Abstract
Muscle contusion is one of the most common muscle injuries in sports medicine. Macrophages play complex roles in the regeneration of skeletal muscle. However, the roles of macrophages, especially the mechanisms involved, in the regeneration of muscle contusion are still not fully understood. We hypothesize that the depletion of macrophages impairs skeletal muscle regeneration and that pro-fibrotic factors, inflammation, and oxidative stress may be involved in the process. To test these hypotheses, we constructed a muscle contusion injury and a macrophage depletion model and followed it up with morphological and gene expression analyses. The data showed that fibrotic scars were formed in the muscle of contusion injury, and they deteriorated in the mice of macrophage depletion. Furthermore, the sizes of regenerating myofibers were significantly reduced by macrophage depletion. Pro-fibrotic factors, inflammatory cytokines, chemokines, and oxidative stress-related enzymes increased significantly after muscle injury. Moreover, the expression of these factors was delayed by macrophage depletion. Most of them were still significantly higher in the later stage of regeneration. These results suggest that macrophage depletion impairs skeletal muscle regeneration and that pro-fibrotic factors, inflammation, and oxidative stress may play important roles in the process.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Muscle contusion is one of the most common muscle injuries in sports medicine clinics. The process of healing injured skeletal muscle in animal models is made up of three distinct phases: degeneration and inflammation, regeneration, and fibrosis [1, 2]. The initial phase, which takes place in the first few days post-injury, shows the following signs: local swelling at the injury site, formation of hematoma, necrosis of muscle tissue [3, 4], degeneration, and inflammatory response. Regeneration, which is the next phase, normally takes place 5 to 10 days post-injury and, it comprises phagocytosis of the damaged tissue and regeneration of the injured muscle [5]. In the final phase, fibrosis (scar tissue formation), which seems to be the final result of the muscle repair process, hinders full muscle regeneration.
Several studies have shown that macrophages play complex roles in an injured skeletal muscle and may be involved in all the three phases of regeneration, as described above [2, 6]. Acute damage causes release of chemoattractant molecules that initially attract neutrophils or ‘classically activated’ macrophages (M1) into the muscle. M1 macrophages produce both pro-inflammatory cytokines and nitric oxide, promoting muscle damage. Moreover, M1 macrophages also release cytokines that can promote satellite cell activation and proliferation. Further, M1 macrophages are replaced by an ‘alternatively activated’ M2 phenotype which then promotes muscle repair, differentiation, and growth [2]. Conversely, macrophages are likely to be involved in limiting the efficacy of regeneration, with formation of fibrotic scars and fat replacement of the tissue when the original insult persists [7].
There is a deep understanding of the processes of skeletal muscle regeneration, as mentioned above. However, the roles of macrophages, especially the mechanisms involved, in the regeneration of muscle contusion are still not fully understood. In the present study, we hypothesize that depletion of macrophages impairs skeletal muscle regeneration and that pro-fibrotic factors, inflammation, and oxidative stress may be involved in the process.
METHODS
Mice
One hundred and twelve C57BL/6 male mice (weighing between 18.2 and 22.9 g, and purchased from Shanghai Lab. Animal Research Center, Shanghai, China) were provided food and water adlibitum and maintained on a 12:12-h light-dark cycle. The mice were randomly divided into two groups, muscle contusion group (group S) and macrophage depleted group (group T). The mice of both groups suffered from contusion injury. All experimental protocols were approved by the Ethics Review Committee for Animal Experimentation of Shanghai University of Sports (No. 2014025).
Contusion Model
A simple and reproducible muscle contusion model in mice [8–10] was used with a little modification. In preparing the mice for muscle injury induction, they were anesthetized with 400 mg/kg chloral hydrate administered intraperitoneally. The animals’ hind limbs were positioned on a board dorsiflexing the ankle to 90°. A 16.3-g (diameter: 15.9 mm) stainless steel ball was dropped from the height of 100 cm through a tube (interior diameter of tube: 16 mm) onto an impactor [8] resting with a surface of 28.26 mm2 on the middle of the gastrocnemius muscle (GM) of the mouse. The instantaneous force delivered by the falling object with these characteristics was calculated to equal 0.58 N.m.cm-2, where 1 N.m is equal to the force of an object weighing 100 g falling over a distance of 1 m [11]. The muscle contusion created by this method was a high-energy blunt injury that created a large hematoma, and was followed by a massive muscle regeneration [8, 12], a healing process that is very similar to that seen in humans [13]. The mice that had bone fracture (fracture rate of 3.3 %) were foreclosed. The injured mice in this study had signs of unrelieved pain such as piloerection of fur, reluctance to ambulate, overgrooming of the injured limb, and abnormal gait or posture [14]. At different time points (12 h, 1 day, 3 days, 5 days, 7 days, and 14 days) post-injury, the mice were killed by cervical dislocation while under anesthesia, and gastrocnemius muscles were harvested (eight mice per time point).
Macrophage Depletion
For macrophage depletion, we treated mice with 2 mg clodronate-containing (CL) or control liposomes (purchased at www.clodronateliposomes.com), triggered by intraperitoneal injection 3 days before contusion injury and then 0.5 mg on days 0, 3, 6, 9, and 12 after muscle contusion (contusion group, control liposome injection; macrophage depleted group, CL-liposomes injection). After injection, clodronate liposome was ingested and digested by macrophages followed by intracellular release and accumulation of clodronate. At a certain intracellular concentration, clodronate induced apoptosis of the macrophage. This protocol was based on previous publications [15, 16], as well as discussions at the Web site: www.clodronateliposomes.com.
