Abstract
Propofol has been found to have a protective effect against spinal cord injury (SCI). However, the underlying molecular mechanism of propofol regulating SCI process remains unclear. In this study, lipopolysaccharide (LPS)-induced PC12 cells were used to build SCI cell models. Cell viability and apoptosis were determined by cell counting kit 8 assay, flow cytometry, and caspase-3 activity detection. The protein levels of apoptosis-related markers and TNFAIP3 interacting protein 2 (TNIP2) were assessed using western blot analysis, and the levels of inflammatory factors were detected using ELISA. Cell oxidative stress was evaluated by measuring malondialdehyde (MDA) and reactive oxygen species (ROS) levels. The expression of microRNA (miR)-672-3p was examined by quantitative real-time PCR. SCI rat models were constructed to assess the effect of propofol in vivo. We found that propofol treatment promoted viability, while inhibited apoptosis, inflammation and oxidative stress of LPS-induced PC12 cells. Propofol decreased miR-672-3p expression, and miR-672-3p overexpression eliminated the inhibiting effect of propofol on LPS-induced PC12 cell injury. Besides, miR-672-3p targeted TNIP2, and TNIP2 knockdown reversed the protective effect of miR-672-3p inhibitor or propofol against LPS-induced PC12 cell injury. In vivo experiments, propofol treatment enhanced the motor function recovery and inhibited apoptosis of SCI rat models. In conclusion, propofol increased TNIP2 level by reducing miR-672-3p expression, thereby alleviating LPS-induced PC12 cell injury and improving the motor function of SCI rat models.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Spinal cord injury (SCI) is the structural or functional damage of the spinal cord, resulting in serious functional dysfunction of the limbs below the injured segments (Anjum et al. 2020; Liu et al. 2021b). SCI is considered to be a serious central nervous system trauma with a high rate of disability, which brings huge economic burden to patients (Wang et al. 2021; Xia et al. 2022). Inflammation, oxidative stress and apoptosis induced by SCI are important reasons for the difficulty in recovering spinal cord function, which is very important for the prognosis of patients (Hou et al. 2021a; Li et al. 2022). There is growing evidence that the antioxidant and anti-inflammatory therapy of SCI can promote the recovery of limb function after injury (Fakhri et al. 2022; Heo et al. 2020). Therefore, exploring the molecular mechanism affecting cell injury may provide potential targets for the treatment of SCI.
Propofol is a kind of intravenous sedative anesthetic with quick action, short duration and rapid recovery, which is widely used in clinical practice for the induction and maintenance of anesthesia and the sedation of critically ill ICU patients (Eleveld et al. 2018; Sahinovic et al. 2018). Many studies have shown that propofol has anti-inflammatory and antioxidant effects (Hou et al. 2021b; Zhang et al. 2022). Propofol could reduce neuroinflammation caused by hypoxia via repressing oxidative stress in BV2 microglia (Peng et al. 2020). Zhou et al. showed that propofol combined with BMSCs transplantation could treat SCI in rats (Zhou et al. 2015). However, the underlying molecular mechanism of propofol in treating SCI remains unclear.
MicroRNAs (miRNAs) are non-coding RNAs with a length of about 20–25 nts that affect their translation or degradation by pairing with target mRNA (Correia de Sousa et al. 2019). MiRNA is a potential target for treating human diseases, and its abnormal expression can lead to the occurrence of diseases (He et al. 2020). Previous studies revealed that miR-672-3p was upregulated in SCI rats, and its knockdown had a neurorestorative effects (Wang et al. 2022a), suggesting its potential in targeting SCI mechanism. TNFAIP3 interacting protein 2 (TNIP2), an inhibitor of NF-κB, has been found to be downregulated in SCI rat models and cell models (He et al. 2022). In this, we found that there had binding sites between miR-672-3p and TNIP2. However, it is not clear whether miR-672-3p mediates SCI process by regulating TNIP2.
The aim of our study is to reveal the underlying molecular mechanism of propofol in the treatment of SCI. We found that propofol inhibited miR-672-3p expression and promoted TNIP2 expression. Therefore, the hypothesis that propofol inhibited SCI process through miR-672-3p/TNIP2 pathway was proposed and verified in vivo and in vitro.
