Abstract
IFN-τ, which is a type I interferon with low cytotoxicity, is defined as a pregnancy recognition signal in ruminants. Type I interferons have been used as anti-inflammatory agents, but their side effects limit their clinical application. The present study aimed to determine the anti-inflammatory effects of IFN-τ in a lipopolysaccharide-stimulated acute lung injury (ALI) model and in RAW264.7 cells and to confirm the mechanism of action involved. The methods used included histopathology, measuring the lung wet/dry ratio, determining the myeloperoxidase activity, ELISA, qPCR, and western blot. The results revealed that IFN-τ greatly ameliorated the infiltration of inflammatory cells and the expression of TNF-α, IL-1β, and IL-6. Further analysis revealed that IFN-τ down-regulated the expression of TLR-2 and TLR-4 mRNA and the activity of the NF-κB and MAPK pathways both in a lipopolysaccharide-induced ALI model and in RAW264.7 cells. The results demonstrated that IFN-τ suppressed the levels of pro-inflammatory cytokines by inhibiting the phosphorylation of the NF-κB and MAPK pathways. Thus, IFN-τ may be an optimal target for the treatment of inflammatory diseases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
IFN-τ is a subclass of type I interferons that is secreted by the trophectoderm of ruminant conceptuses [1]. It is also known to be the pregnancy recognition signal [2]. It has been established that type I interferons such as IFN-α, INF-β, and INF-ω play crucial roles in antiviral and antimicrobial defenses and in the pathogenesis of various diseases [3, 4]. All type I interferons share a common heterodimeric IFNα/β receptor that is composed of IFNAR1 and IFNAR2 [5]. IFN-τ is a glycoprotein with a low molecular weight of approximately 20 kDa [6]. It has been shown that IFN-τ exhibits a broad cross-species reactivity and low levels of cytotoxicity in humans and other living animals [7]. In addition, it has been reported that treatment with IFN-τ results in an anti-inflammatory phenotype and increases tolerance in inflammatory diseases [8]. Recent studies have shown that IFN-τ inhibits obesity-associated inflammatory responses and the secretion of IL-1β [9, 10].
Acute lung injury (ALI) and acute respiratory distress syndrome are common complications that arise in the intensive care unit and contribute to remarkable morbidity and mortality [11]. LPS is widely used to induce ALI in animal models for the preclinical evaluation of anti-inflammatory drug candidates [12]. Recent studies have revealed that IFN-α could protect against ALI [3, 13] and exert numerous biological functions. Although IFN-α and IFN-β have been used as anti-inflammatory and viral therapies, adverse effects and cytotoxicity limit their clinical applications [14]. It had been reported that IFN-τ is able to inhibit the development of experimental allergic encephalomyelitis just as effectively as IFN-β, but without the associated toxicity [15].
ALI is usually induced with infection. It is well known that inflammation is essential for the host defense against invading pathogens and that the inflammatory response must be resolved after the pathogens are cleared because unchecked inflammation can cause tissue damage and organ failure in the host [16, 17]. The inflammatory response induces multiple negative feedback regulators [18]. For example, it activates intracellular signaling through MyD88 and TLRs and thereby leads to the activation of major MAP kinases (MAPKs) and the translocation of nuclear factor-κB (NF-κB) [19]. However, few studies have shown the effects of IFN-τ on LPS-induced inflammation. Thus, we aimed to investigate whether IFN-τ could protect against LPS-induced inflammation in a mouse model of ALI and in RAW264.7 macrophages. The present study reveals a potential clinical application for IFN-τ in the treatment of inflammatory diseases.
MATERIALS AND METHODS
Reagents
Recombinant ovine interferon-tau (rOvIFN-τ) [>97 % high-performance liquid chromatography (HPLC) purity] was purchased from Creative Bioarray (NY, USA). LPS (Escherichia coli 055:B5) was obtained from Sigma-Aldrich (St. Louis, USA). The IL-1β, IL-6, and TNF-α enzyme-linked immunosorbent assay (ELISA) kits were purchased from BioLegend (Camino Santa Fe, CA, USA). All of the antibodies were purchased from Cell Signaling Technology.
