Abstract
Pterostilbene (PTB) has been suggested to protect against myocardial ischemia/reperfusion (MI/R) injury. Gas6/Axl signaling has been suggested to play an important role in cell survival. However, the interaction between PTB and Gas6/Axl signaling in MI/R remains unclear. This study aims to evaluate the role of Gas6/Axl signaling in the protective effects of PTB against MI/R injury. In experiment 1, the rats were subjected to 30 min of ischemia, followed by 3, 6, and 12 h of reperfusion, respectively. In experiment 2, the rats were administered intraperitoneally with PTB or vehicle and subjected to MI/R injury. The results suggested that the expression of Gas6 and Axl decreased significantly after MI/R injury. PTB treatment conferred a cardioprotective effect with an improved post-ischemic cardiac function, a reduced myocardial infarct size, and decreased lactate dehydrogenase and creatine kinase-MB in the serum, a decreased oxidative stress and inflammation, and a reduced number of apoptotic cardiomyocytes. Moreover, PTB treatment up-regulated the expression of Gas6, Axl, and Bcl-2 and down-regulated Bax expression. Our findings suggest that PTB treatment exerts cardioprotection against MI/R injury via attenuating inflammatory response, oxidative stress, and apoptosis and up-regulating the expression of Gas6 and Axl. The application of PTB may be a new strategy for the treatment of MI/R injury.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Myocardial ischemia/reperfusion (MI/R) injury is the primary cause of cardiac failure and has a morbidity and mortality worldwide [1, 2]. Accumulating evidence suggests that the oxidative stress induced by the overproduction of reactive oxygen species (ROS) during the acute reperfusion phase plays a pivotal role in the pathogenesis of MI/R injury [3, 4]. In addition, ROS induces inflammation, leading to severe myocardial injury [3, 5]. Therefore, diminishing ROS production is a novel target for attenuating MI/R injury.
Pterostilbene (PTB, Fig. 1), a natural dimethylated analog of resveratrol from blueberries, has been reported to exert various pharmacological effects, such as anti-cancer, anti-inflammation, and anti-oxidant [6]. Due to methoxyl substitution-induced hyperlipophilicity, PTB may present higher bioactivity than resveratrol [7]. In animal studies, PTB was suggested to have 80% bioavailability compared to 20% for resveratrol, making it potentially advantageous as a therapeutic agent [8]. It has been indicated that PTB attenuates the inflammatory reaction induced by ischemia/reperfusion [9]. In another study, PTB was shown to protect against atherosclerosis via regulation of vascular smooth muscle cells and vascular endothelial cells [10]. PTB inhibits rat aortic vascular smooth muscle cell proliferation by inhibiting Akt-dependent pathway [10]. However, whether PTB attenuates the oxidative stress induced by MI/R injury remains unclear.
Growth arrest specific gene 6 protein (Gas6) is a vitamin K-dependent protein and binds to receptor tyrosine kinases including Axl, Mer, and Tyro3, known as TAM receptors [11]. Gas6 has the highest affinity for Axl among its three receptors, and Gas6/Axl signaling pathway has been suggested to play a critical role in various diseases such as breast cancer [12], hypertension [13], and chronic liver disease [14]. Lijnen et al. showed that oral administration of R428, an Axl antagonist, contributes to adipocyte hypotrophy, increased macrophage infiltration, and apoptosis [15]. Llacuna et al. suggest that Gas6 is hepatoprotective against ischemia/reperfusion injury [16]. However, the role of Gas6/Axl signaling in myocardial ischemia remains unclear.
Therefore, this study was aimed to investigate the cardioprotective effect of PTB against MI/R injury and the possible molecular mechanisms involved in the protective effects of PTB was also investigated.
MATERIALS AND METHODS
Animals
Male Sprague–Dawley (SD) rats (200–250 g) were obtained from the Experimental Animal Center of the Central South University (Changsha, China). All the rats had free access to food and water. All of the procedures were approved by the Committee of Experimental Animals of the Central South University and conformed to the Guide for the Care and Use of Laboratory Animals published by the United States National Institutes of Health (NIH Publication No. 85-23, revised 1996).