Materials and Methods
Cell Culture, Treatment and Transfection
Rat pheochromocytoma cells (undifferentiated PC12; CRL-1721, ATCC, Manassas, VA, USA) were cultured at 37 °C with 5% CO2 in RPMI-1640 medium (Gibco, Grand Island, NY, USA) containing 10% heat-inactivated horse serum (Gibco), 5% FBS (Gibco) and 1% penicillin/streptomycin (Invitrogen, Carlsbad, CA, USA). PC12 cells were treated with 10 µg/mL lipopolysaccharide (LPS) (Sigma-Aldrich, St. Louis, MO, USA) for 12 h to mimic SCI cell model. In addition, PC12 cells were treated with 50 µM/L propofol for 12 h before LPS treatment. For cell transfection, Lipofectamine 3000 (Invitrogen) was used to transfect with miR-672-3p mimic, inhibitor (anti-miR-672-3p), siRNA against TNIP2 (si-TNIP2) and their negative controls into PC12 cells.
Quantitative Real-time PCR (qRT-PCR)
Total RNAs were extracted with Trizol reagent (Invitrogen) and reverse-transcribed to cDNA using Prime-Script RT Reagent Kit (Takara, Tokyo, Japan). Then, qRT-PCR was performed by SYBR green (Takara) with specific primers (miR-672-3p, F 5’-TCGGCAGACACACAGTCGCCAT-3’, R 5’-CTCAACTGGTGTCGTGGA-3’; U6, F 5’-CTCGCTTCGGCAGCACA-3’, R 5’-AACGCTTCACGAATTTGCGT-3’). Relative expression of miR-672-3p was calculated using 2−ΔΔCt method with U6 as internal control.
Cell Counting kit 8 (CCK8) Assay
PC12 cells seeded in 96-well plates were cultured for 48 h. Next, cells were treated with CCK8 solution (Dojindo, Kumamoto, Japan). Absorbance was detected at 450 nm to analyze cell viability.
Flow Cytometry
PC12 cells were suspended by binding buffer and dyed with Annexin V-FITC and PI (Beyotime, Shanghai, China). Lastly, flow cytometry was employed to analyze cell apoptotic rate.
Caspase-3 Activity Detection
Collected PC12 cells were centrifuged at 16,000 g for 15 min. Cell supernatant was incubated with buffer solution and Ac-DEVD-pNA solution (Beyotime) for 2 h, and the absorbance at 405 nm was analyzed with a microplate reader to calculate caspase-3 activity.
Western Blot (WB) Analysis
RIPA buffer (Beyotime) was employed to extract total proteins, which were then electrophoresed on SDS-PAGE gel and transferred to PVDF membranes. Membrane was incubated with anti-Bax (1:1000, ab32503, Abcam, Cambridge, CA, USA), anti-Bcl-2 (1:1000, ab32124), anti-TNIP2 (ab155513, 1:1000) or anti-GAPDH (1:2500, ab9485), and hatched with secondary antibodies (1:50000, ab205718) after blockage. Protein signals were visualized by ECL reagent (Beyotime).
ELISA
Basing on the instructions of Rat TNF-α, IL-1β and IL-6 ELISA Kits (Abcam), the levels of TNF-α, IL-1β and IL-6 in the culture supernatant of PC12 cells were determined, respectively.
Oxidative Stress Detection
According to the kit instructions, ROS and MDA levels in PC12 cells were measured by ROS and MDA Assay Kits (Beyotime), respectively.
Dual-luciferase Reporter Assay
The wild-type and mutant-type fragments of TNIP2 3’UTR were integrated into the pGL3 basic vector to construct TNIP2 3’UTR-WT and TNIP2 3’UTR-MUT vectors. PC12 cells were co-transfected with above vectors and miR-672-3p mimic/miR-NC. Relative luciferase activity was determined by Dual-Lucy Assay Kit (Solarbio, Beijing, China).
Animals
Female SD rats (about 300 g; Hunan SJA Laboratory Animal Co., Ltd, Hunan, China) were randomly divided into 3 groups (n = 10/group). All rats were anesthetized with 10% chloral hydrate, exposed the spines of T9-11, and then resected T10 spines. For sham group, rats could be sutured after disinfection and hemostasis. For SCI group, the exposed spinal cord segment of rats were impinged by Infinite Horizon Impactor (80 Kdyn; Precision Systems and Instrumentation, Lexington, KY, USA). Then, rats were sutured. For SCI + propofol group, rats were injected with 2 mL/kg propofol via the tail vein after SCI surgery. BBB score was performed at 1, 3, and 7 days after SCI. 7 days later, 5 rats in each group were randomly selected for sacrifice, and their spinal cord tissues were collected for qRT-PCR and WB analysis. 28 days later, the remaining 5 rats in each group were monitored for the evaluation of motor function. This study was approved by the Animal Ethics committee of First People’s Hospital of Lianyungang.
