Abstract
The aim of the present study was to determine the effects of carboxymethyl chitosan (CMC) on titanium particles-induced oxidative stress in mouse RAW264.7 macrophages. The mouse RAW264.7 macrophages were divided into four groups: (i) the control group; (ii) the CMC group received stimulation of CMC for 4 h; (iii) the titanium particles group received stimulation of titanium particles for 12 h; and (iv) the CMC group received pre-stimulation of CMC hydrogels for 4 h followed by treatment of titanium particles for 12 h. Afterwards, reactive oxygen species (ROS) level in the cells was measured by flow cytometry. A spectrophotometer was used to measure the activities of oxidases and antioxidant enzymes. Fluorescence quantitative PCR was performed to analyze mRNA levels of enzymes and tumor necrosis factor α (TNF-α). ELISA was used to detect the mass concentration of TNF-α after indicated treatment. CMC effectively suppressed titanium particles-induced oxidative stress in RAW264.7 cells, as evidenced by the decrease in intracellular ROS level, the transcription of oxidases, and TNF-α concentration as well as the increase in the transcription of antioxidant enzymes. CMC exerts a protective impact against wear particles-induced oxidative stress and reduces the release of TNF-α in RAW264.7 cells.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Every year, millions of people around the world suffer mobility issues due to end-stage joint diseases such as osteoarthritis and rheumatoid arthritis (Cho et al. 2021; Mustonen et al. 2021). Artificial joint replacement surgery remains to be the most effective method for these diseases and can significantly improve the patient’s life quality after surgery (Dodd et al. 2022; Günther et al. 2021). However, aseptic loosening of joint prosthesis is still the main complication following joint replacements, hindering the development of artificial joints (Wooley and Schwarz 2004; Cherian et al. 2015). Around the loose prosthesis, there are not only inflammatory factors produced by macrophages swallowing wear particles, but also a large number of oxides (Steinbeck et al. 2014). Inflammation together with oxidative stress stimulate the proliferation and activation of osteoclasts, and ultimately result in osteolysis and the loosening of prosthesis (Peng et al. 2018).
Carboxymethyl chitosan (CMC) is a high-molecular polymer that forms following deacetylation from keratin (Younes and Rinaudo 2015). Keratin is widely found in the cell walls of fungi and the shells of crustaceans and insects in nature. Keratin molecules form CMC through N-terminal deacetylation (LogithKumar et al. 2016), which is similar in structure to glycosaminoglycans in the extracellular matrix, and can promote cell-to-cell adhesion, proliferation and differentiation. The introduction of carboxymethyl into CMC not only retains the excellent characteristics of CMC, but also has stronger water solubility and biological activity (Fiamingo and Campana-Filho 2016). CMC has been demonstrated to have antioxidant effects, as the hydroxyl and carboxyl groups in the polymer chain can easily absorb hydrogen atoms in the free radicals to form stable polymer groups (Ramasamy et al. 2017).
The aim of the present study was to investigate whether pretreating macrophages with CMC can inhibit titanium particles-induced oxidative stress in macrophages. The results of the present study may provide a theoretical basis for exploring the role of CMC as a free radical scavenger in preventing prosthesis loosening.
Materials and methods
Preparation of titanium particles
Titanium particles were purchased from Alfa Aesar (Shanghai, China). First, the titanium particles were added with 75% ethanol and then were placed on a horizontal shaker for 24 h. Next, the particles were centrifugated at 111.8 × g for 5 min at room temperature and then suspended in phosphate-buffered saline (PBS) solution. Afterwards, high temperature (180 °C) and high-pressure sterilization was performed for 6 h. Subsequently, the titanium particles were subjected to the same centrifugal and autoclaving conditions followed by resuspension in 0.1 g/l of PBS at the concentration of 10 g/l (Luo et al. 2016). A Limulus assay kit (Houshiji, Xiamen, China) was used to test these particles, and those with endotoxin levels less than 0.1 EU/ml were regarded as uncontaminated and were used in the current work. A scanning electron microscope with a magnification of 2000 × was used to capture images to detect the diameter of titanium particles. For cell treatment, RAW264.7 cells were stimulated with titanium particles for 12 h.