Animals and Treatment
A total of 90 BALB/c mice (6 weeks, 18–22 g) were obtained from the Experimental Animal Center of Wuhan University (Wuhan, China). All of the experiments followed the guidelines for the care and use of laboratory animals published by the US National Institutes of Health. All of the mice were maintained on a 12-h light/dark cycle and fed ad libitum.
The mice were randomly divided into six groups: a control group, an LPS groups, three LPS + IFN-τ groups (2, 4, and 8 μg/kg), and an LPS + dexamethasone (DEX, 5 mg/kg) group. The IFN-τ was dissolved in sterile water and given to the mice according to weight (the final concentrations were 2, 4, and 8 μg/kg). The creation of the LPS-induced ALI model was described previously [20]. Briefly, the mice were intranasally administered 50 μL of LPS (1 μg/μL) to induce ALI, and the mice in the control group were administered equal volumes of saline. After 24 h, the DEX group was intraperitoneally injected with 5 mg/kg DEX three times every 6 h. The control group and LPS group were given equal volumes of saline intraperitoneally. The LPS + IFN-τ groups were intraperitoneally injected with 2, 4, or 8 μg/kg IFN-τ three times every 6 h. The mice were euthanized with CO2, and the lung tissues were collected and stored at −80 °C until analysis.
Histological Assessment of the Lung Tissue
The lung tissues were collected and fixed with 10 % neutral-buffered formalin. The lung samples were embedded in paraffin, sliced, and then stained with hematoxylin and eosin (H&E). After staining, the pathological changes in the lung tissues were observed under a light microscope.
Lung W/D Ratio
The lung tissues were collected to calculate the ratio of wet lung to dry lung. The wet lung tissues were separated from the mice, and their weights were recorded. The lung tissues were subsequently incubated at 80 °C for 24 h to obtain the dry weight. The wet and dry weights were then used to calculate the wet/dry (W/D) ratio.
Myeloperoxidase Assay in Lung Tissues
The activity of MPO reflects the parenchymal infiltration of neutrophils and macrophages. The right lungs from each group were collected, homogenized, and analyzed with an assay kit (Jiangcheng Company, Nanjing, China) according to the manufacturer’s protocol. MPO activity was measured with a spectrophotometer at 460 nm.
Cell culture and treatment
RAW264.7 cells were purchased from the American Type Culture Collection (ATCC TIB-71™). The cells were cultured in DMEM containing 10 % FBS and incubated at 37 °C and 5 % CO2. The cells were pre-treated with IFN-τ (10, 20, or 40 ng/mL) for 1 h and then stimulated with LPS (0.5 μg/mL) for 24 h. Untreated cells served as control.
Cell Viability and Proliferation Assays
Cell viability and proliferation were examined using the 3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide (MTT) and CCK-8 methods, respectively. The cells (1 × 105 cells/mL) were plated in 96-well plates and incubated at 37 °C for 1 h. The cells were treated with IFN-τ (0–40 ng/mL) for 24 h. Following incubation with 20 μL of MTT (5 mg/mL) for 4 h at 37 °C, the supernatants were removed, and 100 μL of dimethyl sulfoxide was added to each well. The absorbance was read at 570 nm with a microplate reader. The proliferation of RAW264.7 cells was assessed using the Cell Counting Kit-8 (CCK-8) according to the manufacturer’s protocol. The cells were treated with IFN-τ (40 ng/mL) and LPS (0.5 μg/mL) for 0, 6, 12, or 24 h. The absorbance was read at 450 nm with a microplate reader (Thermo Scientific Multiskan MK3, USA).
