Abstract
Implant-related infection (IRI) is closely related to the local immunity of peri-implant tissues. The generation of reactive oxygen species (ROS) in activated macrophages plays a prominent role in the innate immune response. In previous studies, we indicated that implant wear particles promote endotoxin tolerance by decreasing the release of proinflammatory cytokines. However, it is unclear whether ROS are involved in the damage of the local immunity of peri-implant tissues. In the present study, we assessed the mechanism of local immunosuppression using titanium (Ti) particles and/or lipopolysaccharide (LPS) to stimulate RAW 264.7 cells. The results indicate that the Ti particles induced the generation of a moderate amount of ROS through nicotinamide adenine dinucleotide phosphate oxidase-1, but not through catalase. Pre-exposure to Ti particles inhibited ROS generation and extracellular-regulated protein kinase activation in LPS-stimulated macrophages. These findings indicate that chronic stimulation by Ti particles may lead to a state of oxidative stress and persistent inflammation, which may result in the attenuation of the immune response of macrophages to bacterial components such as LPS. Eventually, immunosuppression develops in peri-implant tissues, which may be a risk factor for IRI.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Total joint replacement (TJR) is widely used to alleviate pain and to improve the quality of life. However, this operation is associated with complications such as aseptic failure or implant-related infection (IRI) [1]. IRI occurs in 1.5–2.5 % of all cases of primary hip or knee arthroplasty, despite the rigorous use of perioperative antimicrobial prophylaxis [2, 3]. Recent studies have suggested that certain cases of aseptic failure, particularly those involving aseptic loosening, may have been infected by bacteria that cannot be detected using conventional microbial cultures [4–8]. Inflammatory reactions associated with implant wear particles are believed to be the major cause of long-term failure of TJR [9, 10]. The surface of wear particles serves as a site for the adherence of bacteria, bacterial biofilms, or bacterial structural components such as lipopolysaccharide (LPS), which may promote the development of persistent infections and chronic inflammation [10–12].
The reactive oxygen species (ROS) include superoxide, hydrogen peroxide, and hydroxyl radicals, as well as a variety of their reaction products [13]. ROS play an important role in the innate immunity of macrophages. Following infection with a pathogen, macrophages begin to produce large amounts of ROS and mediate powerful microbicidal activity through nicotinamide adenine dinucleotide phosphate (NADPH) oxidases (NOX)—this phenomenon is known as “respiratory burst” [13–15]. However, ROS are double-edged swords in that they can also be toxic to cells and can cause tissue damage [14, 16]. Previous studies have indicated the presence of oxidative stress in particle-stimulated cells in vitro [17, 18] and pre-implant tissue in vivo [19], which may lead to nuclear factor-κB (NF-κB) activation, proinflammatory cytokine release, and persistent inflammation [13, 20, 21]. Furthermore, ROS have been reported to activate extracellular-regulated protein kinases (ERK) 1/2 [22–24], which is a kinase of the MAPK family and primarily stimulates cell proliferation [25].
Two main families of opposing enzymes regulate the generation of ROS—oxidative enzymes such as NOX and anti-oxidative enzymes such as catalase (CAT), glutathione peroxidase, and superoxide dismutase [14, 16, 25]. NOX enzymes serve as a major source for the deliberate generation of ROS in response to inflammatory signals [14]. In particular, NOX-1 is an important enzyme of the NOX family, and has been shown to be transcriptionally induced by LPS in different cells [25, 26]. Furthermore, CAT is essential in defending cells against oxidative damage by degrading hydrogen peroxide [27].
In the present study, we examined the generation of ROS and their related enzymes in response to titanium (Ti) particles in vitro to elucidate the signal pathway underlying oxidative stress around implants. In addition, we tested the hypothesis that wear particles may affect respiratory burst and macrophage proliferation, which may be a risk factor for IRI.
