Abstract
Accumulating evidence shows that immune cells play an important role in carotid atherosclerotic plaque development. In this study, we assessed the association of 6 different natural killer T (NKT) cell subsets, based on CD57 and CD8 expression, with risk for development of carotid atherosclerotic plaque (CAP). Molecular expression by peripheral NKT cells was evaluated in 13 patients with high-risk CAP and control without carotid stenosis (n = 18). High-risk CAP patients, compared with healthy subjects, had less percentage of CD57+CD8− NKT cell subsets (8.64 ± 10.15 versus 19.62 ± 10.8 %; P = 0.01) and CD57+CD8int NKT cell subsets (4.32 ± 3.04 versus 11.87 ± 8.56 %; P = 0.002), with a corresponding increase in the CD57−CD8high NKT cell subsets (33.22 ± 11.87 versus 18.66 ± 13.68 %; P = 0.007). Intracellular cytokine staining showed that CD8+ NKT cell subset was the main cytokine-producing NKT cell. Cytokine production in plasma was measured with Bio-Plex assay. The expression levels of pro-inflammatory mediators (IFN-γ, IL-17, IP-10) were significantly higher in CAP patients as compared to that from controls. These data provide evidence that NKT cell subset compartment reconfiguration in patients with carotid stenosis seems to be associated with the occurrence of carotid atherosclerotic plaque and suggest that both pathogenic and protective NKT cell subsets exist.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Atherosclerotic complications of strokes remain as the leading causes of morbidity and mortality globally [1]. These lethal clinical syndromes are caused by the compromise of blood flow in arteries by rupture-initiated thrombus [2]. Carotid atherosclerosis is a chronic inflammatory disease of the arterial wall and its development and progression is a strong risk for ischemic stroke. Patients with carotid disease also demonstrate a higher than normal systemic inflammation [3]. Research has delineated atherosclerosis as a multifactorial disease in which immune and inflammatory responses are key pathogenetic factors [4]. Inflammation is involved in the atherosclerotic process by recruiting leukocytes, promoting plaque growth, and inducing plaque destabilization. The major role of immune and inflammatory responses in the development and progression of atheromatous plaques has prompted a search for circulating immunological and inflammatory moieties that reflect atherosclerosis and may serve as diagnostic and prognostic markers of disease.
Most attention has focused on the role of different lymphocyte subsets in carotid atheroma [2, 5, 6], whereas no study has been reported about the possible involvement of natural killer T (NKT) cells. NKT cells are a heterogeneous group that share properties of both T cells and natural killer cells, which function at the interface between innate and adaptive immunity. Many of these cells recognize the CD1d molecule-presenting molecule that binds self and foreign lipids and glycolipids. NKT cells include both NK1.1+ and NK1.1−, as well as CD4+, CD4−, CD8+, and CD8− cells [7, 8]. Upon stimulation, NKT cells are able to produce large amounts of IFN-γ and granulocyte-macrophage colony-stimulating factor, as well as multiple other cytokines and chemokines (such as IL-2, IL-13, IL-17, IL-21, and TNF-α). This makes NKT cells a unique T cell population with potentially both pro- and anti-inflammatory properties [9]. Elevation of these factors has been observed in serum from patients with carotid atherosclerosis, particularly from patients with complicated plaque [10]. These findings suggested that percentages of NKT cells could be influential in carotid atherosclerotic plaque development. In the present study, the relationship of NKT-cell compartment reconfiguration with carotid atherosclerotic plaque (CAP) occurrence was assessed and development of carotid atherosclerotic plaque. We also assessed the association between cytokine content in plasma and carotid disease. We found increased CD57-CD8high NKT cell subsets in CAP patients and less CD57+CD8− NKT cell subsets and CD57+CD8int NKT cell subsets in CAP patients, as well as related cytokine changes in plasma that were associated with subclinical inflammation.
MATERIALS AND METHODS
Ethical Considerations
The study was approved by the ethics committee, and written informed consent was obtained from all participants. All experiments were performed in accordance with relevant guidelines and regulations, and the plasma was stored at −80 °C until analysis in the approved biobank. All authors declare no conflict of interest in the present study.
