Abstract
Objective and design
To determine alternate therapeutic measures to combat Staphylococcus aureus induced arthritis. Thus, azithromycin was combined with riboflavin, which may combat the ROS production and inflammation.
Methods
An in vivo model of S. aureus infection-induced arthritis was set up by infecting mice with 5 × 106 bacterial cell/mouse. S. aureus was administered intravenously. Azithromycin and riboflavin was injected intraperitoneally at a single dose of 100 and 20 mg/kg body, respectively. The mice were sacrificed at 3, 9, 15 days post infection (dpi). TNF-α, IFN-γ, IL-6 and IL-10 from serum and SOD, catalase and reduced glutathione concentration were observed in hepatic, cardiac, renal and splenic tissue.
Results
CFU was found very prominent in spleen and joints and reduced in blood at 3 and 9 dpi. However, treatment with azithromycin and riboflavin completely eradicated the bacteria from blood and spleen. TNF-α, IFN-γ, IL-6, and MCP-1 were induced due to infection which were downregulated by treatment with azithromycin and riboflavin. Infected mice were also found to have altered antioxidant status, measured in terms of reduced glutathione and anti-oxidant enzymes such as SOD and catalase.
Conclusion
These changes were found to be ameliorated when the animals were co-treated with azithromycin and riboflavin.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Staphylococcus aureus (S. aureus) causes a range of bone and joint pathologies such as infection of prostheses, osteomyelitis, septic arthritis and septic bursitis [1]. In case of staphylococcal arthritis, persistence of S. aureus DNA was observed in the synovial fluid till 10 weeks post infection, despite adequate antibiotic treatment [2]. Various risk factors for development of septic arthritis, such as host factors (TNF-α, IL-1, IL-10), bacterial proteins, toxins and enzymes, are reported to be an important determinant of pathogenesis in mouse models [3]. It was reported that inoculation of mice with a TSST-1-producing S. aureus strain induced a higher frequency and severity of arthritis than the strain which lacks the gene for TSST-1, indicating its potential arthritogenic role [4]. However, despite the recognition of this plethora of virulence factors, we are still far from identifying exactly which factors are necessary for infection and which ones may be targeted by new therapies [5].
The current treatment for S. aureus infection of bones and joints relies on the use of antibiotics such as flucloxacillin, oxacillin, ciprofloxacin and methicillin, etc. [6]. However, with the advent of methicillin and vancomycin-resistant S. aureus, it is imperative to determine alternate therapeutic measures to combat S. aureus induced arthritis [7]. One such promising strategy involves the combined use of antibiotics and immunomodulatory agents in order to kill the bacteria and simultaneously downregulate the undesired activation of the immune system [8]. A study in mice using corticosteroids, in conjunction with antibiotics, indicates that this approach does lead to decreased morbidity and mortality [9]. By combining antibiotics with biological agents for therapies, we envision alternatives to direct microbial killing, such as blocking disease progression by neutralizing specific virulence factors or boosting key innate immune defenses [10].
There are many pieces of evidence implicating free radicals in the pathogenesis of arthritis such as the presence of different cell types in the inflamed joint which are known to the same [11]. It has been reported that arthritic patients are exposed to oxidant stress and lipid peroxidation (LPO) because of a reduced antioxidant defense system [12]. The highly reactive hydroxyl radicals cause most damage, attacking a wide range of targets, including hyaluronic acid in the synovial fluid, causing loss of lubricant properties and consequently mechanical damage to the joint [13].
Azithromycin has been reported to be highly bactericidal against strains of S. pneumonia, H. influenza, L. monocytogenes and S. aureus. It is found to be well absorbed when administered orally and is also found to have a good level of tissue distribution in mice, rats, dogs and monkeys, due to its stability in acidic pH [14]. A single dose of azithromycin (100 mg/kg body weight, a fair concentration of the drug and greater than the MIC of the S. aureus strain used) has been reported in the extravascular fluid even after 5 days [15]. Moreover, reports suggest macrophages and monocytes also uptake azithromycin well in both in vivo and in vitro conditions [16].
The role of riboflavin as an antioxidant has been recently recognized [17], although the mechanism underlying such antioxidant activity is poorly understood. Riboflavin is important water-soluble B-vitamin available through food, and co-enzymes like FAD and FMN derived from it regulate the activity of many different metabolic enzymes of the redox pathways associated with energy production. Pharmacological doses of the vitamin are well tolerated in humans [18]. The antimicrobial properties of riboflavin against common pathogens such as S. aureus have been reported for infective keratitis [19].
In the current investigation, we hypothesize that riboflavin in combination with the antibiotic azithromycin (AZI) might be an effective therapy in an experimental model of septic arthritis where ROS might modulate synovial inflammation by stimulating the synthesis of cytokines. In our earlier studies, we have demonstrated the success of antibiotic–antioxidant co-therapeutic approach in not only reducing the stress burden in the infected tissues but also in producing a reduced level of inflammation [20], even though no ameliorating effect of riboflavin administration on arthritis development has been reported to date.
Materials and methods
Animal model
Male Swiss albino mice, 6–8 weeks of age with body weight 20 ± 4 g, were purchased from the regular animal suppliers to our department. Upon arrival, the mice were randomized into plastic cages with filter bonnets and sawdust bedding, followed by a 1-week quarantine period. Six mice were housed per cage with food and water ad libitum. Animal holding rooms were maintained at 21–24 °C and 40–60 % humidity with a 12-h light-dark cycle. The mice were fed a normal rodent diet. Six mice were used per experiment in each group.