Evaluate the Motor Function
For open-field test, the BBB score (0–21 points) was performed at 1 days, 3 days, 7 days, 14 days, 21 days, and 28 days after SCI. The rats were placed in a square hard board and their left and right hind limbs were recorded and scored over a 4 min period. For gait analysis, the stride length and stride frequency of locomotion were quantified using Digigait analysis software (Digigait 12.4).
HE and TUNEL Staining
Spinal cord tissues were fixed in 10% formalin and prepared for paraffin sections. The sections were stained with hematoxylin and eosin solution according to the instructions of HE Staining Kit (Beyotime). Besides, the sections were stained with TUNEL solution and DAPI solution using a TUNEL Detection Kit (Beyotime). Cavity area and TUNEL positive cells were observed under a microscope.
Statistical Analysis
The results were presented as mean ± SD. Statistical analysis was performed using GraphpadPrism 7.0 software. Comparisons between groups were conducted by Student’s t-test or ANOVA. P < 0.05 was considered as a significant difference.
Results
Propofol Played Pro-viability, Anti-apoptosis, Anti-inflammation and Anti-oxidative Stress in LPS-induced PC12 Cells
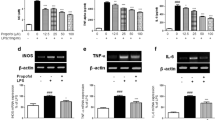
In LPS-induced PC12 cells, we found that LPS treatment inhibited cell viability, promoted cell apoptotic rate and enhanced caspase-3 activity, while propofol treatment abolished these effect (Fig. 1A-C). WB analysis showed that propofol decreased Bax protein level and increased Bcl-2 level in LPS-induced PC12 cells (Fig. 1D). Also, the levels of inflammatory factors (IL-1β, TNF-α and IL-6) and oxidative stress-related markers (MDA and ROS) were enhanced under LPS treatment in PC12 cells, and propofol also reduced the levels of IL-1β, TNF-α, IL-6, MDA and ROS in LPS-induced PC12 cells (Fig. 1E-I). Above data indicated that propofol inhibited LPS-induced PC12 cell apoptosis, inflammation and oxidative stress.
Effects of propofol on LPS-induced PC12 cell injury. PC12 cells were treated with propofol and LPS. (A) CCK8 assay was used to measure cell viability. (B) Cell apoptotic rate was detected by flow cytometry. (C) Caspase-3 activity was examined by Caspase-3 Assay Kit. (D) Protein expression was examined by WB analysis. (E-G) ELISA was performed to measure inflammatory factor levels. (H-I) MDA and ROS levels were detected to assess oxidative stress. *P < 0.05
Propofol Decreased Mir-672-3p Expression in LPS-induced PC12 Cell Injury
MiR-672-3p was upregulated in LPS-induced PC12 cells, and propofol treatment markedly reduced miR-672-3p expression (Fig. 2A). To explore whether propofol regulated cell injury by mediating miR-672-3p expression, miR-672-3p mimic was transfected into LPS-induced PC12 cells to enhance its expression (Fig. 2B). By detecting cell viability and apoptosis, we confirmed that miR-672-3p overexpression reversed propofol-mediated cell viability promotion, apoptotic rate inhibition, and caspase-3 activity reduction in LPS-induced PC12 cells (Fig. 2C-E). Also, miR-672-3p mimic eliminated the decreasing effect of propofol on Bax protein level and the increasing effect on Bcl-2 protein level (Fig. 2F). In addition, the inhibitory effect of propofol on the levels of IL-1β, TNF-α, IL-6, MDA and ROS in LPS-induced PC12 cells were overturned by overexpressing miR-672-3p (Fig. 2G-K). These results showed that propofol might alleviate SCI process by reducing miR-672-3p expression.
