Abstract
The 5-hydroxytryptamine-3 receptor (5-HT3R) antagonist ondansetron has been clinically approved as an anti-emetic agent. Recent findings indicate that ondansetron has anti-inflammatory properties. The aim of the present study was to assess the therapeutic action of ondansetron in cerulein-induced acute pancreatitis model. Male-BALB/c mice were used in the present study. Acute pancreatitis was induced by an hourly injection of cerulein. Ondansetron was administered subcutaneously at a dose of 3 mg/kg. The messenger RNA (mRNA) expression of 5-HT3 R in pancreatic tissue was assessed with RT-PCR. Plasma amylase, lipase, and interleukin (IL)-6 levels were evaluated. Pancreatic injury was histopathologically graded, and myeloperoxidase (MPO)-positive cells were counted. 5-HT3R mRNA was expressed in the pancreas. In acute pancreatitis model mice, amylase, lipase, and IL-6 levels were significantly increased in the blood. With ondansetron treatment, these levels were significantly decreased. Histopathological evaluation revealed that ondansetron attenuated the inflammatory damage in acute pancreatitis. The number of infiltrated neutrophils stained by MPO was decreased by ondansetron treatment. In summary, the 5-HT3R antagonist ondansetron attenuated pancreatic injury through its anti-inflammatory action. These findings suggest that ondansetron may potentially be of use for therapy of acute pancreatitis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Acute pancreatitis (AP) is an inflammatory disorder, which is commonly observed in clinical practice. The activation of pancreatic enzymes in acinar cell causes tissue injury, leading to the recruitment of inflammatory cells and secretion of inflammatory cytokines [1]. The mortality rate associated with the severe form of AP has been reported to be 15–25% [2]. The cause of death in AP is mainly induced by multiple organ dysfunction syndromes, caused by the systemic inflammation [3, 4]. In addition to high mortality rate, AP can be chronic, and may cause complications such as diabetes [5]. To date, there is no specific and effective therapy for AP. There is a need to identify active agents and novel mechanisms for the treatment of AP.
Serotonin (5-hydroxytryptamine; 5-HT) is a well-characterized neurotransmitter in the central nervous system. The 5-HT receptors (5-HTR) are classified into seven families (5-HT1–7R) and play a crucial role in regulating mood, body temperature, sleep, appetite, metabolism, and inflammation [6]. Among these, 5-HT3R is a cys-loop ligand-gated ion channel family that is mainly localized in the central nervous systems and gastrointestinal tract and mediates various physiological functions. In particular, 5-HT3R expressed in the chemoreceptor trigger zone (CTZ) and vomiting center plays an important role in the induction of vomiting reflex. Clinically, 5-HT3R antagonists are used as anti-emetic agents. To date, many 5-HT3R antagonists have been developed, including ondansetron, tropisetron, granisetron, dolasetron, and palonosetron [7]. Among these, ondansetron is one of the commonly used selective 5-HT3R antagonists for the treatment of nausea and vomiting caused by chemotherapy, radiation therapy, gastroenteritis, and surgery [8,9,10,11].
In addition to its anti-emetic action, novel properties of the 5-HT3R antagonist have recently been postulated. It is reported that 5-HT3R is expressed in monocytes, macrophages, and dendritic cells and modulates the production of inflammatory cytokines such as IL-1β and IL-6 [12,13,14,15]. It has been reported that 5-HT3R antagonist ameliorates colitis, acute liver injury, and postoperative ileus through its anti-inflammatory action [12, 13, 16]. In addition, recent findings indicate that the 5-HT3R antagonist inhibits the discharge of pancreatic vagal afferents, which in turn suppress the secretion of pancreatic enzymes such as amylase [17, 18].
In the present study, we investigated the possibility of the 5-HT3R antagonist ondansetron as a potential therapeutic agent against AP. To this end, we explored the anti-inflammatory effect of ondansetron in a rodent AP model.