ELISA Assay
The effects of IFN-τ on the levels of LPS-induced pro-inflammatory cytokines were determined in lung tissues and cells. The lung tissues were homogenized in phosphate-buffered saline and then centrifuged to collect the supernatants. The cell supernatants were also harvested. The levels of TNF-α, IL-1β, and IL-6 in these supernatants were detected by ELISA according to the manufacturer’s directions. The absorbance was read at 450 nm with a microplate reader (Thermo Scientific Multiskan MK3, USA).
qPCR Analysis
Total RNA was extracted from lung tissues and cells using Trizol according to the manufacturer’s recommendation (Invitrogen, USA) and then reverse transcribed into cDNA. The PCR was performed using the SYBR® Select Master Mix kit and a PCR system (Applied Biosystems, CA, USA). The expression level of each target gene was normalized to the corresponding GAPDH threshold cycle (Ct) values using the 2−ΔΔCt comparative method (Table 1).
Western Blotting
Total protein was harvested from lung tissues and cells using RIPA lysis buffer supplemented with a protease inhibitor. The protein concentrations were determined using the BCA protein assay kit. The proteins were then separated by SDS-PAGE and transferred to a PVDF membrane. After blocking for 2 h with 5 % nonfat dry milk, the membrane was incubated with the primary antibody for 12 h at 4 °C. The membrane was then incubated with the secondary antibody for 1 h at room temperature. The bound antibodies were detected using the ECL Plus Western Blotting Detection System (ImageQuant LAS 4000mini, USA).
Statistical Analysis
The SPSS15.0 software was used to analyze the data. The results are presented as the mean ± S.D. The comparisons between the groups were performed by ANOVA followed by Dunnett’s test. A p value of <0.05 was considered to be statistically significant.
RESULTS
IFN-τ Alleviates LPS-Induced Lung Injury
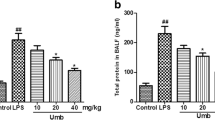
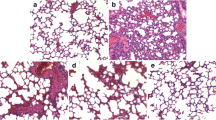
The lung tissues were collected, and lung injury was assessed by pathological sectioning, the W/D ratio, and the MPO assay. The results of the histopathology showed that the control group displayed a normal structure with no histopathological changes (Fig. 1a). The LPS group showed inflammatory cell infiltration, alveolar congestion, and incrassation in alveolar walls (Fig. 1b). In contrast, the IFN-τ treatment groups and the DEX group exhibited reduced inflammatory cell infiltration, alveolar congestion, and alveolar wall thickness (Fig. 1c–f). Figure 1g shows that the lung W/D ratio in the LPS group was significantly increased compared with the control group. The lung W/D ratio was significantly reduced in the DEX group. Compared with the LPS group, the IFN-τ groups showed a dose-dependent decrease in the lung W/D ratio. To confirm the effects of IFN-τ on LPS-induced ALI, we also measured MPO activity. The result showed that MPO activity in the LPS group was greatly increased compared with the control group. In contrast, MPO activity was significantly decreased in the DEX and IFN-τ treatment groups compared with the LPS group (Fig. 1h).
Effects of IFN-τ on LPS-induced lung injury. Histopathology of lung tissues after LPS stimulation (HE, ×200). a Control group. b LPS group. c LPS + DEX group. d–f IFN-τ (2, 4, and 8 μg/kg) treatment groups. g The lung W/D ratio. h The MPO assay. CG indicates the control group; LPS indicates the LPS-stimulated group; and L, M, and H indicate the groups administered 2, 4, and 8 μg/kg IFN-τ, respectively. The data were represented as the mean ± S.D. *p < 0.05 vs. the control group, and ##p < 0.01 vs. the LPS group.
Cell viability and proliferation are not affected by IFN-τ treatment
The potential cytotoxicity of IFN-τ in RAW264.7 cell was evaluated using the MTT assay. The results showed that cell viability was not affected by IFN-τ treatment (Fig. 2a). The result of the CCK-8 assay revealed that cell proliferation was not affected by IFN-τ treatment (Fig. 2b).
