot Analysis
BV2 cells were treated with 10 ng/ml LPS and 50 μM propofol for 24 h. The cells were washed with ice-cold PBS, followed by homogenization in 0.2-ml ice-cold lysis buffer (50 mM Tris-HCl (pH 7.4), 1% NP-40, 0.25% sodium deoxycholate, 150 mM NaCl, and 1 mM EGTA) containing protease inhibitor cocktail (1 mM phenylmethylsulfonyl fluoride, 1 μg/ml aprotinin, 5 μg/ml leupeptin, 1 mM Na3VO4, and 1 mM NaF). Cell lysates were centrifuged at 12,000×g for 15 min at 4 °C. The supernatant was collected, and the protein concentration was determined by using a BCA protein assay kit. Samples of the lysates were separated by 10% SDS-PAGE and then transferred onto PVDF membranes. After being placed in blocking buffer, the membranes were incubated first with the anti-SOCS1 primary antibodies (1:1000 dilution) and then with horseradish peroxidase-conjugated secondary antibodies. ECL Western blotting detection reagents were used for visualization of the protein bands.
Cell Transfection
Transfection was done by using Lipofectamine RNAiMAX (Invitrogen) according to the manufacturer’s instructions. BV2 microglia cells were transfected with the miR-155 hairpin inhibitor (GenePharma) to knock down miR-155 or with SOCS1-specific siRNA (GenePharma) to silence SOCS1. In brief, sub-confluent BV2 cells were transfected with the desired final concentration of siRNA or negative control with Lipofectamine reagent for 20 min in antibiotic and serum-free medium. After 6 h of transfection, the cells were washed with normal medium and treated with LPS and propofol, followed by an additional 24 h of incubation. Then the culture medium and cells were collected for further experiments.
Statistical Analyses
The data are expressed as mean ± standard error of the mean (SEM). Statistical differences were determined by Student’s t test or one-way analysis of variance (ANOVA) by using the Graphpad Prism 5 software, followed by the Newman-Keuls post hoc test. P values <0.05 were considered to be statistically significant.
RESULTS
Propofol Attenuates LPS-Induced Production of Nitric Oxide and Inflammatory Cytokines
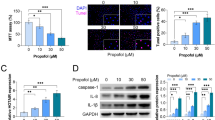
To explore the anti-inflammatory effects of propofol on LPS-induced microglial activation, BV2 cells were treated with various concentrations of propofol (12.5–100 μM) and stimulated with 10 ng/ml LPS for 24 h. The results showed that the exposure of BV2 cells to LPS increased the production of NO, TNF-α, and IL-6, respectively, by 5-, 11.4-, and 12.2-fold over the basal level (n = 6; P < 0.001 versus control; Fig. 1a–c). However, these pro-inflammatory mediators were significantly inhibited by propofol in a concentration-dependent manner. Propofol at 100 μM remarkably decreased the levels of NO, TNF-α, and IL-6 to 53.9, 65.6, and 49.3%, respectively (n = 6; P < 0.001 versus LPS).
Propofol inhibits LPS-induced expression of pro-inflammatory mediators. BV2 microglia cells were treated with LPS (10 ng/ml) and propofol (0–100 μM) for 24 h. The production of a nitric oxide in the culture medium was quantified by Griess assay. The production of b TNF-α and c IL-6 in the culture medium was quantified by ELISA. The transcript levels of d iNOS, e TNF-α, and f IL-6 were then quantified by RT-PCR. Data are mean ± SEM; N = 6 independent measurements. ### P < 0.001 versus control; *P < 0.05, **P < 0.01, and *** P < 0.001 versus LPS group. Statistical analyses were done by using one-way ANOVA with the Student-Newman-Keuls post hoc test.
