Abstract
The main objective of this study was to investigate the potential efficacy of carvacrol (CAR) in mitigating bleomycin (BLM)-induced pulmonary fibrosis (PF). Sixty-six male Wistar rats were assigned into two main groups of 7 and 21 days. They were divided into the subgroups of control, BLM, CAR 80 (only for the 21-day group), and CAR treatment groups. The CAR treatment groups received CAR (20, 40, and 80 mg/kg, orally) for 7 or 21 days after an instillation of BLM (5 mg/kg, intratracheally). Results indicated that BLM significantly increased total cell count in bronchoalveolar lavage fluid and the percentages of neutrophils and lymphocytes, and reduced the percentage of macrophages. CAR dose-dependently decreased total cell count and the percentage of neutrophils and lymphocytes. CAR significantly reduced thiobarbituric acid reactive substances and hydroxyproline levels and elevated the total thiol level and catalase, superoxide dismutase, and glutathione peroxidase activities in BLM-exposed rats. Furthermore, CAR decreased the transforming growth factor-β1, connective transforming growth factor, and tumor necrosis factor-α on days 7 and 21. BLM increased interferon-γ on day 7 but decreased its level on day 21. However, CAR reversed interferon-γ levels on days 7 and 21. Based on histopathological findings, BLM induced inflammation on days 7 and 21, but for induction of fibrosis, 21-day study showed more fibrotic injuries than the 7-day group. CAR showed the improvement of fibrotic injuries. The effect of CAR against BLM-induced pulmonary fibrosis is possibly due to its antioxidant, anti-inflammatory, and antifibrotic activity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pulmonary fibrosis (PF) is the serious adverse effect of bleomycin therapy in up to 10% of patients and leads to death in 1–3% of them. Proliferation of fibroblasts, excessive deposition of collagen, and loss of lung function are all symptoms of this condition (Liu et al. 2017; Shariati et al. 2019b; Zhang et al. 2019). Bleomycin (BLM)-induced PF occurs probably due to oxidative stress, low level of BLM hydrolase in the lung, genetic susceptibility, and amplification of fibrotic cytokines (Ge et al. 2018; Shariati et al. 2019a). Since the Food and Drug Administration (FDA) approved drugs for the PF have poor efficacy in most patients, further investigations are essential to find antifibrotic drugs with high efficacy and low adverse effects (Canestaro et al. 2016). According to some research, inflammatory cells can generate reactive species, pro-inflammatory cytokines, proteases, peroxidases, and growth factors like transforming growth factor beta-1 (TGF-β1) can harm lung tissue (Lee et al. 2010; Zhang et al. 2015; Rangarajan et al. 2018). The most critical cytokines in the pathogenesis of PF include TGF-β1, connective tissue growth factor-β (CTGF-β), tumor necrosis factor-α (TNF-α), and interferon-γ (IFN-γ). The imbalance of these mediators leads to the proliferation of fibroblasts and activation of myofibroblasts, which causes massive collagen accumulation, lung tissue remodeling, and respiratory impairment (Zaghloul et al. 2019; Zhao et al. 2019). Therefore, suppression of inflammatory cytokines secretion by anti-inflammatory agents is considered an important therapeutic target in PF (Zhao et al. 2019).
Carvacrol [C6H3CH3 (OH) (C3H7)] (CAR) is a phenolic monoterpene that has shown many pharmacological activities, including anti-inflammatory, antioxidant, antispasmodic, and tracheal muscle-relaxing effects (Sharifi‐Rad et al. 2018, Silva et al. 2018).
CAR, a monoterpenoid phenolic compound found in plants like oregano and thyme, exhibits wide therapeutic effects, including reducing respiratory inflammation. In Iranian traditional medicine, Zataria multiflora Boiss (Labiatae) is utilized to manage respiratory conditions like colds and bronchitis, with CAR being a key component in Zataria multiflora, thyme oil, and oregano oil. Studies have confirmed CAR's anti-proliferative and anti-cancer properties in human lung cancer (Dai et al. 2016; Khazdair and Boskabady 2019).
Furthermore, CAR showed a protective effect in ovalbumin-induced airway inflammation, chronic obstructive pulmonary (COPD) disease in guinea pigs (Boskabady et al. 2014). In a similar study, the protective effect of CAR on the COPD model caused by cigarette smoke in guinea pigs has been proven (Mahtaj et al. 2015). A clinical trial study found that CAR was effective in improving asthma and reducing respiratory symptoms due to its anti-inflammatory effect (Alavinezhad et al. 2018). According to the antioxidant and anti-inflammatory effects observed in different experimental models and in humans (Alavinezhad et al. 2018; Khazdair and Boskabady 2019; Ezz-Eldin et al. 2020), this research is focused on investigating the potential effects of CAR on BLM-induced PF in rats.
Materials and methods
Drugs
BLM sulfate was purchased from Nippon Kayaku (Tokyo, Japan). CAR and other reagents were purchased from Sigma–Aldrich. CAR and BLM were dissolved in normal saline (NS) immediately before administration.
Animals
Male Wistar rats (weighing 200–250 g) were obtained from Research Center & Experimental Animal House of Ahvaz Jundishapur University of Medical Sciences (AJUMS) Ahvaz, Iran. The study protocol was approved by the Institutional Animal Ethical Committee of AJUMS (IR.AJUMS.REC.1396.256).
Induction of PF by BLM
The BLM-induced PF model is a reproducible and practical in vivo model in rats (Moeller et al. 2008).This model generates an initial inflammatory phase (day 7) and a late fibrotic phase (day 21) in the lung (Chaudhary et al. 2006). A pilot study was performed to determine the appropriate dose of BLM to produce typical fibrosis without changing the survival rate. After induction of anesthesia with ketamine and xylazine, the PF was induced in rats by single-dose intratracheal (i.t.) administration of BLM, 5 mg/kg BW (body weight), in 0.25 mL fresh NS on day 1. The control group received NS (i.t.) with an equal volume (Şener et al. 2007; Egger et al. 2013).