Preparation of bacteria
S. aureus (strain # AG-789) was obtained from Apollo Gleneagles Hospital, Calcutta, and was maintained in our laboratory and tested for antibiotic sensitivity. We have extensively used a few clinical isolates of S. aureus in a mouse model of arthritis with a short-term but non-lethal infection [20–22]. Bacteria were cultured on blood agar (5 % human erythrocytes) for 24 h and then re-incubated on blood agar for another 24 h. Before experimentation, bacteria were grown overnight at 37 °C in 5 ml of Luria–Bertani (LB) broth, and were then diluted in fresh broth and cultured until the mid-logarithmic phase of growth. Bacteria were harvested, washed in sterile phosphate buffered saline (PBS), and adjusted to the desired inoculums [23] spectrophotometrically before infection (OD620 = 0.2 = 5 × 107 cells/ml for S. aureus). The colony forming units (CFU) were confirmed by serial dilution and culture on blood agar.
Treatment of S. aureus infected mice with antibiotic (AZI) followed by riboflavin
In a separate set of experiments, starting at day zero after intravenous injection of S. aureus (AG-789) (5 × 106 cells/ml), AZI (dissolved in sterile PBS) was injected intraperitoneally into mice at a single dose of 100 mg/kg after 24 h of infection [24]. AZI was chosen as an antibiotic since it was shown that a single dose of erythromycin or penicillin G could not prevent arthritis,. whereas AZI was known to give the best results. A fresh solution of riboflavin was then prepared on the day of antibiotic treatment, and 160–200 μl of riboflavin, corresponding to 20 mg/kg body weight in mice (approx 19 mg of vitamin B2), were given intraperitoneally to the same mice after 2 h of antibiotic treatment [25]. Then these mice were sacrificed at 3, 9, and 15 days post infection.
Determination of number of viable S. aureus cells in blood and organs (spleen and synovial tissue)
Blood samples from mice of different groups were obtained by cardiac puncture under ether anesthesia at selected intervals. Spleen tissues were aseptically removed and homogenized with 3 ml of sterile RPMI-1640. All wrist and ankle joints from each mouse were removed, weighed, and homogenized in RPMI-1640 medium (1 ml/100 mg joint weight). Blood and tissue homogenates were then spread on mannitol agar plates and viability of S. aureus was determined by the method described earlier [21].
Induction and assessment of septic arthritis
Individual mice were observed daily for up to 15 days, blind to genotype or infection status. Swelling of wrist and ankle joints were used to determine the level of the inflammatory response in mice challenged with S. aureus. Prior to experimentation, the paws of randomly selected and age-matched mice were measured to determine the baseline paw size. After infection, the mice were measured every other day for 15 days with a dial-type vernier caliper, graduated 0.1 cm increments, by carefully measuring the width and thickness of each wrist and ankle joint. The daily mean value for each of the groups was determined by the number of wrists or ankles measured in each group. This average value represented the severity of wrist and ankle joint swelling [26].
Percentage reduction in arthritis per group of treated animals was calculated as follows: [(Mean diameter of swelling of wrist or ankle on day 15 – swelling of wrist or ankle on day 15 after treatment)/Mean diameter of swelling of wrist or ankle on day 15] × 100].
Differences in the reduction of joint (wrist or ankle) swelling between groups of drug treated and untreated arthritic mice were evaluated statistically [27].
Sample preparation for cytokine measurement
Blood samples from mice of different groups were obtained by cardiac puncture under ether anesthesia at selected intervals. In each experiment, the mice were coded to ensure that the observer was blinded. The serums from different groups were normalized to the protein content by the Bradford method before the assay and the levels of cytokines were determined. A mouse cytokine bead array (CBA) kit (BD Biosciences, CA, USA) was used as per manufacturers’ instructions to quantitatively measure proinflammatory cytokine/chemokine levels in serum from all treatment groups. Data were acquired using Cell Quest Pro Software in FACSCalibur and analyzed using BD CBA software [21].
Articular neutrophil accumulation
Myeloperoxidase (MPO) enzyme activity was analyzed as an index of neutrophil infiltration in the synovial tissue, using O-dianisidine dihydrochloride as substrate by a method described in our earlier study [21]. Protein levels in the tissue homogenates were determined by Bradford method. MPO enzyme activity has been defined as the concentration of enzyme degrading 1 μM of peroxide/min at 37 °C and was expressed as change in absorbance/min mg of protein.
Measurement of LPO level
The weighed amount of the cardiac, hepatic, renal and splenic tissue was homogenized (10 %) in ice-cold 0.9 % saline (pH 7.0) with a Potter Elvejhem all-glass homogenizer (Belco Glass Inc., Vineland, NJ, USA) for 30 s, and the levels of the lipid peroxidation products in the homogenate was determined by thio-barbituric acid reactive substances (TBARS) assay. In brief, the homogenates were mixed with trichloro acetic acid–thiobarbituric acid–hydrochloric acid (TBA–TCA–HCl) reagent and heated for 20 min at 80 °C. The tubes containing the samples were then cooled to room temperature. The absorbance of the pink chromogen present in the clear supernatant after centrifugation at 1,200 × g for 10 min at room temperature was measured at 532 nm using a UV–Vis spectrophotometer (SmartSpec Plus, BioRad, CA, USA). Tetraethoxypropane (TEP) was used as standard. The values were expressed as nmoles of TBARS per mg protein [28].