Flow Cytometry
The GMs from the mice treated by clodronate-containing or control liposomes were surgically removed on days 1 and 3 after injury for evaluation (six mice per group). Collagenase, dispase, and trypsin were used to digest the tissue matrix and isolate the cells. Debris was removed via filtration with 70 μm filters. Flow cytometry was carried out using the following antibodies, PE-CD11b and FITC-F4/80 (BioLegend, San Diego, CA, USA) [17, 18]. The percent of CD11b+ F4/80+ cell population (macrophage) was calculated using Cytomics™ FC 500 (Beckman Coulter).
Hematoxylin and Eosin (H & E) Staining
At the time point of 14 days post-injury, the right gastrocnemius muscles were collected and embedded in paraffin (six mice per group). Cross sections were cut 8 μm from the midbelly of each gastrocnemius muscle and were stained with hematoxylin and eosin for morphological analysis. Using a 40 lens objective, images were captured for each muscle section (Labphot-2, Nikon, Tokyo, Japan). The Northern Eclipse software was used to measure the minor axis diameters of centronucleated regenerating myofibers (i.e., the smallest diameter of a myofiber across the central nucleus; 200 random myofibers were obtained from six samples/group) [19].
Masson’s Trichrome Staining
To measure the area of fibrotic tissue in the injury sites, Masson’s trichrome staining (total collagen staining) was performed (six mice per group). After Masson’s trichrome staining, the ratio of the fibrotic area to the total cross-sectional area was calculated to estimate the fibrosis formation by using Image J software. The ratio of the fibrotic area within the injury sites was quantified using a previously described protocol [20, 21]. The measurement was obtained by a blinded independent investigator in order to ensure the objectivity of the results. To measure the total collagen positive area under the microscope, 10 random fields were selected for each sample.
RNA Extraction and cDNA Synthesis
Approximately 50 mg of tissue (from the middle of left gastrocnemius muscle, strike site) was homogenized using an Ultra-Turrax homogenizer (IKA, Germany) in a solution of TRIzol Reagent (Invitrogen, America) (eight mice per group). Total RNA was isolated using a modified guanidinium isothiocyanate-CsCl method [22], and the concentration and purity were determined by measuring the absorbance at 260 and 280 nm in a spectrophotometer (NanoDrop 2000, Thermo Scientific). Total RNA was reverse-transcribed into cDNA using the RevertaidTM First Strand cDNA Synthesis Kit from Fermentas. cDNA was synthesized using 2 μg of total RNA, 0.2 μg of random primers, 20 mM dNTP mix, 5× Reaction buffer, 20U RiboLockTM RNase Inhibitor and 200 U of RevertaidTM M-MuLV Reverse Transcriptase in a total volume of 20 μl. The reaction was carried out at 25 °C for 5 min followed by another 60 min at 42 °C and was terminated by the deactivation of the enzyme at 70 °C for 5 min. Control reactions lacking either reverse transcriptase or template were included to assess carryover of genomic DNA and non-specific contamination, respectively.
Real-Time Polymerase Chain Reaction (PCR)
Quantitative PCR was carried out in triplicates in reactions consisting of 12.5 μl 2 × Maxima SYBR Green/ROX qPCR Master mix (Thermo Scientific), 1 μl cDNA, nuclease-free water and 300 nM of each primer. Primer specifications are listed in Table 1. Amplifications were performed on a StepOnePlus™ PCR-Cycler (Life Technologies) with the following parameters: activation at 95 °C for 10 min, 40 cycles of denaturation at 95 °C for 15 s, and annealing/extension at 60 °C for 1 min. The threshold cycle (CT, the number of cycles to reach threshold of detection) was determined for each reaction, and the levels of the target mRNAs were quantified relative to the level of the housekeeping gene GAPDH using 2−△△CT method [23].
Statistical Analysis
The data were analyzed using SPSS 19.0 for windows. The data of regenerating myofibers or fibrosis were compared using independent samples t test. Mean values of gene data were compared using repeated-measure analysis. Post hoc multiple comparisons were performed using the Bonferroni test. Correlations were calculated according to Pearson. All values are expressed as mean ± S.D., and statistical significance was set at P < 0.05.
RESULTS
The Effects of CL-Liposomes on the Number and the Specific Markers of Macrophages in Injured GMs
The number of macrophage in injured skeletal muscle usually peaks from 1 to 3 days post-injury [2]. The percent of CD11b+ F4/80+ cell population (macrophages) in injured GM was calculated using flow cytometry. The data showed that macrophages were significantly reduced by 55.77 % (S1, 0.052 ± 0.014; T1, 0.023 ± 0.004; p < 0.01) and 63.16 % (S3, 0.019 ± 0.007; T3, 0.007 ± 0.003; p < 0.05) in the CL-liposomes treatment mice as compared to the control mice (Fig. 1).
Effects of CL-liposomes on the percent of macrophages in injured GMs. The percent of CD11b+ F4/80+ cells (macrophages) in injured GMs of group S (a) and group T (b) was tested by flow cytometry. (c) The percent of macrophages in injured GMs of group S and T. S: muscle contusion group; T: muscle contusion and macrophage depleted group. Data are means ± S.D., n = 6. aSignificant difference from S3d, P < 0.05. aaSignificant difference from S1d, P < 0.01.