Experimental design
The study was conducted at two-time end points of 7 and 21 days to distinguish the anti-inflammatory and antifibrotic mechanisms. Sixty-six rats were assigned into two main groups at random:
-
A)
7- day duration with five subgroups (n = 6):
-
I. The control group received a single dose of NS (i.t.) + NS (5 mL/kg/day) by gavage for 7 days;
-
II. BLM group received a single dose of BLM (i.t.) + NS (5 mL/kg/day) by gavage for 7 days;
-
III-V. Treatment groups received a single dose of BLM (i.t.) + CAR at the doses of 20, 40 and 80 mg/kg/day by gavage for 7 days.
-
-
B)
21- day duration with six subgroups (n = 6):
-
I. The control group received a single dose of NS (i.t.) + NS (5 mL/kg/day) by gavage for 21 days;
-
II. BLM group received a single dose of BLM (i.t.) + NS (5 mL/kg/day), by gavage for 21 days;
-
III-V. Treatment groups received a single dose of BLM (i.t.) + CAR at the doses of 20, 40 and 80 mg/kg/day by gavage for 21 days;
-
VI. The CAR control group only received CAR (80 mg/kg/day) by gavage for 21 days. CAR control group evaluates the possible pulmonary toxicity effect of CAR on the lung.
-
CAR doses were selected based on a previous study showing that CAR at these doses was well tolerated in rats and significantly attenuated acute pulmonary injury (Feng and Jia 2014).
Animals were euthanized on days 7 and 21 after BLM administration, and lung tissue was harvested. During the experiment, the BW of the rats was measured, and weight changes were analyzed. Lung weight was normalized with BW as pulmonary index of all groups. The schematic diagram of the experimental design is illustrated in Fig. 1.
Lung sample and bronchoalveolar lavage fluid (BALF) collection
The lungs were lavages four times with 5 mL of phosphate-buffered saline solution. After that, the BALFs were centrifuged at 1500 × g for 10 min at 4 °C, and total leukocyte count and differential percentages of neutrophils, macrophages, and lymphocytes were calculated using the Wright-Giemsa staining and hemocytometer. To prepare a 10% homogenate (w/v), the right lobe of lung tissue was excised and homogenized in the radioimmunoprecipitation (RIPA) lysis buffer solution. The supernatants were kept at –80 °C after centrifugation for further analysis. The total protein content of tissue homogenates was determined using the Bradford technique (Bradford 1976).
Lung index calculation
The lung index (%) was calculated as the ratio of wet lung weight (g) to body weight (g) multiplied by 100.
Hydroxyproline (HP) content measurement
The colorimetric assay of Edwards and O’Brien was carried out to measure the lung tissue HP concentration (Edwards and O'Brien Jr 1980). The wavelength of 550 nm was used to measure the absorbance of the red chromophore complex. Finally, the HP content was measured and reported as mg/g lung tissue.
Thiobarbituric acid reactive substances (TBARS) level estimation
The thiobarbituric acid method was used to determine the TBARS level in lung tissue homogenates (Uchiyama and Mihara 1978). A pink luminous complex is formed at high temperatures and low pH. The absorbance was read at 532 nm and the results were reported as nmol/mg protein.
Determination of total thiol (TT) content
The TT content in lung tissue homogenates was determined using Ellman’s method (Ellman 1959). The amount of TT was calculated using extrapolation from standard curve, then the TT content was reported as µmol/mg protein. Briefly, concentrations of 0.2–0.8 mM glutathione (GSH) were prepared from a 1 mM GSH stock solution (15.4 mg of GSH in 50 mL of 0.1 N HCL solution). Next, 20 μl of standard samples and tissue homogenate samples were mixed with 230 μl of phosphate buffer (pH 7.6) and 50 μl of 1 mM 5,5'-dithiobis-(2-nitrobenzoic acid) (DTNB), and incubated for 5 min at room temperature. The absorbance of the samples was read at a wavelength of 412 nm.
Determination of antioxidant enzyme activity
The RANSOD (Cat. No. SD125, Randox Labs, Crumlin, UK) and RANSEL (Cat. No. RS 504, Randox Labs, Crumlin, UK) kits were used to measure, respectively, SOD and GPx activity in tissue homogenates as per the manufacturer’s instructions. The GPx and SOD activity was reported as U/mg protein. Goth's colorimetric assay was conducted to measure the catalase (CAT) activity (Goth 1991). The absorbance of the yellow complex was read at 410 nm, and the results were reported as µmol/min/mg protein.
Measurement of cytokines
IFN-γ, TNF-α, and TGF-β tissue levels were evaluated using French commercial ELISA kits (Diaclone Research) according to the manufacturer's protocol. The CTGF-β concentration was detected by an ELISA kit (MyBioSource). The cytokine levels of tissue homogenates were reported as pg/mg protein.
Histological studies
The left lobe of the lungs was fixed in buffer formaldehyde (10%). The specimens were embedded in paraffin, cut into 5 µm sections, and stained with Masson's trichrome and hematoxylin and eosin (H&E). An experienced histologist conducted a single-blind examination by a light optical microscope. The inflammatory and fibrosis score were defined according to the Szapiel scoring method (0–3) (Szapiel et al. 1979) and modified numerical Ashcroft scoring system (0–8) (Ashcroft et al. 1988), respectively. Ten randomly selected fields from each of the three slides per animal were examined for grading fibrosis and inflammation. Magnificence of all histopathological figures was 100 x.
Statistical analysis
All results were reported as mean ± SD. The BW changes between groups were analyzed by the Kruskal–Wallis test followed by Dunn’s test, and other results were analyzed by one-way ANOVA followed by Tukey’s Post hoc test. The statistical analysis was performed using the GraphPad Prism version 9.