To further assess the effect of propofol on the transcriptional level of the expression of pro-inflammatory mediators, BV2 microglia cells were stimulated with LPS and treated with 50 μM propofol. The mRNA levels of iNOS, TNF-α, and IL-6 were analyzed by using RT-PCR. As shown in Fig. 1d–f, the iNOS, TNF-α, and IL-6 mRNA levels were significantly increased after LPS stimulation compared with the untreated control (n = 6; P < 0.001 versus control). As expected, the treatment with propofol significantly inhibited the LPS-induced iNOS, TNF-α, and IL-6 mRNA expression (n = 6; P < 0.05 or P < 0.001 versus LPS).
The cytotoxic effects of propofol were evaluated by measuring the viability of BV2 cells incubated with LPS in the presence or absence of propofol with the use of the MTT assay. Consistent with a previous report [13], the cell viability was not significantly altered by propofol at concentrations of 12.5–100 μM (data not shown).Take together, the data indicate that propofol exerts anti-inflammatory effects by inhibiting the LPS-induced production of pro-inflammatory mediators in BV2 cells.
Propofol Suppresses LPS-Induced miR-155 Expression
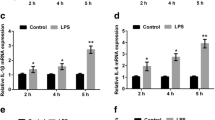
Recent studies have shown that miR-155 is involved in the regulation of inflammation and innate immune responses [24]. Thus, we investigated the expression of miR-155 on LPS stimulation by using a real-time PCR assay. The treatment of BV2 cells with increasing concentrations of LPS (0, 10, 100, and 1000 ng/ml) for 24 h showed a significant dose-dependent induction of miR-155 expression, with miR-155 levels reaching an 18-fold increase at the highest LPS concentration (n = 4; P < 0.001 versus control; Fig. 2a). To establish a time course for this event, the changes in miR-155 level were monitored at different time points (4, 12, and 24 h) after the stimulation of BV2 cells with the lowest concentration of LPS (10 ng/ml). The levels of miR-155 continued to go up, reaching a 10-fold increase at 24 h (n = 4; P < 0.001 versus LPS).
Propofol inhibits miR-155 expression after microglia activation with LPS. BV2 microglia cells were treated with LPS and propofol. RNA was extracted, and the miR-155 levels were determined by quantitative real-time PCR. a BV2 cells were incubated with LPS at 0, 10, 100, and 1000 ng/ml for 24 h. b BV2 cells were incubated with 10 ng/ml LPS for different periods of time (4, 12, and 24 h). c BV2 cells were treated with LPS and propofol for 24 h. Data are mean ± SEM; N = 4 independent measurements. ### P < 0.001 versus control; * P < 0.01 and ***P < 0.001 versus LPS group. Statistical analyses were done by using one-way ANOVA with the Student-Newman-Keuls post hoc test.
To test the hypothesis that propofol downregulates inflammation by targeting miR-155, microglial cells were treated with various concentrations of propofol (12.5–100 μM) and stimulated with LPS (10 ng/ml). The results showed that, compared with that of LPS-activated cells, the expression of miR-155 decreased to 77, 60, and 51% when the cells were treated with 25, 50, and 100 μM propofol, respectively (n = 3; P < 0.05 or P < 0.001 versus LPS).
MiR-155 is Required for Propofol Inhibition of Inflammation
To further evaluate the role of miR-155 in the anti-inflammatory action of propofol, a miR-155 inhibitor was used to knockdown miR-155 in BV2 microglia cells by using lipofectamine. The cells were transfected with the miR-155 hairpin inhibitor or inhibitor negative control, and the knockdown was assessed by real-time PCR. The miR-155 expression was significantly reduced in the cells treated with the miR-155 inhibitor (46 ± 7%) compared with the inhibitor negative control-treated cells (Fig. 3a; n = 3; P < 0.05), confirming that the knockdown was successful.
miR-155 is required for propofol suppression of inflammatory reaction. BV2 cells were incubated with negative control or specific miR-155 inhibitors as described in Materials and methods, followed by treatment with LPS and propofol. a miR-155 expression as determined by quantitative real-time PCR. b NO, c TNF-α, and d IL-6 levels in negative control and miR-155 knockdown BV2 cells treated with LPS (10 ng/ml) in the presence or absence of propofol (50 μM). N = 3–6 independent measurements. ### P < 0.001 versus LPS; *P < 0.05 and ***P < 0.001 versus control inhibitor.