Measurement of reduced glutathione level
Reduced glutathione content (as acid soluble sulfhydryl) was estimated by its reaction with DTNB (Ellman’s reagent) following the method of Sedlac and Lindsey with some modifications. The weighed amounts of the cardiac, hepatic, renal and splenic tissue were homogenized (10 %) in 2 mM ice-cold ethylenediaminetetraacetic acid (EDTA). The homogenates were mixed with Tris–HCl buffer, pH 9.0, followed by the addition of DTNB for colour development. The absorbance was measured at 412 nm using a UV–Vis spectrophotometer to determine GSH content. The values were expressed as nmoles of GSH per mg protein [29].
Measurement of activity of antioxidant enzymes
Superoxide dismutase (Cu–Zn SOD) activity was measured by hematoxylin auto oxidation method of Martin et al. In brief, the weighed amounts of the hepatic tissue were homogenized (10 %) in ice-cold 50 mM phosphate buffer containing 0.1 mM EDTA pH 7.4. The homogenates were then centrifuged at 12,000 × g for 15 min and the supernatant was carefully collected. The inhibition of hematoxylin auto oxidation by the cell free supernatant was measured at 560 nm using a UV–Vis spectrophotometer. The enzyme activity was expressed as units per mg tissue protein [30].
Catalase was assayed by measuring the breakdown of hydrogen peroxide (H2O2) according to the method of Beers and Sizer [31]. The weighed amounts of the hepatic tissue were homogenized in 5 % ice-cold 50 mM phosphate buffer pH 7.2. The homogenates were then centrifuged at 12,000 × g for 12 min. The supernatant thus obtained was then carefully collected and incubated with 0.01 ml of absolute ethanol at 4 °C for 30 min. Thereafter, 10 % Triton X-100 was added to have a final concentration of 1 %. The sample, thus obtained, was used to determine the catalase activity by measuring the breakdown of H2O2 spectrophotometrically at 240 nm. The enzyme activity was expressed as micromoles of H2O2 consumed per min per mg of protein.
Expression of cyclooxegenase-2 in synovial tissue
Expression of cyclooxegenase-2 (Cox-2) in synovial tissues was determined by immunoblotting. Protein levels in the tissue homogenates were determined by the Bradford method. Samples containing 20 μg of protein were electrophoresed on polyacrylamide gel and transferred onto nitrocellulose membrane. After blocking with 7 % skimmed milk, the blots were incubated overnight at 4 °C with primary antibodies against Cox-2 (1:1,000; Chemicon, USA). After extensive washes in PBS–Tween, blots were incubated with appropriate secondary antibodies conjugated with peroxidase (Vector Laboratories). The blots were again washed in PBS–Tween and processed for development using chemiluminescence reagent (Millipore, USA). The images were captured and analyzed using Chemigenius, Bioimaging System (Syngene, Cambridge, UK). The blots were then stripped (30 min at 50 °C in 62.5 mmol/l Tris–HCl pH 6.8, 2 % sodium dodecyl sulfate, and 100 mM β-mercaptoethanol) and reprobed with anti-β-tubulin (Santa Cruz Biotechnology, CA, USA) to determine equivalent loading of samples [32].
DNA isolation from S. aureus recovered from synovial tissue
Bacterial cultures were grown in brain heart infusion broth prior to extraction of total DNA. Total DNA was isolated from 2.5 ml of brain heart infusion broth culture grown overnight for all the bacterial strains used in the study. The procedure used for DNA isolation has been described previously [33]. DNA samples were dissolved in Tris–EDTA buffer (10 mM Tris chloride, 1 mM EDTA [pH 8.0]), and the concentration was determined as micrograms per milliliter according to A 260 values. Template DNA in amounts ranging from 10 to 1,000 ng was used in the study.
Polymerase chain reaction amplification and agarose gel electrophoresis
Primers for polymerase chain reaction (PCR) were synthesized by Osmium Biosolutions on sequence published by Becker et al., 1998 for Coa gene. The sequence of forward primer used for amplification was 5′CGAGACCAAGATTCAACAAG and the reverse primer was 5′AAAGAAAACCACTCACATCA. The PCR amplifications were performed in a volume of 25 μl containing 20–90 ng/μl DNA, 1 × PCR buffer, 3 mM MgCl2, 200 μM dNTPs, 20 pmols primers and 1.25 IU TAQ polymerase. An initial cycle of 96 °C for 5 min was followed by 35 cycles of 94 °C, 2 min at 54 °C and 1 min at 72 °C. Final extension was performed at 72 °C for 7 min. The tubes were placed in a BioRad, MJ-Mini thermocycler. PCR products were visualized on a 2 % agarose gel stained with ethidium bromide and the size of the product was estimated using 100 bp DNA ladder [34].
Statistical analysis
One-way model 1 ANOVA was performed between the groups. In ANOVA observed variance is partitioned into components due to different explanatory variables. A level of P < 0.05 or P < 0.001 was considered significant. Significant differences of the means between the groups were performed by one-way ANOVA. Scheffe’s F test had been done as post hoc test for multiple comparisons of means of different groups when significant F value was found [35].