Results
Effect of CAR on BW and lung index
The effect of CAR on BW (on days 7, 14, and 21) and lung index (on days 7 and 21) in BLM-treated rats is indicated in Fig. 2A and B, respectively. BLM significantly reduced BW at all time points and increased lung index compared to the control group on day 7 (p < 0.05 and p < 0.001, respectively). While treatment with CAR did not cause a significant increase in BW compared to the BLM group at any time, a dose of 80 mg/kg of CAR led to a significant decrease in lung index compared to the BLM group (p < 0.001). In addition, on day 21, the lung index significantly increased in the BLM-treated group compared to the control group (p < 0.001), and CAR treatment (40 and 80 mg/kg) significantly decreased it compared to the BLM group (p < 0.001).
Effect of CAR (carvacrol) (20, 40 and 80 mg/kg/day, orally) on the (A) body weight (on days 7, 14, and 21) and (B) lung index (on days 7 and 21) after intratracheal instillation of BLM (bleomycin) (5 mg/kg) in rats. Data are reported as mean ± SD (n = 6). *p < 0.05 and ***p < 0.001 vs. respective control group. ###p < 0.001 vs. respective BLM group
Effect of CAR on inflammatory cells in lung BALF
On day 7, BLM markedly elevated total cell count (Fig. 3A, p < 0.001) and the percentages of neutrophils (Fig. 3B, p < 0.001) and lymphocytes (Fig. 3D, p < 0.001), and reduced the percentage of macrophages (Fig. 3C, p < 0.001) in BALF in comparison with the control group. However, on day 7, CAR at doses of 40 and 80 mg/kg significantly decreased total cell count (Fig. 3A) and the percentages of neutrophils (Fig. 3B) and lymphocytes (Fig. 3D), and at a dose of 80 mg/kg, it remarkably elevated the percentage of macrophages (Fig. 3C, p < 0.001) in BALF compared to the BLM-treated group. Also, on day 21, BLM significantly increased total cell count (Fig. 3A, p < 0.001) and the percentages of neutrophils (Fig. 3B, p < 0.001) and lymphocytes (Fig. 3D, p < 0.001), and diminished the percentage of macrophages (Fig. 3C, p < 0.001) in BALF compared to the control group. However, on day 21, CAR at doses of 40 and 80 mg/kg markedly decreased total cell count (Fig. 3A) and the percentages of neutrophils (Fig. 3B) and lymphocytes (Fig. 3D) in BALF compared to the BLM-treated group. In addition, CAR at a dose of 80 mg/kg was able to increase the percentage of macrophages in the BALF compared to the BLM group (Fig. 3C. p < 0.05).
Effect of CAR (carvacrol) (20, 40, and 80 mg/kg/day, orally) on inflammatory cells in lung BALF (bronchoalveolar lavage fluid) on days 7 and 21 after intratracheal instillation of BLM (bleomycin) (5 mg/kg) in rats. A total cells, B neutrophil, C macrophage, and (D) lymphocytes. Data are reported as mean ± SD (n = 6). ***p < 0.001 vs. respective control group. #p < 0.05, ##p < 0.01, and ###p < 0.001 vs. respective BLM group
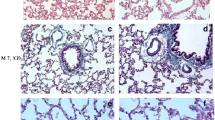
Effect of CAR on BLM-induced lung histological changes
The effect of CAR on lung histological changes induced by BLM on days 7 and 21 is shown in Fig. 4A and B, respectively. The results of lung histopathological study using H&E and Masson’s trichrome staining on day 7 indicated that the control group had normal lung structure (a thin alveolar wall and no collagen deposition). Administration of BLM led to severe pathological changes, such as inflammatory cell infiltration in alveolar spaces, destruction of alveoli, mild fibrosis, and edematous alveolar walls (Fig. 4A). These extensive histological changes induced by BLM were mildly ameliorated by CAR 40 mg/kg and significantly decreased by CAR 80 mg/kg. On day 21, control group demonstrated normal lung appearance. In the BLM-treated group, there was an accumulation of inflammatory cells (predominantly lymphocytes) and collagen deposition in the interstitial space (Fig. 4B). The significant histological changes induced by BLM were slightly improved with a dose of 40 mg/kg of CAR and significantly resolved with a dose of 80 mg/kg of CAR. In Masson's trichrome staining, C represents collagen accumulation (blue strings). In H&E staining, I and E represent the infiltration of inflammatory cells and exudate, respectively.
Effect of CAR (carvacrol) (20, 40, and 80 mg/kg/day, orally) on lung histological changes in rats stained with H&E (hematoxylin and eosin) and Masson’s trichrome on days (A) 7 and (B) 21 after intratracheal instillation of BLM (bleomycin) (5 mg/kg). The magnification of all histopathological figures was 100x. C (collagen accumulation), I (infiltration of inflammatory cells), and E (exudate)
Effect of CAR on the scores of alveolitis and fibrosis
As illustrated in Fig. 5A and B the scores of alveolitis and fibrosis on day 7 significantly increased in the BLM group in comparison with the control group, respectively (p < 0.001). However, CAR (40 and 80 mg/kg) significantly reduced the alveolitis score (Fig. 5A, p < 0.05 and p < 0.001, respectively), and at a dose of 80 mg/kg, it also decreased the fibrosis score (Fig. 5B, p < 0.001). Moreover, the results of day 21 showed that the administration of BLM increased the scores of alveolitis and fibrosis compared to the control group (Fig. 5A, p < 0.001) and treatment with CAR (40 and 80 mg/kg) remarkably reversed these increases.