Cell culture
Mouse RAW264.7 macrophage cell line was purchased from Procell (Procell Life Science & Technology Co.,Ltd, Wuhan, China). RAW264.7 cell line was incubated with High-glycemic DMEM (GIBCO, Grand Island, NY, USA) containing 10% fetal bovine serum (GIBCO) in the environment of 5% CO2 at 37 °C. Cells were removed gently with a cell scraper every 48 h for passage.
Preparation of CMC solution
High-concentration CMC (Yishengtang Medical Supplies Co., Ltd., Shijiazhuang, Hebei, China) was diluted in equal proportions and stored at 4 °C in the dark. For cell treatment, CMC was used to pretreat mouse macrophages for 4 h.
Detection of the effect of CMC on cell viability
RAW264.7 cells that were in the logarithmic growth phase were used for the experiments. The cell density was adjusted to 5 × 103/ml with DMEM (GIBCO). A total of 0 (control group), 0.2, 0.5, 1, 5, or 25 mg/ml CMC (Yishengtang Medical Supplies Co., Ltd.) was added to cells in each well of 96-well plates, and the plate was placed in an incubator for 24 h. A total of 10 μL of CCK8 reagent (MedChem Express, Monmouth Junction, NJ, USA) was added to each well, and the plate was incubated at 37 °C with 5% CO2 for 4 h. After incubation, the solution was mixed thoroughly, and the absorbance at 450 nm was measured with a microplate reader. Cell proliferation rate = absorbance value of experimental group-absorbance value of blank group / absorbance value of the control group-absorbance value of the blank group. The concentration with the least effect on cell activity was chosen for subsequent experiments.
Detection of ROS level in RAW264.7 cells
RAW264.7 cells that were in the logarithmic growth phase and a Cell Reactive Oxygen Detection Kit (Beyotime Biotechnology, Shanghai, China) were used for the experiments. The cell density was adjusted to 5 × 105/ml with DMEM (GIBCO). After added to 6-well plates with 1 ml cell suspension per well, cells were incubated overnight at 37 °C and divided into four groups: (i) The non-stimulated negative control group was routinely cultured; (ii) the CMC group received 1 mg/ml CMC for 4 h; (iii)the titanium particles group received 0.1 g/l titanium particles to stimulate the cells for 12 h; and (iv) the CMC group received pre-stimulation of 1 mg/ml CMC for 4 h and treatment of 0.1 g/l titanium particles for 12 h. RAW264.7 cells were collected following intervention and resuspended in 1 ml of DCFH-DA solution (diluted with serum-free culture medium to 10 µmol/l), followed by incubation at 37 °C for 20 min in the dark, with mixing every 3 min during the incubation. It was ensured that the probe and cells made full contact, and they were then washed three times with serum-free cell culture medium. Finally, the cells were resuspended in PBS, and the fluorescence value was detected with a flow cytometer using a wavelength of 488 nm, and an emission wavelength of 525 nm.
Measurement of activities of oxidative stress-associated enzymes
After centrifugation, the supernatant was taken for testing. Activities of enzymes were measured following the recommendations of the enzyme activity test kit (Nanjing Jiancheng Institute of Biological Engineering, Nanjing, China). In brief, after the test solution and the supernatant were fully mixed, the absorbance value was recorded with a spectrophotometer. For each ml of cell supernatant, the molecular weight of the decomposition product of the enzyme produced every min was taken as an enzyme activity unit.