MATERIALS AND METHODS
Animals
Thirty-three male BALB/c mice aged 7 weeks were obtained from a commercial vendor (BALB/c SLC: Japan SLC Inc., Shizuoka Japan). The animals were acclimated to the facility for 1 week before experimentation and kept in polycarbonate cages (CL-0106-1, CLEA Japan, Tokyo, Japan) with wood shavings. Animals were kept in a room equipped with a barrier system at the Research Institute of Biosciences, Azabu University. The room was air-conditioned at a temperature of 22 ± 1 °C and a humidity of 55 ± 5% and was lit 12 h each day from 06:00 to 18:00 h. Mice were fed a pelleted mouse diet (mouse and rat chow; MC-2, CLEA Japan, Tokyo, Japan) ad libitum and had unrestricted access to sterilized drinking water provided in a water bottle. Animals were examined and deemed clinically healthy before the study, and experiments were conducted when mice were 8 weeks of age. All procedures in the present study were conducted in accordance with the guidelines approved by the Animal Research Committee of Azabu University.
Experimental Procedure
Thirty-three mice were randomly assigned to control or experimental groups. Cerulein-induced AP induced hourly intraperitoneal injection of cerulein (Bachem AG, Switzerland) at a dose of 50 μg/kg for 7 h. Six hours after the last cerulein administration, the mice were euthanized by cervical dislocation under pentobarbital anesthesia (60 mg/kg, ip) after blood collection. Heparinized blood was centrifuged; the plasma was collected and stored at −20 °C until use. Autopsy was performed to obtain the pancreatic tissue, which was divided into three portions: head, body, and tail. The head portion was immediately frozen using liquid nitrogen and stored at −80 °C until histochemical staining with myeloperoxidase (MPO). The body portion was used for histopathological grading. The tail portion was used for RT-PCR.
mRNA Expression of 5-HT3R
Total RNA was extracted with a commercially available kit (RNAspin Mini RNA Isolation Kit; GE Healthcare UK Ltd., Buckingham shire, England) according to the manufacturer’s instructions. Reverse transcription was performed using a PrimeScript RT Reagent Kit (Takara Bio Inc). Cyber green quantitative PCR was performed to assess the messenger RNA (mRNA) expression level of 5-HT3R (SYBR premix EX taq II, Takara Bio Inc). The PCR amplification involved the following steps: pre-denaturing (95 °C for 10 s) and 40 cycles of denaturation (95 °C for 5 s), annealing (60 °C for 30 s), and extension (95 °C for 15 s). The following primer pairs were used: 5-HT3R (forward primer: 5′-ATATCCAGGCGTGAAGACGTT-3′, reverse primer: 3′-CTAACACGTTGGTGGAAGT-5′), and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (forward primer: 5′-TGTCCCCACCCCCAATGTATC-3′, reverse primer: 3′-CTCCGATGCCTGCTTCACTACCTT-5′). The PCR products were electrophoresed in 7.5% acrylamide, stained with ethidium bromide, and visualized using a UV transilluminator. The expression of the 5-HT3 receptor in the brain was also confirmed as the positive control.
Measurement of Pancreatic Enzyme and Inflammatory Cytokine Levels
Plasma amylase and lipase activities and levels were evaluated according to the manufacturer’s instruction (cobas 6000, Roche Diagnostic, North America). Plasma IL-6 level was measured using a commercially available sandwich ELISA kit (Quantikine IL-6 ELISA kit, R&D Systems Inc., MN). The absorbance of each sample was measured at 450 nm using a plate reader (Power Scan HT, DS Pharma Biomedical, Co., Ltd., Osaka), and the IL-6 levels were calculated using a four-parameter logistic curve fit.
Histopathological Assessment
The body portion of the pancreas was fixed with 4% paraformaldehyde/PBS. Then, the tissues were embedded in paraffin and cut into 4-μm-thick sections, which were stained with hematoxylin and eosin. Histopathological grading was performed as previously described [19]. The severity of AP was graded by semiquantitative evaluation of edema, inflammatory cell infiltrate, and acinar cell necrosis (Table 1). Each histopathological parameter was assessed for each individual, and the total histopathological score (range 0–9) was calculated.