As shown in Fig. 3b–d, LPS (10 ng/ml) stimulation resulted in a considerable increase in nitrite, TNF-α, and IL-6 production in the inhibitor negative control-transfected cells (n = 6; P < 0.001). Consistent with our observation in untransfected BV2 cells, the increase in NO, TNF-α, and IL-6 levels was markedly suppressed by propofol (50 μM). In miR-155 knockdown BV2 cells, however, the LPS induction of these cytokines was less robust compared with the inhibitor negative control-transfected cells (n = 6; P < 0.001); however, propofol had little effect on the production of these cytokines. The data indicate that miR-155 is critical to the anti-inflammatory action of propofol under these experimental conditions.
Propofol Upregulates SOCS1 by Suppressing miR-155
SOCS1 is a proven downstream target of miR-155. Thus, we investigated whether propofol inhibition of miR-155 would release the suppression on SOCS1. The protein expression of SOCS1 in microglial cells was evaluated under different treatments. In cells transfected with inhibitor negative control, the SOCS1 levels were slightly increased with LPS (n = 4; P < 0.05 versus control), as shown in Fig. 4. In the presence of propofol (50 μM), the SOCS1 expression was significantly higher than that with LPS alone (n = 4; P < 0.01), suggesting that propofol was able to upregulate SOCS1 expression. In cells transfected with the miR-155 hairpin inhibitor, no increase in SOCS1 protein expression was observed compared with the inhibitor negative control group. The LPS induction of SOCS1 was much more robust in miR-155 knockdown cells (n = 4; P < 0.001). Whereas propofol markedly increased SOCS1 in the inhibitor negative control-transfected cells in response to LPS stimulation, it failed to induce SOCS1 in miR-155 knockdown cells.
Propofol upregulates SOCS1 by suppressing miR-155. BV2 cells were incubated with negative control or specific miR-155 inhibitors as described in Materials and methods. SOCS1 protein expression (top) and quantitation (bottom) in negative control and miR-155 knockdown BV2 cells treated with LPS (10 ng/ml) in the presence or absence of propofol (50 μM). N = 4 independent measurements. # P < 0.05 and ### P < 0.001 versus control; **P < 0.01 versus LPS.
SOCS1 Knockdown Attenuates the Anti-inflammatory Effects of Propofol
To assess whether SOCS1 is required for the anti-inflammatory effect of propofol in microglial cells, we measured the nitrite and cytokine levels in microglial cells in which SOCS1 was downregulated. The cells were transfected with the SOCS1 siRNA or a control siRNA, and the knockdown was assessed by Western blot. As shown in Fig. 5, the SOCS1 expression was significantly decreased in the cells treated with the SOCS1 siRNA (44 ± 2.9%) compared with the control siRNA-treated cells (n = 4; P < 0.01), confirming that the knockdown was successful.
SOCS1 knockdown attenuates the anti-inflammatory action of propofol. BV2 cells were incubated with control siRNA or the SOCS1 siRNA as described in Materials and methods. a SOCS1 protein levels and b NO, c TNF-α, and d IL-6 levels in control siRNA and SOCS1 knockdown BV2 cells treated with LPS (10 ng/ml) in the presence or absence of propofol (50 μM). N = 4–6 independent measurements. ### P < 0.001 versus LPS; **P < 0.01 and ***P < 0.001 versus control siRNA.
In SOCS1 knockdown cells, the NO, TNF-α, and IL-6 production in response to LPS was significantly increased compared with the cells transfected with control siRNA (n = 6; P < 0.001; Fig. 5b–d). Propofol potently decreased the LPS-induced supernatants NO, TNF-α, and IL-6 in the control siRNA-treated cells (n = 6; P < 0.001). However, in SOCS1 knockdown cells, propofol hardly decreased the nitrite and cytokine levels compared with those treated with LPS alone. Thus, SOCS1 plays an important role in the anti-inflammatory activity of propofol.
