Abstract
Background
The underlying molecular mechanism that leads to development of chronic pancreatitis remains elusive. The aim of this study is to understand the downstream inflammatory signaling involved in progression of chronic pancreatitis, and to use withaferin A (WA), a small molecule inhibitor of nuclear factor κB (NFκB), to prevent progression of chronic pancreatitis.
Methods
Two different protocols were used to induce pancreatitis in mice: standard and stringent administration of cerulein. The severity of pancreatitis was assessed by means of pancreatic histology and serum amylase levels. Immunohistochemistry and flow-cytometric analysis was performed to visualize immune cell infiltration into the pancreas. Real-time PCR and Western blot were used to analyze the downstream signaling mechanism involved in the development of chronic pancreatitis.
Results
The severity of cerulein-induced pancreatitis was reduced significantly by WA, used as either preventive or curative treatment. Immune cell infiltration into the pancreas and acinar cell death were efficiently reduced by WA treatment. Expression of proinflammatory and proapoptotic genes regulated by NFκB activation was increased by cerulein treatment, and WA suppressed these genes significantly. Sustained endoplasmic reticulum stress activation by cerulein administration was reduced. NLRP3 inflammasome activation in cerulein-induced pancreatitis was identified, and this was also potently blocked by WA. The human pancreatitis tissue gene signature correlated with the mouse model.
Conclusions
Our data provide evidence for the role of NFκB in the pathogenesis of chronic pancreatitis, and strongly suggest that WA could be used as a potential therapeutic drug to alleviate some forms of chronic pancreatitis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chronic pancreatitis (CP) is an inflammatory disease characterized by irreversible structural alterations in the pancreas due to inflammation, fibrosis, and acinar atrophy, leading to debilitating abdominal pain, exocrine and endocrine insufficiency, and severely compromised quality of life [1, 2]. CP is primarily a result of repeated episodes of acute pancreatitis (AP) involving intra-acinar trypsinogen activation, vacuole formation, necrosis, and apoptosis of acinar cells and severe inflammation [3]. Currently, there is no treatment for CP nor a unified approach to control disease progression.
Several reports have identified the significant role played by nuclear factor κB (NFκB) in the pathogenesis of CP [4, 5], but the downstream mechanisms associated with disease progression remain unclear. Tamoxifen-induced activation of NFκB and/or overexpression of the active form of inhibitor of κB (IκB) kinase β (IKKβ) in the pancreatic acinar cell increased the severity of CP [6, 7]. A high-fat-diet-induced CP model demonstrated the requirement of increased NFκB activity for disease development [8]. A cerulein-induced experimental mouse model of pancreatitis has been widely used to investigate the mechanism of CP [9, 10].
Endoplasmic reticulum (ER) stress or unfolded protein response is a cellular mechanism to overcome protein overload and regulate protein folding more efficiently to regain homeostasis [11]. Sustained or uncontrolled ER stress leads to apoptosis of the cell, which is mainly regulated by the transcription factor C/EBP homologous protein (CHOP) [12, 13]. During the pathogenesis of pancreatitis, acinar cell death is prominent, and this may lead to an overwhelming amount of protein production in remaining acinar cells, which could result in ER stress. ER stress has been identified as an early event in pancreatic injury, and prolonged administration of cerulein to mice results in sustained activation of ER stress, leading to CP [14].
Cells of the innate immune system use special receptors such as Toll-like receptors (TLRs) and/or nucleotide-binding oligomerization domain like receptors (NLRs) to sense damage or pathogen invasion. NLRP3 (also known as NALP3) is an extensively investigated member of the NLR family that is responsible for the formation and activation of an inflammasome. The NLRP3 inflammasome has been reported to play a role in chronic inflammatory diseases such as cryopyrin-associated periodic syndromes, gout, atherosclerosis, and type 2 diabetes [15].
Withaferin A (WA) is a natural steroidal lactone used in ancient ayurvedic medicine as an anti-inflammatory drug. It has been shown to inhibit NFκB activation by binding to IKKβ and preventing phosphorylation of IκB [16]. In this study, we investigated the downstream mechanisms leading to CP, including ER stress and inflammasome formation. Furthermore, we used WA in vitro and in vivo to investigate whether blocking of NFκB would reduce progression of CP.
Materials and methods
Mice
C57BL/6 mice purchased from Jackson Laboratories were used throughout the study, and all experiments were conducted on age- and sex-matched littermates. All mice were housed in filter-topped shoe box cages with autoclaved food and water. Mice were randomly assigned to control and experimental groups. All experiments were performed in accordance with Baylor Research Institute’s Institutional Animal Care and Use Committee regulations.
Reagents
Cerulein was purchased from Sigma-Aldrich (St Louis, MO, USA), and WA was purchased from Enzo Life Sciences (Farmingdale, NY, USA). The amylase activity kit, picro Sirius red staining kit, and anti-neutrophil and anti-Ki-67 antibodies were obtained from Abcam (Cambridge, MA, USA). Mouse CD45, CD11b, CD14, and Ly6g antibodies were purchased from BioLegend (San Diego, CA, USA). Anti-NFκB antibody was bought from Santa Cruz Biotechnology (Dallas, TX, USA). RNAlater and TRIzol were purchased from Life Technologies (Carlsbad, CA, USA). The high-capacity complementary DNA reverse transcription kit was obtained from Applied Biosystems (Waltham, MA, USA), and the qRT-PCR SYBR Green/ROX master mix and custom-made human inflammatory gene array kit were obtained from Qiagen (Valencia, CA, USA). The IL-6 and monocyte chemotactic protein 1 Luminex multiplex kit was obtained from Millipore (Darmstadt, Germany).
Acute pancreatitis
The mice were made to fast overnight before induction of pancreatitis by cerulein; water was given ad libitum. Cerulein (50 μg/kg intraperitoneally) or saline was injected into the mice every hour for a total of seven injections and the mice were sacrificed 1 h after the last injection. WA (1.25 mg/kg) was administered 1 h before the first cerulein injection (five mice per group) (Fig. 1a). Blood was collected from the inferior vena cava for analysis of serum markers of pancreatitis, and the pancreas was harvested immediately afterward. The pancreas was divided for immunohistochemistry, RNA extraction, protein extraction, and immune cell infiltration analysis.