Effects of propofol and miR-672-3p on LPS-induced PC12 injury. (A) MiR-672-3p expression was detected by qRT-PCR in PC12 cells treated with propofol and LPS. (B) MiR-672-3p expression in LPS-induced PC12 cells transfected with miR-672-3p mimic/miR-NC was measured using qRT-PCR. (C-K) PC12 cells were transfected with miR-672-3p mimic/miR-NC and then treated with propofol and LPS. (C) Cell viability was examined using CCK8 assay. (D) Flow cytometry was used to measure cell apoptotic rate. (E) Caspase-3 Assay Kit was performed to examine caspase-3 activity. (F) WB analysis was employed to detect protein expression. (G-I) Inflammatory factor levels were detected by ELISA. (J-K) Oxidative stress was assessed by measuring MDA and ROS levels. *P < 0.05
MiR-672-3p Directly Targeted TNIP2
Targetscan software predicted the existence of complementary sites between miR-672-3p and TNIP2 3’UTR, and the binding sites were shown in Fig. 3A. Dual-luciferase reporter assay results revealed that only the luciferase activity of TNIP2 3’UTR-WT vector could be inhibited by miR-672-3p mimic (Fig. 3B), confirming the interaction between miR-672-3p and TNIP2. WB results indicated that TNIP2 protein level was downregulated in LPS-induced PC12 cells (Fig. 3C), which was contrary to the expression trend of miR-672-3p. Moreover, anti-miR-672-3p was transfected into PC12 cells to reduce miR-672-3p expression (Fig. 3D). The detection of TNIP2 expression suggested that TNIP2 protein level could be inhibited by miR-672-3p overexpression and promoted by miR-672-3p inhibition (Fig. 3E). Above all, we pointed out that TNIP2 was targeted by miR-672-3p.
MiR-672-3p targeted TNIP2. (A) The sequences of TNIP2 3’UTR-WT/MUT are shown. (B) Dual-luciferase reporter assay was used to assess the interaction between miR-672-3p and TNIP2. (C) TNIP2 protein level was detected by WB analysis in PC12 cells treated with or without LPS. (D) The transfection efficiency of anti-miR-672-3p was confirmed by qRT-PCR. (E) TNIP2 protein level was examined using WB analysis in PC12 cells transfected with miR-672-3p mimic or inhibitor. *P < 0.05
MiR-672-3p Inhibitor Reduced LPS-induced PC12 Cell Injury by Upregulating TNIP2
In addition, si-TNIP2 was constructed to reduce TNIP2 protein level in PC12 cells (Fig. 4A). To further investigate whether miR-672-3p regulated cell injury by targeting TNIP2, PC12 cells were co-transfected with anti-miR-672-3p and si-TNIP2 followed by treated with LPS. The results indicated that miR-672-3p inhibitor promoted cell viability and Bcl-2 protein level, while reduced cell apoptotic rate, caspase-3 activity and Bax protein level in LPS-induced PC12 cells. However, these effects were abolished by TNIP2 knockdown (Fig. 4B-E). Meanwhile, miR-672-3p inhibitor also decreased the levels of IL-1β, TNF-α, IL-6, MDA and ROS in LPS-induced PC12 cells, while TNIP2 downregulation could partially reverse these effects (Fig. 4F-J). The above data illuminated that miR-672-3p might aggravate SCI process by targeting TNIP2.
Effects of anti-miR-672-3p and si-TNIP2 on LPS-induced PC12 injury. (A) The transfection efficiency of si-TNIP2 was confirmed by WB analysis. (B-J) PC12 cells were transfected with anti-miR-NC, anti-miR-672-3p, anti-miR-672-3p + si-NC or anti-miR-672-3p + si-TNIP2, followed by treated with LPS. (B) CCK8 assay was performed to detect cell viability. (C) Flow cytometry was used to test cell apoptotic rate. (D) Caspase-3 activity was measured using Caspase-3 Assay Kit. (E) WB analysis was employed to test protein expression. (F-H) ELISA was used to detect inflammatory factor levels. (I-J) MDA and ROS levels were assessed to evaluate oxidative stress. *P < 0.05
Propofol Alleviated LPS-induced PC12 Cell Injury through miR-672-3p/TNIP2 Axis
To further explore the regulation of propofol on TNIP2, we detected TNIP2 protein level in LPS-induced PC12 cells transfected with miR-672-3p mimic and treated with propofol. As shown in Fig. 5A, propofol treatment significantly increased TNIP2 protein level, and this effect could be reduced by miR-672-3p mimic transfection. For revealing that propofol regulated cell injury by mediating TNIP2 expression, LPS-induced PC12 cells were transfected with si-TNIP2 and treated with propofol. Through assessing cell functions, we found that TNIP2 knockdown reversed the enhancing effect of propofol on cell viability and Bcl-2 protein level, as well as the repressing effect on cell apoptotic rate, caspase-3 activity and Bax protein level in LPS-induced PC12 cells (Fig. 5B-E). Not only that, downregulation of TNIP2 also partially abolished the decreasing effect of propofol on the levels of IL-1β, TNF-α, IL-6, MDA and ROS in LPS-induced PC12 cells (Fig. 5F-J). Above all, we confirmed that propofol suppressed SCI process by sponging miR-672-3p to increase TNIP2 expression.