In addition, we tested the mRNA levels of specific markers of macrophages of muscle. The data showed that CD68 mRNA increased significantly on days 1 and 3 post-injury. CL-liposomes injection significantly inhibited CD68 mRNA level at the time points of 0 (decreased by 51.00 %, p = 0.003), 1 day (decreased by 62.46 %, p = 0.000) and 3 days (decreased by 32.82 %, p = 0.014), as compared to the muscle contusion group. However, interestingly, CD68 mRNA in injured muscles from the mice treated with CL-liposomes was significantly higher than that of the contusion group at days 5, 7, and 14 post-injury (1.81-fold, p = 0. 011; 1.81-fold, p = 0.007; 5.28-fold, p = 0.000; respectively) (Fig. 2a).
Effects of CL-liposomes on the specific markers of macrophages of muscle post-injury. a CD68. b CD163. c CD206. S: muscle contusion group; T: muscle contusion and macrophage depleted group. S0: uninjured control mice; T0: uninjured and macrophage depleted mice. Data are means ± S.D., n = 8; aSignificant difference from S0, P < 0.05; aa P < 0.01. bSignificant difference from T0, P < 0.05; bb P < 0.01. cSignificant difference from the same time points of group S, P < 0.05; cc P < 0.01. CD68: the specific marker of M1 macrophages; CD163 and CD206: the indicators of M2 macrophages.
On the other hand, CD163, the molecule marker of M2 macrophages (M2c) [2, 24], increased significantly on days 1, 3, and 5 post-injury (2.69-fold, p = 0.014; 2.69-fold, p = 0.008; 2.86-fold, p = 0.010; respectively). Similarly, CD206, another marker of M2 macrophages (M2a and M2c) [2, 24], increased significantly on 3 days post-injury. However, CL-liposomes treatment significantly inhibited CD163 (all time points of regeneration) and CD206 (1, 3, 5, and 7 days post-injury) mRNA levels as compared to the contusion group (Fig. 2b, c).
Macrophage Depletion Decreased the Sizes of Regenerating Myofibers
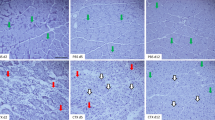
After hematoxylin and eosin staining, the sizes of regenerating myofibers (central nucleation) were evaluated. The size of the regenerating myofiber of the CL-liposomes treated group (T14, 39.50 ± 6.80) was significantly smaller than that of the control liposome group (S14, 48.83 ± 6.97) at 14 days after contusion injury (Fig. 3).
The sizes of regenerating myofibers 14 days post-injury. a Uninjured control group. b Muscle contusion group (14 days post-injury). c Muscle contusion and macrophage depleted group (14 days post-injury). d Quantification of the sizes of regenerating myofibers in GMs. Data are means ± S.D., n = 6. aSignificant difference from S14, P < 0.05. Scale bars = 50 μm.
Macrophage Depletion Aggravated the Fibrosis of Injured Skeletal Muscle
After Masson’s trichrome staining, the area of fibrotic scar tissue was evaluated. The CL-liposomes treated group (T14, 15.61 ± 5.42) showed significantly more fibrotic area than the control liposome group (S14, 6.40 ± 3.21) at 14 days after contusion injury (Fig. 4).
Histologic evaluation of the formation of scar tissue at 14 days after contusion injury by Masson’s trichrome staining of injured GMs treated with CL-liposomes. Scar tissues are shown in blue and muscles are in red. a Uninjured control group. b Muscle contusion group (14 days post-injury). c Muscle contusion and macrophage depleted group (14 days post-injury). d Quantification of the scar tissue area in injured GMs. Scale bars = 50 μm. Data are means ± S.D., n = 6. aaSignificant difference from S14, P < 0.01.
Macrophage Depletion Increased the Pro-fibrotic Factors in the Later Stage of Regeneration
TGF-beta 1 plays a key role in the fibrosis of many tissues [25]. Therefore, we assessed the TGF-beta 1 mRNA levels of the muscles post-injury. The data showed that TGF-beta 1 mRNA increased significantly at 1, 3, and 7 days post-injury. In the macrophages depleted group, TGF-beta 1 mRNA increased significantly at 3, 5, 7, and 14 days. As compared to the contusion group, macrophage depletion significantly inhibited TGF-beta 1 mRNA levels at 1 day post-injury. However, the muscle of the mice from the macrophage depleted group showed a higher expression of TGF-beta 1 mRNA at 7 days (1.56-fold, p = 0.006) and 14 days (2.76-fold, p = 0.000) than those of the contusion group (Fig. 5a).
Effects of macrophages depletion on the pro-fibrotic factors of muscle post-injury. a TGF-beta 1. b myostatin. S: muscle contusion group; T: muscle contusion and macrophage depletion group. Data are means ± S.D., n = 8; aSignificant difference from S0, P < 0.05; aa P < 0.01. bSignificant difference from T0, P < 0.05; bb P < 0.01. cSignificant difference from the same time points of group S, P < 0.05; cc P < 0.01.
Moreover, we tested the expression of myostatin, a member of TGF-beta family, which may be involved in the fibrosis of skeletal muscle [26]. The data revealed that that myostatin did not change in the whole process of regeneration after muscle injury. However, macrophage depletion significantly increased the expression of myostatin at 1 and 3 days post-injury. Furthermore, myostatin in the muscle of mice from the macrophage depletion group was significantly higher than that of the contusion group at 1 day post-injury (Fig. 5b).