Results
Mortality rate end frequency of arthritis induction after in vivo infection of S. aureus (AG-789)
Mortality and incidence of arthritis in mice infected with 5 × 106 CFU/mouse of S. aureus AG-789 were determined. Over a 15-day period, 100 % of the animals survived in all groups of mice, except at 15 dpi in which case S. aureus AG-789 infected mice showed 66.67 % survival (data not shown).
Recovery of bacteria from blood, spleen and synovial tissue after single in vivo injection of S. aureus (AG-789) followed by AZI and riboflavin treatment
In isolates obtained at 3 dpi, growth of S. aureus was noted not only in the joints but also in the blood and spleen. In contrast, in the isolates obtained 9 dpi, growth of bacteria was still prominent in the spleen and joint as compared to blood. No detectable amount of bacteria were found either in blood, spleen or synovial tissue at 15 dpi. However, treatment of mice with AZI after infection followed by riboflavin completely eradicates the bacteria from the blood and spleen and also significantly reduces the bacterial burden in the synovial tissue both at 3 and 9 dpi (P < 0.05) (Table 1).
Experimental evaluation of arthritis: effect of AZI alone or in combination with riboflavin on S. aureus (AG-789) infection-induced swelling of joints
The results showed that there was significantly increased swelling of wrist and ankle joints in pathogenic strain S. aureus (AG-789) infected mice after 3, 9 and 15 dpi compared to the uninfected control group (P < 0.05). Administration of AZI after S. aureus infection followed by riboflavin treatment showed significant reduction in the swelling of joints at day 9 when compared with S. aureus-alone infected mice (Table 2). Treatment of mice with AZI alone or in combination with riboflavin after S. aureus infection showed significant gross reduction in arthritis as compared to S. aureus induced arthritis at 15 dpi (Table 2).
Serum levels of TNF-α, IFN-γ, IL-6 and IL-10, IL-12p70, MCP-1 of different groups of mice at 3, 9 and 15 days post infection
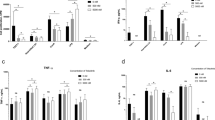
Serum TNF-α, IFN-γ, MCP-1, IL-6 levels, but not IL-12p70 and IL-10, were increased significantly after S. aureus infection (P < 0.05). Treatment of mice with AZI alone or in combination with riboflavin after infection could significantly downregulate the serum TNF-α, IFN-γ, MCP-1, IL-6 at day 3, 9 and 15. However, AZI alone or in combination with riboflavin could also increase the serum IL-12p70 and IL-10 significantly at day 3, 9and 15 (Fig. 1).
Serum levels of MCP-1, TNF-∝, IL-6, IFN-ϒ, IL-12p70 and IL-10 in different groups of mice at 3, 9 and 15 days post infection levels of MCP-1 (a), TNF-α (b), IL-6 (c), IFN-γ (d), IL-12 p70 (e) and IL-10 (f) in serum from S. aureus-infected mice treated with AZI alone or in combination with riboflavin after Staphylococcal infection and from non-infected control animal were determined by utilizing CBA according to the manufacturer’s recommendations and were expressed from triplicate experiments as mean ± SD. Without infection control versus S. aureus AG-789 alone, significant increase in MCP-1, TNF-α and IFN-γ (^^P < 0.05), but decrease in IL-12p70 and IL-10 (# P < 0.05; S. aureus AG-789 alone versus S. aureus AG-789 + azithromycin, significant decrease in MCP-1, TNF-α, IL-6, IFN-γ (*P < 0.05) and increase in IL-12p70IL-10 (^P < 0.05); S. aureus AG-789 alone versus S. aureus AG-789 + azithromycin + riboflavin, significant decrease in MCP-1, TNF-α, IL-6, IFN-γ (*P < 0.05) and increase in IL-12p70 and IL-10 (^P < 0.05)
Synovial tissue MPO enzyme activity
The activity of MPO enzyme which is an indicator for neutrophil infiltration, it was observed that, at 3 and 9 dpi, synovial tissue MPO enzyme activity was significantly higher only for the pathogenic strain—S. aureus (AG-789) than the vehicle group. When AZI was administered alone or in combination with riboflavin it caused significant (P < 0.05) reduction of tissue MPO enzyme activity in case of the pathogenic strain SCRL-28 infected group only at day 9 after infection (Fig. 2).
Articular neutrophil accumulation determined by estimation of myeloperoxidase (MPO) activity. MPO activity was analyzed as index of neutrophil infiltration in the synovial tissue. The rate of change in absorbance was measured spectrophotometrically at 405 nm. MPO activity has been defined as the concentration of enzyme degrading 1 μmole of peroxide/min at 37 °C and was expressed as change in absorbance/min mg of protein. Without infection control versus S. aureus (AG-789) alone, significant increase, ^^P < 0.05 at 3, 9 and 15 dpi; S. aureus AG-789 alone versus S. aureus AG-789 + azithromycin + riboflavin, significant decrease, *P < 0.05 at day 3 and 9 post infection
Effect of AZI and riboflavin treatment on synovial tissue cyclooxygenase-2 level in the S. aureus-infected mice
Immunoblot analysis of synovial tissue homogenate showed that Cox-2 level was significantly increased after 3 dpi in case of the AG-789 S. aureus. After AZI treatment Cox-2 was found to be significantly decreased. After AZI treatment in combination with riboflavin the Cox-2 level was significantly decreased on day 3 (Fig. 3).
