Abstract
Streptococcus suis serotype 2 (SS2) is an emerging zoonosis, and meningitis is the most frequent clinical manifestation, but mechanism of its virulent factor, enolase (Eno), is unknown in meningitis. In this study, Eno was inducibly expressed and added to an in vitro Transwell co-culture model of the blood–brain barrier (BBB) consisted of porcine brain microvascular endothelial cells (PBMECs) and astrocytes (ACs), the results showed that Eno induces a significant increase in BBB permeability and promotes the release of IL-8 et al. cytokines. Furthermore, IL-8 could significantly destroy the integrity of the BBB model in vitro. In mice models administered Eno for 24 h, Eno could significantly promote Evans blue (EB) moving from the blood to the brain and significantly increased the serum and brain levels of IL-8, as detected by ELISA. While G31P (IL-8 receptor antagonist) significantly decreased the concentration of EB in the brains of mice injected with Eno. The present study demonstrated that SS2 Eno may play an important role in disrupting BBB integrity by prompting IL-8 release.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Streptococcus suis (S. suis) is a Gram-positive bacterium. Of the 35 known serotypes, serotype 2 is considered to be the most widely distributed and the most pathogenic [1]. Streptococcus suis serotype 2 (SS2) is an emerging zoonotic pathogen that infects humans and pigs. Meningitis and sepsis are the most common clinical manifestations and are associated with significant mortality and morbidity [2]. S. suis crosses the blood–brain barrier (BBB) and enters the central nervous system (CNS), which are the key steps in meningitis [3].
Enolase (Eno), a potential virulence factor of SS2, was originally identified as a key glycolytic enzyme that catalyzes the dehydration of 2-phosphoglycerate to phosphoenolpyruvate in the last steps of the catabolic glycolytic pathway [4, 5]. Eno is present in a wide range of species, from archaebacteria to mammals, and its sequence is highly conserved [6]. It has been considered to be an ancient protein with a single function. However, recent studies have found that Eno plays an important role in many physiological and pathological processes such as promoting pathogen invasion into the host [7–9], cancer metastasis [10–14], and apoptosis [15, 16]. It was reported that Eno is expressed on the surface of S. suis and promotes SS2 invasion into the CNS [9]. Eno can facilitate the pathogen’s ability to infect the host cell by interacting with the plasminogen receptor on the surfaces of several diverse cell types [17–20]. The Eno interaction with plasminogen can activate the fibrinolysis system, and fibrils and extracellular matrix (ECM) components such as laminin or fibronectin were degraded to disrupt the tissue barrier, contributing to bacterial invasion of the host cells and systemic infection [8]. Although many lines of evidence have shown that Eno might be related to the ability of S. suis to penetrate the BBB, the immunopathological effect of Eno on the BBB remains unclear.
The BBB is a structural and functional barrier formed by brain microvascular endothelial cells (BMECs), astrocytes (ACs) and pericytes and is the protective interface regulating the molecular, ionic and cellular traffic between the blood and the CNS [21, 22]. Numerous models of the BBB have been constructed over the past few years using rat primary cells or rat and human cell lines to construct a mono-model or co-culture model. In most of the available in vitro BBB models, the endothelial cells originate from the bovine [23, 24], rat [25] or human brain [26]; whereas, the astrocytes are predominantly isolated from the brain tissue of newborn rats [27, 28]. The porcine BBB model is not used as frequently. However, porcine cells provide the most suitable in vitro BBB system because porcine brains are readily available in sufficient amounts, and porcine physiology is closely related to human physiology. The use of the same species as the source of both cell types for the construction of a co-culture BBB system is rare and has usually been reported for human BBB models. The co-culture model is more difficult to set up, but it is a more realistic imitation of the BBB in vivo.
In this study, primary porcine brain microvascular endothelial cells (PBMECs) and astrocytes (ACs) are used to construct a Transwell co-culture model of the BBB. The experiments were conducted under in vitro (BBB co-culture model of PBMECs and ACs) and in vivo conditions (mice received a tail vein administration of Eno) to study the potential effects and mechanism of Eno on BBB permeability. The results first showed that Eno can significantly increase BBB permeability by promoting IL-8 secretion in vitro and in vivo. It provides a theoretical and practical basis for the effective diagnosis and the prevention of SS2 meningitis; it also provides a new target for the treatment of SS2 meningitis.