Macrophage Depletion Delayed Inflammatory Response of Injured Skeletal Muscle
We evaluated some inflammatory cytokines which may be involved in the fibrosis of skeletal muscles after injury. The data showed that some inflammatory cytokines (i.e., TNF-alpha, IL-1beta, IL-6 and IL-10) increased significantly at the early stage of regeneration, especially at the first 3 days (Fig. 6). Moreover, this phenomenon was also observed in the mice of the macrophage depletion group, though the extent was small (Fig. 6). In the contusion group, these inflammatory cytokines returned to normal at the 14 days post-injury. However, a high expression of TNF-alpha and IL-1beta was observed in the mice of the macrophage depletion group at the 14 days post-injury. This was significantly higher than that of the contusion group (TNF-alpha, 2.38-fold, p = 0.001; IL-1beta, 6.95-fold, p = 0.000; respectively) (Fig. 6a, b). This phenomenon even existed in the 5 and 7 days post-injury.
Effects of macrophages depletion on the inflammatory cytokines of muscle post-injury. a TNF-alpha. b IL-1beta. c IL-6. d IL-10. S: muscle contusion group; T: muscle contusion and macrophage depleted group. Data are means ± S.D., n = 8; aSignificant difference from S0, P < 0.05; aa P < 0.01. bSignificant difference from T0, P < 0.05; bb P < 0.01. cSignificant difference from the same time points of group S, P < 0.05; cc P < 0.01.
Nonetheless, we tested the expression of CC chemokines after muscle injury. The data showed that some CC chemokines (i.e., CCL2, CCL3 and CCL4) increased significantly at the first 3 days of regeneration (Fig. 7), similarly to some of the inflammatory cytokines observed above. This phenomenon was also observed in the mice of macrophage depletion. However, CC ligand-5 (CCL5) and CCL8 had different gene expression patterns. They peaked at 3 days in both the contusion and the macrophages depleted groups. In the contusion group, the CC chemokines returned to normal at the 14 days post-injury. However, a high expression was observed for all these CC chemokines in the mice of macrophage depletion at the 14 days post-injury; and this was significantly higher than that of the contusion group (CCL2, 4.34-fold, p = 0.000; CCL3, 6.21-fold, p = 0.000; CCL4, 5.32-fold, p = 0.000; CCL5, 4.36-fold, p = 0.002; CCL8, 4.01-fold, p = 0.000; respectively) (Fig. 7a–e).

Furthermore, we investigated the CXC chemokines. The data showed that CXC ligand-10 (CXCL10) mRNA increased significantly at the first 3 days of regeneration, similarly to some of the CC chemokines (i.e., CCL2, CCL3, and CCL4). However, different from CXCL10, the mRNA levels of CXCL9 and CXCL12 tended to decline after muscle contusion. Interestingly, all of these CXC chemokines in the muscle of mice from the macrophage depletion group were significantly higher than that of the contusion group at 5, 7, and 14 days post-injury (Fig. 7f–h).
Macrophage Depletion Increased the Gene Expression of NADPH Oxidases in the Later Stage of Regeneration
NADPH oxidases (Nox) have the unique function of producing reactive oxygen species (ROS), which may be involved in the fibrosis of skeletal muscles [27, 28]. We tested the mRNA levels of gp91phox, which is the key subunit of NADPH oxidases. The data showed that gp91phox mRNA levels increased significantly at the time point of 3 days (11.44-fold, p = 0.000), 5 days (3.51-fold, p = 0.020) and 7 days (3.75-fold, p = 0.045) post-injury. And it returned to normal at 14 days post-injury. Although the increase of gp91phox in the mice of macrophage depletion was smaller than that of the contusion group at the 3 days post-injury, the oxidative stress maintained a longer time after macrophage depletion (3, 5, 7, and 14 days). Further, the gp91phox mRNA levels of the mice from macrophage depletion was significantly higher than that of the contusion group at the 14 days post-injury (3.87-fold, p = 0.000) (Fig. 8).
Effects of macrophages depletion on the expression of gp91phox of muscle post-injury. S: muscle contusion group; T: muscle contusion and macrophage depleted group. Data are means ± S.D., n = 8; aSignificant difference from S0, P < 0.05; aa P < 0.01. bSignificant difference from T0, P < 0.05; bb P < 0.01. cSignificant difference from the same time points of group S, P < 0.05; cc P < 0.01. gp91phox: the key subunit of NADPH oxidases (NOX).
Correlation Between the Specific Markers of Macrophages and the Pro-fibrotic Factors, Inflammatory Cytokines, Chemokines, and Oxidative Stress-Related Enzymes
To comprehend the relationship between the macrophages and the fibrosis-related factors, the Pearson correlations were calculated (all data of group S and group T). The results showed that there was significant correlation between the M1 macrophages (CD68) and the pro-fibrotic factors (TGF-beta), and between the inflammatory cytokines (TNF-alpha, IL-1beta, IL-6 and IL-10) and the chemokines (CCL2, CCL3, CCL4, CCL8 and CXCL10). However, different from M1 macrophages, the significant correlation only existed between M2 macrophages (CD163, CD206) and IL-10, CCL8 and gp91phox (only in CD206), but not in others (Table 2).