Expression of Cox-2 after treatment with azithromycin in combination with riboflavin in synovial tissue. Expression of Cox-2 was measured in terms of fold change over control. Highest level of Cox-2 was found on day 3 of infection. Reduction in Cox-2 level was found after treatment with AZI alone or in combination with riboflavin at 3 dpi. Control versus S. aureus AG-789 at 3 dpi (^P < 0.01 with respect to control); S. aureus AG-789 alone versus S. aureus + azithromycin, S. aureus AG-789 alone versus S. aureus + riboflavin, S. aureus AG-789 alone versus S. aureus + azithromycin + riboflavin (*P < 0.01 with respect to 3 dpi-infected sample)
Effects of AZI-riboflavin co-therapy against S. aureus-infection induced oxidative stress in various tissues of mice and its relation with progression of septic arthritis
Cardiac tissue: Figure 4a demonstrates that there occurred a significant decrease in the level of cardiac GSH in mice infected with S. aureus compared to control at 3, and 15 dpi indicating severe oxidative stress following infection with S. aureus. However, when the mice were treated with AZI along with riboflavin, the GSH level of the cardiac tissue at 3 dpi was found to be increased severalfold compared to the S. aureus treated group. The tissue GSH level was also found to be increased significantly at days 9 and 15 in the AZI and riboflavin co-treated group compared to S. aureus alone. The results indicate that the AZI and riboflavin combination is more effective than when they were administered alone.
Alteration in the anti-oxidant status like reduced glutathione level and the activities of the anti-oxidant enzymes such as SOD and catalase cardiac tissue of mice a GSH, without infection control versus S. aureus alone, significant decrease at 3, 9 and 15 dpi (^P < 0.05); S. aureus alone versus S. aureus + azithromycin, significant increase at 3, 9, and 15 dpi ($P < 0.05); S. aureus alone versus S. aureus + riboflavin, significant increase at 3, 9, 15 dpi ($P < 0.05); S. aureus alone versus S. aureus + azithromycin + riboflavin, significant increase at 3, 9, 15 dpi ($P < 0.05). b LPO, without infection control versus S. aureus alone, significant increase at 3, 9, 15 dpi (*P < 0.05); S. aureus alone versus S. aureus + azithromycin, significant decrease at 3, 9 and 15 dpi (#P < 0.05); S. aureus alone versus S. aureus + riboflavin, significant decrease at 3, 9 and 15 dpi (#P < 0.05); S. aureus alone versus S. aureus + azithromycin + riboflavin, significant decrease at 3, 9 and 15 dpi (#P < 0.05). c SOD, without infection control versus S. aureus alone, significant increase at 3, 9 dpi (*P < 0.05); S. aureus alone versus S. aureus + azithromycin, significant decrease at 3, 9 and 15 dpi (#P < 0.05); S. aureus alone versus S. aureus +riboflavin, significant decrease at 3, 9 and 15 dpi (#P < 0.05); S. aureus alone versus S. aureus + azithromycin + riboflavin, significant decrease at 15 dpi (#P < 0.05). d CAT, without infection control versus S. aureus alone, significant increase at 3, 9 and 15 dpi (*P < 0.05); S. aureus alone versus S. aureus + azithromycin, significant decrease at 3, 9 and 15 dpi (#P < 0.05); S. aureus alone versus S. aureus + riboflavin, significant decrease at 3, 9 and 15 dpi (#P < 0.05); S. aureus alone versus S. aureus + azithromycin + riboflavin, significant decrease at 3, 9 and 15 dpi (#P < 0.05)
Figure 4b demonstrates the severalfold increase in the level of lipid peroxidation products in the cardiac tissue of mice at 3, 9 and 15 dpi compared to control. This elevation in the level of LPO was significantly decreased when mice were treated with either AZI or riboflavin, but not to the basal levels. However, when the S. aureus infected mice were treated with AZI along with riboflavin, the level of tissue LPO returned to that observed in the control mice, indicating that the combination is more effective in decreasing oxidative stress in the infected mice.
Infection of mice with S. aureus caused an about threefold increase in the activity of cardiac SOD, one of the key antioxidant enzymes, at 3, 9, and, 15 dpi indicating the generation of oxidative stress Fig. 4c. Here again, when the infected mice were treated with AZI and riboflavin, the enzyme activity was restored significantly, although they did not reach the control value indicating that the combination is more effective.
In Fig. 4d, an elevation of catalase activity, another important antioxidant enzyme, was observed to several fold at each time-point following infection of the mice with S. aureus. Here also, when the mice were treated with a combination of AZI and riboflavin, the catalase activity was restored almost toward control level at 3, 9, and, 15 dpi. Although, catalase activity was significantly restored at each time-point, the combination seems to be more effective.
Hepatic tissue: In Fig. 5a an about twofold decrease in the level of hepatic GSH at 3, 9 and 15 dpi was observed in mice infected with S. aureus. AZI and riboflavin co-treated groups were found to have higher hepatic GSH compared to the group of infected mice, indicating that the combination can promote the synthesis of GSH, although the antibiotic and the riboflavin individually can also stimulate the GSH biosynthesis, but to a lesser degree.