Study Participant
The demographic characteristics and clinical features of the studied group are presented in Table 1. The majority of patients were male, and the average age was 60 years (range 49–69 years). All patients presented stenosis larger than 70 % evaluated by duplex ultrasound. The most common comorbidities were hypertension, dyslipidemia, smoking, diabetes mellitus, and obesity. Medications used before surgery and their respective frequencies are also described. Peripheral blood was obtained from 13 patients submitted to carotid endarterectomy for stenosis ≥70 % and 18 control subjects. The patients were enrolled at the Stroke Center of Anzhen Hospital in Beijing. Patients’ sample was compared with healthy control (HC) subjects. The control group was made of 18 donors without clinical and duplex ultrasound signs of carotid stenosis. The list of exclusions covered the following: an active malignancy, chronic renal failure and dialysis treatment, chronic viral infections, cold or flu, acute respiratory infection, intestinal infection, dental problems, connective tissue diseases, any type of surgery during the previous week, and any anti-inflammatory treatment.
Flow Cytometry Analysis
Peripheral blood were obtained from for the expression of cell-surface antigens using fluorescein isothiocyanate (FITC)-conjugated and phycoerythrin (PE)-conjugated monoclonal antibodies (mAbs) by flow cytometric analysis (Canto II, BD). Briefly, 100 μl of peripheral blood was incubated with 10 μl of FITC-conjugated anti-human CD57 mAb (BD Pharmingen), 10 μl of PerCP Cy5.5-conjugated anti-human CD3 mAb (BD Pharmingen), 10 μl of antigen-presenting cell (APC)-conjugated anti-human CD56 mAb (BD Pharmingen), and 10 μl of APC-H7-conjugated anti-human CD8 mAb (BD Pharmingen) at saturating concentrations for both reagents. Control isotypes were obtained from mouse immunoglobulins IgG1 (BD Pharmingen) at 5 μg/ml concentration. The number of peripheral blood cells positive for these reagents was determined by a two-dimensional side-scatter fluorescence dot-plot analysis of the sample after gating on the lymphocyte population (Fig. 1a). Different NKT cell subsets staining were characterized according to the expression of CD57 and CD8 molecules (Fig. 1a). Intracellular detection of with anti-IL-4-and anti-IL-17 was performed on fixed and permeabilized cells via Cytofix/Cytoperm (BD Biosciences). For detection of intracellular cytokine production, PBMC cells were stimulated with 20 ng/ml PMA and 1 mM ionomycin in the presence of GolgiStop (BD Biosciences) for 5 h and then stained with anti-IL-4-BV711 and anti-IL-17-BV650 (BD Pharmingen) at 5 μl after fixation and permeabilization. Data acquired by BD LSRFortessa (Becton Dickinson) were analyzed with Diva software. Statistical comparisons were performed with the nonparametric Mann-Whitney U test.
Imbalance of NKT cell subsets proportion in CAPs. Comparison of circulating NKT cell subset compartment between CAPs and HCs. a Leukocytes were gated in the forward (FS)/side-scatter (SS) plot, where the NKT cells were included in the lymphocyte gate. Thereafter, NKT cells were gated as CD3+CD56+ cells and in the next step identified by the expression of CD57 and CD8. b Significant perturbation of NKT cell subsets was registered in CAPs in comparison with controls. There was no change for total NKT cells between CAPs and HCs. The percentage of CD57-CD8high NKT cell subsets was significantly increased in CAPs, whereas CD57+CD8− and CD57+CD8int NKT cell subsets reduced in CAPs (*P < 0.05 respectively, one-way ANOVA test).
Chemokine and Cytokine Detection
A volume of 5 ml of whole blood was collected. The plasma samples were aliquoted after centrifugation at 400×g for 10 min. Concentrations of plasma cytokines were analyzed using Bio-Plex Suspension Array Systems (Bio-Plex Pro Human Cytokine Group 27-Plex Panel) to cover the range of the cytokines potentially involved in the pathophysiology of CAP. Assays were performed in duplicate by following the standard operating protocol provided by the Bio-Plex Multiplex Cytokine Assay.