MATERIALS AND METHODS
Reagents
Plasmid Miniprep Kit was purchased from BIOMIGA (San Diego, USA). Ni-NTA column was obtained from Genscript (NJ, USA). PierceTM BCA Protein Assay Kit was provided by Thermo Scientific (MA, USA). Porcine IL-8 enzyme-linked immunosorbent assay (ELISA) kits were obtained from Biolegend (San Diego,CA, USA)
Cloning, Expression and Purification of Eno
The coding region of the Enolase gene (GenBank: FJ895346.1) from S. suis CVCC 606 was amplified by PCR using the primers up-Eno and down-Eno (up-Enolase: 5′-GGGAATTCCATATGATGTCAATTACTGATG-3′; down-Enolase: 5′- CGCGGATCCTTATTTTTTCAAGTTGTAGAATGAG-3′). The ends of the fragment were modified with NdeI and BamHI, and the fragment was then inserted into the pET-28a vector, which had been cut with NdeI and BamHI. The upstream primer was designed so that the Eno-coding region was fused in-frame to the 6-histidine tag of the vector, resulting in the pEno plasmid. The plasmid DNA was amplified in E. coli DH5-α cells, and the plasmid was extracted with plasmid Miniprep Kit (BIOMIGA, San Diego, USA). The S. suis CVCC 606 Eno gene was expressed in E. coli BL21 (DE3) cells and induced with IPTG. The protein was purified on a Ni-NTA column (Genscript, New Jersey, USA). The protein concentration was measured with the PierceTM BCA Protein Assay Kit (Thermo Scientific, MA, USA). The protein was stored at 4 °C in buffer. The polyclonal mouse anti-rEno antibody was generated using the purified protein, and the titer was approximately 1:12,800, as determined by ELISA.
Construction of the In Vitro BBB Co-culture Models
The BBB co-culture model was prepared as described elsewhere. However, our procedure is slightly modified and contains the following steps: the inserts were inverted with the basal sides facing upwards and placed in a deep glass dish. Culture media was carefully added into the deep dish. ACs at passage 4 were seeded at a concentration of 50,000 cells/filter (1-cm2 surface area) on the bottom side of the Transwell insert, which was positioned upside down. ACs are allowed to adhere for 3 h before being carefully transferred into the incubator. ACs were incubated at 37 °C in an atmosphere with 5 % CO2 for 3 days. After 3 days of incubation, the insert was inverted and placed in a 12-well cell culture plate. Then, 50,000 PBMECs were seeded onto the lumenal side of each insert. The cells were fed with culture medium (0.5 mL in the upper chamber and 1.5 mL in the lower chamber). All of the plates were incubated at 37 °C in an atmosphere with 5 % CO2. The experiments with the BBB co-culture model were performed within 5–7 days after PBMECs were seeded. Inserts without any cells served as negative controls for the barrier integrity studies.
Electrical Resistance Measurements
BBB integrity was assessed by TEER measurements. The TEER (Ωcm2) values were recorded by an Endohm chamber connected to an EVOM resistance meter, and the resistance of a blank filter was subtracted to calculate the final TEER values (Ωcm2). All experiments were performed in triplicate.
Permeability Assay
Permeability was measured by adding 650 μL of HRP to the upper chamber of the BBB co-culture model. After a 30-min incubation, 50 μL of medium was collected from the lower chamber, and the amount of HRP that had passed through the barrier was determined by the OD450. The relevant value of a ‘blank’ cell-free insert was subtracted from all data. The results were presented as the HRP concentration that permeated the BBB model during the incubation.
The Effect of Eno on BBB Permeability In Vitro
After we successfully constructed the in vitro BBB co-culture model, the co-cultured cells were treated with Eno, which was added to the upper chamber of the Transwell at concentrations of 200 and 0 μg/mL for 24 h. DMEM/F12 was the negative control, and CVCC606 (2 × 108 CFU) was the positive control. The TEER values were detected at 0, 1, 2, 3, 6, 9, 12, 18 and 24 h. In addition, the medium was collected from the lower chamber and, the HRP content was analysed at 24 h to assess BBB permeability.
Antibody Microarray
After we successfully constructed the in vitro BBB co-culture model, the model was exposed to Eno, which was added to the upper chamber of the Transwell at concentrations of 100 and 0 μg/mL for 6 h, 12 and 24 h. Then, 500 μL of medium was collected from the lower chamber at each time point and analysed by Ray-Bio Porcine Cytokine Antibody Array (QAP-CYT-1 chip). The assay sensitivity is pg/mL.
