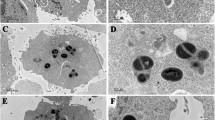
ABSTRACT
We hypothesized that if internalization of Staphylococcus aureus could be blocked by using cytochalasin D (an inhibitor of phagocytosis and phagolysosome fusion), then the intracellular entry and survival of the pathogen in host’s phagocytic cells recruited to the inflammatory site can be restricted. At the same time, if we use antimicrobial agents (e.g., ciprofloxacin and azithromycin) having potent intracellular and extracellular microbicidal activity against the bacterium that have not entered into the phagosome and remains adhered to the phagocytic cell membrane, then they can be eradicated from the site of infection without compromising the host cell. To validate this, role of ciprofloxacin (CIP) and azithromycin (AZM) in eliminating S. aureus by suppressing the phagocytic activity of macrophages with cytochalasin D before infection was investigated. CIP and AZM were used either alone or in combination with cytochalasin D. Supernatant and lysate obtained from the culture of macrophages were used for quantification of reactive oxygen species, lysozymes, antioxidant enzymes, and cytokines produced. Azithromycin was better than ciprofloxacin in combination with cytochalasin D for eradicating S. aureus and regulating cytokine release. Further studies are required for ensuring proper delivery of this combination at the site of infection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Staphylococcus aureus is a major human pathogen causing significant morbidity and mortality in both community- and hospital-acquired infections [1]. S. aureus which often causes chronic or relapsing diseases has been reported to persist as an opportunistic intracellular organism both in vitro and in vivo [2]. Localized S. aureus infections have been followed by bacterial invasion of the vascular system, leading to bacteremia and sepsis. Whether an infection is contained or spreads depends on a complex interplay between S. aureus virulence determinants and host defense mechanisms [3]. S. aureus has been reported to express a wide array of secreted and cell surface-associated virulence factors to help evade immune responses [4]. The treatment of S. aureus infections has been increasingly problematic due to the high prevalence of multi-antibiotic-resistant strains, such as methicillin-resistant S. aureus (MRSA) [5], and the emergence of and vancomycin-resistant S. aureus strains (VRSA) [6].
S. aureus have been reported to survive within phagocytic cells both in polymorphonuclear neutrophil (PMN) and monocytes [7]. However, recent experiments assessing invasion and the intracellular survival of S. aureus in endothelial, epithelial cells, macrophages, and osteoblasts [8–14] suggested that such events may contribute to the persistence of S. aureus during infections such as septic arthritis [15–17]. Moreover, it has long been known that professional phagocytes may serve as intracellular reservoirs of S. aureus [18]. In order to survive and induce infection, pathogenic bacteria have to cope with their changing environment, as well as continuous attacks of the host antimicrobial defense system. In keeping with this idea, recent in vitro studies have confirmed high-level resistance by S. aureus to neutrophil- [19] and macrophage- [20] mediated killing via several virulence factors such as surface proteins that support colonization on host tissues, like coagulase, staphylokinase, leukocidins, etc., that promote bacterial spreading, protein A that inhibits phagocytic engulfment, and toxins, like TSST, hemolysins, leukotoxins, exotoxins, etc., that damage host cell membranes.
It has been suggested by an expert panel of the Infectious Diseases Society of America (IDSA) that vancomycin dosing should be monitored for treatment of infections due to MRSA strains with reduced susceptibility to vancomycin, but the question that still remains to be addressed is what would be the optimal therapy in case of vancomycin resistance. It may be suggested that alternative antibiotic regimen might be used or to find out the role of suitable combination therapy to treat such infections [21]. Thus, there is a need of designing some alternative strategy to treat infections due to MRSA that can eradicate these pathogens completely and ameliorates the progression of the disease processes.
S. aureus may occasionally become intracellular, at least within monocytes, macrophages, and PMN when host defense mechanisms are activated [22]. The facultative intracellular persistence of staphylococci may play an important role in the pathogenesis, because this localization protects them from both humoral and cell-mediated immune responses. The intracellular habitat of S. aureus calls for antibiotics with intracellular activity toward S. aureus. However, antibiotics have routinely been tested only for their in vitro activity on extracellular bacteria. Obviously, the intracellular activity of antibiotics can significantly differ from that exerted extracellularly. In general, it seems impossible to deduce the intracellular antibacterial activity of antibiotics from standard susceptibility tests [23]. Moreover, it has been reported that approximately one third of clinical S. aureus isolates not only survived in but also killed their eukaryotic host cells, which was accompanied by increased in vivo virulence [24]. Therefore, a successful anti-staphylococcal therapy should include the elimination of intracellular bacteria and the rescue of host cells from staphylococci-induced cell death.
The invading bacterium initially adheres to the cell membrane and following transient actin polymerization at sites proximal to the site of entry is thought to trigger ingestion by the formation of a phagocytic vesicle enclosing bacterium [25]. It has been reported that cytochalasin D, a fungal metabolite and specific actin polymerization inhibitor, which binds to actin and modifies its polymerization reversibly has been used as an inhibitor of bacterial uptake and an inducer of cell–cell contact disruption by a variety of cell types [26]. Cytochalasin D permeates cell membrane and causes cells to stop ruffling, stop translocating, and stop rounding up. Functionally, cytochalasin D inhibits microfilament function and polymerization by blocking actin monomer addition at the rapidly growing end of the actin filament [27]. Azithromycin (AZM) belongs to macrolide group of antibiotics that interfere with function of neutrophils and macrophages, disrupting the process of chemotaxis, migration, and cellular activity [28, 29]. This disruption affects cell survival as they induce apoptosis of lymphocytes and neutrophils [30–33]. These antibiotics are also involved in the processes of adherence and diminish the expression of the molecules needed for adherence [34, 35]. Pathogenic bacteria and other infectious agents can stimulate monocytes or macrophages directly, initiating a release of pro-inflammatory cytokines to sustain inflammation and the immunological response. Tumor necrosis factor-alpha (TNF-α), interleukin (IL)-1, IL-6, and IL-8 are biologically active peptides produced by phagocytic macrophages, PMN, eosinophils, and monocytes induced by the pathogen organisms, endotoxin, and other stimuli [36, 37]. Antibiotics are also capable of reducing the production of pro-inflammatory cytokines like IL-1, IL-6, IL-8, TNF-α, and interferon-gamma (IFN-γ) during acute phase inflammatory processes [38, 39]. Furthermore, various oxidizing species that take part in the innate immunity of the host phagocytic cells, such as superoxide anion and nitric oxide, are also diminished by the action of these groups of antibiotics. Their activity is not only restricted within the cytosol of the cell, and they can cause pronounced diminishing effects on several nuclear transcription factors such as NF-κB and activator protein-1 (AP-1), and it is speculated that glutathione deficiency could activate this mechanism [40–43]. Ciprofloxacin (CIP) which belongs to group of fluoroquinolones is a potent synthetic agent active against a variety of bacterial species in vitro. These drugs appear to antagonize the A subunit of the bacterial enzyme DNA gyrase [44–46] and thus block DNA replication. Additional incompletely understood processes typically result in the rapid killing of bacteria [47].
Recent investigation conducted to figure out the role of intracellularly active antibiotics in elimination of intracellular S. aureus reveals that withdrawal of antibiotics leads to cytotoxic activity of the surviving population of S. aureus inside the phagocytic cells which eventually leads to killing of host cells. Thus, uses of antibiotics that are active against intracellular pathogen do not confer full protection from them. This situation demands some alternative strategy which may help to eliminate intracellular pathogen load and also concurrently aid in survival of the phagocytic cells. We, therefore, investigated the role of antibiotics either alone or in combination in eliminating intracellular or extracellular S. aureus by suppressing the phagocytic activity of murine peritoneal macrophages with cytochalasin D before infection to address whether internalization of S. aureus is required or not for antibiotic-mediated killing. This novel strategy might be helpful in reducing intracellular persistence of S. aureus in pathogenesis of several disease processes. In order to address this problem, we hypothesized that if internalization of S. aureus could be blocked by using cytochalasin D (an inhibitor of phagocytosis and phagolysosome fusion), then the intracellular entry and survival of the pathogen in host’s phagocytic cells recruited to the inflammatory site can be restricted. At the same time, if we use antimicrobial agents (e.g., CIP and AZM) having potent intracellular and extracellular microbicidal activity against the bacterium that has not entered into the phagosome and remains adhered to the phagocytic cell membrane, then they can be eradicated from the site of infection without compromising the host cell. Moreover, it has been reported that combination therapy is beneficial over monotherapy. Since no synergistic interaction has been obtained in the in vitro killing of S. aureus, we have omitted the CIP + AZM combination in the cytochalasin D-treated macrophages during S. aureus infection. Thus, we choose combination therapy with an antibiotic (CIP or AZM) and cytochalasin D combination in treatment of S. aureus infection in vitro in isolated murine peritoneal macrophages. Azithromycin was found to be more beneficial in action compared to ciprofloxacin in combination with cytochalasin D in eradicating S. aureus from the infected macrophages. Azithromycin alone was also good enough to decrease the pathogen burden within the macrophages, but owing to intracellular persistence after azithromycin therapy, several diseases posed difficulties in treatment using only antimicrobial therapy. Taken together, it could be suggested that actions of CIP and AZM are enhanced in the presence of cytochalasin D indicating that internalization of S. aureus by macrophages is not essential for antibiotic-mediated killing at least in this in vitro study. Although both the tested antibiotics got access inside the cell as evident from effective pathogen killing, further in vitro studies are required for ensuring proper delivery of this combination at the site of infection or within the phagocytic cells for considering this mode of therapy in cases of intracellular persistence of S. aureus.
MATERIALS AND METHODS
Biochemical Analysis and Biotyping for Characterization of the S. aureus (AG-789) Isolate
Catalase Test
Catalase test was performed according to the protocol given by Cowan [48]. Single colonies of the S. aureus (AG-789) maintained on tryptic soya agar plates were used for the test.