Briefly, anti-cytokine antibody-conjugated beads were added to individual wells of a 96-well filter plate and adhered using vacuum filtration. After washing, 50 μl prediluted standards and serum samples were added into the respective wells and the filter plates were shaken at 300 rpm for 30 min at room temperature. Thereafter, the filter plates were washed and 25 μl prediluted multiplex biotin-conjugated detection antibody was added and incubated for another 30 min. After washing, 50 μl prediluted streptavidin-conjugated PE was added for 10 min followed by an additional wash and the addition of 125 μl Bio-Plex assay buffer to each well. Then, filter plates were analyzed using the Bio-Plex Protein Array System, and concentrations of each cytokine were determined using software (Bio-Plex Manager version 6.0). Standard curves were generated for each biomarker. The line of best fit was determined for standard curves by standard recovery methods and by calculating the concentration of each standard.
Statistical Analysis
Fisher’s exact test was used to test differences in CV risk factors and medical treatment between two groups. Percentage of different NKT cell subsets is given as mean values ± SEM. Univariate comparisons between groups were done with nonparametric Wilcoxon test. P values less than 0.05 were considered statistically significant. Results on serum cytokine levels are expressed as means and ranges of values. Mann-Whitney and Wilcoxon nonparametric tests were used to investigate the significance of data. P values less than 0.05 were considered statistically significant.
All the statistical procedures were performed by SPSS software.
RESULTS
Clinical Characteristics of Patients and Controls
The characteristics of the CAPs and HCs are presented in Table 1. In addition to the carotid disease, a significant number of CAPs presented with other CV related diseases, including HBP, DM, and HL. In addition, CV drugs were commonly used by CAPs. All patients were taken for carotid endarterectomy. There were nine patients with hypertension, seven with hypercholesterolemia, and three with diabetes mellitus. Although a lower percentage was observed in HCs (six with hypertension, four with hypercholesterolemia, and three with diabetes mellitus), there is no statistical difference about CV risk factors between controls and patients. For the medical treatment, all subjects in CAP group are taken anti-atherosclerosis treatment. There are no control subjects that have taken anti-atherosclerosis treatment. Most CAPs are under anti-hypertensive treatment in contrast to that in HCs (P < 0.001).
NKT-Cell Compartment Reconfiguration Is Related with Carotid Atherosclerotic Plaque Occurence
In the present study, proportion of CD57+CD8−, CD57+CD8int, CD57+CD8high, CD57−CD8−, CD57−CD8int, and CD57−CD8high NKT cell subsets were investigated (Fig. 1a). No difference was detected for CD57+CD8high, CD57−CD8−, and CD57−CD8int NKT cell subsets, but low percentage of CD57+CD8− and CD57+CD8int NKT cell subsets was detected in CAP patients (P = 0.011 and P = 0.002). Correspondingly, CD57−CD8high NKT cell subsets showed a higher fraction in CAP patients compared with controls (P = 0.007). Figure 1b presents the mean ± SEM. percentages of different NKT cell subsets in the peripheral blood of CAPs and HCs.
CD8+NKT Cell Subset Was the Main Cytokine-Producing NKT Cell
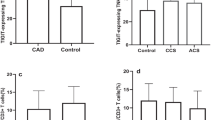
A representative analysis of purified mononuclear cells is demonstrated in Fig. 2. NKT cells were divided into two groups according to CD8 molecules (Fig. 2). The analysis of cytokine production by different cell subsets after stimulation showed that the CD8+ NKT cells were the higher producer of IL-17 (12.0 %) and IL-4 (9.1 %) compared with CD8+ NKT cell subsets. Less IL-17 (2.3 %) and IL-4 (1.6 %) were produced by CD8− NKT cell subsets (Fig. 2). Assessment of cytokine production revealed that CD8+ NKT cell subsets were the main cytokine-producing population for the NKT cells.