The Effect of IL-8 on BBB Permeability In Vitro
After we successfully constructed the in vitro BBB co-culture model, the model was exposed to IL-8, which was added to the upper chamber of the Transwell at concentrations of 200 and 0 μg/mL for 24 h. The TEER values and the HRP content of lower chamber were determined to assess BBB permeability.
In Vivo Permeability Assay
The BBB permeability in mice was investigated using Evans blue extravasation, according to Uyama’s method. Female BALB/c mice (8–10 weeks old) were purchased from the Center of Experimental Animals of Baiqiuen Medical College of the Jilin University (Jilin, China). The animals were maintained on a 12-h light/dark cycle with free access to food and water. The experimental protocol was conducted with the approval of the Institutional Animal Care and Use Committee of the Jilin University under the approved protocol number JLUA-1309. Thirty-six mice were randomly divided into six groups (six mice per group), as follows: 400-μg Eno, 400-μg Eno + 10 μg G31p, 2 × 108 CFU CVCC606, 2 × 108 CFU CVCC606 + 10 μg G31p, 10-μg G31p and PBS. After 24 h, the whole brain was removed. At 23 h, Evans blue (EB, 1 % in saline) was injected via the tail vein. After decapitation, the brain was placed in formamide for 48 h at 37 °C in the dark. The ratio of the EB concentration in brain was determined at OD630. The brain and blood were quickly collected, respectively, for further analyses.
Cytokine Assay
The brains were weighed and homogenized in phosphate-buffered saline (w/v,1:9) on ice and then centrifuged at 2000×g for 40 min at 4 °C. The supernatant was collected. Blood samples were collected from the euthanized animals and were stored in the refrigerator for 30 min before centrifugation. The clear, nonhemolyzed sera were stored at −80 °C until the measurements were collected. The expression levels of the IL-8 protein were determined using enzyme-linked immunosorbent assay (ELISA) kits according to the manufacturer’s protocol.
Statistical Analysis
The data were statistically analysed using SPSS software (ver. 19 for Windows; SPSS Inc., Chicago, IL, USA). The differences between the mean values of normally distributed data were assessed with one-way ANOVA (Dunnett’s test) and two-tailed Student’s t test. P ≤ 0.05 was considered statistically significant.
RESULTS
Cloning, Expression and Purification of Enolase
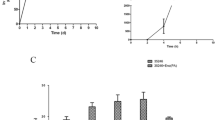
The coding region of the Eno gene (GenBank: FJ895346.1) from the S. suis CVCC606 strain was amplified by PCR and cloned into the pET-28a vector at the NdeI and BamHI sites. The Eno gene was fused in frame with the 6-histidine tag of pET-28a. The resulting plasmid, pEno, was sequenced and identified by double digestion with NdeI and BamHI. The Eno gene in the pEno plasmid was expressed in the E.coli BL21 (DE3) host strain and induced with IPTG. The protein (rEno) was purified on a Ni-NTA resin column and characterized by SDS-PAGE and Western blotting. Figure 1a, b shows that rEno can be stably expressed in E.coli; it is mainly present in the supernatant, and its molecular weight is 54 KDa.
SDS-PAGE and Western blotting analysis of S. suis Enolase (Eno). a SDS-PAGE was used to analyse the expression of rEno in the supernatant of the E.coli expression system. pET28a-Eno were transformed into E. coli BL21 (DE3) and induced with 1-mM IPTG for 5 h at 25 °C. Lane Marker, molecular mass marker in KDa; Lane 1, non-induced E.coli BL21 (DE3) containing pET-28a: Eno; Lane 2, induced E. coli BL21 (DE3) containing pET-28a: Eno; and Lanes 3–4, the precipitate and supernatant of the induced E.coli BL21 (DE3) containing pET-28a: Eno. b Western blotting was used to analyse the expression of rEno. Lane 1, purified rEno protein. The mouse anti-rEno antibody (1:12800) was used to detect the protein.
Eno Enhances BBB Permeability
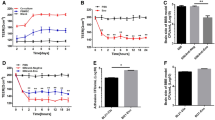
To accurately mimic the BBB in vivo, we will correctly identify and obtain high purity primary PBMECs and ACs to construct the BBB co-culture model in vitro. The TEER value indicates the function of the barrier. The higher the TEER value, the better the barrier function. Figure 2a showed that the TEER value of the BBB co-culture model was significantly higher than that in the control group, and the TEER value rapidly increased beginning at 4 days and peaked at 5 days after co-culture (206 Ω · cm−2) and then stabilized, showing that the in vitro BBB co-culture model was constructed successfully.