Materials
PTB, triphenyltetrazolium chloride (TTC), and 4,6-diamino-2-phenylindole (DAPI) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Lactate dehydrogenase (LDH) assay kit, creatine kinase-MB (CK-MB), malondialdehyde (MDA), superoxide dismutase (SOD), superoxide generation, TNF-α (detection range, 6.5–2000 pg/mL), and IL-1β (detection range, 7.3–2000 pg/mL) assay kits were purchased from Jiancheng Bioengineering Institute (Nanjing, China). Terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) kits were purchased from Roche (Mannheim, Germany). The anti-Gas6, anti-Bcl-2, anti-Bax, and anti-β-actin were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). The gp91phox antibody, IL-1β antibody, and TNF-α antibody were obtained from Abcam (Cambridge, UK). The Axl antibody was purchased from Cell Signaling Technology (Beverly, MA, USA).
Experimental Protocol and Drug Administration
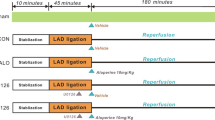
Our experiments included two steps. In step 1, SD rats were randomly divided into four experimental groups: (1) Sham group, the rats received sham operation; (2) MI/R 3 h group, the rats were subjected to 30 min of ischemia, followed by 3 h reperfusion; (3) MI/R 6 h group, the rats were subjected to 30 min of ischemia, followed by 6 h reperfusion; (4) MI/R 12 h group, the rats were subjected to 30 min of ischemia, followed by 12 h reperfusion. In step 2, SD rats were randomly divided into four experimental groups: (1) Sham group; (2) MI/R + vehicle group, the rats were subjected to 30 min of ischemia, followed by 12 h reperfusion, and the rats received the same volume of vehicle as the rats in group (3) at the same time point; (3) MI/R + PTB group, the rats received PTB before MI/R. PTB was first dissolved in 0.05% dimethyl sulfoxide (DMSO) and then diluted with normal saline. PTB was administered intraperitoneally at a dose of 10 mg/kg body weight once a day for five consecutive days.
Myocardial Ischemia/Reperfusion Model
Myocardial ischemia/reperfusion model was carried out as described previously [17]. Briefly, rats were anesthetized by 3% pentobarbital sodium. Then, a left thoracic incision was made, and myocardial ischemia was induced by exteriorizing the heart using a 6-0 silk suture. A slipknot around the left anterior descending coronary artery (LAD) was made. After 30 min of ischemia, the slipknot was released and the myocardium underwent reperfusion. Sham group underwent the same procedures without LAD occlusion.
Evaluation of Cardiac Function by Invasive Hemodynamic Assessment
A microcatheter was inserted into the left ventricle through the right carotid artery to measure the left ventricular pressure (LVP). The artery pressure was measured by right femoral artery intubation. ECG and LVP were simultaneously recorded on a polygraph (RM-6200C; Chengdu, Instrument, Chengdu, China). Left ventricular systolic pressure (LVSP), left ventricular end diastolic pressure (LVEDP), and the instantaneous first derivation of LVP (± dp/dtmax) were recorded by computer algorithms.
Myocardial Infarct Size Measurement
After reperfusion, the slipknot around the LAD was retied, and 1 mL Evans blue (1%) dye was injected into the aorta. The heart was then quickly removed and frozen at −80 °C. Then, the heart was sliced transversally into 1-mm-thick sections and incubated at 37 °C for 30 min in 2% TTC solution. Digital pictures were taken. TTC-unstained pale area (infarct zone), TTC-stained red area (ischemic but viable myocardium), and Evans blue-unstained regions (area-at-risk, AAR) were analyzed by using an image analysis system (Image Pro Plus 6.0; Media Cybernetics). Myocardial infarct size was calculated as INF/AAR × 100%.
Evaluation of Serum CK-MB and LDH
After the 3-h reperfusion, blood was collected from the carotid artery. Serum CK-MB and LDH levels were measured using the commercial assay kits. The activities were expressed as units/liter.
Myocardial Apoptosis Measurement
Myocardial apoptosis was evaluated by using terminal deoxynucleotidyl nick-end labeling (TUNEL) and caspase-3 activity assay. TUNEL staining was performed by using an in situ cell death detection kit according to the instructions. Briefly, the slides were incubated with TUNEL reaction mixture and then with DAPI to detect the nuclei. The apoptotic index was calculated as a percentage of the number of TUNEL-positive apoptotic cells over the total number of nucleated cells. Myocardial caspase-3 activity was determined by using a caspase colorimetric assay kit (Chemicon, Temecula, CA, USA) according to the manufacturer’s instructions.