Effects of propofol and si-TNIP2 on LPS-induced PC12 cell injury. (A) TNIP2 protein level was detected by WB analysis in LPS-induced PC12 cells transfected with miR-672-3p mimic/miR-NC and treated with propofol. (B-J) PC12 cells were transfected with si-TNIP2/si-NC and treated with propofol and LPS. (B) Cell viability was determined using CCK8 assay. (C) Cell apoptotic rate was examined using flow cytometry. (D) Caspase-3 activity was detected by Caspase-3 Assay Kit. (E) Protein expression was tested by WB analysis. (F-H) ELISA was employed to measure inflammatory factor levels. (I-J) Oxidative stress was evaluated to detect MDA and ROS levels. *P < 0.05
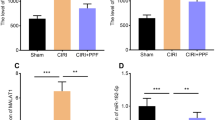
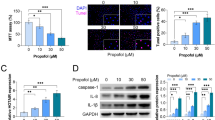
Propofol Promoted the Recovery of Motor Function and Inhibited Apoptosis of SCI Rat Models
To further confirm that propofol regulated miR-672-3p/TNIP2 axis to mediate SCI process, we constructed SCI rat models in vivo. After SCI for 7 days, we collected the spinal cord tissues of rat and detected miR-672-3p and TNIP2 expression. The results showed that miR-672-3p expression was increased and TNIP2 protein level was decreased in SCI models, while propofol treatment remarkably reduced miR-672-3p expression and promoted TNIP2 protein level (Fig. 6A-B). Then, open-field test and gait analysis were used to evaluate the motor function of rat. The results indicated that the BBB scale was higher in propofol treatment group after SCI for 7, 14, 21 and 28 days (Fig. 6C). Also, the stride length were enhanced, while the stride frequency was reduced in propofol treatment group (Fig. 6D-E). Moreover, the results of HE staining and TUNEL staining showed that cavity area and TUNEL positive ratio in the SCI group were higher than those in the sham group, while propofol treatment could reduce cavity area and TUNEL positive ratio in SCI rat models (Fig. 6F-G). These data revealed that propofol treated improved the motor function and suppressed apoptosis of SCI rat models via enhancing TNIP2 expression by decreasing miR-672-3p expression. Above all, we confirmed that propofol could relieve SCI process, which repressed LPS-induced PC12 cell apoptosis, oxidative stress and inflammation through regulating miR-672-3p/TNIP2 axis (Fig. 7).
Effects of propofol on the recovery of motor function of rat after SCI. (A) MiR-672-3p expression was measured by qRT-PCR. (B) TNIP2 protein level was examined by WB analysis. (C) Open field test was used to assess BBB scale. (D-E) Stride length and frequency were detected to perform gait analysis. (F) HE staining was used to evaluate the pathological condition of SCI rats. (G) TUNEL staining was performed to assess spinal cord tissue cell death. *P < 0.05
Discussion
SCI is a serious complication of spinal fracture with high disability rate and great cost. A lot of research has been carried out on the pathological mechanism of SCI. In this study, we pointed out that propofol inhibited SCI process by the regulation of miR-672-3p/TNIP2 axis.