Enolase (Eno) increases blood–brain barrier (BBB) permeability in vitro. a Time course of the changes in the transendothelial electrical resistance (TEER) for the in vitro BBB co-culture model. The porcine brain microvascular endothelial (PBMECs) and astrocytes (ACs) were seeded on Transwell inserts in 12-well plates, and the TEER was measured every day for a week. b Time course of the changes in the TEER values following Eno treatment. Co-cultures of PBMECs and ACs on Transwell inserts in 12-well plates. Eno (200 μg/mL) was added to the upper chamber for 24 h. The untreated co-cultures served as controls. The TEER values were measured at 0, 1, 2, 3, 6, 9, 12, 18 and 24 h. c The changes in HRP permeability were measured at 24 h. Co-cultures of PBMECs and ACs on Transwell inserts in 12-well plates. Eno (200 μg/mL) was added to the upper chamber for 24 h. The untreated co-cultures served as controls. **p < 0.01, *p < 0.05 ns p > 0.05.
To study the effect of Eno on BBB permeability, 200-μg/mL Eno was added to the upper chamber of the BBB model, and the change in the TEER value and the dynamics of the HRP concentrations in the lower chamber were during monitored for 24 h. The TEER value indicates the function of the paracellular barrier. As shown in Fig. 2a, the TEER value of the Eno group was significantly lower than that of the negative control group and was basically the same as that of the CVCC606 group. These indicated that both Eno and CVCC606 can increase the permeability of the in vitro BBB model and that Eno may be an important virulence factor in promoting the infection of the brain. In addition, transcellular barrier is represented by the presence of the 40-KDa HRP protein; the higher the final concentration of HRP, the worse the transcellular barrier. As shown in Fig. 2b, the HRP content in the Eno group was significantly higher than that in the control group (p < 0.01). The CVCC606 group was not significantly different from the Eno group, which indicates that Eno also damaged the transcellular barrier of the BBB, increasing its permeability to HRP. The above results suggest that Eno induces a significant increase in BBB permeability by altering the paracellular barrier and the transcellular barrier.
Eno Promotes Cytokine Expression in the BBB
Previous studies indicated that the S. suis pathogenic process produces a large number of inflammatory factors and chemokines, which play an important role in the destruction of the BBB. Therefore, the BBB co-culture model was constructed, and the effect of Eno on cytokine release from the BBB was determined. The sample was hybridized with Porcine Cytokine Antibody Array Ray-Bio (QAP-CYT-1). The scan results were shown in Fig. 3. The changes in the average concentrations and fold increases of various cytokines are shown in Tables 1 and 2. As shown in Table 1, compared to the control group, the IL-8, IL-10, IFNγ, and TNF-a levels in the Eno group were significantly different at 6 h (p < 0.01). At 12 h, the levels of secreted IL-1β, IL-8, IL-10, and IFNγ in the Eno group were increased compared to the control group, but only the levels of secreted IL-8 and IL-10 were significantly higher than those of the control group (p < 0.01). However, the secretion of these cytokines was not significantly different from the control group at 24 h. Meanwhile, Table 2 shows that the expression levels of IL-8 at 6 h were two times higher in the Eno group than those in the control group. At the same time, the expression of IL-8 is increasing (Fig. 3), ultimately reaching at 25.650 pg/mL (Table 1). These results indicate that Eno can stimulate the BBB to produce a large number of cytokines, increasing the permeability of the BBB.
Scannogram of antibody microarrays after the interaction between Enolase (Eno) and the blood–brain barrier (BBB) co-culture model. Co-cultures of porcine brain microvascular endothelial cells (PBMECs) and astrocytes (ACs) on Transwell inserts in 12-well plates. Eno (100 μg/mL) was added to the upper chamber for 6, 12 and 24 h. The untreated co-cultures served as controls. Then, 500 μL of medium was collected from the lower chamber each time point and analysed by the Ray-Bio Porcine Cytokine Antibody Array (QAP-CYT-1 chip). The assay sensitivity is pg/mL. The boxed cytokines changed greater than twofold and were significantly different compared to the control groups.