Slide Test
Single colony from the solid medium was transferred to a drop of 30 % hydrogen peroxide (H2O2)
Tube Test
About 3 % aqueous H2O2 solution was poured on growth containing slant or broth and observed for bubbling.
Coagulase Test
The procedure for coagulase testing was followed such as single colony of S. aureus (AG-789) was directly placed in diluted plasma [49] and was placed in water bath and monitored at 2, 4, 6, 8, and 24 h, respectively, for clot formation [50] and examined according to the recommendation of American Public Health Association.
Growth on Glucose-Containing Media
Twenty-hour-old culture of S. aureus (AG-789) in tryptic soy broth was transferred to semisolid Brewer’s fluid thioglycolate media which was steamed and cooled to 50 °C. After solidification at room temperature, tubes were incubated at 37 °C for 72 h [51]. Growth was observed in the anaerobic zone by looking at the tubes through a light source.
Bovine Plasma Coagulation
Eighteen to twenty four hour broth culture of S. aureus (AG-789) was prepared in which 0.5 ml of bovine plasma (diluted) was added in an aluminum-capped test tube. The contents of the tube were mixed and placed in water bath at 37 °C in a water bath. Readings were made at an interval of 30 min to determine the exact time of clotting up to 8 h. Controls containing no S. aureus were prepared to determine the clot formation [52].
Growth on Crystal Violet Agar
Crystal violet agar was prepared by adding crystal violet in different dilutions to nutrient agar. Single colonies of S. aureus were taken and transferred to crystal violet agar plates and incubated overnight at 37 °C, and the resulting growth was observed and examined as purple or white colonies [53].
Antimicrobial Agents and Chemicals
The study drugs which included AZM, CIP, methicillin (MET), and chloramphenicol (CLO) (HiMedia, Bombay, India) were used for all in vitro testing. Ciprofloxacin was dissolved in sterile double-distilled water, whereas azithromycin was dissolved in absolute alcohol to prepare a stock solution of 100 μg/ml, which was further diluted in sterile water before use for in vitro assays. Cytochalasin D was obtained from Enzo Life Sciences, USA. Cytochalasin D was prepared to the desired dose by dissolving the chemical in 2 mM dimethyl sulfoxide (DMSO) Merck Ltd, Mumbai, India. The solution was stored at −20 °C until used.
In Vitro Susceptibility Tests
In vitro susceptibilities of the isolate were performed per National Committee for Clinical Laboratory Standards (NCCLS) guidelines. Minimum inhibitory concentrations (MICs) and minimal bactericidal concentrations (MBCs) were determined by the microtitre broth dilution method in MHB (HiMedia, Bombay, India), and disk agar diffusion (DAD) test was performed using Mueller-Hinton agar supplemented with 5 % sheep blood following NCCLS guidelines [54].
Test for Synergism and Antagonism
Checkerboard assay was performed according to the method described earlier [55]. For each combination, a synergy test was performed in a 96-well microtitre plate containing two antimicrobial agents in twofold dilutions (4 × MIC to 1/32 × MIC) dispensed in a checkerboard fashion on the day of the assay. ∑ Fractional inhibitory concentrations (FICs) were calculated and were used to classify the effect of combination of antimicrobial agents as the following: synergistic, for FIC indexes ≤0.5; no interaction, for FIC indexes >0.5–4; and antagonistic, for FIC indexes >4.
Maintenance of Animals and Cells
All experiments involving animals were conducted according to the protocols that had been approved by the Institutional Animal Ethics Committee (IAEC), Department of Physiology, University of Calcutta, under the guidance of Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA) [Approval Number 820/04/ac/CPCSEA dated August 5, 2013], Ministry of Environment and Forest, Government of India. Wild-type male Swiss albino mice were used throughout the study. To minimize the feeling of hypoxia or discomfort before and during mouse dissection and tissue collection, mice were anesthetized with inhaling anesthetics (ether) before terminal surgery. Euthanasia was performed by general anesthesia followed by vital tissue removal using 2–3 % ether for induction and 1 % for maintenance. Macrophages were prepared from peritoneal fluids of thioglycolate-administered mice. The S. aureus strain AG-789 was obtained from Apollo Gleneagles Hospital, Calcutta, West Bengal, India. The strain AG-789 was found to be methicillin-resistant and catalase-positive.
Preparation of Bacteria for In Vitro Infection to Peritoneal Macrophages
The S. aureus strain AG-789 was obtained from Apollo Gleneagles Hospital, Calcutta, West Bengal, India. S. aureus strain (AG-789) grown overnight in Luria-Bertani broth (HiMedia, Bombay, India) was diluted with fresh broth and cultured until mid-logarithmic phase of growth. Bacteria were harvested, washed twice with sterile saline, and adjusted to the desired inoculums spectrophotometrically before infection (OD620 = 0.2 for 5.0 × 107 cells/ml for S. aureus), and the colony-forming unit (CFU) count of the desired inoculum was confirmed by serial dilution and culture on blood agar [15–17].
Isolation and Stimulation of Peritoneal Macrophages
The mice, used at 6 to 8 weeks of age and fed standard laboratory chow and water, were injected intraperitoneally with 2 ml of 4 % sterile thioglycolate broth, and the resulting peritoneal exudates were harvested by lavage of the peritoneal cavities of mice with endotoxin-free Hanks’ solution 4 to 5 days later. Peritoneal macrophages were suspended in 0.83 % ammonium chloride solution containing 10 % (v/v) Tris buffer (pH 7.65) to lyse erythrocytes. The cells were resuspended in Roswell Park Memorial Institute (RPMI) medium supplemented with 10 % fetal bovine serum (FBS), 100 IU/ml penicillin, and 100 μg/ml streptomycin and then were allowed for plastic adherence. Nonadherent cells were removed by aspiration and washing with RPMI 1640 medium before the addition of S. aureus. The adherent macrophages, more than 95 % of which appeared to be typical macrophages by light microscopy, were used for each experiment [11–13]. The peritoneal macrophages collected were suspended in Hank’s balanced salt solution (HBSS) solution. Murine peritoneal macrophages (5 × 106 cells/ml) were infected with S. aureus (5 × 106 CFU/ml) for 30, 60, and 90 min at 37 °C, in humidified 5 % CO2 incubator (Heal Force, Model HF-151, China) in the presence or absence of antibiotics and cytochalasin D at a dose of 5 μg/ml [28] either alone or in combination with azithromycin or ciprofloxacin at a dose of four times the MIC for the S. aureus strain (AG-789).
Assay for Intracellular Killing
Murine peritoneal macrophages (5 × 106 cells/ml) were mixed with S. aureus (5 × 106 CFU/ml) in a 1:1 cell/bacterium ratio [11–13] in RPMI-FBS (5 %) and incubated at 37 °C cell culture incubator for different times in the presence and absence of antibiotics and cytochalasin D either alone or in combination. After centrifugation, cell culture supernatants were collected and stored for further assay. Phagocytosis was stopped by adding cold (4 °C) RPMI-1640, and extracellular S. aureus were removed by washing the suspension in RPMI. The pellets were disrupted in sterile water containing 0.01 % bovine serum albumin (BSA) by vigorously vortexing to release intracellular bacteria in the lysate. The lysates containing bacteria were plated at serial dilutions on mannitol agar plates. The plates were incubated at 37 °C for a day or two, and the number of colonies was determined.
H2O2 Production
After time-dependent phagocytosis, supernatants were collected, and cell lysates were prepared from the pellet. H2O2 assay of the supernatant and lysate was performed according to the method as described earlier with slight modification [56]. Briefly 70 μl of supernatant or lysate, 20 μl horseradish peroxidase (HRP) (500 μg/ml), 70 μl of phenol red (500 μg/ml), and 40 μl medium were added in each of the microtiter plate and was allowed for incubation for 2 h at 37 °C. The reaction was stopped by adding 25 μl of 2 (N) NaOH, and the absorbance reading was taken at 620 nm. Control set received 40 μl of HBSS in place of supernatant/lysate. A standard H2O2 curve was plotted, and H2O2 release in supernatants and lysate was evaluated and expressed in micromolar per 106 cells.
Assay for Determination of Superoxide Anion
Superoxide anion release assay measures the change in color of cytochrome C (cytC), when reduced by O2− released from the stimulated macrophages. Cell-free supernatants recovered from S. aureus-stimulated macrophages in the presence or absence of antibiotics and cytochalasin D either alone or in combination were incubated in the presence of cytochrome C (100 μl at 2 mg/ml in HBSS). The reaction was terminated by placing the tubes in ice for 5 min. The production of superoxide anion was monitored spectrophotometrically (UV-1800 UV–VIS spectrophotometer, Shimadzu, Japan) at 550 nm in reference to the blank. The amount of superoxide production was calculated by the following formula: nanomoles of superoxide anion = (mean absorbance at 550 nm × 15.87) [57].
Determination of Antioxidant Enzyme Activity in Supernatant and Lysate After Time-Dependent Phagocytosis of S. aureus in the Presence or Absence of Antibiotics and Cytochalasin D Either Alone or in Combination
Assay of Catalase Enzyme Activity
Catalase enzyme activity in the supernatant or cell-free lysate was determined spectrophotometrically by measuring the decrease in H2O2 concentration at 240 nm. At time 0, 10 μl of the supernatant or cell-free lysate was added separately to 2.89 ml of potassium phosphate buffer (pH 7.4) taken in a quartz cuvette. To it, 0.1 ml of 300 mM H2O2 was added and absorbance was taken at 240 nm for 5 min at 1-min intervals. Catalase activity was expressed in terms of millimole per minute per milligram protein [15–17].