In addition to anesthesia, other functions of propofol have been discovered. Propofol had been shown to restrain gastric cancer cell growth and metastasis to hinder cancer malignant process (Y. P. Liu et al. 2021a). Also, propofol was considered to use for preeclampsia treatment, which could inhibit LPS-induced toxicity in HTR-8/SVneo cells (Y. Wang et al. 2022a). Previous reports have shown that propofol has a protective effect on ischemia reperfusion damage to the brain and renal (Li et al. 2021; Zhang et al. 2021). In LPS-induced SCI cell models, we confirmed that propofol suppressed cell apoptosis, inflammation and oxidative stress, and promoted cell viability in vitro. Also, propofol facilitated the motor function recovery of rat after SCI, which was consistent with the results reported by Zhou et al. (Zhou et al. 2015). These findings provide new evidence for the treatment of SCI with propofol.
Propofol mediates disease progression by regulating the expression of miRNA, such as miR-125b-5p (Y. P. Liu et al. 2021b), miR-216-5p (Y. Wang et al. 2022b), and miR-126-5p (Li et al. 2021). In this, we found that propofol had an inhibition on miR-672-3p expression in vitro and in vivo. MiR-672-3p might be involved in regulating stroke progression, and its expression was associated with functional recovery in rats after stroke (Huang et al. 2022). Importantly, miR-672-3p, as well as miR-672-5p, had been discovered to promote functional behavior recovery in SCI models (Wang et al. 2022b; Zhou et al. 2022). MiR-672-3p was overexpressed in LPS-induced PC12 cells and SCI rat models, and its inhibitor suppressed LPS-induced cell injury. Moreover, miR-672-3p overexpression negated the protective effect of propofol on cell injury, indicating that propofol decreased miR-672-3p expression to restrain SCI process.
TNIP2, a hub protein in the NF-κB network, regulates the inflammatory activator NF-κB by binding to the anti-inflammatory signaling molecule A20 (Ma et al. 2012; Ventura et al. 2018). TNIP2 has protective function in multiple organ dysfunction syndrome development via repressing inflammation and oxidative stress (Gong et al. 2019). TNIP2 level was associated with sepsis process, which could decrease the releasing of inflammatory factors in LPS-induced macrophages (Lou et al. 2020). TNIP2 mitigated inflammation and apoptosis in OGD/R-induced neuronal to play neuroprotective functions (Yan et al. 2021). In SCI, downregulation of TNIP2 had been confirmed to aggravate apoptosis and inflammation in LPS-induced PC12 cells (He et al. 2022). Consistent with this results, our data showed that TNIP2 knockdown abolished the suppressive functions of miR-672-3p inhibitor or propofol on LPS-induced PC12 cell injury, confirming the anti-apoptosis, anti-inflammation and anti-oxidative stress of TNIP2 in SCI.
In conclusion, propofol could be used for SCI treatment, which inhibited LPS-induced cell injury and improved motor function in SCI rats via the miR-672-3p/TNIP2 axis. Our work offers a functional assessment of propofol in SCI, providing a novel potential target for SCI treatment.
Data Availability
Not applicable.
References
Anjum A, Yazid MD, Fauzi Daud M, Idris J, Ng AMH, Selvi Naicker A, Ismail OHR, Athi Kumar RK, Lokanathan Y (2020) Spinal cord Injury: pathophysiology, Multimolecular interactions, and underlying recovery mechanisms. Int J Mol Sci 21. https://doi.org/10.3390/ijms21207533
Correia de Sousa M, Gjorgjieva M, Dolicka D, Sobolewski C, Foti M (2019) Deciphering miRNAs’ Action through miRNA Editing. Int J Mol Sci 20. https://doi.org/10.3390/ijms20246249
Eleveld DJ, Colin P, Absalom AR, Struys M (2018) Pharmacokinetic-pharmacodynamic model for propofol for broad application in anaesthesia and sedation. Br J Anaesth 120:942–959. https://doi.org/10.1016/j.bja.2018.01.018