DISCUSSION
To investigate the roles of macrophages in the regeneration of muscle contusion and the mechanisms involved, we constructed a contusion injury and a macrophage depletion model. The number of macrophage in injured skeletal muscle usually peaks from 1 to 3 days post-injury [2]. Therefore, the percent of CD11b+ F4/80+ cell population in GM was calculated using flow cytometry [29, 30]. Our data showed that CL-liposomes treatment decreased the percent of macrophages significantly at 1 and 3 days post-injury. Our result conforms to Shen et al., who reported that CL-liposomes injection decreased macrophage infiltration by 73.2 % (1 day post-injury) and 77.4 % (3 days post-injury) respectively [19]. In addition, we tested the mRNA levels of specific markers of macrophages by qRT-PCR [24]. We found that CD68, the specific marker of M1macrophages [2, 24, 31], was inhibited in the early stage of regeneration by CL-liposomes treatment, however, it increased significantly in the later stage of regeneration. In contrast with CD68, CD163 and CD206 as the specific marker of M2 macrophages [2, 24] were inhibited in the whole process of regeneration by CL-liposomes treatment. The result is similar to the study of Villalta SA et al. [24], who found that CL-liposomes caused significant reduction in the expression of CD163 and CD206 in the quadriceps muscles of mdx mice, but not in CD68.
Histologically, the sizes of regenerating myofibers were evaluated, and the total collagen staining was performed to detect fibrosis in the injured muscle as previously described [1, 19, 32]. The muscle treated with CL-liposomes showed a smaller size of regenerating myofiber as well as a more fibrotic scar formation than that of the control liposomes group at 14 days after contusion injury (Figs. 3 and 4). This result conforms to Segawa M et al. [33]. However, the mechanisms involved are still not fully understood. Due to the potent roles of pro-fibrotic factors in the fibrosis of tissues [34], we speculated that the pro-fibrotic factors may be involved in the fibrosis of injured skeletal muscles after macrophage depletion. Enough evidence has proved that transforming growth factor-beta 1 (TGF-beta 1) plays a key role in the fibrosis of skeletal muscles [25]. TGF-beta 1 is expressed in injured muscle, which is a major factor in triggering the fibrotic cascade within injured skeletal muscle [35, 36]. TGF-beta 1 has the potential to induce fibrosis around myofibers, probably by stimulating fibroblasts to produce ECM proteins such as collagen and fibronectin [25]. Owing to the central role of TGF-beta 1 in skeletal muscle fibrosis, anti-TGF-beta 1 therapy was a strategy used to minimize or ameliorate the effects of fibrosis [20, 37]. In this study, the data showed that TGF-beta 1 mRNA increased significantly after muscle contusion. And the expression of TGF-beta 1 was delayed in the mice of macrophage depletion. On the 14 days post-injury, TGF-beta 1 in the muscle of the mice from macrophage depletion was still significantly higher than T0 and S14d. In addition, we tested another pro-fibrotic factor, myostatin, also known as growth differentiation factor (GDF) 8. Myostatin is a TGF-beta family member that is specifically expressed in the skeletal-muscle lineage, where its most characteristic role is negative regulation of muscle growth [25]. However, myostatin regulates not only the growth of muscle cells but also muscle fibroblast activation, and hence, the progression of fibrosis [26]. Myostatin directly promoted fibroblast proliferation resulting in muscle fibrosis in vivo [26]. Additionally, TGF-beta increases myostatin in a co-regulatory manner with fibrosis [37]. Although myostatin did not change in the whole process of regeneration after muscle injury in our study, it significantly increased at 1 and 3 days after macrophage depletion. The upregulation of myostatin, the well-known negative regulator of muscle growth, could increase the fibrosis of skeletal muscle. These data suggest that TGF-beta and myostatin may be involved in the fibrosis of injured skeletal muscles induced by macrophage depletion.
Moreover, we investigated the inflammatory cytokines and chemokines. The data showed that many inflammatory cytokines and chemokines increased significantly after muscle contusion. Although the extent of increase of some of the inflammatory cytokines and chemokines was smaller in the mice of macrophage depletion group than that of the contusion group, it is interesting to note that this tendency was completely reversed in the later stage of regeneration. All of the inflammatory cytokines and chemokines returned to normal at the 14 days post-injury; however, some of the inflammatory cytokines (TNF-alpha and IL-1beta) and all the chemokines were still significantly higher than T0 or S14d in the mice of macrophage depletion at 14 days. It means that the expression of these factors was delayed by macrophage depletion. These data suggest that inflammatory cytokines and chemokines may be involved in the fibrosis of skeletal muscles induced by macrophage depletion. Although inflammation triggers regenerative processes, it also causes pain, decreases skeletal muscle function, and contributes to fibrosis [38]. Frequently, muscle fibrosis is preceded by inflammation, which means that inflammation plays a significant role in muscle fibrosis [39]. Studies have shown that inhibition of inflammation could ameliorate fibrosis. For example, long-term administration of the TNF blocking drug to mdx mice could reduce the fibrosis of skeletal muscle [40]. Two weeks of anti-TNF treatment could increase overall grip strength and decrease fibrosis of skeletal muscles [41].