DISCUSSION
BV2 cells are derived from raf/myc-immortalized murine neonatal microglia and are the most frequently used substitute for primary microglia. It has been suggested that, in response to LPS, 90% of the genes induced in BV2 cells are also induced in primary microglia [25]. Our results show that propofol (12.5–100 μM) potently decreases the LPS-induced production of pro-inflammatory mediators, such as NO, TNF-α, and IL-6, at both the transcriptional and translational levels in BV2 microglia. The blood plasma concentrations of propofol are reportedly 40–60 μM (7.12–10.68 μg/ml) at anesthesia induction and 10–25 μM (1.78–4.45 μg/ml) during anesthesia maintenance [26, 27]. Therefore, propofol at clinically relative concentrations may exert excellent anti-inflammatory potency in the central nervous system.
microRNAs have been shown to play crucial roles in gene regulation and diverse biological processes. However, their possible role in anesthetic-induced anti-inflammation has yet to be examined. miR-155 is of particular interest to us due to its well-established ability to shape the transcriptome of activated myeloid cells that control inflammation responses [24]. miR-155 promotes the inflammatory response of macrophages by modifying their inflammatory capacity. The expression of miR-155 has been reported to be upregulated by 25-fold in ischemic cerebral tissues of middle cerebral artery occlusion mice; a similar result has been obtained in oxygen–glucose deprivation-treated BV2 cells [28]. In vivo miR-155 inhibition after ischemia supports the brain microvasculature, reduces brain tissue damage, and improves animal functional recovery [29]. Similarly, suppression of miR-155 attenuates inflammatory signaling in macrophages, reduces macrophage-mediated neuron toxicity, and improves locomotor function in mice with spinal cord injury [30]. These findings indicate that miR-155 is an important therapeutic target in CNS injury.
In the present study, miR-155 was found to be upregulated on LPS stimulation in both a dose- and time-dependent manner in BV2 microglia cells in vitro. Importantly, propofol treatment markedly suppressed the expression of miR-155. Knockdown of miR-155 could attenuate the LPS-induced expression of TNF-α, IL-6, and NO in BV2 microglia cells. Additionally, miR-155 knockdown was found to compromise the protective effects of propofol. Taken together, these results show, for the first time, that propofol exerts its inhibitory action on LPS-induced neuroinflammation partly by suppressing miR-155 expression.
miR-155 has been shown to be capable of promoting inflammatory macrophage activation in vitro by targeting the negative regulator SOCS1 [31]. We therefore hypothesized that SOCS1 might also be involved in the anti-inflammatory action of propofol. By using an miR-155 inhibitor, knockdown of miR-155 was shown to promote the expression of SOCS1 on microglia activation. The data clearly confirm that SOCS1 may be an important target of miR-155. Propofol is capable of upregulating SOCS1 expression in LPS-activated microglia. However, it failed to upregulate the expression of SOCS1 in LPS-activated BV2 cells after miR-155 knockdown. To further examine whether the inhibitory effect of propofol on LPS-induced inflammation is related to its regulation of SOCS1 expression, we determined the effect of SOCS1 knockdown on NO, TNF-α, and IL-6 expression. As expected, silencing of SOCS1 greatly attenuated the protective effect of propofol on the expression of NO, TNF-α, and IL-6 in LPS-activated BV2 cells, suggesting that miR-155 mediates the anti-inflammatory activity of propofol by regulating the SOCS1 expression.