MPO Staining
MPO staining in pancreatic tissue was performed using commercially available kit (New PO-K Kit, Muto Pure Chemicals, Co., Ltd., Tokyo), according to the manufacturer’s instructions. The frozen tissue was sliced using cryostat (CM3050 S, Leica Biosystems, Germany) at 6 μm. Counter staining was performed using Giemsa stain. MPO-positive cells in the pancreas were evaluated as previously described [14]. Positive cells were counted in 20 consecutive high-power fields (HPFs) on each slide at a magnification of ×400.
Statistical Analysis
One-way ANOVA was performed to compare the values for each group. When data were significant, the differences between the treatments at each group were analyzed using the Tukey–Kramer test. Data were expressed as the mean ± SD. A P value of <0.05 was considered significant. All analyses were performed using commercially available software (StatMate IV; ATMS Co., Ltd., Tokyo, Japan).
RESULTS
At first, we evaluated the messenger mRNA expression of 5-HT3R by RT-PCR. 5-HT3R mRNA expression was detected in the pancreas as well as the brain. The expression of 5-HT3R in the pancreas was 0.87-fold of that in the brain (Fig. 1).
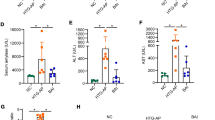
Next, the effect of ondansetron on pancreatic enzymes and cytokine levels in the blood was investigated in the cerulein-induced AP model. In mice treated with cerulein alone, a marked increase in amylase, lipase, and IL-6 levels was observed compared to those treated with saline. Ondansetron significantly decreased the blood levels of amylase and lipase compared with that in mice administered cerulein alone (Fig. 2a, b). Furthermore, IL-6 level significantly decreased in the ondansetron-treated group (Fig. 2c).
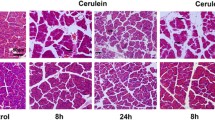
Finally, the histopathological severity of AP was evaluated in each treatment group. The typical histopathological findings in each treatment group are presented in Fig. 3a. Mice treated with cerulein alone showed diffuse edema, vacuolization, inflammatory cell infiltration, and acinar necrosis in the pancreas. The administration of ondansetron decreased the histopathological score compared with that in mice treated with cerulein alone (Table 2). In particular, the scores of edema and inflammatory cell infiltration markedly decreased, indicating the anti-inflammatory effect of ondansetron. In addition, the number of MPO-positive neutrophils in mice treated with cerulein increased, and mice administered ondansetron showed a significant reduction in MPO-positive cells in the pancreas (Fig. 3b).
Ondansetron inhibits histopathological damage and cell infiltration in AP. Typical histopathological findings in each treatment group. Histopathological changes such as necrosis, edema, and cell infiltration were attenuated by ondansetron. b Number of MPO-positive cells in each treatment group. **Significant difference at P < 0.01.
DISCUSSION
To the best of our knowledge, the present study demonstrated for the first time that ondansetron ameliorates pancreatic injury in AP through its anti-inflammatory action. Ondansetron significantly attenuated pancreatic histological damage, diminished infiltration of MPO-positive neutrophils, and reduced plasma IL-6 levels. These findings suggested that 5-HT3R antagonists may act as clinically useful therapeutic agents against AP through anti-inflammatory action.