Protocol for development and treatment of pancreatitis in mice. a Acute pancreatitis was induced by seven hourly injections of cerulein (50 µg/kg). One group of mice received withaferin A (WA) 1 h before the first cerulein injection. The control group received vehicle and saline injection. b Preventive approach. Chronic pancreatitis was induced by repeated episodes of acute pancreatitis for 4 weeks. As a preventive approach, mice were given WA once a week 1 h before the first cerulein injection. c Curative approach. The severity of chronic pancreatitis was increased by induction of acute pancreatitis twice per week for 8 weeks. Mice received WA starting in the fifth week 1 h before cerulein administration. i.p. intraperitoneal
Chronic pancreatitis
Mice were subjected to repeated episodes of AP caused by a supramaximal dose of cerulein. In the preventive model, CP was induced when cerulein was injected intraperitoneally (50 μg/kg) every hour for a total of seven injections. This process was repeated once a week for 4 weeks. Saline was injected into control mice as sham treatment. Another group of mice received WA (1.25 mg/kg) 1 h before the first cerulein injection every week (six mice per group) (Fig. 1b). In the curative model, CP was induced when cerulein was injected intraperitoneally (50 μg/kg) every hour for a total of seven injections. This process was repeated twice a week (Mondays and Thursdays) for 8 weeks. WA (0.625 mg/kg) was injected 1 h before the first cerulein injection, but administration was initiated at week 5 and continued until the end of the experiment (Fig. 1c). In both models, mice were sacrificed and tissue was harvested 1 week after the last cerulein injection.
Amylase activity assay
After treatment, serum samples were harvested from mice and stored at –80 °C until further analysis. Serum was analyzed for amylase activity as per the manufacturer’s protocol (Abcam, Cambridge, MA, USA). Serum collected from mice undergoing AP was used to measure trypsinogen activation peptide as per the manufacturer’s protocol (Cloud-Clone Corp., Houston, TX, USA).
Histology and immunohistochemistry
Pancreas was placed in 10 % formalin immediately after being harvested and fixed for at least 48 h at 4 °C before it was processed. Tissue was embedded in paraffin, and 5-μm sections were taken and mounted on slides for staining. Hematoxylin and eosin staining was performed for histopathological evaluation, and a pathologist blinded to the study groups scored the tissue from 0 (normal) to 5 (severely inflamed). Sirius red staining was performed according to the manufacturer’s protocol on sections to evaluate fibrotic change. The sections were deparaffinized in xylene and rehydrated with ethanol. The slides were incubated in a citrate buffer of pH 6 at 97 °C for 20 min. Following an antigen retrieval process, slides were carefully washed three times for 5 min in Tris(hydroxymethyl)aminomethane-buffered saline (TBS) containing 0.025 % Triton X-100. Slides were blocked in 1 % bovine serum albumin (BSA) in TBS for 2 h at room temperature to block nonspecific binding of the antibodies. Then slides were incubated overnight with primary antibodies, which were diluted in TBS with 1 % BSA. The next day, slides were gently rinsed three times for 5 min in TBS containing 0.025 % Triton X-100. Tetramethylrhodamine-labeled or fluorescein isothiocyanate labeled secondary antibodies from Invitrogen were diluted in TBS with 1 % BSA. The slides were counterstained with 4′,6-diamidino-2-phenylindole and mounted for microscopic analysis. ImageJ was used to perform semiquantitative analysis of the staining.
Immune cell infiltration assay
A single cell suspension was prepared from the pancreas after thorough digestion with collagenase (2 mg/mL) at 37 °C for 15 min. Tissue was further mechanically digested by pipetting several times. The suspension was then washed and filtered to obtain only the single cell fraction. The cell suspension obtained was used for flow-cytometric analysis of infiltrated immune cells. Pancreatic cells (1 × 106) were labeled with anti-CD45, anti-CD11b, anti-CD14, and anti-Ly6 g antibodies. Stained cells were detected with a FACSCanto II system, and data were analyzed with FlowJo.
Luminex assay
Pancreas was homogenized, and the total lysate concentration was measured. Lysate was analyzed directly according to the manufacturer’s protocol, and the results were normalized to the total lysate concentration.
Western blotting
Pancreatic tissues were sonicated in radioimmunoprecipitation assay lysis buffer containing phenylmethylsulfonyl fluoride at 0.2 mg/mL , 0.1 M NaF, 2 mM Na3VO4, aprotinin at 10 μg/mL , pepstatin A at 5 μg/mL , and leupeptin at 5 μg/mL. Equal amounts of protein lysates were subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis, electrotransferred to nitrocellulose membranes, and blocked with 5 % milk in TBS containing 0.1 % Tween 20 (pH 7.4). Primary antibodies for CHOP (Thermo Fisher Scientific, MA, Waltham, USA) or β-actin (Cell Signaling Technology, Danvers, MA, USA) were then added, and membrane was incubated overnight at 4 °C and washed three times with TBS containing 0.1 % Tween 20. Membranes were then incubated with horseradish peroxidase secondary antibodies (Abcam, Cambridge, MA, USA) to detect proteins by SuperSignal West Dura enhanced chemiluminescence (Thermo Fisher Scientific, Waltham, MA, USA) with a G:BOX system (Syngene, Cambridge, UK).
Quantitative real-time PCR
Pancreas was stored in RNAlater at −80 °C until the extraction procedure. Total RNA from the pancreas was extracted with TRIzol reagent. Tissue was homogenized with a handheld homogenizer, and debris was removed by centrifugation. Chloroform was added to separate the RNA from other biomolecules and it was further precipitated with 2-propanol. The RNA pellet was washed with ethanol and quantified with a NanoDrop2000 system. Complementary DNA synthesis was performed with a high-capacity complementary DNA reverse transcription kit as per the manufacturer’s protocol. PCR primers for 18S ribosomal RNA, proline-rich extensin-like receptor kinase, activating transcription factor 6, endoplasmic reticulum to nucleus signaling 1, activating transcription factor 4, X-box binding protein 1, CHOP, high mobility group box 1 (HMGB-1), PYCARD, NLRP3, IL-18, IL-1β, IL-6, tumor necrosis factor α (TNFα), nitric oxide synthase 2, caspase 3, and Bax were purchased from Qiagen. qRT-PCR was performed with a Stratagene 3000p QPCR system (Stratagene, La Jolla, CA, USA) with use of the SYBR Green/ROX master mix from Qiagen as per the manufacturer’s protocol. Data were analyzed by the ΔΔCt method.