Reverse transcription-quantitative polymerase chain reaction
After stimulation, RNA from each group of cells was extracted to perform RT-qPCR detection. Total RNA was extracted using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Waltham, MA, USA). Microspectrophotometers measure the OD260, OD280 and OD260/OD280 values to calculate the purity and concentration of RNA. A ratio between 1.8 and 2.0 was required to meet the experimental requirements. The concentration of total RNA was determined and the ratio of A260/280 was established to ensure that the ratio was between 1.8 and 2.0; then, reverse transcription was performed to obtain cDNA, which was then stored at −20 °C. Quantitative PCR detection was then performed. The reaction conditions are 95 °C 10 min (one cycle), then 95 °C 15 s, 60 °C 1 min (40 cycles), 95 °C 15 s, 60 °C 1 min, 95 °C 15 s (one cycle). After the quantitative PCR, GAPDH was used as an internal reference to analyze and calculate the results. The primers were synthesized by Sangon Biotech (Shanghai, China), and primer sequences are shown in Table 1.
ELISA detection of TNF-α secretion
After stimulation, the cell supernatant was collected, and the ELISA reaction was performed using the Tumor Necrosis Factor α ELISA Kit (R&D systems, Minneapolis, MN, USA) according to the instructions. After termination of the reaction, the color changes were immediately measured using a microplate reader to measure the optical density of each well at a wavelength of 450 nm. At the same time, 540 nm was set as the calibration wavelength for dual-wavelength measurement, and a standard curve was prepared according to the instructions of the manual. The secretion level of TNF-α in the sample was calculated to be tested according to the standard curve.
Statistical analysis
All experimental results were statistically analyzed using SPSS 19.0 software, and the results are expressed as X ± SD; the experimental results of each group were compared by one-way ANOVA. Values of p < 0.05 was considered to indicate statistically significant differences.
Results
RAW264.7 cell morphology
The cultured RAW264.7 cells were round in morphology. The RAW264.7 cells in the control group and the CMC group were concentrated, while the cells in the titanium particle group were more dispersed (Fig. 1).
RAW264.7 macrophages under the microscope at 100 × magnification. A Control RAW264.7 cells conventionally cultured without any treatments. B Titanium particles were used to stimulate RAW264.7 cells for 12 h. C Cells pre-stimulated with carboxymethyl chitosan (CMC) gel for 4 h were then stimulated by titanium particles for 12 h
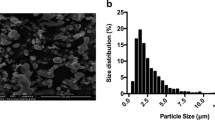
Diameter distribution of titanium particles
According to the image captured by the scanning electron microscopy, the average diameter of titanium particles was 2.4 ± 1.3 μm, and > 90% of the titanium particles were < 3.75 μm. The diameter distribution was similar to that of the wear particles extracted from the loose membrane of the prosthesis (Fig. 2).
Effect of CMC or titanium particles on cell viability
According to results of the CCK-8 test, CMC at 0–1 mg/ml exerts non-toxic effect on RAW264.7 cells after 24 h of incubation (Fig. 3 left panel). Cell viability was significantly inhibited after treatment of 25 mg/ml CMC (Fig. 3 left panel). Thus, 1 mg/ml CMC solution was used for subsequent intervention. In addition, titanium particles induced significant reduction of cell viability, and the alternation was reversed by CMC pretreatment (Fig. 3 right panel).
CMC inhibits titanium particle-induced intracellular reactive oxygen generation
Flow cytometry analysis revealed that the production of intracellular ROS was increased in RAW264.7 cells stimulated with titanium particles (Fig. 4C) compared to that in cells of control group (Fig. 4A). In addition, CMC alone exerted no significant influence on ROS level (Fig. 4B). Moreover, compared with ROS level in titanium group (Fig. 4C), decreased ROS content was detected in RAW264.7 cells pretreated with CMC (Fig. 4D). Quantification of ROS levels in four groups is shown in Fig. 4E.