Effect of IFN-τ on cell viability and proliferation. a RAW264.7 cells were cultured with different concentrations of IFN-τ (0–40 ng/mL) for 24 h. Cell viability was measured using the MTT assay. b RAW264.7 cells were cultured with IFN-τ (40 ng/mL) or LPS (0.5 μg/mL) for 0, 6, 12, or 24 h. The cell proliferation was done through the CCK-8 assay. The data were obtained from three independent experiments and represented as the mean ± S.D.
IFN-τ Decreases the Expression of Pro-Inflammatory Cytokines
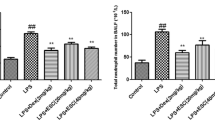
The expression of IL-1β, IL-6, and TNF-α was analyzed by ELISA and qPCR. The results showed that LPS significantly increased the production of IL-1β, IL-6, and TNF-α. IFN-τ attenuated the production of IL-1β, IL-6, and TNF-α in a dose-dependent manner (Fig. 3a). The qPCR results revealed that the levels of IL-1β, IL-6, and TNF-α were significantly increased in the LPS group. IFN-τ greatly decreased the expression levels of pro-inflammatory cytokines. With increasing doses of IFN-τ, the effect gradually became obvious (Fig. 3b). Additional studies performed on RAW 264.7 cells found the same results as in the lung tissues. The results are shown in Fig. 3a, b.
Effects of IFN-τ on cytokine expression in lung homogenates and cellular supernatants. a The LPS-induced expression of IL-1β, IL-6, and TNF-α was detected by ELISA. b The levels of LPS-induced IL-1β, IL-6, and TNF-α were determined by qPCR. GAPDH was used as a control. CG indicates the control group; LPS indicates the LPS-stimulated group; and L, M, and H indicate the tissues treated with 2, 4, or 8 μg/kg IFN-τ, respectively, or the cells treated with 10, 20, or 40 ng/mL IFN-τ, respectively. The data were represented as the mean ± S.D. *p < 0.05 vs. the control group, and #p < 0.05 vs. the LPS group.
IFN-τ Inhibits the Expression of TLR-2 and TLR-4
In this study, the expression of TLR-2 and TLR-4 was determined. The results showed that the expression of TLR-2 and TLR-4 was increased in the LPS group. The expression of TLR-2 and TLR-4 was inhibited by IFN-τ treatment. The levels of TLR-2 and TLR-4 were also detected in RAW264.7 cells. The results were the same as those in lung tissues. The results are shown in Fig. 4.
Effects of IFN-τ on the expression of TLR-2 and TLR-4 in lung tissues and RAW 264.7 cells. a The expression of TLR-2 was detected by qPCR. b The level of TLR-4 was also determined by qPCR. GAPDH was used as a control. CG indicates the control group; LPS indicates the LPS-stimulated group; and L, M, and H indicate the tissues treated with 2, 4, or 8 μg/kg IFN-τ, respectively, or the cells treated with 10, 20, or 40 ng/mL IFN-τ, respectively. The data were represented as the mean ± S.D. *p < 0.05 vs. the control group, and #p < 0.05 vs. the LPS group.
IFN-τ inhibits the LPS-induced activation of the NF-κB pathway
The results showed that LPS treatment significantly increased the phosphorylation of p65 and IκBα. However, the LPS-induced activation of p65 and IκBα was inhibited by IFN-τ in a dose-dependent manner (Fig. 5a). To further confirm the results, the same study was performed using RAW264.7 cells. The results obtained using the cells were consistent with those obtained using the tissues. The results are shown in Fig. 5b.
Effects of IFN-τ on the activation of the NF-κB pathway. a The expression of the p65 and IκBα proteins in lung tissues. b The expression of the p65 and IκBα proteins in RAW 264.7 cells. β-actin was used as a control. CG indicates the control group; LPS indicates the LPS-stimulated group; and L, M, and H indicate the tissues treated with 2, 4, or 8 μg/kg IFN-τ, respectively, or the cells treated with 10, 20, or 40 ng/mL IFN-τ, respectively. The data were represented as the mean ± S.D. *p < 0.05 vs. the control group, and #p < 0.05 vs. the LPS group.