MATERIALS AND METHODS
Preparation of Ti Particles
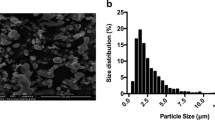
Commercially pure Ti particles were obtained from Alfa Aesar (catalog #00681; Ward Hill, MA, USA). Particle size was measured using scanning electron microscopy and the mean diameter of the particles was 3.2 ± 2.7 μm (Fig. 1). The Ti particles were sterilized by baking at 180 °C for 6 h, followed by treatment with 70 % ethanol for 48 h to remove endotoxin, as described previously [28, 29]. Thereafter, the particles were reconstituted in a stock solution at a concentration of 10 mg/mL in sterile phosphate-buffed saline (PBS) and diluted to different final concentrations in experiments. Prior to the experiments, the particles were tested and confirmed to contain less than 0.1 EU/mL of endotoxin, by using a commercial Limulus assay kit (Chromogenic End-point TAL with a Diazo coupling kit; Xiamen Houshiji, Fujian, China). Previous studies have demonstrated that such commercially available Ti particles are similar to wear debris particles generated in periprosthetic tissues [29, 30].
LPS
LPS from Escherichia coli O111:B4, purchased from Sigma Aldrich (catalog L2630; St. Louis, MO, USA), was solubilized in sterile PBS at a concentration of 1 mg/mL at −20 °C for storage. For the experiment, LPS was diluted to 1 μg/mL in Dulbecco’s modified Eagle’s medium (DMEM) or PBS.
Cell Culture and Treatment
A mouse macrophage cell line RAW264.7 was obtained from the Shanghai Institute of Cell Biology, Chinese Academy of Sciences (Shanghai, China). The cells were cultured in DMEM (Gibco, Grand Island, NY, USA), containing 10 % (v/v) fetal bovine serum (Gibco), at 37 °C in a humidified atmosphere of 5 % CO2 and 95 % air. Cells were removed from 75-cm2 culture flasks and plated in 6-well plates or culture dishes at a density of 106 cells/mL. To examine the dose-dependent effects of Ti particles, the cells were incubated in serum-free DMEM containing different concentrations of Ti particles (0.001, 0.01, 0.1, and 1 mg/mL) for 12 h. Cells were incubated with LPS as a positive control. Furthermore, to assess the time-dependent effects, the RAW264.7 cells were treated with Ti particles at 0.1 mg/mL for 0.5, 1, 3, 6, 12, 24, and 48 h. To study the role of Ti particles in LPS-activated macrophages, RAW264.7 cells were pre-exposed to Ti particles for 12 h and incubated in medium containing 1 μg/mL LPS for 30 min, and were evaluated in comparison with RAW264.7 cells that were not pre-exposed to Ti particles.
Measurement of Intracellular ROS Levels in RAW 264.7 Cells by Flow Cytometry
Intracellular ROS production was measured using the fluorescence probe 2′,7′-dichlorodihydrofluorescein diacetate (H2DCFDA; Invitrogen Co., Carlsbad, CA, USA). The RAW 264.7 cells were grown in 6-well plates. Following treatment with Ti particles or LPS, the cells were collected and re-suspended in pre-warmed PBS, containing 5 μmol/L H2DCFDA, and incubated for 20 min at 37 °C in darkness. Thereafter, the extracellular probe was removed by washing cells in PBS. We then determined the fluorescence intensity by flow cytometry (Beckman-Coulter, Fullerton, CA, USA), with an excitation wavelength of 488 nm and emission wavelength of 525 nm. In total, 50,000 cells were collected and analyzed in each sample. The data obtained were analyzed using Kaluza software (Beckman-Coulter).
Western Blotting
Total cell extracts of RAW264.7 cells were obtained and lysed using a lysis solution containing protease inhibitors and phosphatase inhibitors. Following centrifugation at 12,000 rpm for 20 min at 4 °C, the protein in the supernatants was collected and quantified. Equal amounts of total proteins (range, 30–70 μg) were separated using 12 % sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes. The membranes were blocked for 30 min in Tris-buffered saline containing 5 % skim milk at room temperature, after which probes—including anti-NOX-1(1:1000) from Santa Cruz Biotechnology (Santa Cruz, CA, USA) and anti-CAT (1:1000), anti-ERK1/2 (1:1000), and anti-glyceraldehyde 3-phosphate dehydrogenase (GAPDH; 1:5000) from Cell Signaling Technology (Beverly, MA, USA) antibodies—were added and incubated overnight at 4 °C. The anti-GAPDH antibody was used as an internal control for protein loading. The membranes were then incubated with the corresponding horseradish peroxidase-labeled secondary antibody (1:3000; Jackson Immunoresearch, West Grove, PA, USA) at room temperature for 2 h. The bands were visualized using an enhanced chemiluminescence detection system, and the band density was determined by ImageJ software (National Institutes of Health, Bethesda, MD, USA).