Characterization of cytokine production by peripheral NKT cells. After stimulation with 20 ng/ml of PMA plus 1 mM of ionomycin for 5 h in the presence of GolgiStop, the cells were fixed, permeabilized, stained with the indicated antibodies, and analyzed by flow cytometry. Leukocytes were gated in the forward (FS)/side scatter (SS) plot, where the NKT cell subsets were included in the CD3+CD56+ gate. Thereafter, NKT cells were gated as CD8− and CD8+ cells, and cytokine production was identified by intracellular staining.
Plasma Cytokine Altered in CAP Patients
We assessed NKT cell-related cytokines in the serum samples of healthy control and CAP subjects by Bio-Plex assays. The concentrations of cytokines in picograms per milliliter were measurable. Mean comparison procedures show that serum levels of three biomarkers (IFN-γ, IL-17, IP-10; P < 0.05) are significantly higher in CAP patients compared with control subjects. Nevertheless, interleukin 6 (IL-6) is apparently lower in CAP patients (Fig. 3).
Inflammatory-related mediators in the peripheral of CAPs. The figure compares the mean ± SEM expression levels of the pro-inflammatory mediators between CAPs and HCs. The pro-inflammatory mediators included IFN-γ, IP-10, IL-17, and IL-6. The asterisks show significant differences in the expression levels between CAPs and HCs. *P < 0.05 (one-sided Student’s t test)
DISCUSSION
NKT cell, a potential lymphocyte subset recognizing lipid antigen, can be pro- or anti-inflammatory. The role of NKT cells in carotid atheroma remains not to be reported. In this study, the results showed for the first time the composition imbalance of NKT cell subsets in peripheral blood from CAP patients compared to those cells in peripheral blood from control subjects. We evaluated the percentage of NKT cell subsets based on CD57 and CD8 molecules expression. NKT cells can be apparently divided into six groups, namely, CD57+CD8−, CD57+CD8int, CD57+CD8high, CD57−CD8−, CD57−CD8int, and CD57−CD8high NKT cell subsets, as shown in Fig. 1. We found the decreased fractions of CD57+CD8− and CD57+CD8int NKT cell subsets in peripheral blood from CAP patients. Meanwhile, CD57−CD8high NKT cell subsets increased in CAPs compared to those in control subjects.
Several studies pointed to an important role of NKT cells in atherosclerosis [11–13], and our data demonstrated the reconfiguration of NKT cell compartment in peripheral blood in CAP patients. Increased levels of invariant NKT lymphocytes worsen atherosclerosis in obese mice [12]. CD4+ NKT cells potently promote atherosclerosis by perforin and granzyme B-dependent apoptosis that increases postapoptotic necrosis and inflammation [11]. In human atherosclerosis, invariant NKT (iNKT) cells promoted plaque neovascularization and destabilization; iNKT cell lines produced pro-inflammatory cytokines when stimulated by CD1d-expressing APC-presenting α-galactosylceramide lipid antigen [13].
CD1d-restricted natural killer T (NKT) cells are involved in atherogenesis. CD1d, responsible for the presentation of lipid antigens, is expressed in atherosclerotic lesions and its expression is restricted to dendritic cells. NKT cells accumulate in rupture-prone shoulders of atherosclerotic plaques in human atherosclerotic lesions and directly contact with dendritic cells in rupture-prone regions of the plaque [14]. iNKT cell activation by antigen has pro-angiogenic effects as shown by enhanced microvascular sprout formation in an in vitro assay of angiogenesis. This effect was associated with EC migration as demonstrated by enhanced EC motility in both wound healing and transmigration Boyden chamber assays [13]. NKT cells can also mediate their effects indirectly by activating NK cells; adoptive transfer of NKT cells into lymphocyte-deficient ApoE−/−Rag2−/−γc−/− mice also augmented atherosclerosis development by ≈75 % that was ≈30 % the size of lesions of ApoE−/− mice [15, 16]. Mature NKT cells from mice and humans can be divided into functionally distinct CD4+CD8− and CD4−CD8− subsets, and humans also have a CD4−CD8+ NKT cell subset that is not found in mice. However, there is marked heterogeneity in the expression of functionally important cell surface receptors and cytokines by CD4+ and CD4− NKT cells [17–19]. Our results also show the existence of CD8high, CD8int, and CD8− NKT cell subsets in peripheral blood (Fig. 1a). We report that in peripheral blood from patients with CAPs, although the percentage of NKT cell in lymphocytes is relatively stable as compared with control subjects, different NKT cell subsets changes (Fig. 1b). Furthermore, our results also indicated that CD8+ NKT cells were the main cytokine-producing NKT cells (Fig. 2). Cell population shift may initiate the functional changes, including cytokines production and the effect of interaction with surrounding cells.