Gene array
Healthy human pancreatic tissue was obtained from cadaveric donors. CP tissue was obtained from patients undergoing total pancreatectomy with islet autotransplant who consented to provide samples for research. All protocols were performed according to the regulations of Baylor Research Institute’s Institutional Review Board. Pancreatic tissue was used for RNA extraction by TRIzol and complementary DNA synthesis, as previously described. A custom PCR array for inflammatory and NFκB-regulated genes was obtained from Qiagen, with performance and analysis as per the manufacturer’s recommendations.
Statistical analysis
Statistical analysis was performed with GraphPad Prism6 (GraphPad Software, La Jolla, CA, USA). Statistical significance for more than two groups was determined by one-way analysis of variance and Tukey’s multiple comparison test. Statistical analysis of two groups was determined by an unpaired Student’s t test. Differences were considered significant when p values were less than 0.05.
Results
Effect of WA on the AP model
Administration of the cholecystokinin analog cerulein has been widely established as a model for induction of AP and CP [17, 18]. AP was induced in C57BL/6 mice by seven hourly intraperitoneal injections of cerulein (50 μg/kg) (Fig. 1a). The mice were sacrificed 1 h after the last injection to harvest plasma and pancreas for analysis. The severity of AP was determined by analysis of serum amylase levels and histopathological examination of the pancreatic sections.
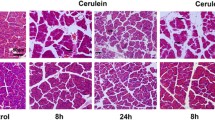
To test the ability of WA to inhibit cerulein-induced pancreatitis, WA was administered 1 h before cerulein injection. Histopathological examination revealed significant necrosis of the pancreatic parenchyma and increased infiltration of inflammatory cells in cerulein-treated mice (Fig. 2a, b). WA treatment prevented damage to the pancreatic parenchyma and also reduced inflammatory cell infiltration significantly. Flow-cytometric analysis revealed a significant increase in the percentage of leukocytes present in the pancreas 1 h after the last cerulein injection compared with controls, which was effectively reduced by WA (Fig. 2c, d). The levels of myeloid cells, neutrophils, and macrophages were found to be increased in the mouse pancreas after cerulein administration, but this increase was abolished by WA pretreatment. Cerulein administration increased serum amylase levels by almost four-fold compared with controls (498.4 ± 87.05 mU/mL vs 1894.04 ± 72.91 mU/mL; p < 0.001). WA significantly reduced cerulein-induced serum amylase level elevation (Fig. 2e). Activation of intra-acinar trypsinogen has been regarded as a pathological event in pancreatitis. We measured levels of trypsinogen activation peptide in serum of mice to see if WA administration affects trypsinogen activation. We found that trypsinogen activation peptide level was unaffected by treatment with WA (Fig. S1),suggesting that WA has no effect on intra-acinar trypsinogen activation.
Withaferin A (WA) reduces the severity of acute pancreatitis and abolishes immune cell infiltration. a Hematoxylin and eosin staining of pancreatic sections from control, cerulein-treated, and cerulein-plus-WA-treated mice. Representative sections of pancreases from at least five mice per group. Microscopic images were taken at ×200 magnification. b A histological score of 0 to 5 was assigned in a blinded fashion to the study groups, where 0 represents healthy pancreas morphology and 5 represents severe pancreatitis morphology. At least four different loci per pancreas were visually inspected and scored; the data are represented as the mean ± standard deviation (SD) of five independent experiments. c For flow-cytometric analysis, pancreatic cells were stained with antibodies to CD45, CD11b, Ly6G, and CD14. The data are representative of five independent experiments. d Representative bar graph showing the percentage of CD45-positive leukocytes infiltrating the pancreas. The data are expressed as the mean ± SD of five mice per group. e Serum amylase was measured by ELISA. The data are expressed as the mean ± SD of five independent experiments. One asterisk P < 0.05, three asterisks P < 0.001, four asterisks P < 0.0001 (one-way analysis of variance with multiple comparison)
CP progression was attenuated by WA
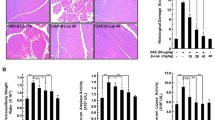
Two different models of CP were investigated, a preventive and a curative model. In the preventive model, CP was induced by seven hourly injections of cerulein (50 μg/kg) performed once a week for 4 weeks, and WA was administered 1 h before the cerulein injections every week (Fig. 1b). Vehicle-treated control mice exhibited a normal pancreatic morphology, and WA administration alone did not cause any structural change in the pancreatic tissue compared with controls (Fig. 3a). The WA dose (1.25 mg/kg) was well tolerated by the mice. CP induced by repeated episodes of AP caused a significant morphological change in the pancreatic parenchyma, as evidenced by hematoxylin and eosin staining of pancreas. The acinar tissue was atrophic with significant infiltration of inflammatory cells, ductal metaplasia, and hemorrhage.
Withaferin A (WA) can prevent development and abrogate progression of chronic pancreatitis (CP). a–c Preventive CP model. d–e Curative CP model. a Hematoxylin and eosin (H&E) staining of pancreas from control, cerulein-, WA-, or cerulein-plus-WA-treated mice. Representative sections of pancreas from at least six mice per group. Microscopic images were taken at ×100 magnification. b, e The histological score was evaluated as described in the legend for Fig. 2 from at least five different loci per pancreas, with a total of six independent experiments per group. c Serum amylase was measured by ELISA after cerulein injections for 4 weeks. The data are expressed as the mean ± standard deviation (SD) of six independent experiments. d H&E staining of pancreatic sections from stringent-CP-induced mice. Fibrosis is a hallmark of severe CP, and Sirius red staining was used to visualize fibrotic changes in the pancreatic parenchyma. Microscopic images were taken at ×100 magnification. ImageJ was used to quantitatively determine the percentage of fibrosis area by measurement of the red stained area divided by the total tissue area. f Luminex analysis of IL-6 and monocyte chemotactic protein 1 (MCP-1) present in the pancreatic homogenate was performed and the data were normalized to the total protein concentration in the lysate. The data are expressed as the mean ± SD of six independent experiments. The images are representative of six different experiments. NS not significant, one asterisk P < 0.05, two asterisks P < 0.01, three asterisks P < 0.001, four asterisks P < 0.0001 (one-way analysis of variance with multiple comparison)
WA administration 1 h before cerulein injection significantly abolished progression of CP. Histological examination confirmed that WA protected the pancreatic parenchyma from cerulein-induced damage and maintained pancreas morphology. Inflammatory cell infiltration and acinar atrophy were reduced or completely absent in the WA-treated group (Fig. 3b).