There are several possibilities to demonstrate the anti-inflammatory effect of 5-HT3R antagonists. The first possibility is that immune reactive cells that express 5-HT3R may regulate acute inflammation. Durk et al. reported that 5-HT3R expressed in immune cells, such as monocytes, and dendritic cells, promotes the secretion of IL-6 and IL-1β to accelerate cellular inflammatory responses [20]. In addition, the 5-HT3R antagonist inhibited the production of TNF-α and IL-1βin human monocytes [21]. An increasing number of findings provide evidence that suggest the anti-inflammatory action of 5-HT3R antagonist in various inflammatory models. Ondansetron inhibited the infiltration of CD68-positive macrophages and decreased the mRNA expression of MCP-1, TNF-α, IL-1β, IL-6, and iNOS in a post-operative ileus model [13]. In an acute liver injury model, ondansetron treatment reduced the mRNA expression of TNF-α and IL-6 via p-38 MAPK phosphorylation [16]. Based on these findings, the inhibition of cell infiltration and cytokine production observed in the present study may be achieved through 5-HT3R expressed in inflammatory cells such as macrophages. In the present study, 5-HT3R mRNA was highly expressed in pancreatic tissue. However, commercially available antibodies against 5-HT3R may not have enough selectivity for the receptor, indicating the requirement for extreme caution to study the distribution of 5-HT3R.
The second possibility is that the target cells that induce therapeutic action through ondansetron are acinar cells in the pancreas. The present study demonstrated that ondansetron treatment decreased amylase and lipase levels in the blood. The pathogenesis of AP involves the extracellular leakage of pancreatic enzymes, followed by the disruption of acinar cells, which flow into the circulating blood [3]. The leakage of amylase and lipase is a major trigger for the onset of AP. A recent study revealed that pancreatic inflammation was significantly decreased in the AP model of tryptophan hydroxylase-1 knockout mice [22]; therefore, 5-HT is required for the onset of pancreatic inflammation. Previous findings indicate that 5-HT directly regulates the secretion of pancreatic enzymes. 5-HT originating from intestinal EC cells activated 5-HT2R and 5-HT3R on vagal afferent fibers to mediate luminal factor-stimulated pancreatic secretion [18]. An in vitro and ex vivo study demonstrated that the reduction in intracellular levels of 5-HT resulted in the decrease of zymogen granule secretion by altering the cytoskeleton dynamics [22]. Furthermore, a previous study demonstrated that the 5-HT3R antagonist MDL72222 inhibited pancreatic exocrine secretion [23]. Taken together, ondansetron may inhibit the secretion of zymogen, leading to the attenuation of pancreatic injury. Further studies may be warranted to clarify the exact mechanism underlying the decrease in blood pancreatic enzyme levels by ondansetron.
The final possibility is that the anti-inflammatory action of 5-HT3R antagonists in AP may be mediated through the activation of α7 nicotinic acetylcholine receptors (α7AChR) in macrophages. A typical 5-HT3R antagonist, tropisetron, has a partial agonistic ability for α7AChR [24]. The stimulation of α7nAChRs in leukocytes induces the anti-inflammatory action in animal models [25,26,27]. However, we demonstrated that the ondansetron-induced anti-inflammatory action was reduced in a POI model using 5-HT3R null mice [13], which indicated that ondansetron can ameliorate pancreatic injury in AP through 5-HT3R inhibition without α7nAChR activation.
The present study suggests that ondansetron may be clinically effective in the treatment of AP by modulating acute inflammatory process. Along with its anti-inflammatory action, ondansetron exhibits potent anti-emetic action through 5-HT3R [28]. Gastrointestinal clinical symptoms such as nausea and vomiting are common clinical symptoms of AP. It is reported that nausea and vomiting are observed in 75–90% of patients [29]. Collectively, 5-HT3R antagonists such as ondansetron may be useful therapeutic agents for AP owing to their anti-inflammatory action in addition to their anti-emetic action. The anti-inflammatory action of the 5-HT3R antagonist may also be clinically useful in the treatment of other inflammatory diseases as a drug repositioning concept, which explores the novel therapeutic indications of conventional clinical agents [30]. As the repositioned drug has already passed a significant number of toxicity and other tests, its safety is known and the risk of failure for reasons of adverse toxicology is reduced. Further clinical studies are warranted to investigate the clinical efficacy of ondansetron in the treatment of AP.
References
Russell, M.K. 2004. Acute pancreatitis: a review of pathophysiology and nutrition management. Nutrition in Clinical Practice 19: 16–24.
Steer, M. 2002. Pancreatitis severity: who calls the shots? Gastroenterology 122: 1168–1172.