Effect of CAR (carvacrol) (20, 40, and 80 mg/kg/day, orally) on (A) alveolitis score and (B) fibrosis score in the lung tissue of rats on days 7 and 21 after intratracheal instillation of BLM (bleomycin) (5 mg/kg). Data are reported as mean ± SD (n = 6). ***p < 0.001 vs. respective control group. #p < 0.05 and ###p < 0.001 vs. respective BLM group
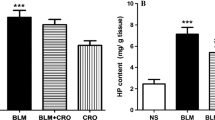
Effect of CAR on HP content in the lung tissue
The effect of CAR on HP levels on days 7 and 21 after administration of BLM was demonstrated in Fig. 6. BLM significantly increased the lung HP content compared to the control group (p < 0.001) on day 7, and treatment with CAR (40 and 80 mg/kg) markedly reduced the amount of HP in comparison with the BLM group (p < 0.05 and p < 0.001, respectively). Additionally, on day 21, the levels of HP remarkably elevated in BLM group compared to the control group (p < 0.001) and CAR treatment (40 and 80 mg/kg) significantly decreased the levels of HP in comparison with the BLM group (p < 0.01 and p < 0.001, respectively).
Effect of CAR (carvacrol) (20, 40, and 80 mg/kg/day, orally) on hydroxyproline levels in the lung tissue of rats on days 7 and 21 after intratracheal instillation of BLM (bleomycin) (5 mg/kg). Data are reported as mean ± SD (n = 6). ***p < 0.001 vs. respective control group. #p < 0.05, ##p < 0.01, and ###p < 0.001 vs. respective BLM group
Effect of CAR on oxidative stress markers in the lung tissue
On day 7, BLM-treated rats showed a significant elevation in TBARS level in comparison with the control group (Fig. 7A, p < 0.001). Also, the levels of total thiol and the activities of CAT, GPx, and SOD enzymes significantly decreased compared to the control group (Fig. 7B, C, D, and E, respectively, p < 0.001). However, treatment with CAR (40 and 80 mg/kg) markedly reversed the changes in oxidative stress indices compared to the BLM group (p < 0.001). Moreover, on day 21, administration of BLM led to a marked increase in TBARS level compared to the control group (Fig. 7A, p < 0.001). Also, the levels of total thiol and the activities of CAT, GPx, and SOD enzymes significantly decreased in comparison with the control group (Fig. 7B, C, D, and E, respectively, p < 0.001). However, CAR at a dose of 80 mg/kg significantly reduced TBARS level (p < 0.001) and at doses of 40 and 80 mg/kg, it restored total thiol content and the activities of CAT, GPx, and SOD enzymes compared to the BLM group.
Effect of CAR (carvacrol) (20, 40, and 80 mg/kg/day, orally) on oxidative stress markers in the lung tissue of rats on days 7 and 21 after intratracheal instillation of BLM (bleomycin) (5 mg/kg). A thiobarbituric acid reactive substances, B total thiol content, C CAT (catalase) activity, D GPx (glutathione peroxidase) activity, and (E) SOD (superoxide dismutase) activity. Data are reported as mean ± SD (n = 6). ***p < 0.001 vs. respective control group. ##p < 0.01 and ###p < 0.001 vs. respective BLM group
Effect of CAR on cytokines in lung tissue
The effect of CAR on cytokines in lung tissue is presented in Fig. 8. Results showed that on day 7, BLM significantly increased TNF-α, INF-γ, TGF-β1, and CTGF-β levels compared to the control group (Fig. 8A, B, C, and D, respectively, p < 0.001). However, CAR (80 mg/kg, as the maximum effective dose in this study) significantly reduced the levels of these cytokines in comparison with the BLM group (p < 0.001). In addition, on day 21, the levels of TNF-α (Fig. 8A, p < 0.05), TGF-β1 (Fig. 8C, p < 0.001), and CTGF-β (Fig. 8D, p < 0.001) remarkably enhanced, and INF-γ level (Fig. 8B, p < 0.01) significantly reduced in the BLM-treated group compared to control group. Treatment with CAR markedly reversed the BLM-induced alterations in cytokine levels.
Effect of CAR (carvacrol) (20, 40, and 80 mg/kg/day, orally) on the levels of (A) TNF-α (tumor necrosis factor-α), B INF-γ (interferon-γ), C TGF-β1 (transforming growth factor-β1), and (D) CTGF-β (connective tissue growth factor-β) in the lung tissue of rats on days 7 and 21 after intratracheal instillation of BLM (bleomycin) (5 mg/kg). Data are reported as mean ± SD (n = 4). *p < 0.05, **p < 0.01, and ***p < 0.001 vs. respective control group. #p < 0.05 and ###p < 0.001 vs. respective BLM group
Discussion
The BLM-induced PF model is one of the most well-known animal models of inflammation and pulmonary fibrosis that has been mentioned in many studies. The inflammatory phase is usually studied in 7 days and the fibrotic phase in 14 or 21 days (Chaudhary et al. 2006; Gauldie and Kolb 2008; Liu et al. 2017). Despite the substantial morbidity and mortality linked to PF, there is currently no effective treatment available that efficiently reverses the underlying pathologies of this disease. Recently, several therapeutic regimens have been discovered that are powerful and effective in the treatment of PF in preclinical stages. However, the non-selectivity and non-specificity of therapeutic molecules also lead to severe side effects (Diwan et al. 2023). Therefore, it is crucial to conduct further research on the therapeutic potential of safe compounds in managing BLM-induced PF. One of these compounds is CAR, which has been approved by FDA as an food additive, confirming its non-toxicity (Mączka et al. 2023). In the present study, during the investigation of the effects of CAR on the alterations of assessed factors in BLM-induced PF, CAR alone group at a dose of 80 mg/kg after 21 days did not cause any significant changes in the investigated factors compared to the control group. This finding showed that CAR had no toxicity on lung tissue. Carvacrol has anti-inflammatory and antioxidant effects, especially in the respiratory system (Şen et al. 2014; Carvalho et al. 2020; de Carvalho et al. 2020b). Therefore, in this study, the effects of CAR on PF caused by BLM were investigated.