Quantification of Superoxide Production
Superoxide production was measured by lucigenin-enhanced chemiluminescence as described previously [18]. Superoxide production was expressed as relative light units (RLU) per second per milligram heart weight (RLU/mg/s).
Assessment of Malondialdehyde Content and Superoxide Dismutase Activity
The MDA content and activity of SOD in heart homogenates were determined spectrophotometrically according to the manufacturer’s instructions.
Assessment of Inflammatory Cytokines
Inflammatory cytokines in the serum and myocardial tissue were assessed by using commercially available TNF-α and IL-1β ELISA kits, under the guidance of the manufacturer’s instructions. The data was analyzed using a microplate reader (Multiskan Spectrum, Thermo Scientific, USA).
Western Blot
The myocardium samples were lysed in lysis buffer. After quantitation of protein concentration with BCA protein assay kit, 30 μg of protein was separated by SDS-PAGE and then transferred to a polyvinylidene difluoride membrane (Millipore, USA). The membranes were blocked with 5% skim milk for 2 h at 37 °C and then incubated with primary antibodies against Gas6, Axl, gp91phox, Bcl-2, Bax, and β-actin overnight at 4 °C. After washing with TBST, the membranes were incubated with secondary antibody for 2 h at 37 °C and then washed using TBST. The protein bands were detected using a chemiluminescent system, and the bands were scanned and quantified using an image analyzer Quantity One System (Bio-Rad, Richmond, CA, USA).
Statistical Analysis
All values are presented as mean ± S.E.M. Statistical tests were performed using GraphPad Prism software version 5.0 (GraphPad Software, Inc., San Diego, CA, USA). Differences among groups were compared with one-way ANOVA followed by Bonferroni corrected t test where appropriate. P < 0.05 was taken as statistically significant.
RESULTS
Gas6/Axl Signaling Was Inhibited During MI/R Injury
First, we detected the expression of Gas6 and Axl during different reperfusion time points. As shown in Fig. 2, the expression of Gas6 and Axl decreased gradually and dramatically during reperfusion, suggesting that the Gas6/Axl signaling was impaired after MI/R injury. Bcl-2 expression decreased and Bax expression increased, indicating myocardial apoptosis was induced during MI/R.
The changes of expression levels of Gas6, Axl, Bcl-2, and Bax during MI/R. a Representative images of Western blots. b Gas6 expression. c Axl expression. d Bcl-2 expression. e Bax expression. The results are expressed as the mean ± S.E.M. (n = 6 for each group). *P < 0.05 in comparison to the sham group.
PTB Treatment Improved Cardiac Function After MI/R
As shown in Fig. 3, MI/R impaired cardiac function greatly, as evidenced by the decrease in LVSP and LV ± dP/dtmax and the increase in LVEDP. PTB treatment dramatically induced an increase in LVSP and LV ± dP/dtmax and a decrease in LVEDP in comparison with the MI/R + vehicle group (P < 0.05), suggesting that PTB treatment improved cardiac function following MI/R injury.
PTB treatment improved cardiac function in MI/R rats. The results are expressed as the mean ± S.E.M. (n = 6 for each group). * P < 0.05 in comparison to the sham group, # P < 0.05 in comparison to the MI/R + vehicle group. PTB, pterostilbene; LVSP, left ventricular systolic pressure; LVEDP, left ventricular end diastolic pressure; LV ± dP/dt max , the instantaneous first derivation of left ventricle pressure.
PTB Treatment Alleviated MI/R Injury and Myocardial Apoptosis
As shown in Fig. 4a, PTB treatment resulted in a significantly decreased myocardial infarct size (versus the MI/R + vehicle group, P < 0.05). Then, the serum levels of CK-MB and LDH were measured. As shown in Fig. 4b, c, MI/R injury triggered a dramatic increase in CK-MB and LDH levels in the serum. PTB administration significantly decreased CK-MB and LDH levels in comparison with the MI/R + vehicle group (P < 0.05). In addition, as apoptosis is a major form of cell death after MI/R, we investigated whether PTB treatment could decrease apoptosis induced by MI/R injury. Compared with the Sham group, the TUNEL-positive cells (Fig. 4d, e) and myocardial caspase-3 activity (Fig. 4f) significantly increased in the MI/R + vehicle group (P < 0.05). PTB treatment significantly reduced myocardial apoptosis in comparison with the MI/R + vehicle group (P < 0.05). Our results suggested that PTB treatment attenuated myocardial injury and apoptosis induced by MI/R injury.