Further, we tested the expression of gp91phox, the key subunit of NADPH oxidase (NOX). The unique function of NOX is to produce reactive oxygen species (ROS), which may be involved in the fibrosis of skeletal muscles [27, 28]. Acute oxidative stress, caused by contusion, is usually determined by measuring gp91phox [42]. The data showed that the expression of gp91phox increased significantly after muscle injury and returned to normal at 14 days post-injury. However, the expression of gp91phox was delayed in the mice of macrophage depletion, which is similar to TGF-beta 1 and some cytokines above. It suggests that ROS mediated by NADPH oxidase may be involved in the fibrosis of skeletal muscles induced by macrophage depletion. It is well-known that ROS generated by NADPH oxidase participate in intracellular signaling processes that regulate cell proliferation and differentiation, as shown in our previous studies [43, 44]. However, there is increasing evidence that oxidant signaling involving NADPH oxidase has other important roles in cell biology. The superoxide generating enzyme of NADPH oxidase is a major cause of oxidative stress. Recent studies also suggest that NADPH oxidase-induced ROS is involved in the fibrosis of skeletal muscles [27, 28], while ROS could enhance fibrotic scar tissue formation in muscle after contusion injury [42].
Macrophages are the master regulators of inflammation and fibrosis [45]. The pro-fibrotic factors, inflammatory cytokines, chemokines, and oxidative stress-related enzymes, usually originate from macrophages or myogenic cells. For example, TGF-beta1 is expressed in injured muscles, and moreover, its major resource is the invaded macrophages [46]. However, we do not know which macrophages phenotypes are the main resources of TGF-beta1, inflammatory cytokines, chemokines, and NADPH oxidase. Therefore, we calculated the Pearson correlations in this study. The results showed that there were significant correlations between the M1 macrophages (CD68) and the pro-fibrotic factors (TGF-beta), and the inflammatory cytokines (TNF-alpha, IL-1beta, IL-6 and IL-10) and the chemokines (CCL2, CCL3, CCL4, CCL8 and CXCL10). However, different from M1 macrophages, the significant correlation only existed between M2 macrophages (CD163, CD206) and IL-10, CCL8 and gp91phox (only in CD206), but not in others (Table 2). These data suggest that pro-fibrotic factors produced by M1 macrophages, oxidative stress-related enzymes from M2 macrophages, and inflammatory cytokines from M1 and M2 macrophages may be involved in the fibrosis of skeletal muscles post-injury. However, this hypothesis still needs further study to be proved.
CONCLUSIONS
Macrophage depletion induced a smaller size of regenerating myofiber as well as a more fibrotic scar formation after muscle contusion. Moreover, macrophage depletion could delay the expression of pro-fibrotic factors, inflammatory cytokines, chemokines, and oxidative stress-related enzymes. Most of them were still significantly higher in the later stage of regeneration. These results suggest that macrophage depletion impairs skeletal muscle regeneration and that pro-fibrotic factors, inflammation, and oxidative stress may be involved in the process.
Abbreviations
- GM:
-
Gastrocnemius muscle
- CL-liposomes:
-
Clodronate-containing (CL) liposomes
- HE staining:
-
Hematoxylin and eosin staining
- PCR:
-
Polymerase chain reaction
- TGF-beta 1:
-
Transforming growth factor-beta 1
- TNF-alpha:
-
Tumor necrosis factor (TNF)-alpha
- IL-1 beta:
-
Interleukin (IL)-1beta
- IL-6:
-
Interleukin (IL)-6
- IL-10:
-
Interleukin (IL)-10
- CCL2:
-
CC ligand-2
- CXCL10:
-
CXC ligand-10
- NADPH-oxidases:
-
Nicotinamide adenine dinucleotide phosphate (NADPH)-oxidases
- ROS:
-
Reactive oxygen species
References
Nozaki, M., Y. Li, J. Zhu, F. Ambrosio, K. Uehara, F.H. Fu, and J. Huard. 2008. Improved muscle healing after contusion injury by the inhibitory effect of suramin on myostatin, a negative regulator of muscle growth. The American Journal of Sports Medicine 36(12): 2354–2362.
Tidball, J.G., and S.A. Villalta. 2010. Regulatory interactions between muscle and the immune system during muscle regeneration. American Journal of Physiology—Regulatory, Integrative and Comparative Physiology 298(5): R1173–R1187.
Honda, H., H. Kimura, and A. Rostami. 1990. Demonstration and phenotypic characterization of resident macrophages in rat skeletal muscle. Immunology 70(2): 272–277.
Hurme, T., H. Kalimo, M. Lehto, and M. Jarvinen. 1991. Healing of skeletal muscle injury: an ultrastructural and immunohistochemical study. Medicine and Science in Sports and Exercise 23(7): 801–810.
Carlson, B.M., and J.A. Faulkner. 1983. The regeneration of skeletal muscle fibers following injury: a review. Medicine and Science in Sports and Exercise 15(3): 187–198.
Summan, M., G.L. Warren, R.R. Mercer, R. Chapman, T. Hulderman, N. Van Rooijen, and P.P. Simeonova. 2006. Macrophages and skeletal muscle regeneration: a clodronate-containing liposome depletion study. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology 290(6): R1488–R1495.
Bosurgi, L., A.A. Manfredi, and P. Rovere-Querini. 2011. Macrophages in injured skeletal muscle: a perpetuum mobile causing and limiting fibrosis, prompting or restricting resolution and regeneration. Frontiers in Immunology 2: 62.
Kasemkijwattana, C., J. Menetrey, G. Somogyi, M.S. Moreland, F.H. Fu, B. Buranapanitkit, S.C. Watkins, and J. Huard. 1998. Development of approaches to improve the healing following muscle contusion. Cell Transplantation 7(6): 585–598.