Assay of Superoxide Dismutase (SOD) Enzyme Activity
One hundred microliters of the supernatant or cell-free lysate was mixed separately with 1.5 ml of a Tris-ethylenediamine hydrochloric acid (Tris–EDTA–HCl) buffer (pH 8.5), then 100 μl of 7.2 mmol/L pyrogallol was added and the reaction mixture was incubated at 25 °C for 10 min. The reaction was terminated by the addition of 50 μl of 1 M HCl and measured at 420 nm. One unit was determined as the amount of enzyme that inhibited the oxidation of pyrogallol by 50 %. The activity was expressed as units per milligram protein [15–17].
Assay of Reduced Glutathione (GSH) Content
Reduced glutathione content (as acid-soluble sulfhydryl) was estimated by its reaction with DTNB (Ellman’s reagent) following the method of Sedlac and Lindsey with some modifications [58]. Of the sample, 0.3 ml was mixed with 0.3 ml 10 % TCA followed by vortexing. Then, the mixture was centrifuged at 5,000 rpm for 10 min at 4 °C. Two hundred fifty microliters of the resultant supernatant was added with 500 μl 0.8 M Tris–HCl followed by the addition of 25 μl of DTNB. The absorbance was measured at 412 nm using a UV–VIS spectrophotometer to determine GSH content. The values were expressed as nanomoles of GSH per milligram protein
Assay of Bactericidal Lysozyme Enzyme Release
The supernatants and cell-free lysates obtained in the phagocytosis assay were used to estimate the lysozyme content after varying phagocytic times. Lysozyme content was estimated according to the method described by Colowick et al. [59]. A suspension of Micrococcus lysodeikticus in 0.15 M potassium phosphate buffer, pH 6.2, was prepared and its optical density was fixed at 0.6–0.7 at 450 nm. Of this suspension, 900 μl was mixed with 50 μl of 1 % Triton X-100, and the reaction was started by adding 50 μl of the supernatants or cell-free lysates. The decrease in optical density was recorded at 450 nm as a function of time. The change in absorbance for the first minute only was used to determine the rate of the reaction. One unit of enzyme is defined as the amount of enzyme that produces a decrease in absorbance of 0.001/min at 450 nm.
Assay for Protein Estimation of the Sample
Protein content of the respective supernatant and lysate was measured by the method [60] as described earlier.
Tumor Necrosis Factor-α, Interferon-Gamma, Interleukin-6, and Interleukin-10 ELISA Assays
Murine peritoneal macrophages (5 × 106 cells/ml) were mixed with S. aureus (5 × 106 CFU/ml) in a 1:1 cell/bacterium ratio [11–13] in RPMI-FBS (5 %) and incubated at 37 °C cell culture incubator for different times in the presence and absence of antibiotics and cytochalasin D either alone or in combination. After incubation, cell culture supernatants were collected and stored at −70 °C prior to analysis. Supernatants from different groups were normalized to the protein content by Bradford method before the assay, and levels of TNF-α, IFN-γ, IL-6, and IL-10 production were assayed using enzyme-inked immunosorbent assay (ELISA) kits according to the instructions provided by the manufacturer (RayBiotech, Inc. USA) in a BioRad ELISA reader.
Statistical Analysis
Isolated peritoneal macrophages from mice were pooled together to obtain the requisite amount of macrophages (5 × 106 cells/ml), and the different parameters were measured. This was repeated for three times for each parameter (e.g., H2O2 production), then the mean value of these triplicate experiments was taken for calculation. Data was expressed as mean ± SD. Means were compared between groups by using analysis of variance (ANOVA). P < 0.05 was considered significant.
RESULTS
Biochemical Analysis and Biotyping for Characterization of the S. aureus (AG-789) Isolate
Catalase Test
Bubbling was observed in case of both slide and tube test upon addition of H2O2 which confirms S. aureus (AG-789) to be catalase-positive.
Coagulase Test
In the second hour, slight coagulation was seen. In the fourth hour, coagulation was seen in one fourth of the volume of the tube. In 24th hour, a slight increase in coagulation than the fourth hour was observed. These observations hold S. aureus (AG-789) to be coagulase-positive. The clot can be described as 2+.
Anaerobic Growth in Glucose-Containing Media
Dense uniform anaerobic growth denoted by ++ was seen in the anaerobic zone of the tubes.
Bovine Plasma Coagulation
Coagulation was observed after incubation for 2 h, and subsequently, the size of clot got increased till the eighth hour after which no increase in the size of the clot was visualized. Diluted plasma coagulated less as compared to plasma without dilution. Thus, it can be said that S. aureus (AG-789) can coagulate bovine plasma.
Growth on Crystal Violet Agar
Positive growth of S. aureus (AG-789) was seen in all dilutions of crystal violet agar except the plate containing lowest dilution of crystal violet (i.e., 100 μg/ml). Maximum growth was visualized in the plate containing highest dilution of crystal violet (1 μg/ml). Thus, it can be said that S. aureus (AG-789) can grow on crystal violet containing agar, but crystal violet inhibits growth at higher concentration.
In Vitro Susceptibility Test
The tested strain AG-789 was found to be resistant to methicillin. This MRSA was found to be sensitive to ciprofloxacin, chloramphenicol, and azithromycin. MBC and zone diameter obtained from DAD test were found to be in compliance with the MIC value for the tested strain. Results of MIC, MBC, and DAD were represented in Table 1.
Evaluation of Synergy by Checkerboard Assay
Table 2 illustrates the interaction between the study drugs in different combination that might be of therapeutic use. The results of this assay showed no synergistic interaction between the antibiotics, ammonium chloride, or cytochalasin D. Antagonism was observed with methicillin interacting with ciprofloxacin or azithromycin. Interaction of these antibiotics with cytochalasin D showed indifference in action. Thus, combination of two antibiotics could not be recommended for use in this experimental setup.
Effect of Cytochalasin D and Antibiotic Treatment Either Alone or in Combination on Phagocytosis of S. aureus by Peritoneal Macrophages
Control macrophages were readily able to phagocytose S. aureus. After 90 min of incubation, the number of viable bacteria within macrophages declined. However, the number bacteria phagocytosed by cytochalasin D-treated macrophages appeared to be lower than that observed for cytochalasin D-untreated macrophages at the same incubation times. Concentration of DMSO used for reconstitution of cytochalasin D did not show any significant alteration of phagocytosis of S. aureus by macrophages (Table 3). Overall, the experimental results indicated that cytochalasin D treatment leads to a reduction in the uptake of S. aureus cells by macrophages presumably by interfering with actin-mediated phagocytosis. CIP and AZM do not affect the viability of macrophages (data not shown) but almost immediately after application to infected macrophages inhibit the S. aureus growth and replication. There were statistically significant differences in the counts of viable bacteria recovered from cytochalasin plus antibiotic-pretreated macrophages before S. aureus infection when compared with cytochalasin D-pretreated plus S. aureus-infected macrophages (Table 3). This result clearly showed that cytochalasin D in the presence of either CIP or AZM reduced intra-macrophage killing of S. aureus.
Effect of Cytochalasin D and Antibiotic Treatment on S. aureus Infection-Induced Hydrogen Peroxide Production in the Supernatant and Lysate by Murine Peritoneal Macrophages
Amount of H2O2 released in the supernatant or lysate was significantly higher (P < 0.05) in S. aureus-infected macrophage than that of control macrophages only at 60 and 90 min after S. aureus infection. Statistically significant increment in the amount of H2O2 production only in the supernatant but not in lysate was observed (P < 0.05) in cytochalasin D-pretreated macrophages before infection at 60 and 90 min post-incubation, when compared with the only S. aureus-infected macrophages (Table 4).
Macrophages pretreated either with CIP or AZM alone before S. aureus infection also showed reduced production of H2O2 in the supernatant or lysate in comparison to the amount released by the only S. aureus-infected macrophages. However, administration of either CIP or AZM to the cytochalasin D-pretreated macrophages before S. aureus infection showed reduced H2O2 release in the supernatant when compared with cytochalasin D plus S. aureus-infected macrophages (Table 4).
Effect of Cytochalasin D and Antibiotic Treatment on S. aureus Infection-Induced Superoxide Anion Release in the Supernatant and Lysate by Murine Peritoneal Macrophages
The amount of superoxide anion (O2 .−) (nanomoles/106 cells) released in the supernatant and lysates was also estimated. The increase in the O2 .− released in supernatant and lysate after different times of phagocytosis in the presence of S. aureus infection was found to be significant (P < 0.05) when compared to the O2 .− release of noninfected control macrophages (Table 5). Statistically significant increase in the amount of O2 ·− released both in the supernatant and lysate was observed (P < 0.05) in cytochalasin D-pretreated macrophages before infection, when compared only with the S. aureus-infected macrophages at 30 and 60 min post-incubation. Treatment of macrophages with CIP alone before S. aureus infection showed increased amount of O2 .− released in the supernatant but decreased in the lysate at 60 and 90 min post-incubation when compared with S. aureus alone-infected macrophages. Treatment of macrophages with AZM alone before S. aureus infection also showed increased amount of superoxide anion released in the supernatant in comparison to the only S. aureus-infected macrophages. However, the administration of either CIP or AZM to the cytochalasin D-pretreated macrophages before S. aureus infection showed significantly reduced amount of O2 .− release in the supernatant as well as in the lysate when compared with cytochalasin D plus S. aureus-infected macrophages after phagocytosis (Table 5).