There was basal amylase level in the serum of control and WA-treated groups. However, cerulein injection caused a significant increase in serum amylase concentration. WA pretreatment reduced the serum amylase levels, suggesting a significant reduction of acinar damage caused by cerulein stimulation (Fig. 3c).
In most cases, CP is diagnosed after several attacks or episodes of AP. Therefore, to make the experiment clinically relevant, the curative potential of WA was tested with a stringent CP protocol in mice. CP was induced by seven hourly injections of cerulein (50 μg/kg) performed twice per week for 8 weeks. Cerulein was injected into mice in the WA treatment group for the first 4 weeks, and WA (0.625 mg/kg) administration was initiated from week 5 until the end of the experiment (Fig. 1c). CP development before WA administration was evaluated; morphological analysis and the fibrosis score in cerulein-treated mice revealed development of CP (Fig. S2). The histopathological examination revealed a normal morphology in pancreas of vehicle-treated mice, whereas pancreas from mice that underwent cerulein administration with a stringent protocol exhibited pronounced destruction of pancreatic parenchyma, which was efficiently attenuated by WA administration (Fig. 3d). The acinar cell number dropped significantly in pancreas of cerulein-treated mice, and WA treatment protected cells from further damage by cerulein
Fibrotic change is one of the hallmarks of advanced CP. To visualize fibrotic changes, pancreatic sections were stained for collagen fibers with Sirius red. Mice into which cerulein alone had been injected demonstrated distinct fibrosis compared with both control and WA-treated mice. Quantification of the fibrotic area revealed noticeable fibrosis in cerulein-treated mice compared with control mice. Treatment with WA significantly reduced the fibrosis area (Fig. 3d). Inflammation in the tissue was scored histologically by a pathologist blinded to sample identity, and the WA treatment group had a significantly lower score than the cerulein treatment group (Fig. 3e).
A major characteristic of advanced CP is the lack of enzyme production due to exocrine insufficiency. Therefore, in a stringent model, the level of pancreatic amylase in serum after cerulein treatment was lower or undetectable (data not shown). Local inflammation was evaluated by measurement of protein levels of IL-6 and monocyte chemotactic protein 1 in pancreas. Both were increased by cerulein injection, and WA treatment restored the levels of both proteins back to control levels. Together these data suggest that WA has a preventive effect on CP progression. A similar effect was seen in a curative pancreatitis model (Fig. 3f).
Cytoprotective and antiinflammatory actions of WA
Cerulein-induced necrotic damage to acinar cells triggers a regenerative response and proliferation of cells to repair and replace dead cells. Ki67, a marker of proliferation, was used to assess cellular response. There were few to no Ki67-positive cells in the pancreas of control mice, but repeated cerulein injections resulted in a significant increase in the number of Ki67-positive cells in the pancreas (Fig. 4a). With WA administration, the percentage of Ki67-positive cells was significantly lower (Fig. 4b), indicating less cellular damage that required a lesser regenerative response. Comparison of the preventive and curative CP models revealed more pronounced damage to the pancreas in the curative model, but WA was able to potently reduce the damage caused even in this model (Fig. 4c, d).
Withaferin A (WA) reduces cerulein-induced acinar damage and blocks neutrophil infiltration. a, c Immunohistochemical staining of mouse pancreatic tissue for Ki67, a marker of proliferation, demonstrates the severity of damage. 4′,6-Diamidino-2-phenylindole (DAPI) was used to stain the nucleus blue and anti-Ki67 antibody with anti-rabbit fluorescein isothiocyanate labeled secondary antibody was used. b, d ImageJ was used to determine the percentage of Ki67-positive cells per frame. The data are expressed as the mean ± standard deviation (SD) representative of three different loci in three independent experiments. e Neutrophil infiltration into the pancreas was visualized by staining for granulocyte marker Ly6G and tetramethylrhodamine isothiocyanate labeled secondary antibody; DAPI was used to stain the nucleus. Microscopic images were taken at ×200 magnification. f ImageJ was used to determine the percentage of neutrophils per frame. The data are expressed as the mean ± SD representative of three different loci in three independent experiments. CP chronic pancreatitis, two asterisks P < 0.01, three asterisks P < 0.001 (one-way analysis of variance with multiple comparison)
Pancreatic tissue was stained for infiltration of neutrophils after CP had been induced. Mice into which cerulein had been injected demonstrated a plethora of granulocytes infiltrating the pancreas, but WA pretreatment significantly reduced neutrophil infiltration (Fig. 4e, f). Neutrophils, one of the first responder cells to reach the site of injury, initiate the inflammatory cascade [19]. Thus, the lack of neutrophils in pancreas of WA-pretreated mice suggested reduced inflammation.
WA blocks translocation of the p65 subunit and prevents inflammatory cytokine transcription
Overexpression or sustained activation of NFκB in acinar cells increases the severity of cerulein-induced pancreatitis [4, 6, 7]. WA is known to bind to IKKβ and inhibit phosphorylation of IκBα, hence preventing activation of NFκB and its translocation into the nucleus [16]. Administration of cerulein to mice resulted in activation and translocation of the NFκB p65 subunit in pancreatic acinar cells as shown with the preventive CP model (Fig. 5a). WA treatment not only reduced expression but also prevented translocation of the active subunit of NFκB into the nucleus. Quantification of the percentage of acinar cells showing p65 translocation revealed cerulein-treated mice had more than 40 % NFκB activation, whereas control and WA-treated groups had significantly lower activation (12 and 14 % respectively) (Fig. 5a). Furthermore, WA reduced messenger RNA expression of proinflammatory cytokines (IL-6, TNFα) and proapoptotic molecules (nitric oxide synthase 2, caspase 3) that were upregulated by cerulein administration (Fig. 5b–e). In the curative model, inhibition of IL-6, TNFα, nitric oxide synthase 2, and Bax by WA was observed in a similar fashion (Fig. S3). Together these data provide substantial evidence for the anti-inflammatory and protective role of WA against cerulein-induced CP.