PTB treatment alleviated MI/R injury and myocardial apoptosis. a Myocardial infarction. b Serum level of CK-MB. c Serum level of LDH. d Representative images of apoptotic cardiomyocytes by TUNEL staining. The green fluorescence shows the TUNEL-positive nuclei; the blue fluorescence shows the nuclei of all cardiomyocytes, original magnification ×400. e Apoptotic index. f Caspase-3 activity. The results are expressed as the mean ± S.E.M. (n = 6 for each group). * P < 0.05 in comparison to the sham group, # P < 0.05 in comparison to the MI/R + vehicle group. PTB pterostilbene, CK-MB creatine kinase-MB, LDH lactate dehydrogenase.
PTB Treatment Ameliorated Oxidative Stress After MI/R
Compared with the Sham group, myocardial superoxide generation and MDA content were significantly increased in MI/R + vehicle group (P < 0.05), and PTB administration inhibited superoxide accumulation and decreased MDA content (Fig. 5a, b). In contrast, PTB administration increased SOD activity in MI/R rats in comparison with the MI/R + vehicle group (P < 0.05, Fig. 5c). It is suggested that gp91phox is a critical part of NADPH oxidase, and it is the main source producing superoxide. PTB administration markedly reduced gp91phox expression in MI/R + PTB group (versus MI/R + vehicle group, P < 0.05, Fig. 5d).
PTB treatment ameliorated oxidative stress in MI/R rats. a Cardiac superoxide generation. b Myocardial MDA contents. c Myocardial SOD activity. d Western blot analysis of gp91phox expression. The results are expressed as the mean ± S.E.M. (n = 6 for each group). * P < 0.05 in comparison to the sham group, # P < 0.05 in comparison to the MI/R + vehicle group. PTB pterostilbene, MDA malondialdehyde, SOD superoxide dismutase.
PTB Treatment Reduced Levels of Inflammatory Cytokines After MI/R
As shown in the Fig. 6, MI/R induced dramatic increases in the levels of TNF-α and IL-1β in the serum and myocardial tissue. However, the levels of the inflammatory cytokines TNF-α and IL-1β in the serum and myocardial tissue significantly decreased in the MI/R + PTB group than those in the MI/R + vehicle group (P < 0.05).
PTB treatment reduced levels of inflammatory cytokines in MI/R rats. a Serum TNF-α. b Myocardial TNF-α. c Serum IL-1β. d Myocardial IL-1β. e Representative image of pro-IL-1β and cleaved-IL-1β detected using Western blot. f Cleaved-IL-1β/β-actin. The results are expressed as the mean ± S.E.M. (n = 6 for each group). * P < 0.05 in comparison to the sham group, # P < 0.05 in comparison to the MI/R + vehicle group. PTB pterostilbene.
Effect of PTB on Gas6/Axl Signaling Pathway Following MI/R
To further investigate the mechanism of PTB’s cardioprotective effects, we detected the Gas6/Axl signaling pathway and apoptotic pathway. As shown in Fig. 7, PTB dramatically up-regulated Gas6 and Axl expression after MI/R (versus MI/R + vehicle group, P < 0.05). Moreover, PTB also markedly decreased Bax expression increased Bcl-2 expression (versus MI/R + vehicle group, P < 0.05). Our results suggest that Gas6/Axl signaling activation was involved in the cardioprotection conferred by PTB.
PTB activated the Gas6/Axl signaling and inhibited myocardial apoptosis. a Representative images of Western blots. b Gas6 expression. c Axl expression. d Bcl-2 expression. e Bax expression. The results are expressed as the mean ± S.E.M. (n = 6 for each group). * P < 0.05 in comparison to the sham group, # P < 0.05 in comparison to the MI/R + vehicle group. PTB pterostilbene.
DISCUSSION
In the present study, our data show that PTB markedly alleviates MI/R injury. The protective effects were demonstrated by improved cardiac function, a decreased myocardial infarct size, a decreased CK-MB and LDH release in the serum, a reduced apoptosis, and decreasing in the oxidative stress and inflammation. Importantly, the PTB treatment can increase the expression of Gas6 and Axl, suggesting that the Gas6/Axl signaling might be involved in the cardioprotective effect of PTB.