Fakhri S, Sabouri S, Kiani A, Farzaei MH, Rashidi K, Mohammadi-Farani A, Mohammadi-Noori E, Abbaszadeh F (2022) Intrathecal administration of naringenin improves motor dysfunction and neuropathic pain following compression spinal cord injury in rats: relevance to its antioxidant and anti-inflammatory activities. Korean J Pain 35:291–302. https://doi.org/10.3344/kjp.2022.35.3.291
Gong H, Sheng X, Xue J, Zhu D (2019) Expression and role of TNIP2 in multiple organ dysfunction syndrome following severe trauma. Mol Med Rep 19:2906–2912. https://doi.org/10.3892/mmr.2019.9893
He B, Zhao Z, Cai Q, Zhang Y, Zhang P, Shi S, Xie H, Peng X, Yin W, Tao Y, Wang X (2020) miRNA-based biomarkers, therapies, and resistance in Cancer. Int J Biol Sci 16:2628–2647. https://doi.org/10.7150/ijbs.47203
He C, Xiao J, Ye Y, Huang S, Zhong Y, Liu L, Liu W, Liu S (2022) Long non-coding RNA-small nucleolar RNA host gene 7 regulates inflammatory responses following spinal cord injury by regulating the microRNA-449a/TNF-alpha-induced protein 3-interacting protein 2 axis. Bioengineered 13:10215–10226. https://doi.org/10.1080/21655979.2022.2061294
Heo SD, Kim J, Choi Y, Ekanayake P, Ahn M, Shin T (2020) Hesperidin improves motor disability in rat spinal cord injury through anti-inflammatory and antioxidant mechanism via Nrf-2/HO-1 pathway. Neurosci Lett 715:134619. https://doi.org/10.1016/j.neulet.2019.134619
Hou Y, Luan J, Huang T, Deng T, Li X, Xiao Z, Zhan J, Luo D, Hou Y, Xu L, Lin D (2021a) Tauroursodeoxycholic acid alleviates secondary injury in spinal cord injury mice by reducing oxidative stress, apoptosis, and inflammatory response. J Neuroinflammation 18:216. https://doi.org/10.1186/s12974-021-02248-2
Hou Y, Xiao X, Yu W, Qi S (2021b) Propofol suppresses Microglia inflammation by targeting TGM2/NF-kappaB signaling. J Immunol Res 2021:4754454. https://doi.org/10.1155/2021/4754454
Huang L, Hua L, Zhang X (2022) The Exosomal MicroRNA Profile is responsible for the Mesenchymal Stromal Cell Transplantation-Induced improvement of functional recovery after stroke in rats. Neuroimmunomodulation 29:151–160. https://doi.org/10.1159/000518637
Li X, Zhang Z, Li A, Hu Y (2021) Propofol attenuates renal ischemia/reperfusion injury by regulating the MALAT1/miR-126-5p axis. J Gene Med 23:e3349. https://doi.org/10.1002/jgm.3349
Li Z, Li Z, Chen Z, Sun H, Yuan Z, Wang X, Wei J, Cao X, Zheng D (2022) Andrographolide contributes to spinal cord injury repair via inhibition of apoptosis, oxidative stress and inflammation. Front Pharmacol 13:949502. https://doi.org/10.3389/fphar.2022.949502
Liu YP, Qiu ZZ, Li XH, Li EY (2021a) Propofol induces ferroptosis and inhibits malignant phenotypes of gastric cancer cells by regulating miR-125b-5p/STAT3 axis. World J Gastrointest Oncol 13:2114–2128. https://doi.org/10.4251/wjgo.v13.i12.2114
Liu WZ, Ma ZJ, Li JR, Kang XW (2021b) Mesenchymal stem cell-derived exosomes: therapeutic opportunities and challenges for spinal cord injury. Stem Cell Res Ther 12:102. https://doi.org/10.1186/s13287-021-02153-8
Lou Y, Huang Z (2020) microRNA-15a-5p participates in sepsis by regulating the inflammatory response of macrophages and targeting TNIP2. Exp Ther Med 19:3060–3068. https://doi.org/10.3892/etm.2020.8547
Ma A, Malynn BA (2012) A20: linking a complex regulator of ubiquitylation to immunity and human disease. Nat Rev Immunol 12:774–785. https://doi.org/10.1038/nri3313
Peng X, Li C, Yu W, Liu S, Cong Y, Fan G, Qi S (2020) Propofol attenuates Hypoxia-Induced inflammation in BV2 microglia by inhibiting oxidative stress and NF-kappaB/Hif-1alpha signaling. Biomed Res Int 2020:8978704. https://doi.org/10.1155/2020/8978704