Statistical Analysis
Data are presented as the mean ± standard deviation of at least three independent experiments. The differences between groups were assessed using one-way analysis of variance. The significance for multiple comparisons among groups was assessed using a post hoc examination with least significant difference tests. A p value of <0.05 was considered to be statistically significant. All data were processed using the SPSS 13.0 software (SPSS Inc., Chicago, IL, USA).
RESULTS
Ti Particles Stimulate a Moderate Amount of NOX-1 Expression and ROS Generation in RAW264.7 Macrophages
The protein levels of NOX-1 or CAT were determined using western blot analyses. The expression of NOX-1 in RAW264.7 macrophages increased in the presence of Ti particles in a dose- and time-dependent manner, whereas the expression of CAT remained unchanged. The addition of LPS significantly increased NOX-1 expression (Fig. 2a), in accordance with previous studies [25]. In cases receiving Ti particle stimulation for 12 h, the NOX-1/GAPDH ratio increased with increasing Ti concentration (0.001–1 mg/mL) (p < 0.05), although the induction of NOX-1 expression by Ti particles was weaker than that with LPS (p < 0.05) (Fig. 2a); however, no significant change in CAT expression was noted (p > 0.05) (Fig. 2b). A Ti particle concentration of 0.1 mg/mL induced NOX-1 expression at all time points, particularly at 6, 12, 24, and 48 h (p < 0.05) (Fig. 3a, b), although it did not have any effect on CAT expression (p > 0.05) (Fig. 3a, c). Thus, the induction of NOX-1 may contribute to ROS generation following treatment with Ti particles. Therefore, we assessed the effect of the treatment of RAW264.7 macrophages with Ti particles for 12 h on ROS generation. We noted that the Ti particles induced a moderate increase in the amount of ROS generated in a dose-dependent manner, particularly for Ti particle concentrations of 0.1 and 1 mg/mL (p < 0.05) (Fig. 4). Similar to that noted for NOX-1 expression, Ti particles at a concentration of ≤0.1 mg/mL induced ROS generation to a lesser extent compared to that noted with LPS stimulation (p < 0.05) (Fig. 4).
Effect of Ti particles and LPS on NOX-1 and CAT expression in RAW 264.7 macrophages at different Ti particle concentrations. RAW 264.7 cells were treated with Ti particles at concentrations of 0, 0.001, 0.01, 0.1, and 1 mg/mL or LPS at 1 μg/mL for 12 h. The NOX-1 and CAT protein levels were examined using western blot assays. a The ratio of NOX-1/GAPDH is presented as means ± standard deviation (from three independent experiments). *p < 0.05, **p < 0.001 vs. negative control (NC, 0 mg/mL Ti group). # p < 0.05, ## p < 0.001 vs. LPS group. b The ratio of CAT/GAPDH is presented as means ± standard deviation (from three independent experiments). No significant differences in the CAT/GAPDH ratio were noted among the groups. NS not significant.
Effect of Ti particles (0.1 mg/mL) on NOX-1 and CAT expression in RAW 264.7 macrophages at different time points. a RAW 264.7 cells were stimulated by Ti particles for 0, 1, 3, 6, 12, 24, and 48 h, respectively. The expressions of NOX-1 and CAT were assayed by western blotting. Statistical analysis of NOX-1 (b) and CAT (c) levels from three independent experiments are shown below. Data are presented as means ± standard deviation. *p < 0.05, **p < 0.001 vs. the 0 h group. NS not significant.
Flow cytometry analysis of the effect of Ti particles and LPS on ROS production. RAW 264.7 macrophages were stimulated with different concentrations of Ti particles (range, 0–1 mg/mL) or 1 μg/mL LPS for 12 h. a Ti particles at a concentration of 0.1 mg/mL or LPS at a concentration of 1 μg/mL significantly increased ROS production. The data related to the stimulation with 0.001, 0.01, and 1 mg/mL Ti particles are not presented. b Statistical results of stimulation with different concentrations of Ti particles (range, 0–1 mg/mL) on ROS production from three independent experiments. Data are presented as means ± standard deviation (from three independent experiments). *p < 0.05, **p < 0.001 vs. negative control (NC, 0 mg/mL Ti group). # p < 0.05, ## p < 0.001 vs. LPS (12 h) group.