PTB exhibits various pharmacological effects and potentially confer cardioprotection [19, 20]. A recent study suggests that PTB attenuates hypoxia-reoxygenation injury in cardiomyocytes by Sirt1 activation [19]. Previous studies have also indicated that PTB exerts inhibitory effects against neutrophil activation and inflammatory mediators production in different models [21, 22]. In the present study, PTB treatment dramatically improved cardiac function, reduced myocardial apoptosis, inflammation, and oxidative stress triggered by MI/R. Importantly, the Gas6/Axl signaling pathway is involved in the protective effects of PTB.
MI/R injury is associated with enhanced inflammation and oxidative stress. The underlying mechanisms include (1) cell injury caused by the release of oxygen free radicals and cytotoxic factors, (2) the released inflammatory cytokines that lead to vascular endothelial cell damage and increased vascular permeability, and (3) activated inflammatory cells that enhance the inflammatory response [23]. Therefore, inhibition of oxidative stress and inflammation response during MI/R injury is of great significance for developing cardioprotective strategies against MI/R injury [24]. In the present study, we found that PTB treatment markedly reduced superoxide generation, MDA content, and gp91phox expression, and increased SOD activity, suggesting that PTB inhibited oxidative stress induced by MI/R injury. Moreover, we found PTB treatment significantly reduced myocardial TNF-α and IL-1β production, indicating that PTB reduced cardiac inflammation. Taken together, these experimental results showed that PTB possibly attenuated inflammation response and reducing oxidative stress during myocardial reperfusion.
Oxidative stress contributes to cardiac injury after MI/R [25]. In the myocardium, the NADPH oxidase is an important source of superoxide, which includes membrane integrated subunits (p22phox and gp91phox), cytosolic subunits (p40phox, p47phox, and p67phox), and the G-protein (Rac 1) [26]. Of these subunits, gp91phox is one of the most important. The superoxide can bring injury to the proteins, lipids, and DNA, leading to cell damage [27]. Additionally, superoxide can react with nitric oxide, leading to the production of peroxynitrite, which can contribute to nitrative stress [28]. MDA is a product and indicator of lipid peroxidation. And SOD is an enzyme-diminishing superoxide. Our results suggest that PTB treatment decreases superoxide generation, MDA content, and gp91phox expression, and increases SOD activity, indicating that PTB protects against MI/R injury via alleviating oxidative stress.
Gas6/Axl signaling pathway has been reported to confer various effects in different diseases. Yan et al. suggest that testosterone delays vascular smooth muscle cell senescence and inhibits collagen synthesis via the Gas6/Axl signaling pathway [29]. Recombinant mouse Gas6 has been reported to confer anti-inflammatory effects in sepsis-induced kidney injury, sepsis-induced lung injury, and renal I/R injury [30–32]. Moreover, it is indicated that Gas6 protects against liver I/R injury and Gas6−/− mice were highly sensitive to hepatic I/R injury [16]. It has been demonstrated that Axl can be activated by Gas6, and the downstream signaling of Axl may include the suppressor of cytokine signaling 1, 3 (SOCS1, SOCS3) [33, 34]. SOCSs have been suggested to suppress cytokines release, such as IL-6, IL-4, and IL-1 [35–37]. SOCS-1 selectively inhibits LPS-induced IL-6 production [35] and SOCS1-deficient mice develop severe inflammatory disease [36]. Additionally, administration of adenovirus overexpressing Gas6 attenuates arthritis inflammation [38]. Gruber et al. also indicated that intraventricular delivery of Gas6 inhibits inflammation in experimental autoimmune encephalomyelitis (EAE) [39]. In the present study, our results suggest that PTB up-regulated Gas6 and Axl expression, possibly leading to the inhibition of inflammation after myocardial ischemia.
In summary, PTB exhibits cardioprotection via alleviating inflammatory reaction, oxidative stress, and apoptosis. Additionally, the molecular mechanisms are associated with the up-regulation of levels of Gas6 and Axl. The study suggests the potential application of PTB in the treatment of ischemic heart disease and promotes the development of a novel therapy against MI/R injury.
References
Hausenloy, D.J., and D.M. Yellon. 2013. Myocardial ischemia-reperfusion injury: a neglected therapeutic target. Journal of Clinical Investigation 123(1): 92–100. doi:10.1172/JCI62874.
Bice, J.S., B.R. Jones, G.R. Chamberlain, and G.F. Baxter. 2016. Nitric oxide treatments as adjuncts to reperfusion in acute myocardial infarction: a systematic review of experimental and clinical studies. Basic Research in Cardiology 111(2): 23. doi:10.1007/s00395-016-0540-y.