Alteration in the antioxidant status like reduced glutathione level and the activities of the antioxidant enzymes such as SOD and catalase hepatic tissue of mice a GSH, without infection control versus S. aureus alone, significant decrease at 3, 9 and 15 dpi (^P < 0.05); S. aureus alone versus S. aureus + azithromycin, significant increase at 3, 9, and 15 dpi ($P < 0.05); S. aureus alone versus S. aureus + riboflavin, significant increase at 3, 9, 15 dpi ($P < 0.05); S. aureus alone versus S. aureus + azithromycin + riboflavin, significant increase at 3, 9, 15 dpi ($P < 0.05). b LPO, without infection control versus S. aureus alone, significant increase at 3, 9, 15 dpi (*P < 0.05); S. aureus alone versus S. aureus + azithromycin, significant decrease at 3, 9 and 15 dpi (#P < 0.05); S. aureus alone versus S. aureus + riboflavin, significant decrease at 3, 9 and 15 dpi (#P < 0.05); S. aureus alone versus S. aureus + azithromycin + riboflavin, significant decrease at 3, 9 and 15 dpi (#P < 0.05). c SOD, without infection control versus S. aureus alone, significant increase at 3, 9 and 15 dpi (*P < 0.05); S. aureus alone versus S. aureus + azithromycin, significant decrease at 3, 9 and 15 dpi (#P < 0.05). S. aureus alone versus S. aureus + riboflavin, significant decrease at 3, 9 and 15 dpi (#P < 0.05); S. aureus alone versus S. aureus + azithromycin + riboflavin, significant decrease at 3, 9 and 15 dpi (#P < 0.05). d CAT, without infection control versus S. aureus alone, significant increase at 3, 9 and 15 dpi (*P < 0.05); S. aureus alone versus S. aureus + azithromycin, significant decrease at 3, 9 and 15 dpi (#P < 0.05). S. aureus alone versus S. aureus + riboflavin, significant decrease at 3, 9 and 15 dpi (#P < 0.05); S. aureus alone versus S. aureus + azithromycin + riboflavin, significant decrease at 3, 9 and 15 dpi (#P < 0.05)
Figure 5b demonstrate more than twofold increase in the level of LPO in the hepatic tissue of mice infected with S. aureus at days 3, 9 and 15 compared to respective controls. However, when these infected mice were co-treated with AZI and riboflavin, the LPO levels came down to the control levels compared to infected mice. This indicates that AZI and riboflavin co-treatment has the ability to reduce the oxidative stress imposed by S. aureus infection.
Figure 5c demonstrates an elevation of twofold in the activity of superoxide dismutase of the hepatic tissue of infected mice at days 3 and 9, and onefold increase of SOD activity at day 15, when compared to respective control. When the infected mice were co-treated with AZI and riboflavin, the level of activity of this important anti-oxidant enzyme was protected from getting increased although individually, the antibiotic or riboflavin appear to be effective but the combination was found more effective.
Similarly, Fig. 5d demonstrates catalase activity at the three time-points. The activity of this enzyme was found to be elevated by twofold in mice infected with S. aureus at days 3, 9, 15 compared to control. Co-treatment of infected mice with AZI and riboflavin caused the restoration of activity of the enzyme to control levels at day 9 and 15. The activity was found to be somewhat decreased compared to control at day 3. The results are indicative of a protective role of this combination against the oxidative stress induced in the organ due to S. aureus infection.
Renal tissue: Fig. 6a demonstrates infection of mice with S. aureus caused oxidative stress in the organ, which is evident from the fact that kidney tissue GSH level was found to decrease severalfold at 3, 9 and 15 dpi compared to control mice. At each time point, the GSH level of the tissue was found to be completely restored when the infected mice were co-treated with AZI and riboflavin. Here again, neither AZI nor riboflavin was found to be effective in complete amelioration of oxidative stress in the infected mice.
Alteration in the antioxidant status like reduced glutathione level and the activities of the antioxidant enzymes such as SOD and catalase renal tissue of mice a GSH, without infection control versus S. aureus alone, significant decrease at 3, 9 and 15 dpi (^P < 0.05); S. aureus alone versus S. aureus + azithromycin, significant increase at 3, 9, and 15 dpi ($P < 0.05); S. aureus alone versus S. aureus + riboflavin, significant increase at 3, 9 and 15 dpi ($P < 0.05); S. aureus alone versus S. aureus + azithromycin + riboflavin, significant increase at 3, 9, 15 dpi ($P < 0.05). b LPO, without infection control versus S. aureus alone, significant increase at 3, 9, 15 dpi (*P < 0.05); S. aureus alone versus S. aureus + azithromycin, significant decrease at 3, 9 and 15 dpi (#P < 0.05); S. aureus alone versus S. aureus + riboflavin, significant decrease at 3, 9 and 15 dpi (#P < 0.05); S. aureus alone versus S. aureus + azithromycin + riboflavin, significant decrease at 3, 9 and 15 dpi (#P < 0.05). c SOD, without infection control versus S. aureus alone, significant increase at 3, 9 and 15 dpi (*P < 0.05); S. aureus alone versus S. aureus + azithromycin, significant decrease at 3, 9 and 15 dpi (#P < 0.05); S. aureus alone versus S. aureus + riboflavin, significant decrease at 3, 9 and 15 dpi (#P < 0.05); S. aureus alone versus S. aureus + azithromycin + riboflavin, significant decrease at 3, 9 and 15 dpi (#P < 0.05). d CAT, without infection control versus S. aureus alone, significant increase at 3, 9 and 15 dpi *P < 0.05; S. aureus alone versus S. aureus + azithromycin, significant decrease at 3, 9 and 15 dpi (#P < 0.05); S. aureus alone versus S. aureus + riboflavin, significant decrease at 3, 9 and 15 dpi (#P < 0.05); S. aureus alone versus S. aureus + azithromycin + riboflavin, significant decrease at 3, 9 and 15 dpi (#P < 0.05)
Figure 6b demonstrates a four-fold increase in the level of LPO in the renal tissue of infected mice compared to control at 3, 9 and 15 dpi. However, when the animals were co-treated with AZI and riboflavin, the level of LPO was restored to control levels at all time points studied indicating that the combination of the antibiotic and the vitamin is capable of ameliorating the oxidative stress caused due to infection. It is interesting to note that either the antibiotic or the vitamin alone is not sufficient to mitigate the level of oxidative stress in the renal tissue following infection with the bacteria.