IL-8 Promotes the Increased BBB Permeability
Based on the above research, which showed that Eno can promote significantly higher expression of IL-8 compared to the control group (p < 0.01), we further analysed the effect of IL-8 on the permeability of the in vitro BBB model. As shown in Fig. 4a, the TEER value of the IL-8 group was significantly lower than that of the negative control group (p < 0.01). It is suggested that IL-8 can destroy the paracellular barrier to disrupt BBB integrity, thus increasing its permeability. At the same time, the HRP permeability assay (Fig. 4b) showed that the HRP content in the lower chamber of the IL-8 group was significantly higher than that of the negative control group (p < 0.01), which shows that IL-8 also damaged the transcellular barrier of the BBB to increase BBB permeability. These results suggest that IL-8 can promote a significant increase in BBB permeability, but the mechanism by which IL-8 enhances BBB permeability needs further exploration.
IL-8 increases blood–brain barrier (BBB) permeability. Co-cultures of porcine brain microvascular endothelial cells (PBMECs) and astrocytes (ACs) on Transwell inserts in 12-well plates. IL-8 (200 μg/L) was added to the upper chamber for 24 h. The untreated co-cultures served as controls. Changes in the transendothelial electrical resistance (TEER) following IL-8 treatment. b Changes in the permeability to HRP following IL-8 treatment. **p < 0.01, *p < 0.05 ns p > 0.05.
Eno-Induced IL-8 Secretion Enhances BBB Permeability In Vivo
Our results have shown that Eno can increase the permeability of the in vitro BBB model. To further verify that Eno can also increase BBB permeability in vivo, a mouse model was employed, and the animals received a tail vein administration of EB to assess the extent of brain damage and BBB permeability [29]. First, the serum levels of IL-8 in the Eno- and CVCC606-treated groups were significantly higher than the PBS-treated group (p < 0.01), and the IL-8 levels in the brain were similar (p < 0.05) (Fig. 5a, b). In addition, although the EB content in the Eno-treated brains was significantly lower than that of the CVCC606-treated group (p < 0.01), the concentration in the Eno-treated group was higher than the PBS-treated group (p < 0.01). However, G31P (IL-8 receptor antagonist) decreased the concentration of EB in the brains of the Eno-injected mice (p < 0.05) (Fig. 5c). These results suggested that Eno can significantly increase BBB permeability by promoting IL-8 secretion from the BBB.
The administration of Enolase (Eno) increases blood–brain barrier (BBB) permeability (in vivo study). Eno was administered through the tail vein and BBB permeability was measured in the brain. IL-8 was detected in the serum and brain. a The serum IL-8 levels were measured by ELISA. b The brain IL-8 levels were measured by ELISA. c The Evans blue (EB) content in the brains of the Eno, treated and blank control groups of mice were analysed at 24 h. **p < 0.01, *p < 0.05 ns p > 0.05.
DISCUSSION
In the present study, we found that Eno induces a significant increase in BBB permeability by altering the paracellular barrier and the transcellular barrier. Moreover, the Eno-induced high levels of IL-8 significantly reduce the TEER value and increase the HRP content in the lower chamber of the Transwell co-culture model, destroying the integrity of the BBB. Furthermore, when Eno was administered to mice via the tail vein, it significantly increased the serum and brain levels of IL-8 and promoted EB leakage from the blood to the brain. However, G31P decreased the concentration of EB in the brains of the Eno-injected mice. Thus, Eno can significantly increase BBB permeability by promoting IL-8 secretion from the BBB. This study sheds light on the mechanism of the pathogenesis of SS2 meningitis and provides a new target for the treatment of SS2 meningitis.
The BBB has mainly been studied in various BMEC cell lines at home and abroad, but previous studies have indicated that primary cells can maintain more of the in vivo characteristics such as the rich tight junctions between BMEC cells, a small amount of pinocytotic vesicles and Fenestration, a large number of mitochondria, and the expression of barrier-related protein markers such as P-glycoprotein (P-gp), ZO-1 and Occludin [30]. Meanwhile, co-cultures of PBMECs and ACs can enhance the expression level of γ-glutamyl transpeptidase and alkaline phosphatase, giving rise to high transendothelial electrical resistance (TEER) [31]. In addition, a single pig brain produces a higher yield of cells compared to rat or mouse brains, and porcine brains are relatively easy to obtain. The porcine genome, anatomy, physiology and disease progression reflect human biology more closely than many established laboratory animals [32]. The availability of miniature pigs and novel porcine transgenic disease models make the pig the most suitable animal model to study human disease [33, 34]. Therefore, this study constructed a Transwell co-culture model for the BBB, with a consistent source of PBMECs and ACs to better mimic the in vivo conditions.