Petrosillo, G., F.M. Ruggiero, N. Di Venosa, and G. Paradies. 2003. Decreased complex III activity in mitochondria isolated from rat heart subjected to ischemia and reperfusion: role of reactive oxygen species and cardiolipin. FASEB Journal 17(6): 714–716. doi:10.1096/fj.02-0729fje.
Csonka, C., M. Sarkozy, M. Pipicz, L. Dux, and T. Csont. 2016. Modulation of hypercholesterolemia-induced oxidative/nitrative stress in the heart. Oxidative Medicine and Cellular Longevity 2016: 3863726. doi:10.1155/2016/3863726.
Zweier, J.L., and M.A. Talukder. 2006. The role of oxidants and free radicals in reperfusion injury. Cardiovascular Research 70(2): 181–190. doi:10.1016/j.cardiores.2006.02.025.
Remsberg, C.M., J.A. Yanez, Y. Ohgami, K.R. Vega-Villa, A.M. Rimando, and N.M. Davies. 2008. Pharmacometrics of pterostilbene: preclinical pharmacokinetics and metabolism, anticancer, antiinflammatory, antioxidant and analgesic activity. Phytotherapy Research 22(2): 169–179. doi:10.1002/ptr.2277.
Cichocki, M., J. Paluszczak, H. Szaefer, A. Piechowiak, A.M. Rimando, and W. Baer-Dubowska. 2008. Pterostilbene is equally potent as resveratrol in inhibiting 12-O-tetradecanoylphorbol-13-acetate activated NFkappaB, AP-1, COX-2, and iNOS in mouse epidermis. Molecular Nutrition & Food Research 52(Suppl 1): S62–S70. doi:10.1002/mnfr.200700466.
Kapetanovic, I.M., M. Muzzio, Z. Huang, T.N. Thompson, and D.L. McCormick. 2011. Pharmacokinetics, oral bioavailability, and metabolic profile of resveratrol and its dimethylether analog, pterostilbene, in rats. Cancer Chemotheraphy and Pharmacology 68(3): 593–601. doi:10.1007/s00280-010-1525-4.
Lv, M., K. Liu, S. Fu, Z. Li, and X. Yu. 2015. Pterostilbene attenuates the inflammatory reaction induced by ischemia/reperfusion in rat heart. Molecular Medicine Reports 11(1): 724–728. doi:10.3892/mmr.2014.2719.
Park, E.S., Y. Lim, J.T. Hong, H.S. Yoo, C.K. Lee, M.Y. Pyo, and Y.P. Yun. 2010. Pterostilbene, a natural dimethylated analog of resveratrol, inhibits rat aortic vascular smooth muscle cell proliferation by blocking Akt-dependent pathway. Vascular Pharmacology 53(1–2): 61–67. doi:10.1016/j.vph.2010.04.001.
Schneider, C., R.M. King, and L. Philipson. 1988. Genes specifically expressed at growth arrest of mammalian cells. Cell 54(6): 787–793. doi:10.1016/S0092-8674(88)91065-3.
Wang, C., H. Jin, N. Wang, S. Fan, Y. Wang, Y. Zhang, L. Wei, et al. 2016. Gas6/Axl axis contributes to chemoresistance and metastasis in breast cancer through Akt/GSK-3beta/beta-catenin signaling. Theranostics 6(8): 1205–1219. doi:10.7150/thno.15083.
Batchu, S.N., A. Hughson, J. Gerloff, D.J. Fowell, and V.A. Korshunov. 2013. Role of Axl in early kidney inflammation and progression of salt-dependent hypertension. Hypertension 62(2): 302–309. doi:10.1161/HYPERTENSIONAHA.113.01382.
Barcena, C., M. Stefanovic, A. Tutusaus, L. Joannas, A. Menendez, C. Garcia-Ruiz, P. Sancho-Bru, et al. 2015. Gas6/Axl pathway is activated in chronic liver disease and its targeting reduces fibrosis via hepatic stellate cell inactivation. Journal of Hepatology 63(3): 670–678. doi:10.1016/j.jhep.2015.04.013.
Lijnen, H.R., V. Christiaens, and L. Scroyen. 2011. Growth arrest-specific protein 6 receptor antagonism impairs adipocyte differentiation and adipose tissue development in mice. Journal of Pharmacol and Experimental Therapeutics 337(2): 457–464. doi:10.1124/jpet.110.178046.