CMC inhibits titanium particle-induced intracellular reactive oxygen generation. A-D ROS content in RAW264.7 cells of control group, CMC group (stimulation time: 4 h), titanium particles group (stimulation time: 12 h), and titanium particles + CMC group (pre-stimulated with CMC for 4 h and then treated with titanium particles for 12 h) was probed using flow cytometry. E Quantification of ROS levels in four groups based on A–D. *p < 0.05. CMC, carboxymethyl chitosan
CMC protects the activities of antioxidant enzymes
The activities of antioxidant enzymes (SOD, GR, and CAT) and oxidases (NADPH oxidase and iNOS) were evaluated by a spectrophotometer. Compared with the control group, activities of antioxidant enzymes in CMC group were not significantly affected and those in titanium particles group were greatly decreased while those of oxidases were elevated (Fig. 5). In addition, decreased activities of antioxidant enzymes and increased activities of oxidases caused by titanium particles were partially reversed by CMC pre-stimulation (Fig. 5).
CMC protects the activities of antioxidant enzymes. A Activities of antioxidant enzymes, including superoxide dismutase (SOD), glutathione reductase (GR), and catalase (CAT), as well as activities of NADPH oxidase, and iNOS in control group, CMC group (stimulation time: 4 h), titanium particles group (stimulation time: 12 h), and titanium particles + CMC group (pre-stimulated with CMC for 4 h and then treated with titanium particles for 12 h) were evaluated by a spectrophotometer. *p < 0.05. CMC, carboxymethyl chitosan
CMC upregulates mRNA levels of antioxidant enzymes in RAW264.7 cells
RT-qPCR was performed to measure mRNA levels of these enzymes in RAW264.7 cells. Compared with the control group, mRNA expression of oxidases, including iNOS and NOS-1, was increased in the titanium particle group while that of antioxidative enzymes, including CAT, CuZnSOD, GR, GSH-PX and Mn-SOD, was reduced (Fig. 6). In addition, CMC alone has not obvious effect on levels of these oxidases and enzymes (Fig. 6). Importantly, the suppressive impact on antioxidant enzyme activities and the promoting impact on oxidase activities exerted by titanium particles were countervailed by CMC pretreatment (Fig. 6).
CMC upregulates mRNA levels of antioxidant enzymes in RAW264.7 cells. The mRNA levels of antioxidant enzymes, including CAT, CuZnSOD, GR, GSH-PX, and Mn-SOD, levels of oxidases, such as iNOS, NOX-1, and NOX-2, as well as TNFα level in RAW264.7 cells of four groups (control group, CMC group, titanium particles group, and titanium particles + CMC group) were subjected to RT-qPCR analysis. For CMC group or titanium particles group, RAW264.7 cells were stimulated with CMC for 4 h or treated with titanium particles for 12 h. For the last group, cells were pre-stimulated with CMC for 4 h and then treated with titanium particles for 12 h. *p < 0.05. CAT: catalase; GR: glutathione reductase; CMC: carboxymethyl chitosan; GSH-PX: glutathione peroxidase; iNOS: nitric oxide synthase; NOX-1: NAPDH oxidase 1
CMC inhibits the mRNA expression and release of pro-inflammatory factor TNF-α
TNF-α mRNA level was subjected to qPCR analysis and TNF-α concentration was measured by ELISA. Compared with the control group, the titanium particle group displayed increased TNF-α mRNA expression and concentration (Figs. 6 and 7). Moreover, CMC counteracted the elevation of TNF-α mRNA level and accumulation in macrophages treated with titanium particle (Figs. 6 and 7). The concentrations of other inflammatory cytokines including IL-6, IL-1b, and IL-1β were also detected using ELISA. Similarly, titanium particles induced high levels of IL-6, IL-1b, and IL-1β, and the alternation was reversed by CMC pretreatment (Fig. 7).