Indeed, the functional versatility of NKT cells is increasingly being attributed to NKT cell subsets with distinct cytokine profiles [20], and there is evidence that alterations to the structure of glycolipid ligands can affect the cytokine responses of NKT cells through selective stimulation of particular NKT cell subsets. iNKT cell activation elicits massive release of Th1 and Th2 cytokines and elevation in plasma levels of IL-6 and monocyte chemoattractant protein 1, which have been proposed to enhance local inflammation [21, 22]. Activated CD4+ NKT cells secrete copious amounts of IFN-γ, IL-4, and IL-21 [23, 24]. IFN-γ and IL-4 augment development of atherosclerosis [25, 26], whereas IL-21 augments cytokine secretion by NKT cells [24] and is expressed in atherosclerotic lesions [27]. Our findings indicated that IFN-γ, IL-17, IP-10, and IL-6 are altered in peripheral blood from CAP patients. Upon activation, NKT cells are also able to produce these factors. IFN-γ, IL-17, and IP-10, as pro-inflammatory mediators, were higher in plasma from CAP patients than that from controls. These alterations may induce inflammatory response in atheromatous lesion. However, IL-6, perceived broadly as pro-inflammatory cytokine, was lower in CAP patients. IL-6 can also act as an anti-inflammatory cytokine. Its role as an anti-inflammatory cytokine is mediated through its inhibitory effects on TNF-α and IL-1, and activation of IL-1RA and IL-10. Its function needs to be explored further in CAP patients.
When the study populations’ pharmacological treatment was taken into account, a study has reported that there was no relation between circulating inflammatory cell, Treg-cell levels, and statin treatment or angiotensin blocker treatment [28]. Several reports also showed that medication could modulate the inflammatory cell distribution, inflammatory status, and atherosclerosis [29–32], which influenced atherosclerosis by regulating inflammatory cell apoptosis, phenotype, function, and modulating systemic blood pressure. In our study, medical treatment was not well matched in either HC or CAP groups; it is not sure whether it might affect the results we measured. This is the limitation in this study.
In conclusion, our data indicated the presence of NKT cell subsets reconfiguration in peripheral from CAP patients, although the exact biological role of these cells has not been elucidated. Our data suggest that the alteration of cytokines may be associated with proportion change of different NKT cell subsets, which may contribute to the atheromatous plaque development. The analyses of NKT cell subsets could have a role in identifying patients at risk for new carotid stenosis events.
References
Mathers, C.D., T. Boerma, and Fat D. Ma. 2009. Global and regional causes of death. British Medical Bulletin 92: 7–32.
Hansson, G.K., and A. Hermansson. 2011. The immune system in atherosclerosis. Nature Immunology 12: 204–212.
Pitocco, D., S. Giubilato, F. Zaccardi, E. Di Stasio, A. Buffon, L.M. Biasucci, et al. 2009. Pioglitazone reduces monocyte activation in type 2 diabetes. Acta Diabetologica 46: 75–77.
Hansson, G.K. 2005. Inflammation, atherosclerosis, and coronary artery disease. New England Journal of Medicine 352: 1685–1695.
Ciccone, M.M., A. Marzullo, D. Mizio, D. Angiletta, F. Cortese, P. Scicchitano, et al. 2011. Can carotid plaque histology selectively predict the risk of an acute coronary syndrome? International Heart Journal 52: 72–77.
Xia, Q., X. Xiang, S. Patel, R. Puranik, Q. Xie, and S. Bao. 2012. Characterisation of IL-22 and interferon-gamma-inducible chemokines in human carotid plaque. International Journal of Cardiology 154: 187–189.