Typically, in animal models, lung index and BALF analysis are used as inflammatory indices (Kenyon et al. 2002; Kadam and Schnitzer 2023). White blood cell infiltration, known as leukocytic infiltration, is a notable feature seen in lung inflammation and fibrosis. It is thought to play a role in the development of fibrosis by triggering or exacerbating the fibrotic response within the lung tissues (Gross and Hunninghake 2001). The administration of BLM in rodent models leads to a cascade of events, starting with the increased recruitment of neutrophils to the affected site. This influx of neutrophils plays a crucial role in the subsequent development of fibrosis, a process characterized by the excessive accumulation of fibrous connective tissue (Herrmann et al. 2017). Myeloperoxidase released by neutrophils has the ability to cause cell damage. Neutrophils release myeloperoxidase, which has the ability to trigger cellular damage (Klebanoff 1988). Fibrosis developed as a result of the activation of fibroblasts caused by the infiltration of leukocytes, leading to a hyperproliferative response that altered the functional properties of the alveoli (Chandler et al. 1983). In our study, the total cell counts, neutrophils, and lymphocyte percentage increased in the BLM groups of two endpoint times, and CAR in different doses was able to reverse them. In many studies these results were consistent with the results of our study (Feng and Jia 2014; Mahtaj et al. 2015). In our study, administration of BLM significantly reduced BW and increased lung index, which may be due to the inflammation and fibrosis, and CAR reversed the changes of BW and lung index by its anti-inflammatory and anti-fibrotic effects. These results were consistent with the results of our previous studies (Mirzaee et al. 2019; Shariati et al. 2019a). In our study, according to the histopathological examination and BALF cell count results, CAR reduced the infiltration of inflammatory cells in the lung. BLM produces reactive oxygen species (ROS), leading to DNA chain breakage and lipid peroxidation. The excess ROS in the lung leads to alveolar epithelial damage, inflammation, and activation of various fibrotic signaling pathways such as TGF-β1 (Zaghloul et al. 2019). Moreover, TGF-β1 induces oxidative stress by producing ROS (Barratt et al. 2018). ROS activate various redox-sensitive signaling cascades that caused activation of the inflammatory and profibrotic cytokines such as TNF-α, interleukin (IL-1), and growth factors. It is argued that this is one of the most important causes of lung injury (Ozer et al. 2017; Cui et al. 2019). GSH is an intracellular thiol that protects the lung against oxidative stress and inflammatory responses (Rahman and MacNee 2000). CAT, GPx, and SOD are essential constituents of the antioxidant enzyme defense system and are responsible for the detoxification of free radicals in the lung (Day 2008). In our study, BLM increased lipid peroxidation and impaired antioxidant defense system. CAR treatment decreased TBARS level and increased the level of TT and the activity of CAT, GPx, and SOD enzymes in both inflammatory and fibrotic phases in the lung. These findings are consistent with prior research demonstrating antioxidant and free radical scavenging action of CAR and other pharmacological interventions such as sumatriptan in pulmonary system (Kianmehr et al. 2016; Bahramifar et al. 2024). The hydroxyl group connected to the aromatic ring in CAR’s molecular structure is thought to be responsible for its antioxidant effect (de Carvalho et al. 2020b; Imran et al. 2022). These findings suggest that anti-inflammatory and antifibrotic activity of CAR is partly due to its antioxidant effect.
Chronic inflammation is a pathological signaling of PF, and an increase in the number of inflammatory cells in both the alveolar space and the interstitial tissue has been well documented in PF. Activated inflammatory cells produce ROS and various cytokines such as TNF-α, IFN-γ, TGF-β1, and CTGF-β, which lead to proliferation, migration of fibroblasts/myofibroblasts, and fibrosis (Barratt et al. 2018). In accordance with previous research, BLM raised the alveolitis score, presence of inflammatory cells, and production of TNF-α and INF-γ in the lung tissue (Kandhare et al. 2015). INF-γ is one of the inflammatory factors that also has anti-fibrotic properties (Gurujeyalakshmi and Giri 1995). This role of INF-γ is evident in the present study. IFN-γ exhibited a pro-inflammatory effect in the inflammatory phase, but has an antifibrotic effect in the fibrosis phase. The antifibrotic effect of IFN-γ is achieved by preventing fibroblasts from transforming into myofibroblasts and consequently reducing the production of collagen and α-smooth muscle actin (α-SMA), potentially by neutralizing the effects of TGF-β1 (Vu et al. 2019). The antifibrotic activity of CAR was confirmed by reduction in HP level, score of fibrosis, TGF-β1, and CTGF-β and reserving pathological changes in the lung induced by BLM. In similar studies, CAR and other antioxidants reduced inflammation and fibrosis (Akgedik et al. 2012; Kianmehr et al. 2016; Shariati et al. 2019a; Bahramifar et al. 2024; Mady et al. 2024). CAR markedly decreased the TNF-α level in the lung tissue at both inflammatory and fibrotic phases, which possibly is due to inhibition of inflammatory cell influx into the lung (Kianmehr et al. 2016; de Carvalho et al. 2020a). TGF-β1 is the most intense fibrotic cytokine, and it is involved in the development of fibrotic illness and cancer. TGF-β1 induces profibrotic effectors such as CTGF-β, α-SMA, and collagen in activated fibroblasts (Fisher et al. 2017). In addition, it increases ROS production and serves as a chemotactic agent for macrophages. CTGF-β is a major mediator of tissue remodeling and fibrosis that is involved in angiogenesis, cell migration, and adhesion, as well as fibroblast activation. There is strong evidence that downregulation of TGF-β1 and CTGF-β decreases the expression of collagen that can be considered as a therapeutic choice for PF and other malignancies (Katsuno et al. 2013; Barratt et al. 2018). In this study, TGF-β1 and CTGF-β levels significantly increased after BLM instillation; meanwhile, CAR could effectively reverse these fibrotic markers. Kianmehr et al. found that CAR inhibited expression of TGF-β1 gene in the splenocytes of ovalbumin-sensitive mice, which is consistent with our findings (Kianmehr et al. 2016). According to these results, it seems that the antifibrotic effect of CAR is due to the downregulation of TGF-β1 and inhibition of its downstream signaling pathways.