CMC inhibits the release of pro-inflammatory factor TNF-α. The concentration of TNF-α, IL-6, IL-1b, and IL-1βin control group, titanium particles group (stimulation time: 12 h), and titanium particles + CMC group (pre-stimulated with CMC for 4 h and then treated with titanium particles for 12 h) was evaluated by ELISA. *p < 0.05. CMC, carboxymethyl chitosan
Discussion
Aseptic loosening is one of the most common causes of artificial joint replacement (Cherian et al. 2015). The specific mechanism has been studied a lot, and the role of oxidative stress is gaining increasing attention. Numerous studies have observed that oxides can also enhance the RANKL signaling pathway (Fontani et al. 2015; Filaire and Toumi 2012), induce the expression of osteoclast genes, destroy bone and eventually cause osteolysis and joint loosening. CMC is a derivative formed by introducing carboxymethyl into the molecular chain of chitosan, which not only retains the excellent characteristics of chitosan, but also has stronger water solubility and biological activity (Fiamingo and Campana-Filho 2016). As a derivative of natural polysaccharide chitosan, CMC has a strong antioxidant effect and has broad application prospects. In addition, it was also found that by decreasing the molecular weight of CMC, its antioxidant activity increased due to the decrease in the number of intra- and intermolecular hydrogen bonds.
Evidence has shown that the mechanism of oxidative stress in prosthesis loosening is that active oxides can promote fibrosis and osteoclast differentiation around the prosthesis. First, particles produced by wear between joint prostheses are swallowed by surrounding macrophages to produce reactive oxygen free radicals (Wang et al. 2002). Previous studies have shown that active oxygen free radicals are positively correlated with the development of fibrosis in the tissues around the joint prosthesis (Riedle and Kerjaschki 1997; Windhager et al. 1998). Free radicals induce the cross-linking of matrix proteins and stimulate fibroblasts to secrete collagen, which further increases the excessive fibrosis around the joint prosthesis, increases the pressure in the joint cavity, expands the effective joint space, and allows inflammatory mediators to enter the prosthesis-bone interface and finally leads to aseptic loosening (Kinov et al. 2006). Secondly, oxidative stress affects the functions of osteoblasts and osteoclasts (Wauquier et al. 2009). The amount of oxygen free radicals is directly proportional to the degree of inflammation of synovial cells (Fiorito et al. 2001). At the same time, too much oxide will cause the cells to undergo lipid peroxide reaction, which will damage the cell membrane and destroy the function and structure of osteoblasts. However, recent studies have suggested that a small amount of reactive oxygen species play the role of a second signal molecule in the complex receptor signaling pathway (Forman et al. 2004; Rhee 1999), promoting the differentiation and maturation of osteoclasts. Ha et al. (2004) found evidence that RANKL mediates the production of reactive oxygen species. After RANKL is stimulated for 5 min, the production of reactive oxygen species can be detected by a reactive oxygen species detector, and antioxidants can attenuate this effect. The specific mechanism is that RANKL binds to TNF receptor-related factor 6, induces NADPH oxidase to produce reactive oxygen species, promotes osteoclast gene expression, and makes it mature. In addition, reactive oxygen species can also inhibit the expression of OPG and promote osteoclast differentiation (Fontani et al. 2015; Lee et al. 2005).
CMC has the effect of antagonizing oxidative stress. First, oxides and free radicals are neutralized. The antioxidant effect of CMC is associated with the hydroxyl and carboxyl groups in its polymer chain. They can easily absorb hydrogen atoms in free radicals to form stable polymer groups (Ramasamy et al. 2017). Second, antioxidant enzymes are protected. The production of reactive oxygen radicals is primarily regulated by oxidative stress-associated enzymes. The oxidases mainly include nicotinamide adenine dinucleotide phosphate oxidase and nitric oxide synthase, and the antioxidant enzymes mainly include catalase, superoxide dismutase, glutathione peroxidase and glutathione reductase (Matés 2000; Sareila et al. 2011). The present study revealed that titanium particle stimulation can induce oxidative stress, secrete oxide free radicals, and increase the expression and activity of oxidase. The CMC pretreatment not only neutralizes the oxides, but also decreases the damaging effect of the titanium particles on antioxidant enzymes and protects the activity of antioxidant enzymes.