Withaferin A (WA) inhibits cerulein-induced nuclear factor κB (NFκB) in acinar cells and downregulates expression of proinflammatory and proapoptotic molecules. a NFκB translocation into the nucleus was visualized by staining of the the nucleus with 4′,6-diamidino-2-phenylindole (DAPI) (blue) and staining of the p65 subunit of NFκB with anti-p65 and fluorescein isothiocyanate labeled secondary antibody (green). Translocation of NFκB into the nucleus was visualized by detection of the overlap of green and blue. Activation of NFκB was quantitatively determined by measurement of the percentage of the nucleus showing a green color with use of ImageJ. Statistical analysis was performed by measurement of NFκB activation in six mice per group. Mice undergoing standard cerulein treatment were used for staining. Microscopic images were taken at ×200 magnification (top panel) and ×600 magnification (bottom panel). b–e Real-time PCR analysis of proinflammatory cytokines and proapoptotic signals was performed on RNA extracted from the pancreases of chronic- pancreatitis-induced mice and control mice. The data are shown as a box and whisker plot, where the top and bottom ends of the box indicate the range, the whiskers indicate the standard deviation, and the line in the middle indicates the median. The data are representative of at least six mice per group. Casp3 caspase 3, IL-6 interleukin-6, Nos2 nitric oxide synthase 2, TNF-α tumor necrosis factor α, one asterisk P < 0.05, two asterisks P < 0.01 (one-way analysis of variance with multiple comparison)
Inhibition of the ER stress pathway during pathogenesis of CP
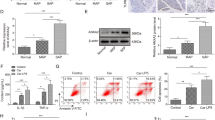
Previous reports have demonstrated the significance of sustained activation of ER stress in the pathogenesis of CP [14]. High ER volume and protein load renders acinar cells susceptible to ER stress [20]. ER stress leads to overexpression of proteins, such as proline-rich extensin-like receptor kinase, activating transcription factor 6, and endoplasmic reticulum to nucleus signaling 1 (also known as IRE1α), in the ER lumen [13]. On administration of cerulein, mouse pancreatic tissue showed significant upregulation of these ER stress proteins (Fig. 6a). WA-treated mice showed significant lower expression of these ER stress proteins, and hence there was reduced damage to the pancreas. Expression of genes encoding downstream signaling molecules in the ER stress pathway, including activating transcription factor 4, X-box binding protein 1, and CHOP, was also upregulated during CP induction, and was significantly abolished by WA administration (Fig. 6b). Similar results were observed in the curative model of CP (Fig. S4).
Withaferin A (WA) reduces expression of endoplasmic reticulum (ER) stress and inflammasome related genes. Real-time PCR analysis of a, b ER-stress-regulated and d, e inflammasome-related genes was performed on RNA extracted from pancreases of chronic pancreatitis (CP)-induced mice and control mice. The data are shown as a box and whisker plot, where the top and bottom ends of the box indicate the range, the whiskers indicate the standard deviation, and the line in the middle indicates the median. The data are representative of at least six mice per group. a Eif2ak3 (which encodes proline-rich extensin-like receptor kinase, PERK), Atf6 (which encodes activating transcription factor 6) and Ern1 (which encodes endoplasmic reticulum to nucleus signaling 1, ERN1), b Atf4 (which encodes activating transcription factor 4), Xbp1 (which encodes X-box binding protein 1), and Ddit3 (which encodes C/EBP homologous protein, CHOP), d Hmgb1 (which encodes high mobility group box 1, HMGB-1), Pycard, and Nlrp3 (which encodes nucleotide-binding oligomerization domain like receptor family pyrin- domain-containing 3, NLRP3), and e Il18 (which encodes interleukin-18, IL18) and Il1b (which encodes interleukin-1β, IL-1β) c Western blot analysis of lysates from pancreases of stringent-CP–induced mice and control mice. Semiquantitative analysis of CHOP in the lysates. The data are expressed as the mean ± standard deviation of three independent experiments. CER cerulein, CTL control, one asterisk P < 0.05, two asterisks P < 0.01, three asterisks P < 0.001, four asterisks P < 0.0001 (one-way analysis of variance with multiple comparison)
CHOP induction during ER stress in most cases leads to cell apoptosis [21]. On continuous injection of cerulein, CHOP expression increased significantly, which may result in apoptosis of the acinar cells. Cerulein-induced CHOP expression was reduced in pancreas of WA-treated mice (Fig. 6c).
Inflammasome signaling in CP and attenuation by WA
The inflammasome is a large multimeric protein complex produced and assembled by myeloid cells in many chronic inflammatory diseases [22]. The formation of the inflammasome and its role in CP have not been investigated. Tissue damage results in the release of molecules called “alarmins” or “damage-associated molecular patterns” (DAMPs) such as HMGB-1, heat shock proteins, double-stranded DNA, and ATP. Cerulein injection caused a significant increase in the expression of the HMGB-1 gene in acinar cells, but WA administration caused a modest reduction of this gene expression (Fig. 6d).
Infiltrating immune cells such as macrophages, neutrophils, or dendritic cells can be primed by DAMPs to initiate production and activation of inflammasome molecules [15]. CP induction by cerulein increased the expression levels of NLRP3, and this expression was efficiently blocked by WA treatment (Fig. 6d). Another important protein required for activation of the inflammasome is apoptosis-associated speck-like protein (ASC), and it has a caspase-binding domain called “CARD”. Expression of ASC (PYCARD) was also significantly increased when cerulein was injected to induce CP but was efficiently inhibited by WA (Fig. 6d). On activation of the inflammasome, the inactive caspase 1 is cleaved to become active caspase 1, and the activated caspase 1 is required for processing and release of mature IL-1β and IL-18. The expression levels of these two cytokines increased in cerulein-treated mice but not in WA-pretreated mice (Fig. 6e). Similar results were obtained when WA was administered after CP had been established in the curative model (Fig. S5). In summary, inflammasome signaling may have been activated during CP development, which had not been described previously, and WA administration effectively inhibited this pathway. Further investigation may be required to identify the role of the inflammasome in the pathogenesis of CP.