The effects of propofol on microRNA expression have been investigated in different systems. In the rat hippocampus, 11 microRNAs known to be involved in stem cell self-renewal, synaptic plasticity, and memory consolidation have been identified as affected by propofol anesthesia [32]. Similarly, propofol anesthesia causes about 39 microRNA expression changes in the rat liver [6]. The functional consequences of miRNA modulation by propofol in these observations remain to be determined. In a recent study in human embryonic stem cell-derived neurons, Twaroski found that propofol-induced cell apoptosis involved the downregulation of miR-21 and subsequently increased the expression of its target gene Sprouty 2 [33]. Overexpression of miR-21 reduced this toxicity [33]. Several other studies have reported that propofol inhibits the invasion and growth of ovarian, pancreatic, and gastric cancer cells by regulating miR-9, miR-21, miR-133, respectively [34,35,36]. Further investigations are needed to determine how these different microRNAs are affected by propofol.
Activation of microglia is one of the universal components of neuroinflammation. Activated microglia secrete various pro-inflammatory mediators that are believed to induce neuronal damage. Thus, controlling their activation may ameliorate immune-mediated central nervous system disorders, including stroke and traumatic brain injury. Current evidence indicates that propofol can protect the brain against ischemic or traumatic injury through the direct action of neurons [37, 38]. Our study adds more evidence that propofol, at clinically relevant concentrations, can reduce inflammatory responses in LPS-activated microglia and thus may have a potential role against neuroinflammation after brain ischemic insult and traumatic injury. Furthermore, the anti-inflammatory effect of propofol may be due partly to the downregulation of miR-155, thus increasing the protein expression of SOCS1 and subsequently inhibiting inflammatory responses.
References
Kettenmann, H., U.K. Hanisch, M. Noda, and A. Verkhratsky. 2011. Physiology of microglia. Physiological Reviews 91 (2): 461–553.
Hanisch, U.K., and H. Kettenmann. 2007. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nature Neuroscience 10 (11): 1387–1394.
Wang, D., R. Couture, and Y. Hong. 2014. Activated microglia in the spinal cord underlies diabetic neuropathic pain. European Journal of Pharmacology 728: 59–66.
Graeber, M.B., W. Li, and M.L. Rodriguez. 2011. Role of microglia in CNS inflammation. FEBS Letters 585 (23): 3798–3805.
Cunningham, C. 2013. Microglia and neurodegeneration: the role of systemic inflammation. Glia 61 (1): 71–90.
Ishikawa, M., S. Tanaka, M. Arai, Y. Genda, and A. Sakamoto. 2012. Differences in microRNA changes of healthy rat liver between sevoflurane and propofol anesthesia. Anesthesiology 117 (6): 1245–1252.
Chen, Y., S.J. Won, Y. Xu, and R.A. Swanson. 2014. Targeting microglial activation in stroke therapy: pharmacological tools and gender effects. Current Medicinal Chemistry 21 (19): 2146–2155.
Suk, K., and J. Ock. 2012. Chemical genetics of neuroinflammation: natural and synthetic compounds as microglial inhibitors. Inflammopharmacology 20 (3): 151–158.
Nelson, L.E., T.Z. Guo, J. Lu, C.B. Saper, N.P. Franks, and M. Maze. 2002. The sedative component of anesthesia is mediated by GABA(A) receptors in an endogenous sleep pathway. Nature Neuroscience 5 (10): 979–984.
Franks, N.P. 2015. Structural comparisons of ligand-gated ion channels in open, closed, and desensitized states identify a novel propofol-binding site on mammalian gamma-aminobutyric acid type A receptors. Anesthesiology 122 (4): 787–794.
Zheng, Y.Y., Y.P. Lan, H.F. Tang, and S.M. Zhu. 2008. Propofol pretreatment attenuates aquaporin-4 over-expression and alleviates cerebral edema after transient focal brain ischemia reperfusion in rats. Anesthesia and Analgesia 107 (6): 2009–2016.
Adembri, C., L. Venturi, A. Tani, A. Chiarugi, E. Gramigni, A. Cozzi, et al. 2006. Neuroprotective effects of propofol in models of cerebral ischemia: inhibition of mitochondrial swelling as a possible mechanism. Anesthesiology 104 (1): 80–89.