Ti Particles Suppress LPS-Induced ROS Production and ERK Phosphorylation in RAW264.7 Macrophages
Short-term LPS stimulation resulted in a large increase in ROS levels. We noted that LPS stimulation (1 μg/mL) for 30 min significantly enhanced ROS generation (p < 0.001). To assess the role of implant wear particles on IRI in vitro, we used RAW264.7 macrophages that were pre-exposed to 0.1 mg/mL Ti particles (LPS-free) for 12 h before they were stimulated with LPS for 30 min. In the cultures of macrophages pre-exposed to Ti particles, in which LPS was subsequently added, we noted that the ROS generation was lower compared to the cultures of macrophages that were not pre-exposed to LPS-free Ti particles prior to LPS stimulation (p < 0.001) (Fig. 5a, b). In addition, we assessed the expression of phosphorylated ERK1/2 (pERK1/2). We noted that phosphorylation of ERK by LPS stimulation was attenuated by Ti particles (p < 0.05) (Fig. 5c).
Flow cytometry and western blot analysis indicating the suppressive effects of Ti particles on LPS-induced ROS generation and ERK1/2 phosphorylation in RAW264.7 cells. a Generation of ROS following stimulation under different conditions. NC (negative control): cells cultured without any additional stimulation; Ti: stimulation with Ti particles for 12 h; LPS 30 min: stimulation with LPS for 30 min. LPS 30 min (Ti-exposed): cells previously exposed to Ti particles for 12 h were then stimulated with LPS for 30 min. b Statistical results of panel a from three independent experiments. c The p-ERK/GAPDH ratio following stimulation under different conditions was also assessed. The data are presented as means ± standard deviation (from three independent experiments). *p < 0.05, **p < 0.001 vs. negative control (NC).
DISCUSSION
Macrophages are known to play a central role in the innate immune responses to implant wear particles in peri-implant tissues [31]. Particle-stimulated macrophages have been shown to release a number of proinflammatory cytokines, including prostaglandin E2, interleukin-1 beta, interleukin-6, interleukin-17C, and tumor necrosis factor-alpha, via upregulation of the transcription factor NF-κB [9, 31–33]. The continuous activation of NF-κB and release of proinflammatory cytokines are the main causes of chronic inflammation in such cases.
During an acute infection, a sharp increase in oxygen uptake is found in macrophages, thus generating large amounts of ROS (respiratory burst) to oxidize and kill the invading pathogens [13, 15]. Moreover, the generation and accumulation of ROS is associated with chronic inflammation, while the amount is relatively low or moderate [34–36]. A moderate amount of ROS cannot eliminate detrimental stimuli thoroughly, they act as second messengers to activate NF-κB [13, 21, 36] and other proinflammatory signals [34], resulting in a low-grade, chronic inflammation [36]. In the present study, we noted that although LPS-free Ti particles stimulate ROS generation, their effect is relatively weaker compared to long-term (12 h) or short-term (30 min) LPS stimulation. Short-term LPS stimulation may be typically representative of an acute infection. Following short-term LPS stimulation, macrophages generate large numbers of ROS to eliminate the invasive pathogens. However, LPS-free Ti particle stimulation induces the generation of a moderate amount of ROS, which could lead to chronic inflammation. To evaluate the reasons underlying ROS accumulation in cells, we examined the expression of two ROS-related enzymes—NOX-1 and CAT—in response to stimulation by Ti particles and LPS. We noted that the upregulation of NOX-1, but not the downregulation of CAT, contributed to ROS accumulation.
The state of chronic inflammation around implant tissues due to stimulation by wear particles is a risk factor for IRI, as the wear particles could induce LPS tolerance and inhibit macrophage response to bacteria [37, 38]. Invasion of bacteria around implants is, to some extent, related to local immunosuppression. However, due to local immunosuppression, even a low level of invasive pathogens cannot be thoroughly eliminated; hence, the pathogens continue to grow and even form biofilms, which may then lead to persistent inflammation and eventually, IRI [7, 11].