CMC has the ability to decrease the production of TNF-α. TNF-α can bind to the TNF receptor on the cell membrane, activate multiple signaling pathways, and cause cell inflammatory response or cell apoptosis (Ma et al. 2015). TNF-α can also promote the overexpression of RANKL, which directly stimulates the production of osteoclasts (Li et al. 2003), and can enhance the aggregation of osteoclasts (Azuma et al. 2000; Bertolini et al. 1986). TNF-α can induce osteoblast apoptosis in vivo (Kitaura et al. 2013), and inhibit osteoblast differentiation (Zheng et al. 2017). In this work, titanium particle stimulation increased the level of TNF-α transcription, while macrophages treated with CMC offset the increase in TNF-α mRNA level. ELISA also proved that CMC decreased the secretion of TNF-α.
The present study demonstrated through in vitro experiments that titanium particles stimulate macrophages to increase ROS level, activities of oxidases, and TNF-α concentration while decreasing activities of antioxidant enzymes. CMC exerts an antioxidant effect to resist the oxidative stress of macrophages and the release of TNF-α caused by the stimulation of wear particles. The results of the present study provide options for non-surgical treatment of aseptic loosening of artificial joint prostheses. However, the stimulating condition of the cell experiment was just one kind, which cannot accurately simulate the complicated pathological conditions in the body. Animal experiments will be performed in future studies to investigate the effect of CMC around artificial joint prostheses.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Azuma Y et al (2000) Tumor necrosis factor-alpha induces differentiation of and bone resorption by osteoclasts. J Biol Chem 275:4858–4864
Bertolini DR et al (1986) Stimulation of bone resorption and inhibition of bone formation in vitro by human tumour necrosis factors. Nature 319:516–518
Cherian JJ et al (2015) What host factors affect aseptic loosening after THA and TKA? Clin Orthop Relat Res 473:2700–2709
Cho BK, An MY, Ahn BH (2021) Comparison of clinical outcomes after total ankle arthroplasty between end-stage osteoarthritis and rheumatoid arthritis. Foot Ankle Int 42:589–597
Dodd A et al (2022) Sex differences in end-stage ankle arthritis and following total ankle replacement or ankle arthrodesis. J Bone Joint Surg Am 104:221–228
Fiamingo A, Campana-Filho SP (2016) Structure, morphology and properties of genipin-crosslinked carboxymethylchitosan porous membranes. Carbohydr Polym 143:155–163
Filaire E, Toumi H (2012) Reactive oxygen species and exercise on bone metabolism: friend or enemy? Joint Bone Spine 79:341–346
Fiorito S et al (2001) Increase in free radicals on UHMWPE hip prostheses components due to inflamed synovial cell products. J Biomed Mater Res 57:35–40
Fontani F et al (2015) Glutathione, N-acetylcysteine and lipoic acid down-regulate starvation-induced apoptosis, RANKL/OPG ratio and sclerostin in osteocytes: involvement of JNK and ERK1/2 signalling. Calcif Tissue Int 96:335–346
Forman HJ, Fukuto JM, Torres M (2004) Redox signaling: thiol chemistry defines which reactive oxygen and nitrogen species can act as second messengers. Am J Physiol Cell Physiol 287:C246–C256
Günther KP et al (2021) Total hip replacement for osteoarthritis-evidence-based and patient-oriented indications. Dtsch Arztebl Int 118:730–736
Ha H et al (2004) Reactive oxygen species mediate RANK signaling in osteoclasts. Exp Cell Res 301:119–127
Kinov P et al (2006) Role of free radicals in aseptic loosening of hip arthroplasty. J Orthop Res 24:55–62
Kitaura H et al (2013) Immunological reaction in TNF-alpha-mediated osteoclast formation and bone resorption in vitro and in vivo. Clin Dev Immunol 2013:181849
Lee NK et al (2005) A crucial role for reactive oxygen species in RANKL-induced osteoclast differentiation. Blood 106:852–859