Effect of Cytochalasin D and Antibiotic Treatment on S. aureus Infection-Induced Alteration in the Catalase Enzyme Activity in the Supernatant and Lysate by Murine Peritoneal Macrophages
Figure 1 shows a marked increase in the catalase enzyme activity in the supernatant or lysate of peritoneal macrophages infected with S. aureus after 30, 60, and 90 min of phagocytosis compared with the noninfected control group. A significant decrease in the catalase enzyme activity was observed in the supernatants of cytochalasin D-treated murine peritoneal macrophages before infection compared with the cytochalasin D-nontreated infected group. Treatment of macrophages with either CIP or AZM alone before S. aureus infection showed decreased amount of catalase enzyme activity in the supernatant when compared with S. aureus alone-infected macrophages. This decrease in the catalase enzyme activity was even more prominent when cytochalasin D-pretreated macrophages were incubated with either CIP or AZM before S. aureus infection in comparison to cytochalasin D plus S. aureus-infected macrophages (Fig. 1).
Effect of cytochalasin D and antibiotic treatment on S. aureus infection-induced alteration in the catalase enzyme activity in the supernatant and lysate by murine peritoneal macrophages. The supernatant and lysate recovered after time-dependent phagocytosis in the presence or absence of cytochalasin D or antibiotic treatment as described in the “MATERIALS AND METHODS” section were used to determine catalase activity in the presence of 15 μmol of H2O2/ml of phosphate buffer. Catalase activity was expressed in terms of millimoles per minute milligram protein. Results were shown as mean ± SD of three independent experiments. CM, control macrophage; SAM, S. aureus-infected macrophages; Cyto D + SAM, cytochalasin D-pretreated + S. aureus-infected macrophages; SAM + CIP, S. aureus-infected macrophages treated with ciprofloxacin; SAM + AZM, S. aureus-infected macrophages treated with azithromycin; Cyto D + SAM + CIP, cytochalasin D-pretreated macrophages infected with S. aureus and then treated with ciprofloxacin; Cyto D + SAM + AZM, cytochalasin D-pretreated macrophages infected with S. aureus and then treated with azithromycin. Asterisk: Significant difference with respect to CM. Number sign: Significant difference with respect to SAM. Dollar sign: Significant difference with respect to SAM plus CIP v ersus SAM plus AZM-treated macrophages. Circumflex accent: Significant difference with respect to either ciprofloxacin (CIP) or azithromycin (AZM) plus S. aureus-infected macrophages versus cytochalasin D plus SAM, at P < 0.05 level of significance.
Effect of Cytochalasin D and Antibiotic Treatment on S. aureus Infection-Induced Alteration in the SOD Enzyme Activity in the Supernatant and Lysate by Murine Peritoneal Macrophages
Figure 2 shows a significant increase in the superoxide dismutase enzyme activity in the supernatant or lysate of peritoneal macrophages infected with S. aureus after 30, 60, and 90 min of phagocytosis compared with the noninfected control group (P < 0.05). A significant decrease in the SOD enzyme activity was observed in the supernatants and lysates of cytochalasin D-treated murine peritoneal macrophages before infection compared with the cytochalasin D-nontreated infected group. Treatment of macrophages with either CIP or AZM alone before S. aureus infection showed decreased amount of SOD enzyme activity in the supernatant and lysate when compared with S. aureus alone-infected macrophages. This decrease in the SOD enzyme activity was even more prominent in the supernatant at 60 and 90 min when cytochalasin D-pretreated macrophages were incubated with either CIP or AZM before S. aureus infection in comparison to cytochalasin D plus S. aureus-infected macrophages (Fig. 2).
Effect of cytochalasin D and antibiotic treatment on S. aureus infection-induced alteration in the superoxide dismutase (SOD) enzyme activity in the supernatant and lysate by murine peritoneal macrophages. The supernatant and lysate which recovered after time-dependent phagocytosis in the presence or absence of cytochalasin D or antibiotics were used to determine SOD activity and were expressed in terms of the unit of SOD per milligram protein. Results were presented as mean ± SD of three independent experiments. Asterisk: Significant difference with respect to CM. Number sign: Significant difference with respect to SAM. Dollar sign: Significant difference with respect to SAM plus CIP versus SAM plus AZM-treated macrophages. Circumflex accent: Significant difference with respect to either ciprofloxacin (CIP) or azithromycin (AZM) plus S. aureus-infected macrophages versus cytochalasin D plus SAM, at P < 0.05 level of significance.
Effect of Cytochalasin D and Antibiotic Treatment on S. aureus Infection-Induced Alteration in the GSH Content in the Supernatant and Lysate by Murine Peritoneal Macrophages
Content of GSH in the supernatant or lysate was significantly higher (P < 0.05) in S. aureus-infected macrophage than that of control macrophages at 30, 60, and 90 min after S. aureus infection. Statistically significant increase in the amount of GSH only in the supernatant but not in lysate was observed (P < 0.05) in cytochalasin D-pretreated macrophages before infection at 30, 60, and 90 min post-incubation, when compared with the only S. aureus-infected macrophages (Fig. 3).
Effect of cytochalasin D and antibiotic treatment on S. aureus infection-induced alteration in the GSH content in the supernatant and lysate by murine peritoneal macrophages. The supernatant and lysate which recovered after time-dependent phagocytosis in the presence or absence of cytochalasin D or antibiotics were used to determine the GSH content and were expressed in terms of micromolar per milligram protein. Results were presented as mean ± SD of three independent experiments. Asterisk: Significant difference with respect to CM. Number sign: Significant difference with respect to SAM. Dollar sign: Significant difference with respect to SAM plus CIP versus SAM plus AZM-treated macrophages. Circumflex accent: Significant difference with respect to either ciprofloxacin (CIP) or azithromycin (AZM) plus S. aureus-infected macrophages versus cytochalasin D plus SAM, at P < 0.05 level of significance.
Macrophages pretreated with CIP alone before S. aureus infection also showed increased level of GSH in the supernatant at 30 and 60 min post-incubation but decreased at 90 min in comparison to the amount released by the only S. aureus-infected macrophages. Macrophages pretreated with AZM alone before S. aureus infection showed decreased amount of GSH content in the supernatant at 60 and 90 min of post-incubation when compared to the amount of superoxide anion released by the only S. aureus-infected macrophages. However, administration of CIP to the cytochalasin D-pretreated macrophages before S. aureus infection showed increased GSH content in the supernatant but decreased in the lysate when compared with cytochalasin D plus S. aureus-infected macrophages at 60 and 90 min post-incubation (Fig. 3). Administration of AZM to the cytochalasin D-pretreated macrophages before S. aureus infection showed reduced GSH level both in the supernatant and in the lysate in comparison to the GSH level of cytochalasin D plus S. aureus-infected macrophages at 60 and 90 min post-incubation (Fig. 3).
Effect of Cytochalasin D and Antibiotic Treatment on S. aureus Infection-Induced Alteration in the Lysozyme Enzyme Activity in the Supernatant and Lysate by Murine Peritoneal Macrophages
Figure 4 shows a significant increase in the lysozyme enzyme activity in the supernatant or lysate of peritoneal macrophages infected with S. aureus after 30, 60, and 90 min of phagocytosis compared with the noninfected control group (P < 0.05). A significant decrease in the lysozyme enzyme activity was observed in the supernatants and lysates of cytochalasin D-treated murine peritoneal macrophages before infection when compared with the cytochalasin D-nontreated infected group. Treatment of macrophages with either CIP or AZM alone before S. aureus infection also showed decreased amount of lysozyme enzyme activity in the supernatant and lysate when compared with S. aureus alone-infected macrophages. However, the lysozyme enzyme activity was restored significantly in the lysate at 30 and 90 min when cytochalasin D-pretreated macrophages were incubated with either CIP or AZM before S. aureus infection in comparison to cytochalasin D plus S. aureus-infected macrophages (Fig. 4).
Effect of cytochalasin D and antibiotic treatment on S. aureus infection-induced alteration in the lysozyme enzyme activity in the supernatant and lysate by murine peritoneal macrophages. The supernatants and cell-free lysates obtained in the phagocytosis assay were allowed to interact with a suspension of Micrococcus lysodeikticus in 0.15 M potassium phosphate buffer, pH 6.2, and optical density fixed at 0.6–0.7 at 450 nm. One unit of lysozyme was defined as the amount of enzyme that produces a decrease in absorbance of 0.001/min at 450 nm, and the lysozyme enzyme activity was expressed in terms of units per milligram protein. Results were presented as mean ± SD of three independent experiments. Asterisk: Significant difference with respect to CM. Number sign: Significant difference with respect to SAM. Dollar sign: Significant difference with respect to SAM plus CIP versus SAM plus AZM-treated macrophages. Circumflex accent: Significant difference with respect to either ciprofloxacin (CIP) or azithromycin (AZM) plus S. aureus-infected macrophages versus cytochalasin D plus SAM, at P < 0.05 level of significance.
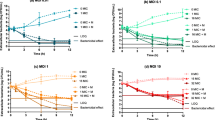
Effect of Cytochalasin D and Antibiotic Treatment on S. aureus Infection-Induced TNF-α, IFN-γ, IL-6, and IL-10 Production by Murine Peritoneal Macrophages
Figure 5 showed a gradual decrease in the IL-6 (Fig. 5c), IL-10 (Fig. 5d), and IFN-γ (Fig. 5b) level in the media (culture supernatant) with an increase in phagocytic time in the presence of cytochalasin D significantly (P < 0.05) when compared to the cytokine production after time-dependent phagocytosis with cytochalasin D-nontreated infected macrophage. Macrophages pretreated either with CIP or AZM alone before S. aureus infection also showed reduced level of IL-6, IL-10, and IFN-γ in the supernatant in comparison to the amount released by the only S. aureus-infected macrophages. However, the level of IL-6, IL-10, and IFN-γ was decreased significantly in the medium at 60 and 90 min when cytochalasin D-pretreated macrophages were incubated with either CIP or AZM before S. aureus infection in comparison to cytochalasin D plus S. aureus-infected macrophages. Level of TNF-α was decreased significantly in the medium at 60 and 90 min when cytochalasin D-pretreated macrophages were incubated with either CIP or AZM before S. aureus infection in comparison to cytochalasin D plus S. aureus-infected macrophages (Fig. 5a).
a–d Cytokine release in the supernatant. Levels of tumor necrosis factor-alpha (TNF-α) (a), interferon-gamma (IFN-γ) (b), interleukin-6 (IL-6) (c), and interleukin-10 (IL-10) (d) in the supernatants collected after 30, 60, and 90 min of Staphylococcus aureus-infected macrophages in the presence or absence of cytochalasin D or antibiotics were determined by utilizing ELISA according to the manufacturer’s recommendations and were expressed from triplicate experiments. Asterisk: Significant difference with respect to CM. Number sign: Significant difference with respect to SAM. Dollar sign: Significant difference with respect to SAM plus CIP versus SAM plus AZM-treated macrophages. Circumflex accent: Significant difference with respect to either ciprofloxacin (CIP) or azithromycin (AZM) plus S. aureus-infected macrophages versus cytochalasin D plus SAM, at P < 0.05 level of significance.