To corroborate the above-mentioned findings of the cerulein-induced CP model as relevant to a clinical setting, we tested pancreatic tissue collected from healthy individuals (deceased organ donors) and CP patients (undergoing total pancreatectomy with islet autotransplant) for proinflammatory gene expression by a real-time polymerase chain reaction array. Most of the proinflammatory genes were highly upregulated (fivefold to 60-fold induction) in pancreases from CP patients compared with those from healthy donors (Fig. 7).
Proinflammatory and proapoptotic genes upregulated in the pancreas of human chronic pancreatitis (CP) patients. a Heat map showing upregulation of proinflammatory and proapoptotic genes in the pancreases of patients with advanced-stage CP (six per group). Pancreatic tissue from healthy individuals was used as a control to determine the fold change. b The table shows genes that were upregulated fivefold or more in CP patient tissue compared with control tissue and that were identified as having statistically significant changes among the genes analyzed in the array. Analysis was performed by and statistics were obtained with SABiosciences-provided software for custom array plates
Discussion
Pancreatitis is characterized by inflammation and parenchymal cell death. These two key pathological parameters determine the severity of the disease [23]. The identity of the initial insult(s) and the molecular mechanism that lead to pancreatitis is still unclear. Damage of acinar cell leads to release of several proinflammatory mediators that instigates a cascade of molecular signaling and inflammatory events [24, 25]. In most cases, inflammation subsides and results in recovery of the pancreas, which is called “acute pancreatitis” (AP). In some other cases, there is persistent chronic inflammation that causes constant damage of the pancreas, or recurrent AP, and both conditions may lead to CP. One of the hallmarks of severe CP is the irreversible replacement of pancreatic acinar cells by fibrotic tissue [26]. In the severest cases, the CP progresses to diabetes or in some instances pancreatic adenocarcinoma [27, 28]. Therefore, it is crucial to identify the trigger and mechanism that causes this life-threatening illness and to develop an effective therapy for the disease.
Although several causes of CP have been identified, including alcohol consumption, autoimmunity, genetic mutation, and pancreas structural abnormality, the permanent damage to the pancreas is inflicted by inflammation. Several mechanisms for pancreatitis pathogenesis have been identified, such as anomalous calcium signaling [29], increase in the levels of intracellular reactive oxygen species caused by oxidative stress [30], activation of the NFκB pathway [31], ER stress [14], autophagy signaling in acinar cells [32], and a pathological role of intracellular and extracellular pH [33]. Intra-acinar trypsinogen activation has been presumed to be major cause of pancreatitis development. However, with use of knockout mice lacking trypsinogen activity, it was established that this theory is only partially true. Lack of active trypsinogen reduced local injury; however, there was no difference in inflammation between trypsinogen knockout mice and wild-type mice, thus suggesting that trypsinogen activation is only an early event independent of the inflammatory pathway that is regulated by NFκB [34]. In this study, using a cerulein-induced AP model, we identified that WA administration reduced disease severity and inflammation in mice. Initially, we tested if WA could also protect mice from recurrent episodes of AP that leads to CP. There were striking histopathological differences between pancreas of WA-treated and cerulein-treated mice compared with pancreas of mice receiving cerulein injections alone. Serum amylase levels, immune cell infiltration, Ki-67 staining, and inflammatory cytokine levels were significantly reduced in the WA-treated group, in contrast to elevations seen in cerulein-treated mice.
Many different experimental models have been devised to understand the mechanism that leads to different types of pancreatitis [35]. Cerulein-induced pancreatitis has been widely accepted as a clinically relevant model for recurrent acute necrotizing idiopathic pancreatitis. Some evidence accrued over the past few years has implicated the role NFκB activation in the pathogenesis of pancreatitis [6, 7, 31], but several reports have contradicted this [36, 37]. Knockout studies revealed that absence of NFκB activity reduced the severity of AP on cerulein injection [9]. When IKKβ was made constitutively active in the acinar cells of mice, cerulein administration worsened the disease. To further bolster this result, overexpression of dominant negative IKKβ relieved CP [7]. Another report published at the same time showed worsening of pancreatitis when Rela was knocked down in the acinar cells of mice [36]. These contrasting studies emphasize that a better understanding of the NFκB signaling pathway is required. Gukovsky and Gukovskaya [38] have judiciously reviewed the role of NFκB in pancreatitis, providing possible theories behind such contradicting reports. It is important to take into consideration that different models and transgenic animals may lead to different outcomes. Nevertheless, the cerulein model has been shown to activate NFκB in acinar cells [10], and continuous stimulation leads to CP in mice [5]. In CP in humans it has been shown that the p65 subunit of NFκB was localized in the nucleus [5]. Therefore, we hypothesized that inhibition of NFκB with use of WA will protect mice from progression to CP.
To make our study clinically relevant, we preestablished CP before WA treatment was initiated using increased doses of cerulein each week. Mice receiving WA showed significantly reduced inflammatory cell infiltration and fibrosis, but mice receiving cerulein injections progressed to severe CP, as demonstrated by fibrotic change. The curative CP model showed an overall increase in the number of Ki67-positive cells compared with the preventive CP model, albeit the WA-treated group had profoundly reduced damage. Together these data support the protective role of WA in the progression of CP.
Recently, ER stress has been recognized as a possible pathological mechanism in CP progression [14]. Loss of pancreatic acinar cells by necroptosis caused by autodigestion of trypsinogen enzymes leads to activation of many inflammatory signaling pathways [39]. This loss may also result in ER stress in remaining cells because of the overwhelming amount of protein synthesis and release required. Cerulein has been shown to activate the ER stress pathway within 30 min of injection, although the levels of the stress molecules subside when the pancreas recovers. It has been shown that repeated injection of cerulein results in chronic ER stress that promotes CP [14]. In concurrence with this finding, we observed an increase in the expression levels of ER stress markers upon cerulein injection to induce CP. More importantly, WA was able to efficiently block the upregulation of all ER stress markers analyzed. It is still unclear if the mechanisms of NFκB and ER stress activation are independent of each other in the pathogenesis of CP, although activation of NFκB and ER stress signaling occurs very soon after cerulein administration [14]. Inhibition of NFκB activation was able to significantly inhibit the ER stress pathway that leads to CP.