In previous studies, we indicated that the Ti particles induced LPS tolerance in RAW 264.7 macrophages and had an immunosuppressive effect by inhibiting the release of proinflammatory cytokines. Furthermore, LPS stimulation can effectively activate RAW 264.7 macrophages to release a large amount of proinflammatory cytokines. However, if the macrophages have been pre-exposed to Ti particles, the stimulation of LPS causes only a moderate production of proinflammatory cytokines [38]. Therefore, although wear particles themselves have proinflammatory effects, they somewhat damage the immune function of macrophages and lead to local immunosuppression.
To further confirm the immunosuppressive effect of Ti particles, we examined ROS generation using methods similar to those used in our previous studies [38]. The present study indicated that pre-exposure to Ti particles can suppress ROS production and the respiratory burst mechanism in macrophages, thus indicating that local immunosuppression, or LPS tolerance, is induced. In addition, we noted that phosphorylation of ERK by LPS was inhibited by pre-exposure to Ti particles, indicating that the ability of macrophages to proliferate was damaged. The damage to both the respiratory burst mechanism and macrophage proliferation could contribute to local immunosuppression in peri-implant tissues. That is, macrophages cannot response rapidly and potently to bacterial components to clear invasive pathogens, which may be a risk factor for IRI.
In conclusion, the results of the present study support the hypothesis that the presence of wear particles can lead to chronic inflammation and LPS tolerance around implants through the suppression of ROS generation and macrophage proliferation. In such cases, immune function is eventually impaired. These tissues would thus be more susceptible to infections. Therefore, appropriate regulation of ROS and ERK may serve as a potential new strategy in the treatment of IRI.
References
Trampuz, A., K.E. Piper, M.J. Jacobson, A.D. Hanssen, K.K. Unni, D.R. Osmon, J.N. Mandrekar, F.R. Cockerill, J.M. Steckelberg, J.F. Greenleaf, and R. Patel. 2007. Sonication of removed hip and knee prostheses for diagnosis of infection. New England Journal of Medicine 357: 654–663.
Lentino, J.R. 2003. Prosthetic joint infections: bane of orthopedists, challenge for infectious disease specialists. Clinical Infectious Diseases 36: 1157–1161.
Zimmerli, W., A. Trampuz, and P.E. Ochsner. 2004. Prosthetic-joint infections. New England Journal of Medicine 351: 1645–1654.
Tunney, M.M., S. Patrick, S.P. Gorman, J.R. Nixon, N. Anderson, R.I. Davis, D. Hanna, and G. Ramage. 1998. Improved detection of infection in hip replacements. A currently underestimated problem. Journal of Bone and Joint Surgery (British Volume) 80: 568–572.
Clarke, M.T., C.P. Roberts, P.T. Lee, J. Gray, G.S. Keene, and N. Rushton. 2004. Polymerase chain reaction can detect bacterial DNA in aseptically loose total hip arthroplasties. Clinical Orthopaedics and Related Research 132–7.
Nalepka, J.L., M.J. Lee, M.J. Kraay, R.E. Marcus, V.M. Goldberg, X. Chen, and E.M. Greenfield. 2006. Lipopolysaccharide found in aseptic loosening of patients with inflammatory arthritis. Clinical Orthopaedics and Related Research 451: 229–235.
Nelson, C.L., A.C. McLaren, S.G. McLaren, J.W. Johnson, and M.S. Smeltzer. 2005. Is aseptic loosening truly aseptic? Clinical Orthopaedics and Related Research 25–30.
Lee, M.S., W.H. Chang, S.C. Chen, P.H. Hsieh, H.N. Shih, S.W. Ueng, and G.B. Lee. 2013. Molecular diagnosis of periprosthetic joint infection by quantitative RT-PCR of bacterial 16S ribosomal RNA. The Scientific World Journal 2013: 950548.
Ingham, E., and J. Fisher. 2005. The role of macrophages in osteolysis of total joint replacement. Biomaterials 26: 1271–1286.