Llacuna, L., C. Barcena, L. Bellido-Martin, L. Fernandez, M. Stefanovic, M. Mari, C. Garcia-Ruiz, J.C. Fernandez-Checa, P. Garcia de Frutos, and A. Morales. 2010. Growth arrest-specific protein 6 is hepatoprotective against murine ischemia/reperfusion injury. Hepatology 52(4): 1371–1379. doi:10.1002/hep.23833.
Ji, L., F. Fu, L. Zhang, W. Liu, X. Cai, L. Zhang, Q. Zheng, H. Zhang, and F. Gao. 2010. Insulin attenuates myocardial ischemia/reperfusion injury via reducing oxidative/nitrative stress. American Journal of Physiology, Endocrinology and Metabolism 298(4): E871–E880. doi:10.1152/ajpendo.00623.2009.
Su, H., L. Ji, W. Xing, W. Zhang, H. Zhou, X. Qian, X. Wang, F. Gao, X. Sun, and H. Zhang. 2013. Acute hyperglycaemia enhances oxidative stress and aggravates myocardial ischaemia/reperfusion injury: role of thioredoxin-interacting protein. Journal of Cellular and Molecular Medicine 17(1): 181–191. doi:10.1111/j.1582-4934.2012.01661.x.
Guo, Y., L. Zhang, F. Li, C.P. Hu, and Z. Zhang. 2016. Restoration of sirt1 function by pterostilbene attenuates hypoxia-reoxygenation injury in cardiomyocytes. European Journal of Pharmacology 776: 26–33. doi:10.1016/j.ejphar.2016.02.052.
Li, J., Ruzhi Deng, X. Hua, L. Zhang, F. Lu, T.G. Coursey, S.C. Pflugfelder, and D.Q. Li. 2016. Blueberry component pterostilbene protects corneal epithelial cells from inflammation via anti-oxidative pathway. Scientific Reports 6: 19408. doi:10.1038/srep19408.
Perecko, T., K. Drabikova, A. Lojek, M. Ciz, S. Ponist, K. Bauerova, R. Nosal, J. Harmatha, and V. Jancinova. 2013. The effects of pterostilbene on neutrophil activity in experimental model of arthritis. BioMed Research International 2013: 106041. doi:10.1155/2013/106041.
Qureshi, A.A., X.Q. Guan, J.C. Reis, C.J. Papasian, S. Jabre, D.C. Morrison, and N. Qureshi. 2012. Inhibition of nitric oxide and inflammatory cytokines in LPS-stimulated murine macrophages by resveratrol, a potent proteasome inhibitor. Lipids in Health and Disease 11: 76. doi:10.1186/1476-511X-11-76.
Lin, J., H. Wang, J. Li, Q. Wang, S. Zhang, N. Feng, R. Fan, and J. Pei. 2013. kappa-Opioid receptor stimulation modulates TLR4/NF-kappaB signaling in the rat heart subjected to ischemia-reperfusion. Cytokine 61(3): 842–848. doi:10.1016/j.cyto.2013.01.002.
Tian, Y., H. Li, P. Liu, J.M. Xu, M.G. Irwin, Z. Xia, and G. Tian. 2015. Captopril pretreatment produces an additive cardioprotection to isoflurane preconditioning in attenuating myocardial ischemia reperfusion injury in rabbits and in humans. Mediators of Inflammation 2015: 819232. doi:10.1155/2015/819232.
Neri, M., I. Riezzo, C. Pomara, S. Schiavone, and E. Turillazzi. 2016. Oxidative-nitrosative stress and myocardial dysfunctions in sepsis: evidence from the literature and postmortem observations. Mediators of Inflammation 2016: 3423450. doi:10.1155/2016/3423450.
Bedard, K., and K.H. Krause. 2007. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiological Reviews 87(1): 245–313. doi:10.1152/physrev.00044.2005.
Seko, Y., T. Fujimura, T. Yao, H. Taka, R. Mineki, K. Okumura, and K. Murayama. 2015. Secreted tyrosine sulfated-eIF5A mediates oxidative stress-induced apoptosis. Scientific Reports 5: 13737. doi:10.1038/srep13737.
Pei, H., Q. Yu, Q. Xue, Y. Guo, L. Sun, Z. Hong, H. Han, E. Gao, Y. Qu, and L. Tao. 2013. Notch1 cardioprotection in myocardial ischemia/reperfusion involves reduction of oxidative/nitrative stress. Basic Research in Cardiology 108(5): 373. doi:10.1007/s00395-013-0373-x.