DISCUSSION
In view of the fact that staphylococcal infections have a tendency to become chronic, the fate of S. aureus within macrophages is an important consideration. Intracellular persistence of S. aureus within the macrophages, especially the antibiotic resistant types, has posed serious problems and difficulties in treatment of several diseases. Moreover, antibiotics with known intracellular bactericidal activity when used have been shown to be uptaken within the host cell, but recent evidences have suggested that they no longer confer protection in vitro. The possible reasons for that discrepancy are numerous: (i) the degree of plasma membrane permeability may determine concentration gradients of the antibiotic inside and outside of the cell, (ii) the subcellular localization of antibiotics and bacteria may segregate in diverse intracellular compartments, (iii) antibiotics may be inactivated by the intracellular milieu, and (iv) differences in the physiological state of the internalized pathogen (resting versus dividing) can modulate the susceptibility to antibiotics [61]. Another complicating factor is the extracellular multiplication of the surface-associated bacteria or those released from lysed infected cells, since these organisms would be subject to repeated endocytic events.
Efforts to obviate this difficulty by adding appropriate antibiotics and by restricting the entry of viable staphylococci associated with the cells surface in vitro require a novel combination of drugs involving an efficacious antibiotic with intracellular bactericidal activity and an inhibitor of phagocytosis, cell adherence, and phagolysosome fusion so that the situation can be controlled by killing the pathogens in the extracellular environment and also ameliorating their intracellular multiplication within phagocytic cells. Thus, the aim of our study was to formulate an efficacious combination using in vitro susceptibility studies and to observe the effect of such combination on uptake of staphylococcal cells by isolated murine peritoneal macrophages and their subsequent lysis to reduce the pathogen load within the phagocytic cells of the host. We chose mouse peritoneal macrophages as we could investigate phagocytosis within 2 h of isolation, compared with 1 week, which is required for differentiation of human monocyte-derived macrophages [62, 63]. Moreover, peritoneal macrophages used in the study were elicited with thioglycolate broth but did not potentially affect intracellular killing as evident from our earlier study [11–13]. Therefore, inclusion of data set by using untreated or nonelicited macrophages, such as alveolar macrophages or splenic macrophages, is not essential in our study. When testing the cytochalasin D as an augmenter of the antibiotics, several assays like intracellular killing, production of reactive oxygen species, antioxidant enzymes, lysozyme release, and finally measurement of cytokines released by host cells in response to all these treatments before S. aureus infection and the rationale for performing these above assays have already been mentioned in the “MATERIALS AND METHODS” section. Therefore, the above assays are very much linked and do not stand individually.
In this study, we tested the in vitro susceptibility of the clinical isolate S. aureus (AG-789). This S. aureus strain was found to be methicillin-resistant and was thus termed MRSA. Results of MIC, MBC, and DAD revealed that this strain was sensitive to AZM and CIP both of which have potent intracellular bactericidal activities. We used checkerboard technique to investigate synergism of the combinations studied against the isolated MRSA (AG-789). Since various concentrations of antibiotic combinations can be studied by twofold broth dilutions, the synergistic effect can be detected by determining the summation fractional inhibitory concentration (Σ FIC) from the respective individual FIC value of each antibiotic in combination. Synergy is defined as a decrease in the viable organism as a result of the combination when compared with the most effective antibiotic when tested alone. Our results show that none of the tested combinations showed synergy. Azithromycin showed indifference, whereas ciprofloxacin was resistant to the pathogen in combination with cytochalasin D, respectively.
Another aim of this study was to determine the intracellular anti-staphylococcal activities of antibiotics in the presence of cytochalasin D, an established inhibitor of phagocytosis and phagolysosome fusion. The role of microfilament and cytoskeleton rearrangement in phagocytosis by mammalian cells and other has been documented in a number of published reports [64, 65]. The cellular processes are normally dependent on actin polymerization and are sensitive to actin polymerization inhibitor cytochalasin D. Cytochalasin D is a specific actin polymerization inhibitor [66] that has been extensively used to study actin polymerization-dependent processes in mammalian cells [67]. It is well established that S. aureus invade phagocytic cells like macrophages, and here, we have also observed that in the absence of cytochalasin D phagocytosis process was normal but in presence of cytochalasin D phagocytosis was impaired in the macrophages. It was reported that viability of macrophages over 48 h was not affected by treatment with cytochalasin D [68]; therefore, alteration of macrophage viability after 90 min of incubation with cytochalasin D in our study could be avoided. DMSO (0.1 %) which was used to dissolve cytochalasin D did not affect the growth rate of bacteria. Macrophages that had received no treatment or had been pretreated with DMSO were included as control cultures. These control co-cultures were used to rule out any possible impact of this solvent on phagocytosis of macrophages. Since counts of bacteria recovered from these control co-cultures were similar to those obtained for co-cultures that used untreated macrophages, the concentration of DMSO used was assumed to not have an adverse effect on phagocytosis of S. aureus by macrophages (Table 3). In fact, phagocytosis of macrophages containing DMSO in medium did not show any significant alteration when compared with the phagocytosis of S. aureus by macrophages containing no DMSO in the cell culture medium (Table 3). Moreover, addition of either azithromycin or ciprofloxacin to the medium of culture was able to completely eradicate S. aureus. The distinct subcellular localization of S. aureus might impair, at least in part, the accessibility for antibiotics. A high intracellular concentration of a given antibiotic does not guarantee effective intracellular antimicrobial activity. What counts is the concentration of free active antibiotic where the bacteria are. Thus, the concentrations of azithromycin and ciprofloxacin acting on S. aureus in the cytoplasm may be low in relation to the total amount of drug present in the cell. The rapid lysis of staphylococci, streptococci, and other pyogenic bacteria exposed to cell wall active antibiotics such as beta-lactams and vancomycin results in exaggerated release of bacterial products and an augmented and potentially harmful host inflammatory response. Therefore, optimal treatment of sepsis and other severe bacterial infections and or other mediators that blunt the host inflammatory response and dampen the cytokine cascade could be a choice [32, 69]. In our study, CIP and AZM do not affect the growth of the macrophage but almost immediately after application to infected macrophages inhibit S. aureus growth and replication. This was supported by a recent report which stated that the macrolide antibiotic AZM at low doses significantly improved the phagocytosis of apoptotic bronchial epithelial cells by bronchoalveolar lavage (BAL)-derived alveolar macrophages from normal and chronic obstructive pulmonary disease (COPD) subjects [70]. The current findings of improvement of peritoneal macrophage’s phagocytic ability for S. aureus in the presence of AZM may thus provide a novel approach to supplement existing therapies in septic shock. Importantly, there have been many reports of the anti-inflammatory properties of AZM [71, 72]. As a result, low-dose AZM has already been used for clinical trials in various disease states including sepsis. The current findings suggest that the effects of AZM may be mediated by improved phagocytic ability of macrophages for S. aureus in addition to other anti-inflammatory properties of the antibiotic.
Cells are provided with very strong biological antioxidant enzymatic and nonenzymatic molecules with enormous capabilities to mitigate the deleterious and potentially harmful effects of reactive oxygen species (ROS) and other free radicals. One of the primary antioxidant defense mechanisms is the GSH redox system [73]. Several studies have suggested that catalase serves as a virulence factor due to its ability to cleave hydrogen peroxide, a reactive oxygen intermediate (ROI) responsible for bactericidal activities of phagocytes. Catalases of pathogenic bacteria are important for optimal detoxification of H2O2 and survival in macrophages [11–13].
It has been reported that cytochalasin activates superoxide anion release and exocytosis of beta-N-acetylglucosaminidase and lysozyme from polymorphonuclear leukocytes (neutrophils). Moreover, they can also activate both nicotinamide adenine dinucleotide phosphatase (NADPH) oxidase and selective degranulation of neutrophils during interaction with the cell [74]. Thus, cytochalasin D also aids in the bactericidal function of the leukocytes [75]. Therefore, enhanced bactericidal action of cytochalasin D-treated macrophages is by releasing hydrogen peroxide, superoxide is not surprising as evident from our study.
Ciprofloxacin, apart from being a bactericidal fluoroquinolone, also increases the production of reactive oxygen species in the bacteria. The involvement of superoxide anion (O2 .−) and H2O2 in the antibacterial action of ciprofloxacin has been well established using superoxide dismutase, catalase, and alkyl hydroperoxide reductase knockout strains of Escherichia coli. Our findings are consistent with those reported earlier that glutathione gives protection against fluoroquinolones and not against nonfluoroquinolone antibiotics [76].
Macrolides like AZM are able to inhibit the production of ROS from neutrophils, and it has been reported that this effect is incubation time-dependent [77]. It has been suggested that this is due in part to cell membrane stabilization. Macrolides attenuate the membrane-destabilizing effect of bioactive phospholipids, such as lysophosphatidylcholine, platelet-activating factor (PAF), and lyso-PAF, and this is accompanied by a dose-related inhibition of superoxide production. Interference with phospholipase/phosphatidic acid phosphohydrolase may also decrease superoxide generation by phagocytes [78].