Wright-Carpenter, T., P. Opolon, H. Appell, H. Meijer, P. Wehling, and L. Mir. 2004. Treatment of muscle injuries by local administration of autologous conditioned serum: animal experiments using a muscle contusion model. International Journal of Sports Medicine 25(8): 582–587.
Xiao, W.H., Y. Liu, B.B. Luo, L.L. Zhao, X.G. Liu, Z.G. Zeng, and P.J. Chen. 2016. Time-dependent gene expression analysis after mouse skeletal muscle contusion. Journal of Sport and Health Science 5(1): 101–108.
Fisher, B.D., V.E. Baracos, T.K. Shnitka, S.W. Mendryk, and D.C. Reid. 1990. Ultrastructural events following acute muscle trauma. Medicine and Science in Sports and Exercise 22(2): 185–193.
Crisco, J.J., P. Jokl, G.T. Heinen, M.D. Connell, and M.M. Panjabi. 1994. A muscle contusion injury model biomechanics, physiology, and histology. The American Journal of Sports Medicine 22(5): 702–710.
Diaz, J.A., D.A. Fischer, A.C. Rettig, T.J. Davis, and K.D. Shelbourne. 2003. Severe quadriceps muscle contusions in athletes. A report of three cases. The American Journal of Sports Medicine 31(2): 289–293.
Dobek, G.L., N.D. Fulkerson, J. Nicholas, and B.S. Schneider. 2013. Mouse model of muscle crush injury of the legs. Comparative Medicine 63(3): 227–232.
Tyner, J.W., O. Uchida, N. Kajiwara, E.Y. Kim, A.C. Patel, M.P. O’Sullivan, M.J. Walter, R.A. Schwendener, D.N. Cook, T.M. Danoff, et al. 2005. CCL5-CCR5 interaction provides antiapoptotic signals for macrophage survival during viral infection. Nature Medicine 11(11): 1180–1187.
Popovich, P.G., Z. Guan, P. Wei, I. Huitinga, N. van Rooijen, and B.T. Stokes. 1999. Depletion of hematogenous macrophages promotes partial hindlimb recovery and neuroanatomical repair after experimental spinal cord injury. Experimental Neurology 158(2): 351–365.
Bacci, M., A. Capobianco, A. Monno, L. Cottone, F. Di Puppo, B. Camisa, M. Mariani, C. Brignole, M. Ponzoni, S. Ferrari, et al. 2009. Macrophages are alternatively activated in patients with endometriosis and required for growth and vascularization of lesions in a mouse model of disease. The American Journal of Pathology 175(2): 547–556.
Holt, M.P., L. Cheng, and C. Ju. 2008. Identification and characterization of infiltrating macrophages in acetaminophen-induced liver injury. Journal of Leukocyte Biology 84(6): 1410–1421.
Shen, W., Y. Li, J. Zhu, R. Schwendener, and J. Huard. 2008. Interaction between macrophages, TGF-beta1, and the COX-2 pathway during the inflammatory phase of skeletal muscle healing after injury. Journal of Cellular Physiology 214(2): 405–412.
Foster, W., Y. Li, A. Usas, G. Somogyi, and J. Huard. 2003. Gamma interferon as an antifibrosis agent in skeletal muscle. Journal of orthopaedic research : official publication of the Orthopaedic Research Society 21(5): 798–804.
Fukushima, K., N. Badlani, A. Usas, F. Riano, F. Fu, and J. Huard. 2001. The use of an antifibrosis agent to improve muscle recovery after laceration. The American Journal of Sports Medicine 29(4): 394–402.
Chirgwin, J.M., A.E. Przybyla, R.J. MacDonald, and W.J. Rutter. 1979. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry 18(24): 5294–5299.
Livak, K.J., and T.D. Schmittgen. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods 25(4): 402–408.
Villalta, S.A., C. Rinaldi, B. Deng, G. Liu, B. Fedor, and J.G. Tidball. 2011. Interleukin-10 reduces the pathology of mdx muscular dystrophy by deactivating M1 macrophages and modulating macrophage phenotype. Human Molecular Genetics 20(4): 790–805.
Mann, C.J., E. Perdiguero, Y. Kharraz, S. Aguilar, P. Pessina, A.L. Serrano, and P. Munoz-Canoves. 2011. Aberrant repair and fibrosis development in skeletal muscle. Skeletal Muscle 1(1): 21.
Li, Z.B., H.D. Kollias, and K.R. Wagner. 2008. Myostatin directly regulates skeletal muscle fibrosis. The Journal of Biological Chemistry 283(28): 19371–19378.
Chan, E.C., F. Jiang, H.M. Peshavariya, and G.J. Dusting. 2009. Regulation of cell proliferation by NADPH oxidase-mediated signaling: potential roles in tissue repair, regenerative medicine and tissue engineering. Pharmacology & Therapeutics 122(2): 97–108.
Cabello-Verrugio, C., M.J. Acuna, M.G. Morales, A. Becerra, F. Simon, and E. Brandan. 2011. Fibrotic response induced by angiotensin-II requires NAD(P)H oxidase-induced reactive oxygen species (ROS) in skeletal muscle cells. Biochemical and Biophysical Research Communications 410(3): 665–670.
Nishimura, S., I. Manabe, M. Nagasaki, K. Eto, H. Yamashita, M. Ohsugi, M. Otsu, K. Hara, K. Ueki, S. Sugiura, et al. 2009. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nature Medicine 15(8): 914–920.