Bhatia, M., M. Brady, S. Shokuhi, S. Christmas, J.P. Neoptolemos, and J. Slavin. 2000. Inflammatory mediators in acute pancreatitis. The Journal of Pathology 190: 117–125.
Wang, M., and R. Lei. 2016. Organ dysfunction in the course of severe acute pancreatitis. Pancreas 45: 5–7.
Meier, J.J., and A. Giese. 2015. Diabetes associated with pancreatic diseases. Current Opinion in Gastroenterology 31: 400–406.
Hoyer, D., J.P. Hannon, and G.R. Martin. 2002. Molecular, pharmacological and functional diversity of 5-HT receptors. Pharmacology, Biochemistry and Behaviour 71: 533–554.
Johnston, K.D., Z. Lu, and J.A. Rudd. 2014. Looking beyond 5-HT3 receptors: a review of the wider role of serotonin in the pharmacology of nausea and vomiting. European Journal of Pharmacology 722: 13–25.
Freedman, S.B., M. Adler, R. Seshadri, and E.C. Powell. 2006. Oral ondansetron for gastroenteritis in a pediatric emergency department. The New England Journal of Medicine 354: 1698–1705.
Franzen, L., J. Nyman, H. Hagberg, M. Jakobsson, B. Sorbe, A.L. Nyth, et al. 1996. A randomised placebo controlled study with ondansetron in patients undergoing fractionated radiotherapy. Annals of Oncology 7: 587–592.
Karim, F., S.C. Roerig, and D. Saphier. 1996. Role of 5-hydroxytryptamine3 (5-HT3) antagonists in the prevention of emesis caused by anticancer therapy. Biochemical Pharmacology 52: 685–692.
Tricco, A.C., C. Soobiah, E. Blondal, A.A. Veroniki, P.A. Khan, A. Vafaei, et al. 2015. Comparative efficacy of serotonin (5-HT3) receptor antagonists in patients undergoing surgery: a systematic review and network meta-analysis. BMC Medicine 13: 136.
Kato, S. 2013. Role of serotonin 5-HT3 receptors in intestinal inflammation. Biological and Pharmaceutical Bulletin 36: 1406–1409.
Maehara, T., K. Matsumoto, K. Horiguchi, M. Kondo, S. Iino, S. Horie, et al. 2015. Therapeutic action of 5-HT receptor antagonists targeting peritoneal macrophages in post-operative ileus. British Journal of Clinical Pharmacology 172: 1136–1147.
Muller, T., T. Durk, B. Blumenthal, M. Grimm, S. Cicko, E. Panther, et al. 2009. 5-hydroxytryptamine modulates migration, cytokine and chemokine release and T-cell priming capacity of dendritic cells in vitro and in vivo. PloS One 4: e6453.
Stratz, C., H.S. Bhatia, R.S. Akundi, T. Nuhrenberg, D. Trenk, E. Munoz, et al. 2012. The anti-inflammatory effects of the 5-HT3 receptor antagonist tropisetron are mediated by the inhibition of p38 MAPK activation in primary human monocytes. International Immunopharmacology 13: 398–402.
Liu, F.C., F.W. Liu, and H.P. Yu. 2011. Ondansetron attenuates hepatic injury via p38 MAPK-dependent pathway in a rat haemorrhagic shock model. Resuscitation 82: 335–340.
Mussa, B.M., D.M. Sartor, and A.J. Verberne. 2008. Activation of cholecystokinin (CCK 1) and serotonin (5-HT 3) receptors increases the discharge of pancreatic vagal afferents. European Journal of Pharmacology 601: 198–206.
Li, Y., Y. Hao, J. Zhu, and C. Owyang. 2000. Serotonin released from intestinal enterochromaffin cells mediates luminal non-cholecystokinin-stimulated pancreatic secretion in rats. Gastroenterology 118: 1197–1207.
Van Laethem, J.L., R. Eskinazi, H. Louis, F. Rickaert, P. Robberecht, and J. Deviere. 1998. Multisystemic production of interleukin 10 limits the severity of acute pancreatitis in mice. Gut 43: 408–413.