The kinetics study of O2 .− production in the supernatant of macrophage cultures has revealed higher concentration of this anion in infected cytochalasin D-pretreated and CIP-treated infected macrophages but not in the AZM-treated infected macrophages after 60 min of incubation. The phagocytosis of microorganisms activates oxidase dependence on adenine dinucleotide phosphatase (NADPH), which induces the production of high levels of superoxide, a process commonly denominated as respiratory burst. Herein, the generation of superoxide anion in supernatant of peritoneal macrophages increased in the 60 to 90 min of the incubation period compared to the control setup as well as the S. aureus-infected macrophages [79].
Antioxidant enzymes are considered to be a primary defense that prevents biological macromolecules from oxidative damage. SOD rapidly dismutates superoxide anion to less dangerous H2O2, which is further degraded by catalase to water and oxygen. The results of the present study showed a significant fall in SOD and catalase enzyme activity which may be due to dispose of the free radicals, produced due to S. aureus infection. Incubation of cytochalasin D-treated macrophages with AZM before S. aureus infection shows increased SOD activity both in the supernatant and lysate at 30 min suggesting that azithromycin was better than ciprofloxacin in mediating its bactericidal action in combination with cytochalasin D.
Glutathione system is an important antioxidant system in cell and is involved in protection against free radicals, peroxides, and toxic compounds, and their reactive intermediates formed intracellularly either spontaneously or enzymatically in cellular systems. The depletion of antioxidant enzyme activity was may be due to the inactivation of the enzyme proteins by MRSA infection-induced depletion of the enzyme substrates and/or downregulation of transcription and translational processes [80]. Increased lysozyme released in the supernatant following incubation with azithromycin to cytochalasin D-pretreated macrophages before S. aureus infection may be helpful for increased killing activity of the macrophages with time.
When an infection occurs, the innate immune system is the first line to address the whole bacteria. As infection also implies a contact with whole bacteria and not with purified microbial products, we analyzed the response of murine peritoneal macrophages to viable bacteria. It has been reported that a high ratio of IL-10 to TNF-α was associated with fatal outcome in patients with infection [81], and thus, we focused our study on the production of pro-inflammatory and an anti-inflammatory cytokines. Increased TNF-α from the cytochalasin D plus CIP or cytochalasin D plus AZM-treated macrophages at early infection (at 30 min) indicated bacterial killing and better efficacy of both these antibiotics in the presence of cytochalasin D during early infection. However, suppression of TNF-α level at late stage of infection by both antibiotics in the presence of cytochalasin D indicated that the combination was able to downregulate inflammation with increasing time which was beneficial to the host cell. Suppression of IFN-γ as well as IL-6 by the cytochalasin D- and AZM-treated macrophages at late stage of infection indicated downregulation of inflammation by this combination. Macrolide antibiotics reportedly repress the production of pro-inflammatory cytokines in macrophages [71]. The present study examined the hypothesis that cytochalasin D in combination with AZM could suppress macrophage TNF-α production in response to whole bacteria, in this case clinical isolates of MRSA. Given the important role of TNF-α in sepsis, such suppression by combination therapy could have a therapeutic effect [82]. Invading pathogens are recognized via pathogen recognition receptors such as toll-like receptor (TLR)-2 and TLR-4, scavenger receptors, and mannose receptors and are phagocytosed. It has been reported that S. aureus is able to “attack” or form pores in macrophages, and TNF-α secretion occurs even in the absence of TLR-2 and TLR-4 sensors. Therefore, another mechanism independent of TLR must exist for cytochalasin D- and AZM-mediated anti-inflammatory actions, at least in S. aureus infections. Consistent with other studies, the current authors noted a significant decrease in TNF-α in the presence of AZM [83, 84].
IL-10, an anti-inflammatory cytokine level, remained decreased at all times following combination treatment compared to only S. aureus-infected and antibiotic-treated macrophages. Thus taken together, decreased pro-inflammatory cytokine levels reveal that combination therapy has eradicated not only the bacterial burden within the macrophages but have also led to the inhibition of signalling pathways that lead to the production of pro-inflammatory cytokines, though we have not observed an increase in anti-inflammatory cytokine level (IL-10) following the treatment.
As an alternative to traditional antibiotics in an era of increasing bacterial resistance, new attention has focused upon development of agents that target bacterial virulence factors, effectively disarming the pathogen burden and allowing clearance by the host [85, 86]. Our observation suggests that cytochalasin D in combination with AZM deserves consideration as a therapeutic target because inhibition of phagocytosis not only reduce bacterial uptake but may in specific cases have the dual benefit of increasing susceptibility to commonly used antibiotics including CIP or AZM. It can be concluded from this in vitro study that the action of AZM in mediating bacterial killing is enhanced in the presence of cytochalasin D indicating internalization of S. aureus by macrophages is not essential for killing of bacteria by AZM. Reduction of the amount of pro-inflammatory/toxic pathogen-derived products, avoids overstimulation of resident migrating immune cells including macrophages, and thereby protects the peripheral tissue. It appears desirable to keep the concentration of pro-inflammatory products of pathogens in the tissue low during the whole course of an infection including its treatment. In clinical practice, a favorable outcome depends on the antibiotic treatment after hospital admission. Therefore, the reduction of potentially deleterious pathogen-derived compounds by rapid initiation of an effective antibiotic therapy or by choosing compounds which do not release large amounts of pathogen products is a promising strategy to overstimulation of phagocytic cells and decrease tissue injury. In arthritis, while a diversity of organisms are present in deep infection, the dominant bacteria are Gram-positive pathogens with S. aureus. Established treatments rely on the controlled release of antibiotics. Therefore, nano-conjugation of antibacterial agents (e.g., AZM) along with the inhibitor of phagocytosis (e.g., cytochalasin D) might be validated by in vivo study in the amelioration of arthritis. Moreover, liposome-mediated targeted delivery of cytochalasin D plus AZM to the site of infection can be extrapolated in some in vivo animal models of arthritis for control of overstimulation of phagocytic cells at the site of tissue injury as well as controlled release of AZM to kill the infiltrated S. aureus into the synovial tissue in septic arthritis. Reduction of oxidative stress burden on the S. aureus-infected macrophages may pave the way for better killing of internalized S. aureus by cytochalasin D plus CIP or cytochalasin D plus AZM. However, cytochalasin D plus AZM combination seems to possess better efficacy, at least, in our in vitro incubations.
Abbreviations
- AZM:
-
Azithromycin
- CIP:
-
Ciprofloxacin
- CPCSEA:
-
Committee for the Purpose of Control and Supervision of Experiments on Animals
- DMSO:
-
Dimethyl sulfoxide
- DTNB:
-
5,5′-Dithiobis-2-nitrobenzoic acid
- ELISA:
-
Enzyme-inked immunosorbent assay
- FBS:
-
Fetal bovine serum
- FIC:
-
Fractional inhibitory concentration
- HBSS:
-
Hank’s balanced salt solution
- IFN-γ:
-
Interferon-gamma
- IL:
-
Interleukin
- IU:
-
International unit
- MHB:
-
Mueller-Hinton broth
- MRSA:
-
Methicillin-resistant S. aureus
- NADPH:
-
Nicotinamide adenine dinucleotide phosphatase
- NaOH:
-
Sodium hydroxide
- NCCLS:
-
National Committee for Clinical Laboratory Standards
- NF-κB:
-
Nuclear factor-kappa beta
- OD:
-
Optical density
- PMN:
-
Polymorphonuclear neutrophil
- RPMI:
-
Roswell Park Memorial Institute
- SOD:
-
Superoxide dismutase
- TCA:
-
Trichloroacetic acid
- TLR:
-
Toll-like receptor
- TNF-α:
-
Tumor necrosis factor-alpha
- Tris–EDTA–HCL:
-
Tris-ethylenediamine hydrochloric acid
- TSST:
-
Toxic shock syndrome toxin
- VRSA:
-
Vancomycin-resistant S. aureus strains
REFERENCES
Lowy, F.D. 1998. Staphylococcus aureus infections. The New England Journal of Medicine 339: 520–532.
Brouillette, E., G. Grondin, L. Shkreta, P. Lacasse, and B.G. Talbot. 2003. In vivo and in vitro demonstration of that Staphylococcus aureus is an intracellular pathogen in the presence or absence of fibronectin binding proteins. Microbial Pathogenesis 35: 159–168.
Gordon, R.J., and F.D. Lowy. 2008. Pathogenesis of methicillin-resistant Staphylococcus aureus infection. Clinical Infectious Diseases 46: 50–59.
Foster, T.J. 2005. Immune evasion by staphylococci. Nature Reviews Microbiology 3: 948–958.
Zetola, N., J.S. Francis, E.L. Nuermberger, and W.R. Bishai. 2005. Community-acquired meticillin-resistant Staphylococcus aureus: an emerging threat. Lancet Infectious Diseases 5: 275–286.
Hiramatsu, K. 2001. Vancomycin-resistant Staphylococcus aureus: a new model of antibiotic resistance. Lancet Infectious Diseases 1: 147–155.
Gresham, H.D., J.H. Lowrance, T.E. Caver, B.S. Wilson, A.L. Cheung, and F.P. Lindberg. 2000. Survival of Staphylococcus aureus inside neutrophils contributes to infection. Journal of Immunology 164: 3713–3722.
Hudson, M.C., W.K. Ramp, N.C. Nicholson, A.S. Williams, and M.T. Nousiainen. 1995. Internalization of Staphylococcus aureus by cultured osteoblasts. Microbial Pathogenesis 19: 409–419.