Lin, T.H., Y. Tamaki, J. Pajarinen, H.A. Waters, D.K. Woo, Z.Y. Yao, and S.B. Goodman. 2014. Chronic inflammation in biomaterial-induced periprosthetic osteolysis: NF-kappa B as a therapeutic target. Acta Biomaterialia 10: 1–10.
Costerton, J.W., P.S. Stewart, and E.P. Greenberg. 1999. Bacterial biofilms: a common cause of persistent infections. Science 284: 1318–1322.
Greenfield, E.M., M.A. Beidelschies, J.M. Tatro, V.M. Goldberg, and A.G. Hise. 2010. Bacterial pathogen-associated molecular patterns stimulate biological activity of orthopaedic wear particles by activating cognate Toll-like receptors. Journal of Biological Chemistry 285: 32378–32384.
Lambeth, J.D., and A.S. Neish. 2014. Nox enzymes and new thinking on reactive oxygen: a double-edged sword revisited. Annual Review of Phytopathology 9: 119–145.
Lambeth, J.D. 2004. NOX enzymes and the biology of reactive oxygen. Nature Reviews Immunology 4: 181–189.
Babior, B.M. 1984. The respiratory burst of phagocytes. The Journal of Clinical Investigation 73: 599–601.
Mates, J.M. 2000. Effects of antioxidant enzymes in the molecular control of reactive oxygen species toxicology. Toxicology 153: 83–104.
Yamada, M., T. Ueno, H. Minamikawa, N. Sato, F. Iwasa, N. Hori, and T. Ogawa. 2010. N-acetyl cysteine alleviates cytotoxicity of bone substitute. Journal of Dental Research 89: 411–416.
Raghunathan, V.K., M. Devey, S. Hawkins, L. Hails, S.A. Davis, S. Mann, I.T. Chang, E. Ingham, A. Malhas, D.J. Vaux, J.D. Lane, and C.P. Case. 2013. Influence of particle size and reactive oxygen species on cobalt chrome nanoparticle-mediated genotoxicity. Biomaterials 34: 3559–3570.
Olmedo, D.G., D.R. Tasat, M.B. Guglielmotti, and R.L. Cabrini. 2005. Effect of titanium dioxide on the oxidative metabolism of alveolar macrophages: an experimental study in rats. Journal of Biomedical Materials Research. Part A 73: 142–149.
Cruz, C.M., A. Rinna, H.J. Forman, A.L. Ventura, P.M. Persechini, and D.M. Ojcius. 2007. ATP activates a reactive oxygen species-dependent oxidative stress response and secretion of proinflammatory cytokines in macrophages. Journal of Biological Chemistry 282: 2871–2879.
Gloire, G., S. Legrand-Poels, and J. Piette. 2006. NF-kappaB activation by reactive oxygen species: fifteen years later. Biochemical Pharmacology 72: 1493–1505.
Wang, X., J.Z. Liu, J.X. Hu, H. Wu, Y.L. Li, H.L. Chen, H. Bai, and C.X. Hai. 2011. ROS-activated p38 MAPK/ERK-Akt cascade plays a central role in palmitic acid-stimulated hepatocyte proliferation. Free Radical Biology and Medicine 51: 539–551.
Sano, M., K. Fukuda, T. Sato, H. Kawaguchi, M. Suematsu, S. Matsuda, S. Koyasu, H. Matsui, K. Yamauchi-Takihara, M. Harada, Y. Saito, and S. Ogawa. 2001. ERK and p38 MAPK, but not NF-kappaB, are critically involved in reactive oxygen species-mediated induction of IL-6 by angiotensin II in cardiac fibroblasts. Circulation Research 89: 661–669.
Torres, M. 2003. Mitogen-activated protein kinase pathways in redox signaling. Frontiers in Bioscience : a Journal and Virtual Library 8: d369–d391.
Maitra, U., N. Singh, L. Gan, L. Ringwood, and L. Li. 2009. IRAK-1 contributes to lipopolysaccharide-induced reactive oxygen species generation in macrophages by inducing NOX-1 transcription and Rac1 activation and suppressing the expression of antioxidative enzymes. Journal of Biological Chemistry 284: 35403–35411.