IFN-τ Inhibits the LPS-Induced Activation of MAPK Pathways
To further understand the mechanism of the LPS-induced inhibition of cytokine expression by IFN-τ, MAPKs were detected by western blotting. The results showed that the phosphorylation of JNK, p38, and ERK was greatly increased in the LPS group. However, the phosphorylation of these three proteins was reduced in the IFN-τ groups, especially in the groups treated with a high concentration of IFN-τ (Fig. 6a). To further confirm the results, the same experiment was performed on RAW264.7 cells. These results also showed that IFN-τ inhibited the phosphorylation of JNK, p38, and ERK. The results are shown in Fig. 6b.
Effects of IFN-τ on the activation of the MAPK pathway. a The expression of MAPK proteins in lung tissues. b The levels of MAPK proteins in RAW 264.7 cells. β-actin was used as a control. CG indicates the control group; LPS indicates the LPS-stimulated group; and L, M, and H indicate the tissues treated with 2, 4, or 8 μg/kg IFN-τ, respectively, or the cells treated with 10, 20, or 40 ng/mL IFN-τ, respectively. The data were represented as the mean ± S.D. *p < 0.05 vs. the control group, and #p < 0.05 vs. the LPS group.
DISCUSSION
IFN-τ is a type I interferon that shares a similar structure with the other members of the family. Thus, it can bind the same type I interferon receptor to activate cell signaling pathways and exert its biological functions [21, 22]. Type I interferons such as IFN-α and IFN-β have anti-inflammatory properties [14]. However, there are few studies regarding the anti-inflammatory action of IFN-τ. In this study, we investigated the anti-inflammatory properties of IFN-τ. The pathological sectioning and W/D assays showed that IFN-τ significantly decreased LPS-induced pulmonary edema. MPO plays a vital role in neutrophil antimicrobial responses, and its activity is an important index of tissue damage [23]. The results showed that IFN-τ significantly inhibited MPO activity following LPS-induced ALI. The present study also showed that IFN-τ suppressed the expression of pro-inflammatory cytokines in mice.
Type I interferons have a wide range of immunomodulatory effects in response to bacterial infections or other inflammatory diseases [24]. Thus, IFN-α and IFN-β are often used to treat systemic lupus erythematosus or multiple sclerosis [25, 26]. However, type I interferons also have a wide variety of side effects. They cause acute and chronic morbidity and alter innate and adaptive defenses against other opportunistic infections [27, 28]. Such side effects restrict their clinical application. IFN-τ belongs to the class of type I interferons. Its biological activity is similar to that of IFN-α and IFN-β, but it is less toxic even at a high concentration [14]. Consistently, the results of the MTT assay in this study did not detect cytotoxicity following treatment with IFN-τ. RAW264.7 cells are often used for inflammatory research [29]. The effects of IFN-τ on the LPS-induced expression of pro-inflammatory cytokines in RAW264.7 cells were consistent with those observed in ALI mice.
Inflammation is a vital part of the innate immune response and acts as an endogenous danger signal [30]. LPS is an important inflammatory molecule that activates NF-κB and subsequently increases the production of pro-inflammatory cytokines such as IL-1β, IL-6, and TNF-α that are thought to be important in the generation of ALI [31–33]. In a previous study, the expression of IL-1β and TNF-α was increased in LPS-stimulated mice [34]. IL-6 plays a role in mediating the negative feedback on inflammatory responses [35]. TNF-α can induce infiltration and activate the cascade of other pro-inflammatory cytokines and inflammatory mediators [36]. The results presented here confirmed that IFN-τ has anti-inflammatory activity.
TLRs are evolutionarily conserved molecules that are able to specifically recognize pathogen-associated molecules [37]. It had been reported that TLR-2 and TLR-4 are involved in LPS-mediated signaling [38, 39] through the NF-κB and MAPK pathways. The results of this study showed that IFN-τ could suppress the excessive expression of TLR-2 and TLR-4 following LPS stimulation.