Luo, T., J. Wu, S.V. Kabadi, B. Sabirzhanov, K. Guanciale, M. Hanscom, et al. 2013. Propofol limits microglial activation after experimental brain trauma through inhibition of nicotinamide adenine dinucleotide phosphate oxidase. Anesthesiology 119 (6): 1370–1388.
Young, Y., D.K. Menon, N. Tisavipat, B.F. Matta, and J.G. Jones. 1997. Propofol neuroprotection in a rat model of ischaemia reperfusion injury. European Journal of Anaesthesiology 14 (3): 320–326.
Marik, P.E. 2005. Propofol: an immunomodulating agent. Pharmacotherapy 25 (5 Pt 2): 28S–33S.
Woodbury, M.E., R.W. Freilich, C.J. Cheng, H. Asai, S. Ikezu, J.D. Boucher, et al. 2015. miR-155 is essential for inflammation-induced hippocampal neurogenic dysfunction. The Journal of Neuroscience 35 (26): 9764–9781.
Guedes, J.R., C.M. Custodia, R.J. Silva, and L.P. de Almeida. 2014. Pedroso de Lima MC, Cardoso AL. Early miR-155 upregulation contributes to neuroinflammation in Alzheimer's disease triple transgenic mouse model. Human Molecular Genetics 23 (23): 6286–6301.
Mycko, M.P., M. Cichalewska, H. Cwiklinska, and K.W. Selmaj. 2015. miR-155-3p drives the development of autoimmune demyelination by regulation of heat shock protein 40. The Journal of Neuroscience 35 (50): 16504–16515.
Butovsky, O., M.P. Jedrychowski, R. Cialic, S. Krasemann, G. Murugaiyan, Z. Fanek, et al. 2015. Targeting miR-155 restores abnormal microglia and attenuates disease in SOD1 mice. Annals of Neurology 77 (1): 75–99.
Moore, C.S., V.T. Rao, B.A. Durafourt, B.J. Bedell, S.K. Ludwin, A. Bar-Or, et al. 2013. miR-155 as a multiple sclerosis-relevant regulator of myeloid cell polarization. Annals of Neurology 74 (5): 709–720.
Mayer, A.M. 1998. Therapeutic implications of microglia activation by lipopolysaccharide and reactive oxygen species generation in septic shock and central nervous system pathologies: a review. Medicina (B Aires). 58 (4): 377–385.
Luo, T., J. Qin, M. Liu, J. Luo, F. Ding, M. Wang, et al. 2015. Astragalus polysaccharide attenuates lipopolysaccharide-induced inflammatory responses in microglial cells: regulation of protein kinase B and nuclear factor-kappaB signaling. Inflammation Research 64 (3–4): 205–212.
Fan, K., D. Li, Y. Zhang, C. Han, J. Liang, C. Hou, et al. 2015. The induction of neuronal death by up-regulated microglial cathepsin H in LPS-induced neuroinflammation. Journal of Neuroinflammation 12: 54.
Vigorito, E., S. Kohlhaas, D. Lu, and R. Leyland. 2013. miR-155: an ancient regulator of the immune system. Immunological Reviews 253 (1): 146–157.
Henn, A., S. Lund, M. Hedtjarn, A. Schrattenholz, P. Porzgen, and M. Leist. 2009. The suitability of BV2 cells as alternative model system for primary microglia cultures or for animal experiments examining brain inflammation. ALTEX 26 (2): 83–94.
Adachi, Y.U., M. Satomoto, H. Higuchi, and K. Watanabe. 2005. Rapid fluid infusion therapy decreases the plasma concentration of continuously infused propofol. Acta Anaesthesiologica Scandinavica 49 (3): 331–336.
Murphy, P.G., M.J. Davies, M.O. Columb, and N. Stratford. 1996. Effect of propofol and thiopentone on free radical mediated oxidative stress of the erythrocyte. British Journal of Anaesthesia 76 (4): 536–543.