Figure 6c demonstrate up to threefold increase in the activity of SOD of renal tissue of the infected mice compared to controls, indicating again the elevated levels of oxidative stress following infection with the bacteria at all the three time-points studied. The level of activity of superoxide dismutase was restored to normal at all the three time-points studied when the mice were co-treated with AZI and riboflavin. However, in this particular tissue, AZI or riboflavin alone appears not much effective in the restoration of the SOD activity to the level that seen in the control mice. This again establishes the efficacy of the combined treatment.
Figure 6d demonstrates a twofold increase of the renal catalase activity following infection of mice with S. aureus, compared to control, at the each time-point studied. However, when the mice were co-treated with AZI and riboflavin, the catalase activity was restored to control level. Here again, the combined dose of the antibiotic and the riboflavin seems to be more effective when compared to the values obtained when they were administered alone.
Splenic tissue: Figure 7a demonstrates a more than fourfold decrease in tissue GSH level following infection of mice with S. aureus compared to control mice indicating generation of severe oxidative. On co-treatment of the infected mice with AZI and riboflavin, the tissue level of GSH was found to be restored to that found in the control mice indicating amelioration of oxidative stress. However, here again, neither AZI nor riboflavin alone was found to be effective completely when administered alone.
Alteration in the antioxidant status like reduced glutathione level and the activities of the antioxidant enzymes such as SOD and catalase splenic tissue of mice a GSH, without infection control versus S. aureus alone, significant decrease at 3, 9 and 15 dpi (^P < 0.05); S. aureus alone versus S. aureus + azithromycin, significant increase at 3, 9, and 15 dpi ($P < 0.05); S. aureus alone versus S. aureus + riboflavin, significant increase at 3, 9 and 15 dpi ($P < 0.05); S. aureus alone versus S. aureus + azithromycin + riboflavin, significant increase at 3, 9, 15 dpi ($P < 0.05). b LPO, without infection control versus S. aureus alone, significant increase at 3, 9, 15 dpi (*P < 0.05); S. aureus alone versus S. aureus + azithromycin, significant decrease at 3, 9 and 15 dpi (#P < 0.05); S. aureus alone versus S. aureus + riboflavin, significant decrease at 3, 9 and 15 dpi (#P < 0.05); S. aureus alone versus S. aureus + azithromycin + riboflavin, significant decrease at 3, 9 and 15 dpi (#P < 0.05). c SOD, without infection control versus S. aureus alone, significant increase at 3, 9 and 15 dpi (*P < 0.05); S. aureus alone versus S. aureus + azithromycin decrease significantly at 3, 9 and 15 dpi (#P < 0.05); S. aureus alone versus S. aureus + riboflavin decrease significantly at 3, 9 and 15 dpi (# P < 0.05); S. aureus alone versus S. aureus + azithromycin + riboflavin, significant decrease at 3, 9 and 15 dpi (#P < 0.05). d CAT, without infection control versus S. aureus alone, significant increase at 3, 9 and 15 dpi (*P < 0.05); S. aureus alone versus S. aureus + azithromycin decrease significantly at 3, 9 and 15 dpi (#P < 0.05); S. aureus alone versus S. aureus + riboflavin decrease significantly at 3, 9 and 15 dpi (#P < 0.05); S. aureus alone versus S. aureus + azithromycin + riboflavin, significant decrease at 3, 9 and 15 dpi (#P < 0.05)
Figure 7b demonstrates up to fourfold increase in the level of LPO in the splenic tissue infected with S. aureus the days 3, 9 and 15, compared to respective controls. However, when the mice were co-treated with AZI and riboflavin, the levels of tissue LPO were found to be restored to that observed in the control mice. Particularly on days 9 and 15, the levels of LPO were found even to be below basal level. Although the antibiotic and the vitamin were found not to be effective at least at 3 and 15 dpi when they were administered alone.
Figure 7c demonstrates up to threefold increase in the activity SOD of the S. aureus infected mice, compared to control, at all the time points studied. When the infected mice were co-treated with AZI and riboflavin, the activity of SOD was found to be restored completely to the level observed in the control mice at all the three time-points studied. In this case also, neither AZI nor riboflavin was found to be effective alone in reducing the activity of this crucial anti-oxidant enzyme to the control level indicating and establishing again the efficacy of the co-therapy.
The results presented in the Fig. 7d demonstrates a twofold increase in the activity of splenic catalase in the mice infected with S. aureus, compared to control, at each of the time-points studied. However, when the mice were co-treated with AZI and riboflavin, the level of activity of catalase was found to be restored completely to that observed in the control mice at 9 and 15 dpi. The levels of activity of catalase, at 3 dpi in the co-treated mice were found even to come below the basal level. Here also, the antibiotic or the vitamin alone was found not to be effective to restore the activity of the enzyme to the control level.