O’Leary, D.P., L. Bhatt, J.F. Woolley, D.R. Gough, J.H. Wang, T.G. Cotter, and H.P. Redmond. 2012. TLR-4 signalling accelerates colon cancer cell adhesion via NF-kappaB mediated transcriptional up-regulation of Nox-1. PloS One 7: e44176.
Alfonso-Prieto, M., X. Biarnes, P. Vidossich, and C. Rovira. 2009. The molecular mechanism of the catalase reaction. Journal of the American Chemical Society 131: 11751–11761.
Rakshit, D.S., K. Ly, T.K. Sengupta, B.J. Nestor, T.P. Sculco, L.B. Ivashkiv, and P.E. Purdue. 2006. Wear debris inhibition of anti-osteoclastogenic signaling by interleukin-6 and interferon-gamma. Mechanistic insights and implications for periprosthetic osteolysis. Journal of Bone and Joint Surgery (American) 88: 788–799.
Liu, F., Z. Zhu, Y. Mao, M. Liu, T. Tang, and S. Qiu. 2009. Inhibition of titanium particle-induced osteoclastogenesis through inactivation of NFATc1 by VIVIT peptide. Biomaterials 30: 1756–1762.
Geng, D.C., Y.Z. Xu, H.L. Yang, X.S. Zhu, G.M. Zhu, and X.B. Wang. 2010. Inhibition of titanium particle-induced inflammatory osteolysis through inactivation of cannabinoid receptor 2 by AM630. Journal of Biomedical Materials Research. Part A 95: 321–326.
Hallab, N.J., and J.J. Jacobs. 2009. Biologic effects of implant debris. Bulletin of the NYU Hospital for Joint Diseases 67: 182–188.
Maloney, W.J., R.E. James, and R.L. Smith. 1996. Human macrophage response to retrieved titanium alloy particles in vitro. Clinical Orthopaedics and Related Research. 268–78.
Hou, C., Y. Zhang, S. Yu, Z. Li, Q. Zhai, Z. Li, X. Zhang, J. Xiao, and P. Sheng. 2013. Presence of interleukin-17C in the tissue around aseptic loosened implants. International Orthopaedics 37: 953–959.
Thomas, C.E., and V. Darley-Usmar. 2000. Forum on therapeutic applications of reactive oxygen and nitrogen species in human disease. Free Radical Biology and Medicine 28: 1449–1450.
Bengtsson, A.A., A. Pettersson, S. Wichert, B. Gullstrand, M. Hansson, T. Hellmark, and A.C. Johansson. 2014. Low production of reactive oxygen species in granulocytes is associated with organ damage in systemic lupus erythematosus. Arthritis Research & Therapy 16: R120.
El Assar, M., J. Angulo, and L. Rodríguez-Mañas. 2013. Oxidative stress and vascular inflammation in aging. Free Radical Biology and Medicine 65: 380–401.
Zhang, Y., C. Hou, S. Yu, J. Xiao, Z. Zhang, Q. Zhai, J. Chen, Z. Li, X. Zhang, M. Lehto, Y.T. Konttinen, and P. Sheng. 2012. IRAK-M in macrophages around septically and aseptically loosened hip implants. Journal of Biomedical Materials Research. Part A 100: 261–268.
Zhang, Y., S. Yu, J. Xiao, C. Hou, Z. Li, Z. Zhang, Q. Zhai, M. Lehto, Y.T. Konttinen, and P. Sheng. 2013. Wear particles promote endotoxin tolerance in macrophages by inducing interleukin-1 receptor-associated kinase-M expression. Journal of Biomedical Materials Research. Part A 101: 733–739.
Acknowledgments
This work was financially supported by the grants from the National Natural Science Foundation of China (grant no. 81171710), the High Technological Industrialization Projects of Guangdong Province, China (no. 2011B010500012) and the International Cooperation of Science and Technology of Guangdong Province, China (no. 2013B051000040)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, W., Li, Z., Guo, Y. et al. Wear Particles Impair Antimicrobial Activity Via Suppression of Reactive Oxygen Species Generation and ERK1/2 Phosphorylation in Activated Macrophages. Inflammation 38, 1289–1296 (2015). https://doi.org/10.1007/s10753-014-0099-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-014-0099-4