Van Der Vliet, H.J., N. Nishi, Y. Koezuka, M.A. Peyrat, B.M. Von Blomberg, A.J. Van Den Eertwegh, et al. 1999. Effects of alpha-galactosylceramide (KRN7000), interleukin-12 and interleukin-7 on phenotype and cytokine profile of human Valpha24+ Vbeta11+ T cells. Immunology 98: 557–563.
Vivier, E., and N. Anfossi. 2004. Inhibitory NK-cell receptors on T cells: witness of the past, actors of the future. Nature Reviews Immunology 4: 190–198.
Braunersreuther, V., F. Mach, and S. Steffens. 2007. The specific role of chemokines in atherosclerosis. Thrombosis and Haemostasis 97: 714–721.
Profumo, E., A. Siracusano, E. Ortona, P. Margutti, A. Carra, A. Costanzo, et al. 2003. Cytokine expression in circulating T lymphocytes from patients undergoing carotid endarterectomy. The Journal of Cardiovascular Surgery 44: 237–242.
Li, Y., K. To, P. Kanellakis, H. Hosseini, V. Deswaerte, P. Tipping, et al. 2015. CD4+ natural killer T cells potently augment aortic root atherosclerosis by perforin- and granzyme B-dependent cytotoxicity. Circulation Research 116: 245–254.
Subramanian, S., M.S. Turner, Y. Ding, L. Goodspeed, S. Wang, J.H. Buckner, et al. 2013. Increased levels of invariant natural killer T lymphocytes worsen metabolic abnormalities and atherosclerosis in obese mice. Journal of Lipid Research 54: 2831–2841.
Kyriakakis, E., M. Cavallari, J. Andert, M. Philippova, C. Koella, V. Bochkov, et al. 2010. Invariant natural killer T cells: linking inflammation and neovascularization in human atherosclerosis. European Journal of Immunology 40: 3268–3279.
Bobryshev, Y.V., and R.S. Lord. 2005. Co-accumulation of dendritic cells and natural killer T cells within rupture-prone regions in human atherosclerotic plaques. Journal of Histochemistry and Cytochemistry 53: 781–785.
Smyth, M.J., M.E. Wallace, S.L. Nutt, H. Yagita, D.I. Godfrey, and Y. Hayakawa. 2005. Sequential activation of NKT cells and NK cells provides effective innate immunotherapy of cancer. Journal of Experimental Medicine 201: 1973–1985.
Eberl, G., and H.R. MacDonald. 2000. Selective induction of NK cell proliferation and cytotoxicity by activated NKT cells. European Journal of Immunology 30: 985–992.
Kim, C.H., B. Johnston, and E.C. Butcher. 2002. Trafficking machinery of NKT cells: shared and differential chemokine receptor expression among V alpha 24(+)V beta 11(+) NKT cell subsets with distinct cytokine-producing capacity. Blood 100: 11–16.
Gumperz, J.E., S. Miyake, T. Yamamura, and M.B. Brenner. 2002. Functionally distinct subsets of CD1d-restricted natural killer T cells revealed by CD1d tetramer staining. Journal of Experimental Medicine 195: 625–636.
Montoya, C.J., D. Pollard, J. Martinson, K. Kumari, C. Wasserfall, C.B. Mulder, et al. 2007. Characterization of human invariant natural killer T subsets in health and disease using a novel invariant natural killer T cell-clonotypic monoclonal antibody, 6B11. Immunology 122: 1–14.
Godfrey, D.I., S. Stankovic, and A.G. Baxter. 2010. Raising the NKT cell family. Nature Immunology 11: 197–206.
Major, A.S., S. Joyce, and L. Van Kaer. 2006. Lipid metabolism, atherogenesis and CD1-restricted antigen presentation. Trends in Molecular Medicine 12: 270–278.
Tupin, E., A. Nicoletti, R. Elhage, M. Rudling, H.G. Ljunggren, G.K. Hansson, et al. 2004. CD1d-dependent activation of NKT cells aggravates atherosclerosis. Journal of Experimental Medicine 199: 417–422.