Eno is a key glycolytic enzyme that catalyzes the interconversion between 2-phosphoglycerate and phosphoenolpyruvate. Eno has been found to be expressed on the surface of multifarious pathogens such as parasites [35], Candida albicans [36], Gram-positive cocci [9]. The interaction between Eno and blood plasminogen is involved in the transfer and invasion of the pathogens. However, the role of Eno in SS2 meningitis remained unclear. In this study, Eno induces a significant increase in BBB permeability by altering the paracellular barrier and the transcellular barrier. It is suggested that Eno plays an important role in the process of SS2 migration to the CNS, which ultimately results in meningitis.
IL-8 is a chemokine for neutrophils and basophils that can be synthesized and expressed in many types of cells following stimulation with inflammatory factors such as IL-1, TNF and bacteria; it is also an important proinflammatory factor. One of the important signs of meningitis is the infiltration of inflammatory cells in the subarachnoid cavity. The infiltration of neutrophils and monocytes is important for the identification and elimination of pathogens, but the presence of white blood cells in the cerebrospinal fluid (CSF) may cause neurological damage to the host. IL-8 can promote leukocyte migration from the blood into inflammatory sites. After the white blood cells enter the subarachnoid space, they can substantially increase IL-8 production, which promotes more white cell infiltration. The Bio-plex cytokine assay system analysis found increased expression of IL-4, IL-6, IL-8, TNF-α, IL-17 and GM-CSF in the CSF from bacterial meningitis patients [37]. Meanwhile, the interaction of hBMECs with group B Streptococcus agalactiae promotes the release of IL-8, CXCL-1, CXCL-2 and GM-CSF, suggesting that IL-8 plays an important role in hematogenous meningitis [38]. These results proved that IL-8 plays an important role in hematogenous meningitis. However, its exact role in CNS infection and its possible impact on the pathophysiology of meningitis require further study. This study found that Eno promotes IL-8 release and that IL-8 can significantly reduce the TEER value and increase the HRP content in the lower chamber of the in vitro BBB model, destroying the integrity of the BBB in vitro. In the mouse model, G31P (IL-8 receptor antagonist) significantly decreases the effect of Eno on BBB permeability. The results showed that IL-8 induces a significant increase in BBB permeability by altering the paracellular barrier and the transcellular barrier. It is suggested that IL-8 plays an important role in the increased BBB permeability and provides a new target for the treatment of SS2 meningitis.
In conclusion, this is the first study to confirm that Eno can increase BBB permeability by promoting IL-8 secretion, thus disrupting BBB integrity. However, the mechanism by which IL-8 enhances BBB permeability needs further study. This study sheds light on the mechanism of the pathogenesis of SS2 meningitis and provides a new target for the treatment of SS2 meningitis.
References
Gottschalk, M., M. Segura, and J. Xu. 2007. Streptococcus suis infections in humans: the Chinese experience and the situation in North America. Animal Health Research Reviews 8: 29–45.
Wertheim, H.F., H.D. Nghia, W. Taylor, and C. Schultsz. 2009. Streptococcus suis: an emerging human pathogen. Clinical Infectious Diseases 48: 617–62.
Vanier, G., M. Segura, P. Friedl, S. Lacouture, and M. Gottschalk. 2004. Invasion of porcine brain microvascular endothelial cells by Streptococcus suis serotype 2. Infection and Immunity 72: 1441–1449.
Pancholi, V. 2001. Multifunctional alpha-enolase: its role in diseases. Cellular and Molecular Life Sciences 58: 902–920.
Zhang, E., J.M. Brewer, W. Minor, L.A. Carreira, and L. Lebioda. 1997. Mechanism of enolase: the crystal structure of asymmetric dimer enolase-2-phospho-D-glycerate/enolase-phosphoenolpyruvate at 2.0 A resolution. Biochemistry 36: 12526–12534.
Piast, M., I. Kustrzeba-Wojcicka, M. Matusiewicz, and T. Banas. 2005. Molecular evolution of enolase. Acta Biochimica Polonica 52: 507–513.