To prevent PF induced by BLM in patients receiving this drug, it is important to use agents along with BLM that are both safe and effective, and do not interfere with the anticancer activity of BLM. In a study by Fan et al. (Fan et al. 2015), it was indicated that CAR suppressed proliferation and caused apoptosis in human colon cancer cells. In another study conducted by Ahmad and Ansari (Ahmad and Ansari 2021), CAR reduced the growth of cervical cancer cells by inhibiting cell cycle progression and caspase-dependent apoptosis. Also, studies have demonstrated that CAR enhances the apoptotic effect of 5-FU on the MCF-7 cell line (Azimi et al. 2022). However, the effect of CAR on the anticancer activity of BLM has not been investigated and it can be evaluated in future studies. Considering the antioxidant, anti-inflammatory and anti-fibrotic properties demonstrated by CAR in this study, it can be considered as an adjunctive therapeutic agent in combination with chemotherapy regimens such as BLM in the future. As limitations of the present study, fibrotic factors such as the level of α-SMA and inflammatory markers such as NF-κB and MPO enzyme activity were not investigated, which can be evaluated in future studies. Furthermore, future research could explore potential strategies for targeted delivery of CAR to the lungs.
Conclusions
Our study revealed that CAR alleviated BLM-induced PF, probably through inhibiting oxidative stress, inflammation, and fibrosis (Fig. 9). Therefore, CAR can be considered as a therapeutic approach for the prevention and treatment of PF in patients receiving BLM.
Data availability
The data of the present study are available from the corresponding author upon reasonable request.
References
Ahmad A, Ansari IA (2021) Carvacrol exhibits chemopreventive potential against cervical cancer cells via caspase-dependent apoptosis and abrogation of cell cycle progression. Anti Cancer Agents Med Chem (Formerly Current Medicinal Chemistry-Anti-Cancer Agents) 21:2224–2235
Akgedik R, Akgedik Ş, Karamanlı H, Uysal S, Bozkurt B, Ozol D, Armutcu F, Yıldırım Z (2012) Effect of resveratrol on treatment of bleomycin-induced pulmonary fibrosis in rats. Inflammation 35:1732–1741
Alavinezhad A, Khazdair MR, Boskabady MH (2018) Possible therapeutic effect of carvacrol on asthmatic patients: A randomized, double blind, placebo-controlled, Phase II clinical trial. Phytother Res 32:151–159
Ashcroft T, Simpson JM, Timbrell V (1988) Simple method of estimating severity of pulmonary fibrosis on a numerical scale. J Clin Pathol 41:467–470
Azimi S, Esmaeil Lashgarian H, Ghorbanzadeh V, Moradipour A, Pirzeh L, Dariushnejad H (2022) 5-FU and the dietary flavonoid carvacrol: a synergistic combination that induces apoptosis in MCF-7 breast cancer cells. Med Oncol 39:253
Bahramifar A, Jafari RM, Sheibani M, Manavi MA, Rashidian A, Tavangar SM, Akbariani M, Mohammadi Hamaneh A, Goudarzi R, Shadboorestan A, Dehpour AR (2024) Sumatriptan mitigates bleomycin-induced lung fibrosis in male rats: Involvement of inflammation, oxidative stress and α-SMA. Tissue Cell 88:102349
Barratt SL, Creamer A, Hayton C, Chaudhuri N (2018) Idiopathic pulmonary fibrosis (IPF): an overview. J Clin Med 7:201
Boskabady MH, Tabatabaee A, Jalali S (2014) Potential effect of the extract of Zataria multiflora and its constituent, carvacrol, on lung pathology, total and differential WBC, IgE and eosinophil peroxidase levels in sensitized guinea pigs. J Funct Foods 11:49–61
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Canestaro WJ, Forrester SH, Raghu G, Ho L, Devine BE (2016) Drug treatment of idiopathic pulmonary fibrosis: systematic review and network meta-analysis. Chest 149:756–766
Carvalho FO, Silva ÉR, Nunes PS, Felipe FA, Ramos KP, Ferreira LAS, Lima VN, Shanmugam S, Oliveira AS, Guterres SS (2020) Effects of the solid lipid nanoparticle of carvacrol on rodents with lung injury from smoke inhalation. Naunyn Schmiedebergs Arch Pharmacol 393:445–455
Chandler DB, Hyde DM, Giri S (1983) Morphometric estimates of infiltrative cellular changes during the development of bleomycin-induced pulmonary fibrosis in hamsters. Am J Pathol 112:170
Chaudhary NI, Schnapp A, Park JE (2006) Pharmacologic differentiation of inflammation and fibrosis in the rat bleomycin model. Am J Respir Crit Care Med 173:769–776
Cui Y, Jiang L, Yu R, Shao Y, Mei L, Tao Y (2019) β-carboline alkaloids attenuate bleomycin induced pulmonary fibrosis in mice through inhibiting NF-kb/p65 phosphorylation and epithelial-mesenchymal transition. J Ethnopharmacol 243:112096
Dai W, Sun C, Huang S, Zhou Q (2016) Carvacrol suppresses proliferation and invasion in human oral squamous cell carcinoma. Onco Targets Ther 9:2297
Day BJ (2008) Antioxidants as potential therapeutics for lung fibrosis. Antioxid Redox Signal 10:355–370