To further understand the mechanism of IFN-τ anti-inflammatory action, we investigated the effect of IFN-τ on the LPS-induced activation of the NF-κB and MAPK pathways. NF-κB plays a key role in inflammatory processes, and IκB is needed to interact with NF-κB [40]. The results of the present study showed that IFN-τ greatly suppressed the LPS-induced phosphorylation of p65 and IκBα in tissues and cells. It had been reported that the suppression of the NF-κB pathway could inhibit LPS-induced inflammation [41]. Our results were in agreement with those of previous studies. The MAPK pathway is important in that it plays pivotal roles in many key cellular processes. The results of this study showed that IFN-τ decreased the phosphorylation of MAPKs following LPS stimulation. The results also indicated that IFN-τ decreased the expression of pro-inflammatory cytokines by inhibiting the activation of the NF-κB and MAPK pathways.
In conclusion, the present study demonstrated that IFN-τ has anti-inflammatory activity. IFN-τ could inhibit the expression of LPS-induced inflammatory cytokines such as IL-1β, IL-6, and TNF-α in ALI mice and RAW264.7 cells by suppressing both the expression of TLR-2 and TLR-4 and the activation of the NF-κB and MAPK pathways. Compared with other type I interferons, IFN-τ has the advantage of low cytotoxicity. The present study provided evidence to support the use of IFN-τ as a treatment for inflammatory diseases.
References
Saugandhika, S., V. Sharma, H. Malik, et al. 2015. Expression and purification of buffalo interferon-tau and efficacy of recombinant buffalo interferon-tau for in vitro embryo development. Cytokine 75: 186–196.
Chethan, S.G., S.K. Singh, J. Nongsiej, et al. 2014. IFN-tau acts in a dose-dependent manner on prostaglandin production by buffalo endometrial stromal cells cultured in vitro. Reproduction in Domestic Animals 49: 403–408.
LeMessurier, K.S., H. Hacker, L. Chi, E. Tuomanen, and V. Redecke. 2013. Type I interferon protects against pneumococcal invasive disease by inhibiting bacterial transmigration across the lung. PLoS Pathogens 9, e1003727.
Gough, D.J., N.L. Messina, C.J.P. Clarke, R.W. Johnstone, and D.E. Levy. 2012. Constitutive Type I interferon modulates homeostatic balance through tonic signaling. Immunity 36: 166–174.
Cho, H., and B.L. Kelsall. 2014. The role of type I interferons in intestinal infection, homeostasis, and inflammation. Immunology Reviews 260: 145–167.
Bartol, F.F., R.M. Roberts, F.W. Bazer, G.S. Lewis, J.D. Godkin, and W.W. Thatcher. 1985. Characterization of proteins produced in vitro by periattachment bovine conceptuses. Biology of Reproduction 32: 681–693.
Alexenko, A.P., A.D. Ealy, and R.M. Roberts. 1999. The cross-species antiviral activities of different IFN-tau subtypes on bovine, murine, and human cells: contradictory evidence for therapeutic potential. Journal of Interferon & Cytokine Research 19: 1335–1341.
Bazer, F.W., J. Kim, G. Song, H. Ka, C.D. Tekwe, and G. Wu. 2012. Select nutrients, progesterone, and interferon tau affect conceptus metabolism and development. Annals of the New York Academy of Sciences 1271: 88–96.
Ying, W., S. Kanameni, C.A. Chang, V. Nair, S. Safe, F.W. Bazer, and B.Y. Zhou. 2014. Interferon tau alleviates obesity-induced adipose tissue inflammation and insulin resistance by regulating macrophage polarization. PloS One 9: 1–17.
Hara, K., K. Shirasuna, F. Usui, et al. 2014. Interferon-tau attenuates uptake of nanoparticles and secretion of interleukin-1beta in macrophages. PloS One 9, e113974.