Vanier, G., M. Segura, P. Friedl, S. Lacouture, and M. Gottschalk. 2004. Enolase from Streptococcus sobrinus is an immunosuppressive protein. Cellular Microbiology 6: 79–88.
Chhatwal, G.S. 2002. Anchorless adhesins and invasins of Gram-positive bacteria: a new class of virulence factors. Trends in Microbiology 10: 205–208.
Esgleas, M., Y. Li, M.A. Hancock, J. Harel, J.D. Dubreuil, and M. Gottschalk. 2008. Isolation and characterization of alpha-enolase, a novel fibronectin-binding protein from Streptococcus suis. Microbiology 154: 2668–2679.
Capello, M., S. Ferri-Borgogno, P. Cappello, and F. Novelli. 2011. alpha-Enolase: a promising therapeutic and diagnostic tumor target. The FEBS Journal 278: 1064–1074.
Chang, G.C., K.J. Liu, C.L. Hsieh, T.S. Hu, S. Charoenfuprasert, H.K. Liu, K.T. Luh, et al. 2006. Introduction of in vitro transcribed ENO1 mRNA into neuroblastoma cells induces cell death. BMC Cancer 5: 161.
Feo, S., D. Arcuri, E. Piddini, R. Passantino, and A. Giallongo. 2000. ENO1 gene product binds to the c-myc promoter and acts as a transcriptional repressor: relationship with Myc promoter-binding protein 1 (MBP-1). FEBS Letters 473: 47–52.
Ghosh, A.K., R. Steele, and R.B. Ray. 2005. c-myc Promoter-binding protein 1 (MBP-1) regulates prostate cancer cell growth by inhibiting MAPK pathway. The Journal of Biological Chemistry 280: 14325–14330.
Chang, G.C., K.J. Liu, C.L. Hsieh, T.S. Hu, S. Charoenfuprasert, H.K. Liu, K.T. Luh, et al. 2006. Identification of alpha-enolase as an autoantigen in lung cancer: its overexpression is associated with clinical outcomes. Clinical Cancer Research 12: 5746–5754.
Ucker, D.S., M.R. Jain, G. Pattabiraman, K. Palasiewicz, R.B. Birge, and H. Li. 2012. Externalized glycolytic enzymes are novel, conserved, and early biomarkers of apoptosis. The Journal of Biological Chemistry 287: 10325–10343.
Yang, H.B., W.J. Zheng, X. Zhang, and F.L. Tang. 2011. Induction of endothelial cell apoptosis by anti-alpha-enolase antibody. Chinese Medical Sciences Journal 26: 152–157.
Lopez-Alemany, R., C. Longstaff, S. Hawley, M. Mirshahi, P. Fabregas, M. Jardi, E. Merton, et al. 2003. Inhibition of cell surface mediated plasminogen activation by a monoclonal antibody against alpha-Enolase. American Journal of Hematology 72: 234–242.
Miles, L.A., C.M. Dahlberg, J. Plescia, J. Felez, K. Kato, and E.F. Plow. 1991. Role of cell-surface lysines in plasminogen binding to cells: identification of alpha-enolase as a candidate plasminogen receptor. Biochemistry 30: 1682–1691.
Redlitz, A., B.J. Fowler, E.F. Plow, and L.A. Miles. 1995. The role of an enolase-related molecule in plasminogen binding to cells. European Journal of Biochemistry 227: 407–415.
Dudani, A.K., C. Cummings, S. Hashemi, and P.R. Ganz. 1993. Isolation of a novel 45 kDa plasminogen receptor from human endothelial cells. Thrombosis Research 69: 185–196.
Abbott, N.J., L. Ronnback, and E. Hansson. 2006. Astrocyte-endothelial interactions at the blood–brain barrier. Nature Reviews Neuroscience 7: 41–53.
Dejana, E., M. Corada, and M.G. Lampugnani. 1995. Endothelial cell-to-cell junctions. The FASEB Journal 9: 910–918.
Gaillard, P.J., L.H. Voorwinden, J.L. Nielsen, A. Ivanov, R. Atsumi, H. Engman, C. Ringbom, et al. 2001. Establishment and functional characterization of an in vitro model of the blood–brain barrier, comprising a co-culture of brain capillary endothelial cells and astrocytes. European Journal of Pharmaceutical Sciences 12: 215–222.