Stoneman, V., D. Braganza, N. Figg, J. Mercer, R. Lang, M. Goddard, and M. Bennett. 2007. Monocyte/macrophage suppression in CD11b diphtheria toxin receptor transgenic mice differentially affects atherogenesis and established plaques. Circulation Research 100(6): 884–893.
Xiao, W., P. Chen, and J. Dong. 2012. Effects of overtraining on skeletal muscle growth and gene expression. International Journal of Sports Medicine 33(10): 846–853.
Chan, Y.S., Y. Li, W. Foster, T. Horaguchi, G. Somogyi, F.H. Fu, and J. Huard. 2003. Antifibrotic effects of suramin in injured skeletal muscle after laceration. Journal of Applied Physiology 95(2): 771–780.
Segawa, M., S. Fukada, Y. Yamamoto, H. Yahagi, M. Kanematsu, M. Sato, T. Ito, A. Uezumi, S. Hayashi, Y. Miyagoe-Suzuki, et al. 2008. Suppression of macrophage functions impairs skeletal muscle regeneration with severe fibrosis. Experimental Cell Research 314(17): 3232–3244.
Uezumi, A., T. Ito, D. Morikawa, N. Shimizu, T. Yoneda, M. Segawa, M. Yamaguchi, R. Ogawa, M.M. Matev, Y. Miyagoe-Suzuki, et al. 2011. Fibrosis and adipogenesis originate from a common mesenchymal progenitor in skeletal muscle. Journal of Cell Science 124(Pt 21): 3654–3664.
Li, Y., W. Foster, B.M. Deasy, Y. Chan, V. Prisk, Y. Tang, J. Cummins, and J. Huard. 2004. Transforming growth factor-beta1 induces the differentiation of myogenic cells into fibrotic cells in injured skeletal muscle: a key event in muscle fibrogenesis. The American Journal of Pathology 164(3): 1007–1019.
Li, Y., and J. Huard. 2002. Differentiation of muscle-derived cells into myofibroblasts in injured skeletal muscle. The American Journal of Pathology 161(3): 895–907.
Zhu, J., Y. Li, W. Shen, C. Qiao, F. Ambrosio, M. Lavasani, M. Nozaki, M.F. Branca, and J. Huard. 2007. Relationships between transforming growth factor-beta1, myostatin, and decorin: implications for skeletal muscle fibrosis. The Journal of Biological Chemistry 282(35): 25852–25863.
Urso, M.L. 2013. Anti-inflammatory interventions and skeletal muscle injury: benefit or detriment? Journal of Applied Physiology 115(6): 920–928.
Meyer, G.A., and R.L. Lieber. 2012. Skeletal muscle fibrosis develops in response to desmin deletion. American Journal of Physiology. Cell Physiology 302(11): C1609–C1620.
Ermolova, N.V., L. Martinez, S.A. Vetrone, M.C. Jordan, K.P. Roos, H.L. Sweeney, and M.J. Spencer. 2014. Long-term administration of the TNF blocking drug Remicade (cV1q) to mdx mice reduces skeletal and cardiac muscle fibrosis, but negatively impacts cardiac function. Neuromuscular Disorders 24(7): 583–595.
Abdelmagid, S.M., A.E. Barr, M. Rico, M. Amin, J. Litvin, S.N. Popoff, F.F. Safadi, and M.F. Barbe. 2012. Performance of repetitive tasks induces decreased grip strength and increased fibrogenic proteins in skeletal muscle: role of force and inflammation. PLoS One 7(5): e38359.
Ghaly, A., and D.R. Marsh. 2010. Ischaemia-reperfusion modulates inflammation and fibrosis of skeletal muscle after contusion injury. International Journal of Experimental Pathology 91(3): 244–255.
Xiao, W., P. Chen, R. Wang, and J. Dong. 2013. Overload training inhibits phagocytosis and ROS generation of peritoneal macrophages: role of IGF-1 and MGF. European Journal of Applied Physiology 113(1): 117–125.
Xiao, W., P. Chen, J. Dong, R. Wang, and B. Luo. 2014. Dietary Glutamine Supplementation Partly Reverses Impaired Macrophage Function Resulting From Overload Training in Rats. International Journal of Sport Nutrition and Exercise 25(2): 179–187.
Wynn, T.A., and L. Barron. 2010. Macrophages: master regulators of inflammation and fibrosis. Seminars in Liver Disease 30(3): 245–257.
Long, K.K., M. Montano, and G.K. Pavlath. 2011. Sca-1 is negatively regulated by TGF-beta1 in myogenic cells. FASEB journal : official publication of the Federation of American Societies for Experimental Biology 25(4): 1156–1165.
Acknowledgments
This work was supported by the grants from the National Natural Science Foundation of China (31271273, 31300975), the Doctoral Fund of Ministry of Education of China (20133156120004) and the Key Lab of Exercise and Health Sciences of Ministry of Education (Shanghai University of Sport).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Authors’ Contributions
WX and PC analyzed and interpreted the data. YL performed the histological examination of the skeletal muscle. All authors read and approved the final manuscript.
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Xiao, W., Liu, Y. & Chen, P. Macrophage Depletion Impairs Skeletal Muscle Regeneration: the Roles of Pro-fibrotic Factors, Inflammation, and Oxidative Stress. Inflammation 39, 2016–2028 (2016). https://doi.org/10.1007/s10753-016-0438-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-016-0438-8