Bayles, K.W., C.A. Wesson, L.E. Liou, L.K. Fox, G.A. Bohach, and W.R. Trumble. 1998. Intracellular Staphylococcus aureus escapes the endosome and induces apoptosis in epithelial cells. Infection and Immunity 66: 336–342.
Menzies, B.E., and I. Kourteva. 1998. Internalization of Staphylococcus aureus by endothelial cells induces apoptosis. Infection and Immunity 66: 5994–5998.
Das, D., S.S. Saha, and B. Bishayi. 2008. Intracellular survival of Staphylococcus aureus: correlating production of catalase and superoxide dismutase with levels of inflammatory cytokines. Inflammation Research 57: 340–349.
Das, D., and B. Bishayi. 2010. Contribution of catalase and superoxide dismutase to the intracellular survival of clinical isolates of Staphylococcus aureus in murine macrophages. Indian Journal of Microbiology 50: 375–384.
Das, D., and B. Bishayi. 2009. Staphylococcal catalase protects intracellularly survived bacteria by destroying H2O2 produced by the murine peritoneal macrophages. Microbial Pathogenesis 47: 57–67.
Lowy, F.D. 2000. Is Staphylococcus aureus an intracellular pathogen? Trends in Microbiology 8: 341–343.
Mal, P., K. Dutta, D. Bandyopadhyay, A. Basu, R. Khan, and B. Bishayi. 2013. Azithromycin in combination with riboflavin decreases the severity of Staphylococcus aureus infection induced septic arthritis by modulating the production of free radicals and endogenous cytokines. Inflammation Research 62: 259–273.
Mal, P., S. Dutta, D. Bandyopadhyay, K. Dutta, A. Basu, and B. Bishayi. 2012. Gentamicin in combination with ascorbic acid regulates the severity of Staphylococcus aureus infection-induced septic arthritis in mice. Scandinavian Journal of Immunology 76: 528–540.
Mal, P., D. Ghosh, D. Bandyopadhyay, K. Dutta, and B. Bishayi. 2012. Ampicillin alone and in combination with riboflavin modulates Staphylococcus aureus infection induced septic arthritis in mice. Indian Journal of Experimental Biology 50: 677–689.
Rogers, D.E., and R. Tompsett. 1952. The survival of staphylococci within human leukocytes. Journal of Experimental Medicine 95: 209–230.
Voyich, J.M., K.R. Braughton, D.E. Sturdevant, A.R. Whitney, B. Saıd-Salim, S.F. Porcella, R.D. Long, D.W. Dorward, D.J. Gardner, B.N. Kreiswirth, J.M. Musser, and F.R. DeLeo. 2005. Insights into mechanisms used by Staphylococcus aureus to avoid destruction by human neutrophils. Journal of Immunology 175: 3907–3919.
Kubica, M., K. Guzik, J. Koziel, M. Zarebski, and W. Richter. 2008. A potential new pathway for Staphylococcus aureus dissemination: the silent survival of S. aureus phagocytosed by human monocyte-derived macrophages. PLoS ONE e3: 1409.
Liu, C., A. Bayer, S.E. Cosgrove, R.S. Daum, S.K. Fridkin, R.J. Gorwitz, S.L. Kaplan, A.W. Karchmer, D.P. Levine, B.E. Murray, M. J Rybak, D.A. Talan, and H.F. Chambers. 2012. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clinical Infectious Diseases 54: 132–173.
Underhill, D.M., and H. Goodridge. 2012. Information processing during phagocytosis. Nature Reviews Immunology 12: 492–502.
Pechère, J.C. 1991. The activity of azithromycin in animal models of infection. European Journal of Clinical Microbiology and Infectious Diseases 2: 114–118.
Van den Broek, P.J. 1991. Activity of antibiotics against micro-organisms ingested by mononuclear phagocytes. European Journal of Clinical Microbiology & Infectious Diseases 10: 114–118.
Krut, O., O. Utermohlen, X. Schlossherr, and M. Kronke. 2003. Strain specific association of cytotoxic activity and virulence of clinical Staphylococcus aureus isolates. Infection and Immunity 71: 2716–2723.
Murai, M., K. Seki, J. Sakurada, A. Usui, and S. Masuda. 1993. Effects of cytochalasins B and D on Staphylococcus aureus adherence to and ingestion by mouse renal cells from primary culture. Microbiology and Immunology 37: 69–73.
Schliwa, M. 1982. Action of cytochalasin D on cytoskeletal networks. Journal of Cell Biology 92: 79–91.
Cooper, J.A. 1987. Effects of cytochalasin and phalloidin on actin. Journal of Cell Biology 105: 1473–1478.
Majhi, A., K. Kundu, R. Adhikary, M. Banerjee, S. Mahanti, A. Basu, and B. Bishayi. 2014. Combination therapy with ampicilllin and azithromycin down regulates Streptococcus pneumoniae induced inflammation in mice. Journal of Inflammation 11: 4. doi:10.1186/1476-9255.
Majhi, A., R. Adhikary, A. Bhattacharyya, S. Mahanti, and B. Bishayi. 2014. Levofloxacin and ceftriaxone in combination attenuates lung inflammation in a mouse model of bacteremic pneumonia by multi-drug resistant Streptococcus pneumoniae via inhibition of cytolytic activities of pneumolysin and autolysin. Antimicrobial Agents and Chemotherapy 58: 5164–5180.
Ishimatsu, Y., J. Kadota, T. Isashita, T. Nagata, H. Ishii, C. Shikuwa, H. Kaida, H. Mukae, and S. Kohno. 2004. Macrolide antibiotics induce apoptosis of human peripheral lymphocytes in vitro. International Journal of Antimicrobial Agents 24: 247–253.
Hodge, S., G. Hodge, S. Brozyna, H. Jersmann, M. Holmes, and P.N. Reynolds. 2006. Azithromycin increases phagocytosis of apoptotic bronchial epithelial cells by alveolar macrophages. European Respiratory Journal 28: 486–495.
Jun, Y.T., H.J. Kim, M.J. Song, J.H. Lim, D.G. Lee, K.J. Han, S.M. Choi, J.H. Yoo, W.S. Shin, and J.H. Choi. 2003. In vitro effects of ciprofloxacin and roxithromycin on apoptosis of jurkat T lymphocytes. Antimicrobial Agents and Chemotherapy 47: 1161–1164.
Yamaryo, T., K. Oishi, H. Yoshimine, Y. Tsuchihashi, K. Matsushima, and T. Nagatake. 2003. Fourteen member macrolides promote the phosphatidylserine receptor dependent phagocytosis of apoptotic neutrophils by alveolar macrophages. Antimicrobial Agents and Chemotherapy 47: 48–53.
Lin, H.C., C.H. Wang, C.Y. Liy, C.T. Yu, and H.P. Kuo. 2000. Erytrhomycin inhibits beta-2-integrins (CD11b/CD18) expression, interleukin-8 release and intracellular oxidative metabolism in neutrophils. Respiratory Medicine 94: 654–660.
Matsuoka, N., K. Eguchi, A. Kawakami, M. Tsuboi, Y. Kawabe, T. Aoyagi, and S. Nagataki. 1996. Inhibitory effect of clarithromycin on co-stimulatory molecule expression and cytokine production by synovial fibroblast-like cells. Clinical and Experimental Immunology 104: 501–508.
Ives, T.J., U.E. Schwab, E.S. Ward, and I.H. Hall. 2003. In-vitro anti-inflammatory and immunomodulatory effects of grepafloxacin in zymogen A- or Staphylococcus aureus-stimulated human THP-1 monocytes. Journal of Infection and Chemotherapy 9: 134–143.
Cigana, C., B.M. Assael, and P. Melotti. 2007. Azithromycin selectively reduces tumor necrosis factor alpha levels in cystic fibrosis airway epithelial cells. Antimicrobial Agents and Chemotherapy 51: 975–981.
Culic, O., I. Erakovic, K. Cepelak, K. Barisic, K. Brajsa, Z. Ferencic, R. Galović, I. Glojnarić, Z. Manojlović, V. Munić, R. Novak-Mircetić, V. Pavicić-Beljak, M. Sucić, M. Veljaca, T. Zanić-Grubisić, and M.J. Parnham. 2002. Azithromycin modulates neutrophil function and circulating inflammatory mediators in healthy human subjects. European Journal of Pharmacology 450: 277–289.
Tkalcevic, I., V.B. Bosnjak, B. Hrvacic, M. Bosnar, N. Marjanovic, Z. Ferencic, K. Situm, O. Culić, M.J. Parnham, and V. Eraković. 2006. Anti-inflammatory activity of azithromycin attenuates the effects of lipopolysaccharide administration in mice. European Journal of Pharmacology 539: 131–138.
Aghai, Z.H., A. Kode, J.G. Saslow, T. Nakhla, S. Farhath, G.E. Stahl, R. Eydelman, L. Strande, P. Leone, I. Rahman, S. Farhath, G.E. Stahl, R. Eydelman, L. Strande, P. Leone, and I. Rahman. 2007. Azithromycin suppresses activation of nuclear factor kappa B and synthesis of pro-inflammatory cytokines in tracheal aspirate cells from premature infants. Pediatric Research 62: 483–488.
Huan, L., and K. Neil. 2007. Glutathione depletion down-regulates tumor necrosis factor α-induced NF-κB activity via IκB kinase-dependent and -independent mechanisms. The Journal of Biological Chemistry 282: 29470–29481.
Cho, S., Y. Urata, and T. Ilada. 1998. Glutathione down-regulates the phosphorylation of I kappa B: auto-loop regulation of the NF-kappa B-mediated expression of NF-κB subunits by TNF-alpha in mouse vascular endothelial cells. Biochemical and Biophysical Research Communications 253: 104–108.
Cozzarelli, N.R. 1980. DNA gyrase and the supercoiling of DNA. Science 207: 953–960.