The role of inflammasome in the development of CP has not been investigated. Inflammasome activation has been identified as a major innate inflammatory response in several chronic diseases [40]. Knockdown of key molecules such as NLRP3, ASC, and caspase 1 reduced the severity of AP [41]. We have demonstrated, for the first time, the role of inflammasome in the pathogenesis of CP and have shown that NFκB inhibition significantly reduced inflammasome gene expression and reduced pancreatitis severity. NFκB is required for production of IL-1β, which is processed by the inflammasome. It is plausible that because of chronic inflammation, immune cells infiltrating the pancreas are activated via Toll-like receptor signaling by binding of DAMPs such as HMGB-1, heat shock proteins, or ATP released by damaged acinar cells. This may result in assembly and activation of the inflammasome that escalates inflammation during CP. An analytical study showed strong correlation of serum HMGB-1 with pancreatitis progression [42]. On cerulein administration, we observed significant upregulation of HMGB-1, a damage marker and initiator of inflammasome signaling, which was profoundly inhibited by WA. NLRP3, ASC, IL-1β, and IL-18 levels were also significantly upregulated, suggesting increased inflammasome activity in cerulein-induced CP. WA was able to strongly inhibit the expression levels of all those molecules. Therefore, on the basis of these results, it is likely that the NLRP3 inflammasome may be partly responsible for enhancing the severity of CP. Previous reports on WA have demonstrated antiinflammatory [43], anticancer [44], and also antiangiogenic [45] properties. One of the widely accepted and largely studied targets of WA is IKKβ inhibition causing blockade of NFκB signaling. Other studies have also reported apoptotic activity of WA, but only under in vitro conditions and not in vivo [46]. Both inflammation and cancer development require NFκB activation [47] and this may be the reason that WA has both antiinflammatory and anticancer properties.
Uncontrolled progression of CP leads to the development of diabetes, which could be fatal. Therefore, total pancreatectomy with islet autotransplant has been shown to result in retention of endocrine function in most cases [48]. However, pancreas from advanced CP patients fails to yield an adequate number of islets to prevent the occurrence of diabetes [49]. Treatment of CP patients with WA to reduce inflammation induced islet damage before surgery may be a potential therapeutic strategy for achieving improved endocrine function. We have previously reported the anti-inflammatory role of WA on islets subjected to inflammatory stress [50].
In summary, the present study strongly supports the use of WA for therapeutic intervention to relieve both acute and chronic forms of pancreatitis. Treatment with WA reduces NFκB inflammatory signaling and prevents cerulein-induced damage of acinar cells. WA administration also reduced inflammatory ER stress signaling and inflammasome gene expression in pancreas of CP-induced mice.
References
Ammann RW, Muellhaupt B. The natural history of pain in alcoholic chronic pancreatitis. Gastroenterology. 1999;116(5):1132–40.
Mullady DK, Yadav D, Amann ST, et al. Type of pain, pain-associated complications, quality of life, disability and resource utilisation in chronic pancreatitis: a prospective cohort study. Gut. 2011;60(1):77–84.
Whitcomb DC. Genetic risk factors for pancreatic disorders. Gastroenterology. 2013;144(6):1292–302.
Huang H, Liu Y, Daniluk J, et al. Activation of nuclear factor-κB in acinar cells increases the severity of pancreatitis in mice. Gastroenterology. 2013;144(1):202–10.
Sah RP, Dudeja V, Dawra RK, et al. Cerulein-induced chronic pancreatitis does not require intra-acinar activation of trypsinogen in mice. Gastroenterology. 2013;144(5):1076–85.e2.
Aleksic T, Baumann B, Wagner M, et al. Cellular immune reaction in the pancreas is induced by constitutively active IκB kinase-2. Gut. 2007;56(2):227–36.
Baumann B, Wagner M, Aleksic T, et al. Constitutive IKK2 activation in acinar cells is sufficient to induce pancreatitis in vivo. J Clin Investig. 2007;117(6):1502–13.
Yan MX, Ren HB, Kou Y, et al. Involvement of nuclear factor kappa B in high-fat diet-related pancreatic fibrosis in rats. Gut Liver. 2012;6(3):381–7.
Altavilla D, Famulari C, Passaniti M, et al. Attenuated cerulein-induced pancreatitis in nuclear factor-kappaB-deficient mice. Lab Investig. 2003;83(12):1723–32.
Steinle AU, Weidenbach H, Wagner M, et al. NF-κB/Rel activation in cerulein pancreatitis. Gastroenterology. 1999;116(2):420–30.
Kaufman RJ. Stress signaling from the lumen of the endoplasmic reticulum: coordination of gene transcriptional and translational controls. Genes Dev. 1999;13(10):1211–33.
Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8(7):519–29.
Schröder M, Kaufman RJ. The mammalian unfolded protein response. Annu Rev Biochem. 2005;74:739–89.
Sah RP, Garg SK, Dixit AK, et al. Endoplasmic reticulum stress is chronically activated in chronic pancreatitis. J Biol Chem. 2014;289(40):27551–61.
Schroder K, Tschopp J. The inflammasomes. Cell. 2010;140(6):821–32.
Heyninck K, Lahtela-Kakkonen M, Van der Veken P, et al. Withaferin A inhibits NF-kappaB activation by targeting cysteine 179 in IKKβ. Biochem Pharmacol. 2014;91(4):501–9.
Lara LF, Levy MJ. Idiopathic recurrent acute pancreatitis. MedGenMed. 2004;6(4):10.
Neuschwander-Tetri BA, Burton FR, Presti ME, et al. Repetitive self-limited acute pancreatitis induces pancreatic fibrogenesis in the mouse. Dig Dis Sci. 2000;45(4):665–74.
Mayer-Scholl A, Averhoff P, Zychlinsky A. How do neutrophils and pathogens interact? Curr Opin Microbiol. 2004;7(1):62–6.
Kim I, Xu W, Reed JC. Cell death and endoplasmic reticulum stress: disease relevance and therapeutic opportunities. Nat Rev Drug Discov. 2008;7(12):1013–30.
Walter P, Ron D. The unfolded protein response: from stress pathway to homeostatic regulation. Science. 2011;334(6059):1081–6.
Latz E, Xiao TS, Stutz A. Activation and regulation of the inflammasomes. Nat Rev Immunol. 2013;13(6):397–411.