Wolburg, H., J. Neuhaus, U. Kniesel, B. Krauss, E.M. Schmid, M. Ocalan, C. Farrell, et al. 1994. Modulation of tight junction structure in blood–brain barrier endothelial cells. Effects of tissue culture, second messengers and cocultured astrocytes. Journal of Cell Science 107(Pt 5): 1347–1357.
Utepbergenov, D.I., K. Mertsch, A. Sporbert, K. Tenz, M. Paul, R.F. Haseloff, and I.E. Blasig. 1998. Nitric oxide protects blood–brain barrier in vitro from hypoxia/reoxygenation-mediated injury. FEBS Letters 424: 197–201.
Rubin, L.L., D.E. Hall, S. Porter, K. Barbu, C. Cannon, H.C. Horner, M. Janatpour, et al. 1991. A cell culture model of the blood–brain barrier. The Journal of Cell Biology 115: 1725–1735.
Hurwitz, A.A., J.W. Berman, W.K. Rashbaum, and W.D. Lyman. 1993. Human fetal astrocytes induce the expression of blood–brain barrier specific proteins by autologous endothelial cells. Brain Research 625: 238–243.
Megard, I., A. Garrigues, S. Orlowski, S. Jorajuria, P. Clayette, E. Ezan, and A. Mabondzo. 2002. A co-culture-based model of human blood–brain barrier: application to active transport of indinavir and in vivo-in vitro correlation. Brain Research 927: 153–167.
Epiphanio, S., M.G. Campos, A. Pamplona, D. Carapau, A.C. Pena, R. Ataide, C.A. Monteiro, et al. 2010. VEGF promotes malaria-associated acute lung injury in mice. PLoS Pathogens 6, e1000916.
Scott, P.A., and R. Bicknell. 1993. The isolation and culture of microvascular endothelium. Journal of Cell Science 105(Pt 2): 269–273.
DeBault, L.E., and P.A. Cancilla. 1980. Some properties of isolated endothelial cells in culture. Advances in Experimental Medicine and Biology 131: 69–78.
Walters, E.M., Y. Agca, V. Ganjam, and T. Evans. 2011. Animal models got you puzzled?: think pig. Annals of the New York Academy of Sciences 1245: 63–64.
Bendixen, E., M. Danielsen, K. Larsen, and C. Bendixen. 2010. Advances in porcine genomics and proteomics—a toolbox for developing the pig as a model organism for molecular biomedical research. Briefings in Functional Genomics 9: 208–219.
Lunney, J.K. 2007. Advances in swine biomedical model genomics. International Journal of Biological Sciences 3: 179–184.
Bernal, D., J.E. de la Rubia, A.M. Carrasco-Abad, R. Toledo, S. Mas-Coma, and A. Marcilla. 2004. Identification of enolase as a plasminogen-binding protein in excretory-secretory products of Fasciola hepatica. FEBS Letters 563: 203–206.
Sundstrom, P., and G.R. Aliaga. 1992. Molecular cloning of cDNA and analysis of protein secondary structure of Candida albicans enolase, an abundant, immunodominant glycolytic enzyme. Journal of Bacteriology 174: 6789–6799.
Asano, T., K. Ichiki, S. Koizumi, K. Kaizu, T. Hatori, O. Fujino, K. Mashiko, Y. Sakamoto, T. Miyasho, and Y. Fukunaga. 2010. IL-17 is elevated in cerebrospinal fluids in bacterial meningitis in children. Cytokine 51: 101–106.
Doran, K.S., G.Y. Liu, and V. Nizet. 2003. Group B streptococcal beta-hemolysin/cytolysin activates neutrophil signaling pathways in brain endothelium and contributes to development of meningitis. The Journal of Clinical Investigation 112: 736–744.
Acknowledgments
This study was supported by “Public welfare industry-specific special research (Agriculture): Streptococcus suis disease prevention and control technology research and demonstration” (201303041).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The experimental protocol was conducted with the approval of the Institutional Animal Care and Use Committee of the Jilin University under the approved protocol number JLUA-1309. Moreover, all efforts were made to minimize suffering.
Conflict of Interest
The authors declare that they have no competing interests
Rights and permissions
About this article
Cite this article
Sun, Y., Li, N., Zhang, J. et al. Enolase of Streptococcus Suis Serotype 2 Enhances Blood–Brain Barrier Permeability by Inducing IL-8 Release. Inflammation 39, 718–726 (2016). https://doi.org/10.1007/s10753-015-0298-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-015-0298-7