Wen, Y., X. Zhang, L. Dong, J. Zhao, C. Zhang, and C. Zhu. 2015. Acetylbritannilactone modulates MicroRNA-155-mediated inflammatory response in ischemic cerebral tissues. Molecular Medicine 21: 197–209.
Caballero-Garrido, E., J.C. Pena-Philippides, T. Lordkipanidze, D. Bragin, Y. Yang, E.B. Erhardt, et al. 2015. In vivo inhibition of miR-155 promotes recovery after experimental mouse stroke. The Journal of Neuroscience 35 (36): 12446–12464.
Gaudet, A.D., S. Mandrekar-Colucci, J.C. Hall, D.R. Sweet, P.J. Schmitt, X. Xu, et al. 2016. miR-155 deletion in mice overcomes neuron-intrinsic and neuron-extrinsic barriers to spinal cord repair. The Journal of Neuroscience 36 (32): 8516–8532.
Androulidaki, A., D. Iliopoulos, A. Arranz, C. Doxaki, S. Schworer, V. Zacharioudaki, et al. 2009. The kinase Akt1 controls macrophage response to lipopolysaccharide by regulating microRNAs. Immunity 31 (2): 220–231.
Goto, G., Y. Hori, M. Ishikawa, S. Tanaka, and A. Sakamoto. 2014. Changes in the gene expression levels of microRNAs in the rat hippocampus by sevoflurane and propofol anesthesia. Molecular Medicine Reports 9 (5): 1715–1722.
Twaroski, D.M., Y. Yan, J.M. Olson, Z.J. Bosnjak, and X. Bai. 2014. Down-regulation of microRNA-21 is involved in the propofol-induced neurotoxicity observed in human stem cell-derived neurons. Anesthesiology 121 (4): 786–800.
Huang, X., Y. Teng, H. Yang, and J. Ma. 2016. Propofol inhibits invasion and growth of ovarian cancer cells via regulating miR-9/NF-kappaB signal. Brazilian Journal of Medical and Biological Research 49 (12): e5717.
Liu, Z., J. Zhang, G. Hong, J. Quan, L. Zhang, and M. Yu. 2016. Propofol inhibits growth and invasion of pancreatic cancer cells through regulation of the miR-21/Slug signaling pathway. American Journal of Translational Research 8 (10): 4120–4133.
Wang, Z.T., H.Y. Gong, F. Zheng, D.J. Liu, and T.L. Dong. 2015. Propofol suppresses proliferation and invasion of pancreatic cancer cells by upregulating microRNA-133a expression. Genetics and Molecular Research 14 (3): 7529–7537.
Velly, L.J., B.A. Guillet, F.M. Masmejean, A.L. Nieoullon, N.J. Bruder, F.M. Gouin, et al. 2003. Neuroprotective effects of propofol in a model of ischemic cortical cell cultures: role of glutamate and its transporters. Anesthesiology 99 (2): 368–375.
Bickler, P.E., D.E. Warren, J.P. Clark, P. Gabatto, M. Gregersen, and H. Brosnan. 2012. Anesthetic protection of neurons injured by hypothermia and rewarming: roles of intracellular Ca2+ and excitotoxicity. Anesthesiology 117 (2): 280–292.
Acknowledgements
This study was supported by Natural Science Foundation of China (grant no. 81271205) and Natural Science Foundation of Guangdong province (grant no. 2015A030313781).
Author information
Authors and Affiliations
Corresponding author
Additional information
Xinxun Zheng and Hongbing Huang contributed equally to this work.
Rights and permissions
About this article
Cite this article
Zheng, X., Huang, H., Liu, J. et al. Propofol Attenuates Inflammatory Response in LPS-Activated Microglia by Regulating the miR-155/SOCS1 Pathway. Inflammation 41, 11–19 (2018). https://doi.org/10.1007/s10753-017-0658-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-017-0658-6