Pandol SJ, Saluja AK, Imrie CW, et al. Acute pancreatitis: bench to the bedside. Gastroenterology. 2007;132(3):1127–51.
Grady T, Liang P, Ernst SA, et al. Chemokine gene expression in rat pancreatic acinar cells is an early event associated with acute pancreatitis. Gastroenterology. 1997;113(6):1966–75.
Gukovskaya AS, Gukovsky I, Zaninovic V, et al. Pancreatic acinar cells produce, release, and respond to tumor necrosis factor-alpha. Role in regulating cell death and pancreatitis. J Clin Investig. 1997;100(7):1853–62.
Muniraj T, Aslanian HR, Farrell J, et al. Chronic pancreatitis, a comprehensive review and update. Part I: epidemiology, etiology, risk factors, genetics, pathophysiology, and clinical features. Dis Mon. 2014;60(12):530–50.
Howes N, Neoptolemos JP. Risk of pancreatic ductal adenocarcinoma in chronic pancreatitis. Gut. 2002;51(6):765–6.
Malka D, Hammel P, Maire F, et al. Risk of pancreatic adenocarcinoma in chronic pancreatitis. Gut. 2002;51(6):849–52.
Saluja AK, Bhagat L, Lee HS, et al. Secretagogue-induced digestive enzyme activation and cell injury in rat pancreatic acini. Am J Physiol. 1999;276(4 Pt 1):G835–42.
Booth DM, Murphy JA, Mukherjee R, et al. Reactive oxygen species induced by bile acid induce apoptosis and protect against necrosis in pancreatic acinar cells. Gastroenterology. 2011;140(7):2116–25.
Chen X, Ji B, Han B, et al. NF-kappaB activation in pancreas induces pancreatic and systemic inflammatory response. Gastroenterology. 2002;122(2):448–57.
Mareninova OA, Hermann K, French SW, et al. Impaired autophagic flux mediates acinar cell vacuole formation and trypsinogen activation in rodent models of acute pancreatitis. J Clin Investig. 2009;119(11):3340–55.
Behrendorff N, Floetenmeyer M, Schwiening C, et al. Protons released during pancreatic acinar cell secretion acidify the lumen and contribute to pancreatitis in mice. Gastroenterology. 2010;139(5):1711–20, 20 e1–5.
Sah RP, Dawra RK, Saluja AK. New insights into the pathogenesis of pancreatitis. Curr Opin Gastroenterol. 2013;29(5):523–30.
Lerch MM, Gorelick FS. Models of acute and chronic pancreatitis. Gastroenterology. 2013;144(6):1180–93.
Algul H, Treiber M, Lesina M, et al. Pancreas-specific RelA/p65 truncation increases susceptibility of acini to inflammation-associated cell death following cerulein pancreatitis. J Clin Investig. 2007;117(6):1490–501.
Neuhofer P, Liang S, Einwachter H, et al. Deletion of IκBα activates RelA to reduce acute pancreatitis in mice through up-regulation of Spi2A. Gastroenterology. 2013;144(1):192–201.
Gukovsky I, Gukovskaya A. Nuclear factor-κB in pancreatitis: jack-of-all-trades, but which one is more important? Gastroenterology. 2013;144(1):26–9.
He S, Wang L, Miao L, et al. Receptor interacting protein kinase-3 determines cellular necrotic response to TNF-α. Cell. 2009;137(6):1100–11.
Ozaki E, Campbell M, Doyle SL. Targeting the NLRP3 inflammasome in chronic inflammatory diseases: current perspectives. J Inflamm Res. 2015;8:15–27.
Hoque R, Sohail M, Malik A, et al. TLR9 and the NLRP3 inflammasome link acinar cell death with inflammation in acute pancreatitis. Gastroenterology. 2011;141(1):358–69.
Lin Y, Lin L-J, Jin Y, et al. Correlation between serum levels of high mobility group box-1 protein and pancreatitis: a meta-analysis. BioMed Res Int. 2015;2015:10.
Kaileh M, Vanden Berghe W, Heyerick A, et al. Withaferin a strongly elicits IκB kinase β hyperphosphorylation concomitant with potent inhibition of its kinase activity. J Biol Chem. 2007;282(7):4253–64.
Thaiparambil JT, Bender L, Ganesh T, et al. Withaferin A inhibits breast cancer invasion and metastasis at sub-cytotoxic doses by inducing vimentin disassembly and serine 56 phosphorylation. Int J Cancer. 2011;129(11):2744–55.
Mohan R, Hammers HJ, Bargagna-Mohan P, et al. Withaferin A is a potent inhibitor of angiogenesis. Angiogenesis. 2004;7(2):115–22.
Vanden Berghe W, Sabbe L, Kaileh M, et al. Molecular insight in the multifunctional activities of withaferin A. Biochem Pharmacol. 2012;84(10):1282–91.
Hoesel B, Schmid JA. The complexity of NF-κB signaling in inflammation and cancer. Mol Cancer. 2013;12:86.
Sutherland DE, Radosevich DM, Bellin MD, et al. Total pancreatectomy and islet autotransplantation for chronic pancreatitis. J Am Coll Surg. 2012;214(4):409–24; discussion 424-6.
Bellin MD, Gelrud A, Arreaza-Rubin G, et al. Total pancreatectomy with islet autotransplantation: summary of an NIDDK workshop. Ann Surg. 2015;261(1):21–9.
SoRelle JA, Itoh T, Peng H, et al. Withaferin A inhibits pro-inflammatory cytokine-induced damage to islets in culture and following transplantation. Diabetologia. 2013;56(4):814–24.
Acknowledgments
The authors gratefully acknowledge Morihito Takita, Prathab Balaji Saravanan, and Faisal Kunnathodi. Technical assistance by Nofit Borenstein-Auerbach, Omar Khan, Ana Rahman, and Yoshiko Tamura is also acknowledged. The manuscript editing service provided by Cynthia Orticio is highly appreciated.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kanak, M.A., Shahbazov, R., Yoshimatsu, G. et al. A small molecule inhibitor of NFκB blocks ER stress and the NLRP3 inflammasome and prevents progression of pancreatitis. J Gastroenterol 52, 352–365 (2017). https://doi.org/10.1007/s00535-016-1238-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00535-016-1238-5