To, K., A. Agrotis, G. Besra, A. Bobik, and B.H. Toh. 2009. NKT cell subsets mediate differential proatherogenic effects in ApoE−/− mice. Arteriosclerosis, Thrombosis, and Vascular Biology 29: 671–677.
Coquet, J.M., K. Kyparissoudis, D.G. Pellicci, G. Besra, S.P. Berzins, M.J. Smyth, et al. 2007. IL-21 is produced by NKT cells and modulates NKT cell activation and cytokine production. Journal of Immunology 178: 2827–2834.
Davenport, P., and P.G. Tipping. 2003. The role of interleukin-4 and interleukin-12 in the progression of atherosclerosis in apolipoprotein E-deficient mice. American Journal of Pathology 163: 1117–1125.
Gupta, S., A.M. Pablo, X. Jiang, N. Wang, A.R. Tall, and C. Schindler. 1997. IFN-gamma potentiates atherosclerosis in ApoE knock-out mice. Journal of Clinical Investigation 99: 2752–2761.
Erbel, C., T.J. Dengler, S. Wangler, F. Lasitschka, F. Bea, N. Wambsganss, et al. 2011. Expression of IL-17A in human atherosclerotic lesions is associated with increased inflammation and plaque vulnerability. Basic Research in Cardiology 106: 125–134.
Ammirati, E., D. Cianflone, M. Banfi, V. Vecchio, A. Palini, M. De Metrio, et al. 2010. Circulating CD4+CD25hiCD127lo regulatory T-Cell levels do not reflect the extent or severity of carotid and coronary atherosclerosis. Arteriosclerosis, Thrombosis, and Vascular Biology 30: 1832–1841.
Yamamoto, S., P.G. Yancey, Y. Zuo, L.J. Ma, R. Kaseda, A.B. Fogo, et al. 2011. Macrophage polarization by angiotensin II-type 1 receptor aggravates renal injury-acceleration of atherosclerosis. Arteriosclerosis, Thrombosis, and Vascular Biology 31: 2856–2864.
Nicholls, S.J., E.M. Tuzcu, K. Wolski, O. Bayturan, A. Lavoie, K. Uno, et al. 2011. Lowering the triglyceride/high-density lipoprotein cholesterol ratio is associated with the beneficial impact of pioglitazone on progression of coronary atherosclerosis in diabetic patients: insights from the PERISCOPE (Pioglitazone Effect on Regression of Intravascular Sonographic Coronary Obstruction Prospective Evaluation) study. Journal of the American College of Cardiology 57: 153–159.
Saremi, A., D.C. Schwenke, T.A. Buchanan, H.N. Hodis, W.J. Mack, M. Banerji, et al. 2013. Pioglitazone slows progression of atherosclerosis in prediabetes independent of changes in cardiovascular risk factors. Arteriosclerosis, Thrombosis, and Vascular Biology 33: 393–399.
Babaev, V.R., P.G. Yancey, S.V. Ryzhov, V. Kon, M.D. Breyer, M.A. Magnuson, et al. 2005. Conditional knockout of macrophage PPARgamma increases atherosclerosis in C57BL/6 and low-density lipoprotein receptor-deficient mice. Arteriosclerosis, Thrombosis, and Vascular Biology 25: 1647–1653.
Acknowledgments
This study was supported by the Open Project of Key Laboratory of Ministry of Education (2013XXGB02).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The study was approved by the Ethics Committee and written informed consent was obtained from all participants. All experiments were performed in accordance with relevant guidelines and regulations, and the plasma was stored at −80 °C until analysis in the approved biobank.
Conflicts of Interest
The authors declare that they have no conflict of interest.
Additional information
Lun Cai and Lei Yu contributed equally to this work.
Rights and permissions
About this article
Cite this article
Cai, L., Yu, L., Liu, S. et al. Reconfiguration of NKT Cell Subset Compartment Is Associated with Plaque Development in Patients with Carotid Artery Stenosis. Inflammation 40, 92–99 (2017). https://doi.org/10.1007/s10753-016-0456-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-016-0456-6