Sahinovic MM, Struys M, Absalom AR (2018) Clinical pharmacokinetics and pharmacodynamics of Propofol. Clin Pharmacokinet 57:1539–1558. https://doi.org/10.1007/s40262-018-0672-3
Ventura S, Cano F, Kannan Y, Breyer F, Pattison MJ, Wilson MS, Ley SC (2018) A20-binding inhibitor of NF-kappaB (ABIN) 2 negatively regulates allergic airway inflammation. J Exp Med 215:2737–2747. https://doi.org/10.1084/jem.20170852
Wang TY, Park C, Zhang H, Rahimpour S, Murphy KR, Goodwin CR, Karikari IO, Than KD, Shaffrey CI, Foster N, Abd-El-Barr MM (2021) Management of Acute traumatic spinal cord Injury: a review of the literature. Front Surg 8:698736. https://doi.org/10.3389/fsurg.2021.698736
Wang Y, Lin C, Wang J, Zhou M, Fang T, Miao L, Wei Y (2022a) Propofol rescues LPS-induced toxicity in HRT-8/SVneo cells via miR-216a-5p/TLR4 axis. Arch Gynecol Obstet 305:1055–1067. https://doi.org/10.1007/s00404-021-06316-z
Wang F, Li J, Zhao Y, Guo D, Liu D, Chang S, Qiao H, Li J, Yang Y, Zhang C, Wang R, Li F, Wang D, Li H, He X (2022b) Mir-672-3p promotes functional recovery in rats with Contusive spinal cord Injury by inhibiting ferroptosis suppressor protein 1. Oxid Med Cell Longev 2022(6041612). https://doi.org/10.1155/2022/6041612
Xia M, Zhang Q, Zhang Y, Li R, Zhao T, Chen L, Liu Q, Zheng S, Li H, Qian Z, Yang L (2022) Growth differentiation factor 15 regulates oxidative stress-dependent ferroptosis Post spinal cord Injury by stabilizing the p62-Keap1-Nrf2 signaling pathway. Front Aging Neurosci 14:905115. https://doi.org/10.3389/fnagi.2022.905115
Yan Z, Chen Y, Zhang X, Hua L, Huang L (2021) Neuroprotective function of TNFAIP3 interacting protein 2 against Oxygen and Glucose Deprivation/Reoxygenation-Induced Injury in hippocampal neuronal HT22 cells through regulation of the TLR4/MyD88/NF-kappaB pathway. Neuropsychiatr Dis Treat 17:2219–2227. https://doi.org/10.2147/NDT.S308360
Zhang T, Wang Y, Xia Q, Tu Z, Sun J, Jing Q, Chen P, Zhao X (2021) Propofol mediated Protection of the Brain from Ischemia/Reperfusion Injury through the regulation of Microglial Connexin 43. Front Cell Dev Biol 9:637233. https://doi.org/10.3389/fcell.2021.637233
Zhang Z, Yan B, Li Y, Yang S, Li J (2022) Propofol inhibits oxidative stress injury through the glycogen synthase kinase 3 beta/nuclear factor erythroid 2-related factor 2/heme oxygenase-1 signaling pathway. Bioengineered 13:1612–1625. https://doi.org/10.1080/21655979.2021.2021062
Zhou YJ, Liu JM, Wei SM, Zhang YH, Qu ZH, Chen SB (2015) Propofol promotes spinal cord injury repair by bone marrow mesenchymal stem cell transplantation. Neural Regen Res 10:1305–1311. https://doi.org/10.4103/1673-5374.162765
Zhou Z, Li C, Bao T, Zhao X, Xiong W, Luo C, Yin G, Fan J (2022) Exosome-shuttled mir-672-5p from anti-inflammatory Microglia Repair traumatic spinal cord Injury by inhibiting AIM2/ASC/Caspase-1 signaling pathway mediated neuronal pyroptosis. J Neurotrauma 39:1057–1074. https://doi.org/10.1089/neu.2021.0464
Acknowledgements
None.
Funding
None.
Author information
Authors and Affiliations
Contributions
Chengliang Sun designed and performed the research; Dongzhi Liu, Shunheng Gao, Mingyu Xiu, Zhaojian Zhang analyzed the data; Chengliang Sun wrote the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
This study was permitted by the Animal Ethics Committee of the First People’s Hospital of Lianyungang.
Consent for Publication
Not applicable.
Conflict of Interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sun, C., Liu, D., Gao, S. et al. Propofol Ameliorates Spinal Cord Injury Process by Mediating miR-672-3p/TNIP2 Axis. Biochem Genet (2024). https://doi.org/10.1007/s10528-024-10718-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10528-024-10718-4