Zhang, S.Y., L.T. Xu, A.X. Li, and S.M. Wang. 2015. Effects of ergosterol, isolated from scleroderma polyrhizum pers., on lipopolysaccharide-induced inflammatory responses in acute lung injury. Inflammation 38: 1979–1985.
Conti, G., S. Tambalo, G. Villetti, et al. 2010. Evaluation of lung inflammation induced by intratracheal administration of LPS in mice: comparison between MRI and histology. Magnetic Resonance Materials in Physics 23: 93–101.
Wang, Y., Q. Tu, W. Yan, et al. 2015. CXC195 suppresses proliferation and inflammatory response in LPS-induced human hepatocellular carcinoma cells via regulating TLR4-MyD88-TAK1-mediated NF-kappaB and MAPK pathway. Biochemical and Biophysical Research Communications 456: 373–379.
Damjanovic, D., A. Khera, M.F. Medina, et al. 2014. Type 1 interferon gene transfer enhances host defense against pulmonary Streptococcus pneumoniae infection via activating innate leukocytes. Molecular Therapy Methods & Clinical Development 1(5): 1–11.
Chon, T.W., and S. Bixler. 2010. Interferon-tau: current applications and potential in antiviral therapy. Journal of Interferon & Cytokine Research 30: 477–485.
Soos, J.M., P.S. Subramaniam, A.C. Hobeika, J. Schiffenbauer, and H.M. Johnson. 1995. The IFN pregnancy recognition hormone IFN-tau blocks both development and superantigen reactivation of experimental allergic encephalomyelitis without associated toxicity. Journal of Immunology 155: 2747–2753.
Do-Umehara, H.C., C. Chen, D. Urich, et al. 2013. Suppression of inflammation and acute lung injury by Miz1 via repression of C/EBP-delta. Nature Immunology 14: 461–469.
Kim, Y.J., J.H. Kim, K.J. Lee, et al. 2015. Botulinum neurotoxin type A induces TLR2-mediated inflammatory responses in macrophages. PloS One 10, e0120840.
Xu, X., P. Yin, C. Wan, et al. 2014. Punicalagin inhibits inflammation in LPS-induced RAW264.7 macrophages via the suppression of TLR4-mediated MAPKs and NF-kappaB activation. Inflammation 37: 956–965.
Tao, W.W., Q. Su, H.Q. Wang, et al. 2015. Platycodin D attenuates acute lung injury by suppressing apoptosis and inflammation in vivo and in vitro. International Immunopharmacology 27: 138–147.
Roberts, R.M., A.D. Ealy, A.P. Alexenko, C.S. Han, and T. Ezashi. 1999. Trophoblast interferons. Placenta 20: 259–264.
Roberts, R.M. 2007. Interferon-tau, a Type 1 interferon involved in maternal recognition of pregnancy. Cytokine & Growth Factor 18: 403–408.
Li, D., Y. Fu, W. Zhang, et al. 2013. Salidroside attenuates inflammatory responses by suppressing nuclear factor-kappaB and mitogen activated protein kinases activation in lipopolysaccharide-induced mastitis in mice. Inflammation Research 62: 9–15.
Gonzalez-Navajas, J.M., J. Lee, M. David, and E. Raz. 2012. Immunomodulatory functions of type I interferons. Nature Reviews Immunology 12: 125–135.
Yan, B., S. Ye, G. Chen, M. Kuang, N. Shen, and S. Chen. 2008. Dysfunctional CD4+, CD25+ regulatory T cells in untreated active systemic lupus erythematosus secondary to interferon-alpha-producing antigen-presenting cells. Arthritis and Rheumatism 58: 801–812.
Namdar, A., B. Nikbin, M. Ghabaee, A. Bayati, and M. Izad. 2010. Effect of IFN-beta therapy on the frequency and function of CD4(+)CD25(+) regulatory T cells and Foxp3 gene expression in relapsing-remitting multiple sclerosis (RRMS): a preliminary study. Journal of Neuroimmunology 218: 120–124.