Li P et al (2003) RANK signaling is not required for TNFα-mediated increase in CD11bhi osteoclast precursors but is essential for mature osteoclast formation in TNFα-mediated inflammatory arthritis. J Bone Miner Res 19:207–213
LogithKumar R et al (2016) A review of chitosan and its derivatives in bone tissue engineering. Carbohydr Polym 151:172–188
Luo G et al (2016) Resveratrol protects against titanium particle-induced aseptic loosening through reduction of oxidative stress and inactivation of NF-κB. Inflammation 39:775–785
Ma M et al (2015) Immune system-related differentially expressed genes, transcription factors and microRNAs in post-menopausal females with osteopenia. Scand J Immunol 81:214–220
Matés JM (2000) Effects of antioxidant enzymes in the molecular control of reactive oxygen species toxicology. Toxicology 153:83–104
Mustonen AM et al (2021) Characterization of hyaluronan-coated extracellular vesicles in synovial fluid of patients with osteoarthritis and rheumatoid arthritis. BMC Musculoskelet Disord 22:247
Peng KT et al (2018) Dysregulated expression of antioxidant enzymes in polyethylene particle-induced periprosthetic inflammation and osteolysis. PLoS ONE 13:e0202501
Ramasamy P et al (2017) Characterization of bioactive chitosan and sulfated chitosan from Doryteuthis singhalensis (Ortmann, 1891). Int J Biol Macromol 99:682–691
Rhee SG (1999) Redox signaling: hydrogen peroxide as intracellular messenger. Exp Mol Med 31:53–59
Riedle B, Kerjaschki D (1997) Reactive oxygen species cause direct damage of Engelbreth-Holm-Swarm matrix. Am J Pathol 151:215–231
Sareila O et al (2011) NOX2 complex-derived ROS as immune regulators. Antioxid Redox Signal 15:2197–2208
Steinbeck MJ et al (2014) The role of oxidative stress in aseptic loosening of total hip arthroplasties. J Arthroplasty 29:843–849
Wang ML et al (2002) Exposure to particles stimulates superoxide production by human THP-1 macrophages and avian HD-11EM osteoclasts activated by tumor necrosis factor-alpha and PMA. J Arthroplasty 17:335–346
Wauquier F et al (2009) Oxidative stress in bone remodelling and disease. Trends Mol Med 15:468–477
Windhager R et al (1998) Evidence for the involvement of the hydroxyl radical in the pathogenesis of excessive connective tissue proliferation in patients with tumor-endoprostheses. Life Sci 62:1261–1269
Wooley PH, Schwarz EM (2004) Aseptic loosening. Gene Ther 11:402–407
Younes I, Rinaudo M (2015) Chitin and chitosan preparation from marine sources: structure, properties and applications. Mar Drugs 13:1133–74
Zheng L et al (2017) Role of autophagy in tumor necrosis factor-α-induced apoptosis of osteoblast cells. J Investig Med 65:1014–1020
Acknowledgements
Not applicable.
Funding
This work was supported by grants from the Wuhan Municipal Health Commission (Grant Nos. WX21Q53).
Author information
Authors and Affiliations
Contributions
FL and YZ Wang designed the experiments. HP conducted experiments on the clinical samples and provided material support. FL and HP performed most of the experiments. HP drafted the manuscript. MX performed the bioinformatics analysis. HX to the analysis and interpretation of the data. FL performed the animal experiments and modified the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interest
The authors declare that they have no competing interests.
Ethical approval
All experimental protocols were approved by the Animal Care and Use Committee of Puai Hospital affiliated to Huazhong University of Science and Technology.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, F., Pan, H., Xie, M. et al. Carboxymethyl chitosan regulates oxidative stress and decreases the expression levels of tumor necrosis factor α in macrophages induced by wear particles. Cytotechnology 75, 153–163 (2023). https://doi.org/10.1007/s10616-023-00569-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10616-023-00569-z