Durk, T., E. Panther, T. Muller, S. Sorichter, D. Ferrari, C. Pizzirani, et al. 2005. 5-Hydroxytryptamine modulates cytokine and chemokine production in LPS-primed human monocytes via stimulation of different 5-HTR subtypes. International Immunology 17: 599–606.
Fiebich, B.L., R.S. Akundi, K. Lieb, E. Candelario-Jalil, D. Gmeiner, U. Haus, et al. 2004. Antiinflammatory effects of 5-HT3 receptor antagonists in lipopolysaccharide-stimulated primary human monocytes. Scandinavian Journal of Rheumatology 119: 28–32.
Sonda, S., A.B. Silva, K. Grabliauskaite, E. Saponara, A. Weber, J.H. Jang, et al. 2013. Serotonin regulates amylase secretion and acinar cell damage during murine pancreatitis. Gut 62: 890–898.
Nawrot-Porabka, K., J. Jaworek, A. Leja-Szpak, J. Szklarczyk, S.J. Konturek, and R.J. Reiter. 2013. Luminal melatonin stimulates pancreatic enzyme secretion via activation of serotonin-dependent nerves. Pharmacological Reports 65: 494–504.
Hibbs, R.E., G. Sulzenbacher, J. Shi, T.T. Talley, S. Conrod, W.R. Kem, et al. 2009. Structural determinants for interaction of partial agonists with acetylcholine binding protein and neuronal alpha7 nicotinic acetylcholine receptor. The EMBO Journal 28: 3040–3051.
Pena, G., B. Cai, J. Liu, E.P. van der Zanden, E.A. Deitch, W.J. de Jonge, et al. 2010. Unphosphorylated STAT3 modulates alpha 7 nicotinic receptor signaling and cytokine production in sepsis. European Journal of Immunology 40: 2580–2589.
Costa, R., E.M. Motta, M.N. Manjavachi, M. Cola, and J.B. Calixto. 2012. Activation of the alpha-7 nicotinic acetylcholine receptor (alpha7 nAchR) reverses referred mechanical hyperalgesia induced by colonic inflammation in mice. Neuropharmacology 63: 798–805.
Tsuchida, Y., F. Hatao, M. Fujisawa, T. Murata, M. Kaminishi, Y. Seto, et al. 2011. Neuronal stimulation with 5-hydroxytryptamine 4 receptor induces anti-inflammatory actions via alpha7nACh receptors on muscularis macrophages associated with postoperative ileus. Gut 60: 638–647.
Patka, J., D.T. Wu, P. Abraham, and R.M. Sobel. 2011. Randomized controlled trial of ondansetron vs. prochlorperazine in adults in the emergency department. The Western Journal of Emergency Medicine 12: 1–5.
Munoz, A., and D.A. Katerndahl. 2000. Diagnosis and management of acute pancreatitis. American Family Physician 62: 164–174.
Jin, G., and S.T. Wong. 2014. Toward better drug repositioning: prioritizing and integrating existing methods into efficient pipelines. Drug Discovery Today 19: 637–644.
Acknowledgments
This study was partially supported by a project grant (Young Scientist Research Training Award personal type) funded by the Azabu University Research Services Division and by a Grant-in-Aid for Scientific Research provided by the Japan Society for the Promotion of Science (MH, 24248050).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
All procedures in the present study were conducted in accordance with the guidelines approved by the Animal Research Committee of Azabu University.
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Atsushi Tsukamoto and Masatoshi Hori contributed equally to this work.
Rights and permissions
About this article
Cite this article
Tsukamoto, A., Sugimoto, T., Onuki, Y. et al. The 5-HT3 Receptor Antagonist Ondansetron Attenuates Pancreatic Injury in Cerulein-Induced Acute Pancreatitis Model. Inflammation 40, 1409–1415 (2017). https://doi.org/10.1007/s10753-017-0584-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-017-0584-7