Sleijfer, S., M. Bannink, A.R. VanGool, W.H.J. Kruit, and G. Stoter. 2005. Side effects of interferon-alpha therapy. Pharmacy World and Science 27: 423–431.
Trinchieri, G. 2010. Type I interferon: friend or foe? The Journal of Experimental Medicine 207: 2053–2063.
Chen, X., J.S. Miao, H. Wang, et al. 2015. The anti-inflammatory activities of Ainsliaea fragrans Champ. extract and its components in lipopolysaccharide-stimulated RAW264.7 macrophages through inhibition of NF-kappa B pathway. Journal of Ethnopharmacology 170: 72–80.
Rauch, I., M. Muller, and T. Decker. 2013. The regulation of inflammation by interferons and their STATs. JAK-STAT 2, e23820.
Wang, J., Y.T. Liu, L. Xiao, L. Zhu, Q. Wang, and T. Yan. 2014. Anti-inflammatory effects of apigenin in lipopolysaccharide-induced inflammatory in acute lung injury by suppressing COX-2 and NF-kB pathway. Inflammation 37: 2085–2090.
Seifart, C., A. Dempfle, A. Plagens, et al. 2005. TNF-alpha-, TNF-beta-, IL-6-, and IL-10-promoter polymorphisms in patients with chronic obstructive pulmonary disease. Tissue Antigens 65: 93–100.
Xu, D., M. Chen, X. Ren, X. Ren, and Y. Wu. 2014. Leonurine ameliorates LPS-induced acute kidney injury via suppressing ROS-mediated NF-kappaB signaling pathway. Fitoterapia 97: 148–155.
Cao, W.J., W. Zhang, J.J. Liu, et al. 2011. Paeoniflorin improves survival in LPS-challenged mice through the suppression of TNF-alpha and IL-1 beta release and augmentation of IL-10 production. International Immunopharmacology 11: 172–178.
Hopkins, S.J. 2003. The pathophysiological role of cytokines. Legal Medicine 5(Suppl 1): S45–S57.
Wang, G., B. Sun, Y. Gao, Q.H. Meng, and H.C. Jiang. 2007. The effect of emodin-assisted early enteral nutrition on severe acute pancreatitis and secondary hepatic injury. Mediators of Inflammation 2007: 29638.
Noreen, M., and M. Arshad. 2015. Association of TLR1, TLR2, TLR4, TLR6, and TIRAP polymorphisms with disease susceptibility. Immunologic Research 62: 234–252.
Takeuchi, O., K. Hoshino, T. Kawai, et al. 1999. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity 11: 443–451.
Xiang, P., T. Chen, Y. Mou, et al. 2015. NZ suppresses TLR4/NF-kappaB signalings and NLRP3 inflammasome activation in LPS-induced RAW264.7 macrophages. Inflammation Research 64: 799–808.
Yamamoto, Y., and R.B. Gaynor. 2004. IkappaB kinases: key regulators of the NF-kappaB pathway. Trends in Biochemical Sciences 29: 72–79.
Ren, J., and S.H. Chung. 2007. Anti-inflammatory effect of alpha-linolenic acid and its mode of action through the inhibition of nitric oxide production and inducible nitric oxide synthase gene expression via NF-kappaB and mitogen-activated protein kinase pathways. Journal of Agricultural and Food Chemistry 55: 5073–5080.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (NO.31272631, 31472254).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no competing interests.
Additional information
Haichong Wu and Gan Zhao contributed equally to this work.
Rights and permissions
About this article
Cite this article
Wu, H., Zhao, G., Jiang, K. et al. IFN-τ Alleviates Lipopolysaccharide-Induced Inflammation by Suppressing NF-κB and MAPKs Pathway Activation in Mice. Inflammation 39, 1141–1150 (2016). https://doi.org/10.1007/s10753-016-0348-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-016-0348-9