Drlica, K. 1984. Biology of bacterial deoxyribonucleic acid topoisomerases. Microbiological Reviews 48: 273–289.
Gellert, M. 1981. DNA topoisomerases. Annual Review of Biochemistry 50: 879–910.
Wolfson, S.J., and C.D. Hooper. 1985. The fluoroquinolones: structures, mechanisms of action and resistance, and spectra of activity in vitro. Antimicrobial Agents Chemotherapy 28(4): 581–586.
Cowan, S.T., and K.J. Steel. 1974. Cowan and steel’s manual for the identification of medical bacteria, 2nd ed. Cambridge: Cambridge University Press.
Lancette, G.A., and S.R. Tatini. 1992. Staphylococcus aureus. In Compendium of methods for the microbiological examination of foods, 3rd ed, ed. C. Vanderzant and D.F. Splittstoesser, 533–550. Washington: American Public Health Association.
Kloos, W.E., D.W. Lambe Jr., and A. Balows. 1991. Staphylococcus. In Manual of clinical microbiology, 5th ed, ed. A. Balows, W.J. Hausler Jr., K.L. Herrmann, H.D. Isenberg, and H.J. Shadomy, 222–237. Washington D.C.: American Society for Microbiology.
Evans, J.B., and W.E. Kloos. 1972. Use of shake cultures in a semisolid thioglycolate medium for differentiating staphylococci from micrococci. Applied Microbiology 23: 326–331.
Bayliss, B.G., and E.R. Hall. 1965. Plasma coagulation by organisms other than Staphylococcus aureus. Journal of Bacteriology 89: 101–105.
Freeman, R., D. Burdess, and S. Smith. 1994. Crystal violet reactions of coagulase negative staphylococci. Journal of Clinical Pathology 47: 283–285.
NCCLS. 2008. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard, 6th ed. NCCLS document. M7-A6. NCCLS, Wayne, Pa
Odds, F.C. 2003. Synergy, antagonism, and what the chequerboard puts between them. Journal of Antimicrobial Chemotherapy 52: 601–607.
Leigh P.C.J., Van Furth R., Van Zwet T.L. 1986. In vitro determination of phagocytosis and intracellular killing by polymorphonuclear and mononuclear phagocytes. In: Weir D.M., eds. Handbook of experimental immunology. Blackwell Scientific publications: Oxford, pp. 104:46.1-46.26.
Absolom, D.R. 1986. Basic methods for the study of phagocytosis. Methods in Enzymology 132: 95–180.
Sedlak, J., and R.H. Lindsay. 1968. Estimation of total, protein-bound, and non-protein sulfhydryl groups in tissue with Ellman’s reagent. Analytical Biochemistry 25: 192–205.
Colowick, S.P., N.O. Kaplan, and D.R. Absolom. 1986. Basic methods for the study of phagocytosis. In Methods in enzymology: part J. Immunochemical techniques, ed. G.D. Sabato and J. Everse, 160. New York: Academic.
Bradford, M.M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry 72: 248–254.
Tulkens, P.M. 1991. Intracellular distribution and activity of antibiotics. European Journal of Clinical Microbiology and Infectious Diseases 10: 100–106.
Ignatowski, T.A., and J.M. Bidlack. 1999. Differential kappa-opioid receptor expression on mouse lymphocytes at varying stages of maturation and on mouse macrophages after selective elicitation. Journal of Pharmacology and Experimental Therapeutics 290: 863–870.
Polliack, A., and S. Gordon. 1975. Scanning electron microscopy of murine macrophages. Surface characteristics during maturation, activation, and phagocytosis. Laboratory Investigation 33: 469–477.
Aslam, A.F., A.K. Aslam, A.C. Thakur, B.C. Vasavada, and I.A. Khan. 2005. Staphylococcus aureus infective endocarditis and septic pulmonary embolism after septic abortion. International Journal of Cardiology 105: 233–235.
Cossart, P., and P.J. Sansonetti. 2004. Bacterial invasion: the paradigms of enteroinvasive pathogens. Science 304: 242–248.
Weihing, R.R. 1978. In vitro interactions of cytochalasins with contractile proteins. Frontiers of Biology 46: 431–444.
Elliott, J.A., and W.C. Jr. Winn. 1986. Treatment of alveolar macrophages with cytochalasin D inhibits uptake and subsequent growth of Legionella pneumophila. Infection and Immunity 51: 31–36.
Mogensen, T.H. 2009. Pathogen recognition and inflammatory signaling in innate immune defenses. Clinical Microbiology Reviews 22: 240–273.
Nau, R., and H. Eiffert. 2002. Modulation of release of pro-inflammatory bacterial compounds by antibacterials: potential impact on course of inflammation and outcome of sepsis and meningitis. Clinical Microbiology Reviews 15: 95–110.
Labro, M.T. 1998. Anti-inflammatory activity of macrolides: a new therapeutic potential? Journal of Antimicrobial Chemotherapy 41: 37–46.
Tamaoki, J. 2004. The effects of macrolides on inflammatory cells. Chest 125: 41S–50S.
Stortz, G., L.A. Tartaglia, and B.N. Ames. 1990. Transcriptional regulation of oxidative stress inducible genes: direct activation by oxidation. Science 248: 189–194.
Bentley, J.K., and P.W. Reed. 1981. Activation of superoxide production and differential exocytosis in polymorphonuclear leukocytes by cytochalasins A, B, C, D and E. Effects of various ions. Biochemica et Biophysica Acta 678: 238–244.
Okamura, N., K. Hanakura, M. Kodakari, and S. Ishibashi. 1980. Cooperation of cytochalasin D and anti-microtubular agents in stimulating superoxide anion production in polymorphonuclear leukocytes. Journal of Biochemistry 80: 139–144.
Goswami, M., S.H. Mangoli, and N. Jawali. 2006. Involvement of reactive oxygen species in the action of ciprofloxacin against Escherichia coli. Antimicrobial Agents and Chemotherapy 50: 949–954.
Levert, H., B. Gressier, I. Moutard, C. Brunet, T. Dine, M. Luyckx, M. Cazin, and J.C. Cazin. 1998. Azithromycin impact on neutrophil oxidative metabolism dependent on exposure time. Inflammation 22: 191–201.
Kanoh, S., and B.K. Rubin. 2010. Mechanisms of actions and clinical application of macrolide as immunomodulatory medications. Clinical Microbiology Reviews 23: 590–615.
DeMorais, N.G., T.B. da Costa, T.M. de Almeida, M.S. Severo, and C.M.M.B. de Castro. 2013. Immunological parameters of macrophages infected by methicillin resistant/sensitive Staphylococcus aureus. J Bras Patol Med Lab 49: 84–90.
Chakroborty, S.P., and S. Roy. 2014. In vitro Staphylococcus aureus-induced oxidative stress in mice murine peritoneal macrophages: a duration-dependent approach. Asian Pacific Journal of Tropical Biomedicine 4 (suppl1): S298–S304.
Van Dissel, J.T., P. Van Langevelde, R.G. Westendorp, K. Kwappenberg, and M. Frolich. 1998. Anti-inflammatory cytokine profile and mortality in febrile patients. Lancet 351: 950–953.
Spentzas, T., R.K. Shapley, C.A. Aguirre, E. Meals, L. Lazar, M.S. Rayburn, B.S. Walker, and B.K. English. 2011. Ketamine inhibits tumor necrosis factor secretion by RAW264.7 murine macrophages stimulated with antibiotic-exposed strains of community-associated, methicillin-resistant Staphylococcus aureus. BMC Immunology 12: 11. doi:10.1186/1471-2172-12-11.
Akira, S., and S. Sato. 2003. Toll-like receptors and their signalling mechanisms. Scandinavian Journal of Infectious Diseases 35: 555–562.
Plowden, J., M. Renshaw-Hoelscher, C. Engleman, J. Katz, and S. Sambhara. 2004. Innate immunity in aging: impact on macrophage function. Aging Cell 4: 161–167.
Nizet, V. 2007. Understanding how leading bacterial pathogens subvert innate immunity to reveal novel therapeutic targets. Journal of Allergy and Clinical Immunology 120: 13–22.
Escaich, S. 2010. Novel agents to inhibit microbial virulence and pathogenicity. Expert Opinion on Therapeutic Patents 10: 1401–1418.
Clatworthy, A.E., E. Pierson, and D.T. Hung. 2007. Targeting virulence: a new paradigm for antimicrobial therapy. Nature Chemical Biology 9: 541–548.
ACKNOWLEDGMENTS
This work was supported by the Department of Biotechnology (DBT), Government of West Bengal, Calcutta, West Bengal [Grant Number: vide sanction order 786-BT/Estt/RD-4/13 date: 30.10.2013 to BB] for funding this project. The author (Biswadev Bishayi) is indebted to the Department of Science and Technology, Government of India for providing with the instruments procured under the DST-PURSE program to the Department of Physiology, University of Calcutta.
CONFLICT OF INTEREST
For the manuscript entitled “In vitro anti-inflammatory and immunomodulatory effects of ciprofloxacin or azithromycin in Staphylococcus aureus stimulated murine macrophages are beneficial in the presence of cytochalasin D” by Somrita Dey et al., the authors declared that they have no conflict of interest for this manuscript toward submission in Inflammation. The authors also state that they do not have a direct financial relation with the commercial identities mentioned in this manuscript that might lead to a conflict of interest for any of the authors.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dey, S., Majhi, A., Mahanti, S. et al. I n Vitro Anti-inflammatory and Immunomodulatory Effects of Ciprofloxacin or Azithromycin in Staphylococcus aureus-Stimulated Murine Macrophages are Beneficial in the Presence of Cytochalasin D. Inflammation 38, 1050–1069 (2015). https://doi.org/10.1007/s10753-014-0070-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-014-0070-4