de Carvalho FO, Silva ÉR, Gomes IA, Santana HSR, do Nascimento Santos D, de Oliveira Souza GP, de Jesus Silva D, Monteiro JCM, de Albuquerque Júnior RLC, de Souza Araújo AA, Nunes PS (2020a) Anti-inflammatory and antioxidant activity of carvacrol in the respiratory system: A systematic review and meta-analysis. Phytother Res 34:2214–2229
de Carvalho FO, Silva ÉR, Gomes IA, Santana HSR, do Nascimento Santos D, de Oliveira Souza GP, de Jesus Silva D, Monteiro JCM, de Albuquerquejúnior RLC, de Souzaaraújo AA (2020b) Anti-inflammatory and antioxidant activity of carvacrol in the respiratory system: A systematic review and meta-analysis. Phytother Res 34:2214–2229
Diwan R, Bhatt HN, Beaven E, Nurunnabi M (2023) Emerging delivery approaches for targeted pulmonary fibrosis treatment. Adv Drug Deliv Rev 204:115147
Edwards C, O’Brien W Jr (1980) Modified assay for determination of hydroxyproline in a tissue hydrolyzate. Clin Chim Acta 104:161–167
Egger C, Cannet C, Gérard C, Jarman E, Jarai G, Feige A, Suply T, Micard A, Dunbar A, Tigani B (2013) Administration of bleomycin via the oropharyngeal aspiration route leads to sustained lung fibrosis in mice and rats as quantified by UTE-MRI and histology. PLoS ONE 8:e63432
Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82:70–77
Ezz-Eldin YM, Aboseif AA, Khalaf MM (2020) Potential anti-inflammatory and immunomodulatory effects of carvacrol against ovalbumin-induced asthma in rats. Life Sci 242:117222
Fan K, Li X, Cao Y, Qi H, Li L, Zhang Q, Sun H (2015) Carvacrol inhibits proliferation and induces apoptosis in human colon cancer cells. Anticancer Drugs 26:813–823
Feng X, Jia A (2014) Protective effect of carvacrol on acute lung injury induced by lipopolysaccharide in mice. Inflammation 37:1091–1101
Fisher AJ, Cipolla E, Varre A, Gu H, Mickler EA, Vittal R (2017) Potential mechanisms underlying TGF-β-mediated complement activation in lung fibrosis. Cell Mol Med: Open Access 3:14
Gauldie J, Kolb M (2008) Animal models of pulmonary fibrosis: how far from effective reality? Am J Physiol-Lung Cell Mol Physiol 294:L151–L151
Ge V, Banakh I, Tiruvoipati R, Haji K (2018) Bleomycin-induced pulmonary toxicity and treatment with infliximab: A case report. Clin Case Rep 6:2011
Goth L (1991) A simple method for determination of serum catalase activity and revision of reference range. Clin Chim Acta 196:143–151
Gross TJ, Hunninghake GW (2001) Idiopathic pulmonary fibrosis. N Engl J Med 345:517–525
Gurujeyalakshmi G, Giri S (1995) Molecular mechanisms of antifibrotic effect of interferon gamma in bleomycin-mouse model of lung fibrosis: downregulation of TGF-β and procollagen I and III gene expression. Exp Lung Res 21:791–808
Herrmann FE, Wollin L, Wirth J, Gantner F, Lämmle B, Wex E (2017) Olodaterol shows anti-fibrotic efficacy in in vitro and in vivo models of pulmonary fibrosis. Br J Pharmacol 174:3848–3864
Imran M, Aslam M, Alsagaby SA, Saeed F, Ahmad I, Afzaal M, Arshad MU, Abdelgawad MA, El-Ghorab AH, Khames A (2022) Therapeutic application of carvacrol: A comprehensive review. Food Sci Nutr 10:3544–3561
Kadam AH, Schnitzer JE (2023) Characterization of acute lung injury in the bleomycin rat model. Physiol Rep 11:e15618
Kandhare AD, Bodhankar SL, Mohan V, Thakurdesai PA (2015) Effect of glycosides based standardized fenugreek seed extract in bleomycin-induced pulmonary fibrosis in rats: decisive role of Bax, Nrf2, NF-κB, Muc5ac, TNF-α and IL-1β. Chem Biol Interact 237:151–165
Katsuno Y, Lamouille S, Derynck R (2013) TGF-β signaling and epithelial–mesenchymal transition in cancer progression. Curr Opin Oncol 25:76–84
Kenyon NJ, van der Vliet A, Schock BC, Okamoto T, McGrew GM, Last JA (2002) Susceptibility to ozone-induced acute lung injury in iNOS-deficient mice. Am J Physiol-Lung Cell Mol Physiol 282:L540–L545
Khazdair MR, Boskabady MH (2019) The effect of carvacrol on inflammatory mediators and respiratory symptoms in veterans exposed to sulfur mustard, a randomized, placebo-controlled trial. Respir Med 150:21–29
Kianmehr M, Rezaei A, Boskabady MH (2016) Effect of carvacrol on various cytokines genes expression in splenocytes of asthmatic mice. Iran J Basic Med Sci 19:402
Klebanoff S (1988) Phagocytic cell: products of oxygen metabolism. Inflammation: basic principles and clinical correlates 391–444
Lee S-H, Jang A-S, Kim Y-E, Cha J-Y, Kim T-H, Jung S, Park S-K, Lee Y-K, Won J-H, Kim Y-H (2010) Modulation of cytokine and nitric oxide by mesenchymal stem cell transfer in lung injury/fibrosis. Respir Res 11:1–14
Liu T, De Los Santos FG, Phan SH (2017) The Bleomycin Model of Pulmonary Fibrosis. In: Rittié L (ed) Fibrosis: Methods and Protocols. Springer New York, New York, pp 27–42
Mączka W, Twardawska M, Grabarczyk M, Wińska K (2023) Carvacrol—a natural phenolic compound with antimicrobial properties. Antibiotics 12:824
Mady B, Ibrahim HF, ElAziz MA, Basta M, Assem S, Ahmed Ali M, El Mottelib LMMAA (2024) The potential ameliorating effect of vitamin E on bleomycin − induced lung fibrosis in adult albino rats. Int Immunopharmacol 136:112375
Mahtaj LG, Feizpour A, Kianmehr M, Soukhtanloo M, Boskabady MH (2015) The effect of carvacrol on systemic inflammation in guinea pigs model of COPD induced by cigarette smoke exposure. Pharmacol Rep 67:140–145