Chen, Y.Q., J. Zhao, C.W. Jin, Y.H. Li, M.X. Tang, Z.H. Wang, W. Zhang, Y. Zhang, L. Li, and M. Zhong. 2016. Testosterone delays vascular smooth muscle cell senescence and inhibits collagen synthesis via the Gas6/Axl signaling pathway. Age (Dordrecht, Netherlands) 38(3): 60. doi:10.1007/s11357-016-9910-5.
Chen, L.W., W. Chen, Z.Q. Hu, J.L. Bian, L. Ying, G.L. Hong, Q.M. Qiu, G.J. Zhao, and Z.Q. Lu. 2016. Protective effects of growth arrest-specific protein 6 (Gas6) on sepsis-induced acute kidney injury. Inflammation 39(2): 575–582. doi:10.1007/s10753-015-0282-2.
Giangola, M.D., W.L. Yang, S.R. Rajayer, J. Nicastro, G.F. Coppa, and P. Wang. 2013. Growth arrest-specific protein 6 attenuates neutrophil migration and acute lung injury in sepsis. Shock 40(6): 485–491. doi:10.1097/SHK.0b013e3182a588c1.
Giangola, M.D., W.L. Yang, S.R. Rajayer, M. Kuncewitch, E. Molmenti, J. Nicastro, G.F. Coppa, and P. Wang. 2015. Growth arrest-specific protein 6 protects against renal ischemia-reperfusion injury. Journal of Surgical Research 199(2): 572–579. doi:10.1016/j.jss.2015.05.049.
Zagorska, A., P.G. Traves, E.D. Lew, I. Dransfield, and G. Lemke. 2014. Diversification of TAM receptor tyrosine kinase function. Nature Immunology 15(10): 920–928. doi:10.1038/ni.2986.
Rothlin, C.V., S. Ghosh, E.I. Zuniga, M.B. Oldstone, and G. Lemke. 2007. TAM receptors are pleiotropic inhibitors of the innate immune response. Cell 131(6): 1124–1136. doi:10.1016/j.cell.2007.10.034.
Kimura, A., T. Naka, T. Muta, O. Takeuchi, S. Akira, I. Kawase, and T. Kishimoto. 2005. Suppressor of cytokine signaling-1 selectively inhibits LPS-induced IL-6 production by regulating JAK-STAT. Proceedings of the National Academy of Sciences of the United States of America 102(47): 17089–17094. doi:10.1073/pnas.0508517102.
Fenner, J.E., R. Starr, A.L. Cornish, J.G. Zhang, D. Metcalf, R.D. Schreiber, K. Sheehan, D.J. Hilton, W.S. Alexander, and P.J. Hertzog. 2006. Suppressor of cytokine signaling 1 regulates the immune response to infection by a unique inhibition of type I interferon activity. Nature Immunology 7(1): 33–39. doi:10.1038/ni1287.
Yoshimura, A., T. Naka, and M. Kubo. 2007. SOCS proteins, cytokine signalling and immune regulation. Nature Reviews Immunology 7(6): 454–465. doi:10.1038/nri2093.
van den Brand, B.T., S. Abdollahi-Roodsaz, E.A. Vermeij, M.B. Bennink, O.J. Arntz, C.V. Rothlin, W.B. van den Berg, and F.A. van de Loo. 2013. Therapeutic efficacy of Tyro3, Axl, and Mer tyrosine kinase agonists in collagen-induced arthritis. Arthritis and Rheumatism 65(3): 671–680. doi:10.1002/art.37786.
Gruber, R.C., A.K. Ray, C.T. Johndrow, H. Guzik, D. Burek, P.G. de Frutos, and B. Shafit-Zagardo. 2014. Targeted GAS6 delivery to the CNS protects axons from damage during experimental autoimmune encephalomyelitis. The Journal of Neuroscience 34(49): 16320–16335. doi:10.1523/JNEUROSCI.2449-14.2014.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
None declared.
Rights and permissions
About this article
Cite this article
Wu, M., Lu, S., Zhong, J. et al. Protective Effects of Pterostilbene Against Myocardial Ischemia/Reperfusion Injury in Rats. Inflammation 40, 578–588 (2017). https://doi.org/10.1007/s10753-016-0504-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-016-0504-2