Mirzaee S, Mansouri E, Shirani M, Zeinvand-Lorestani M, Khodayar MJ (2019) Diosmin ameliorative effects on oxidative stress and fibrosis in paraquat-induced lung injury in mice. Environ Sci Pollut Res 26:36468–36477
Moeller A, Ask K, Warburton D, Gauldie J, Kolb M (2008) The bleomycin animal model: A useful tool to investigate treatment options for idiopathic pulmonary fibrosis? Int J Biochem Cell Biol 40:362–382
Ozer EK, Goktas MT, Toker A, Bariskaner H, Ugurluoglu C, Iskit AB (2017) Effects of carvacrol on survival, mesenteric blood flow, aortic function and multiple organ injury in a murine model of polymicrobial sepsis. Inflammation 40:1654–1663
Rahman I, MacNee W (2000) Oxidative stress and regulation of glutathione in lung inflammation. Eur Respir J 16:534–554
Rangarajan S, Bone NB, Zmijewska AA, Jiang S, Park DW, Bernard K, Locy ML, Ravi S, Deshane J, Mannon RB (2018) Metformin reverses established lung fibrosis in a bleomycin model. Nat Med 24:1121–1127
Şen HS, Şen V, Bozkurt M, Türkçü G, Güzel A, Sezgi C, Abakay Ö, Kaplan I (2014) Carvacrol and pomegranate extract in treating methotrexate-induced lung oxidative injury in rats. Med Sci Monitor: Int Med J Exp Clin Res 20:1983
Şener G, Topaloğlu N, Şehirli AÖ, Ercan F, Gedik N (2007) Resveratrol alleviates bleomycin-induced lung injury in rats. Pulm Pharmacol Ther 20:642–649
Shariati S, Kalantar H, Pashmforoosh M, Mansouri E, Khodayar MJ (2019a) Epicatechin protective effects on bleomycin-induced pulmonary oxidative stress and fibrosis in mice. Biomed Pharmacother 114:108776
Shariati S, Khodayar MJ, Hemmati A, Goudarzi M, Kiani M, Rezaei A (2019b) The ameliorative effects of allopurinol on paraquat-induced pulmonary fibrosis in rats. Pharm Sci 25:11–16
Sharifi-Rad M, Varoni EM, Iriti M, Martorell M, Setzer WN, del Mar CM, Salehi B, Soltani-Nejad A, Rajabi S, Tajbakhsh M (2018) Carvacrol and human health: A comprehensive review. Phytother Res 32:1675–1687
Silva ER, de Carvalho FO, Teixeira LG, Santos NGL, Felipe FA, Santana HSR, Shanmugam S, Quintans Júnior LJ, de Souza Araújo AA, Nunes PS (2018) Pharmacological effects of carvacrol in in Vitro studies: a review. Curr Pharm Des 24:3454–3465
Szapiel SV, Elson NA, Fulmer JD, Hunninghake GW, Crystal RG (1979) Bleomycin-induced interstitial pulmonary disease in the nude, athymic mouse. Am Rev Respir Dis 120:893–899
Uchiyama M, Mihara M (1978) Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal Biochem 86:271–278
Vu TN, Chen X, Foda HD, Smaldone GC, Hasaneen NA (2019) Interferon-γ enhances the antifibrotic effects of pirfenidone by attenuating IPF lung fibroblast activation and differentiation. Respir Res 20:1–14
Zaghloul MS, Said E, Suddek GM, Salem HA (2019) Crocin attenuates lung inflammation and pulmonary vascular dysfunction in a rat model of bleomycin-induced pulmonary fibrosis. Life Sci 235:116794
Zhang Q, Guo Y, Dong R, Dai R, Zhou M (2015) Suppressor of cytokine signaling 1-modulated metalloproteinases and tissue inhibitor of metalloproteinase in pulmonary fibrosis. Mol Med Rep 12:3855–3861
Zhang H-X, Li Y-N, Wang X-L, Ye C-L, Zhu X-Y, Li H-P, Yang T, Liu Y-J (2019) Probucol ameliorates EMT and lung fibrosis through restoration of SIRT3 expression. Pulm Pharmacol Ther 57:101803
Zhao L, Mu B, Zhou R, Cheng Y, Huang C (2019) Iguratimod ameliorates bleomycin-induced alveolar inflammation and pulmonary fibrosis in mice by suppressing expression of matrix metalloproteinase-9. Int J Rheum Dis 22:686–694
Funding
Acknowledgments and funding: This study was financially supported by the Toxicology Research Center and Vice Chancellor of Research at Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran (grant no. TRC-9602).
Author information
Authors and Affiliations
Contributions
M.P. Writing-Original draft preparation, Conceptualization, Methodology, Writing- Reviewing and Editing. H. RV. Conceptualization, Methodology, Investigation, Writing-Original draft preparation. L.K. Histopathological analysis. S.S. Writing- Reviewing and Editing. S.M. Formal analysis, Writing- Reviewing and Editing. MJ.K. Supervision, Conceptualization, Methodology, Data analysis, Investigation, Project administration, Funding Acquisition. The authors declare that all data were generated in-house and that no paper mill was used.
Corresponding author
Ethics declarations
Ethical approval
The study protocol was approved by the Institutional Animal Ethical Committee of AJUMS (IR.AJUMS.REC.1396.256).
Consent for publication
The authors confirm their consent for publication.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pashmforosh, M., Rajabi Vardanjani, H., Khorsandi, L. et al. Carvacrol protects rats against bleomycin-induced lung oxidative stress, inflammation, and fibrosis. Naunyn-Schmiedeberg's Arch Pharmacol (2024). https://doi.org/10.1007/s00210-024-03273-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00210-024-03273-7