Discussion
Septic arthritis is triggered by the hematogenous spread of bacteria from an initial nidus to the joint spaces. We studied the pathogenesis of S. aureus septic arthritis in a murine model in which bacteria are injected intravenously, seed to the joints and cause the disease that clinically resembles septic arthritis in humans. Since there was no death in the S. aureus infected mice at different time points post infection, it can be inferred that the dose of S. aureus cells were non-lethal yet sufficient to induce arthritis. Circulating staphylococci disseminate to virtually all tissues, but within less than a week they are cleared from the blood, with the exception of the spleen and joints. It has been shown previously that the degree of arthritis and inflammation under normal circumstances are dependent on the amount of bacteria injected and the ability of the host to clear the bacteria [36]. Interestingly, it is not the staphylococci per se but rather the immune response evoked, as measured by the level of proinflammatory cytokines, that are related to the progression and development of arthritis.
S. aureus (AG-789) is found sensitive to AZI and we observed that early administration of antibiotic alone, or in combination with riboflavin, eradicates the bacteria from blood and tissues that also correlated with joint inflammation. The 100 mg/kg dose was selected in order to maintain high antibiotic levels for longer periods of time. AZI, with its prolonged t1/2 and high concentrations in tissue, was reported to be a very promising drug for the cure of acute and chronic Group B type IV Streptococci infections. It has been reported that plasma concentrations of AZI were consistently higher at 2 h interval after i.v. administration [24]. Hence, we administered riboflavin just after 2 h of antibiotic treatment. Although a high intracellular concentration of a given antibiotic does not guarantee effective intracellular antimicrobial activity, what counts is the concentration of free active antibiotic at the site of bacterial presence.
During our study we observed a three-step pathogenic process in S. aureus arthritis: (1) During the first 3 days of infection, bacteria recruited and bind selectively to different target organs, namely spleen and synovial tissue, (2) once in the joint, an unspecific inflammatory process takes during the first 6 days (i.e., 9 dpi), primarily with phagocytosis of bacteria by PMNs and macrophages. The bacterial burden and host phagocytic properties are important for progression of disease at this stage, (3) at 15th dpi, although no bacteria are found in the joint, swelling is still prominent in the infected group. At this stage, coagulase or TSST-1 produced during the initial 6 days could promote T cell proliferation leading to induction of cytokine production. In these experiments performed in mice treated with AZI and riboflavin, the final result is that the staphylococci were cleared faster from the blood and spleen, supporting the combined use of riboflavin and AZI in the prevention of infection produced by S. aureus (AG-789).
As we are unable to ensure initiation of antibiotic therapy based on Gram-stain results of joint fluid from mice, we recovered the bacteria from blood and spleen after time-dependent infection by the colony counting method. A number of bacterial CFU obtained from either blood or spleen were not false positive, since bacterial presence was defined as 15 CFU or more for blood or tissue, and are even higher in our case (6). After 3 and 9 dpi, bacterial burden was completely cleared from blood and spleen due to combinatorial treatment, but were still prominent in the joint, which is again cleared at 15 dpi. Thus early treatment of AZI alone or in combination with riboflavin protects the host from widespread dissemination of bacteria into different tissues and is also correlated with joint inflammation.
Thus, the signs of arthritis suggested that the swelling may not be directly due to the bacterial burden in the blood or tissues, but might be due to the TSST-1 or coagulase induced during the course of infection. Therefore, the occurrence, even in the treated mice, is not surprising, bearing in mind that treatment was started 24 h post inoculation with bacteria. Owing to the fact that there is such a short time difference between infection and antibiotic treatment, it may be questioned whether this has any physiological significance. We believe that currently the focus should be on the effect, rather than the time schedule. If the drug is found to be effective, then its effect may be extended up to longer time periods.
Among the different cytokines implicated in inflammation and septic shock, TNF-α, IFN-γ and IL-6 are pro-inflammatory and IL-10 and IL-12p70 are anti-inflammatory. Indeed, mice inoculated with S. aureus AG-789 displayed significantly higher levels of TNF-α, IFN-γ, IL-6 and MCP-1 than the control animals. Several cytokines, including IL-1α, TNF-α, and IL-6, have been reported to stimulate bone resorption. It was also reported that IL-10 is an important endogenous suppressor of infection-stimulated bone resorption in vivo, likely acting via inhibition of IL-1α expression [37]. Our results are also supported by earlier studies suggesting that the elevated TNF-α, IFN-γ and IL-6 levels induced by S. aureus infection may be related to bone damage mainly in the early phase of infection [38].
Indeed, higher bacterial counts in blood and spleens of infected mice early during infection (3 dpi) support the notion that mice producing TNF-α, IFN-γ and IL-6 may be sicker in the early stages of infection. It may be suggested that non-phagocytosed, synovial-tissue-bound bacteria, or more importantly their exotoxins, activate macrophages and monocytes to release TNF-α, IFN-γ and IL-6 which enter the circulation and trigger a general inflammatory response. Moreover, we found elevated MCP-1 in the serum of S. aureus infected mice, indicating recruitment of monocytes to the synovial tissue to regulate inflammation.
In serum, which reflects the primary site of inflammation in this model, IL-10 continues to increase even at 15 dpi after treatment of mice with AZI and riboflavin. The sustained production of regulatory mediators is likely an important aspect relating to in vivo cytokine balance, which dictates the resolution of inflammation and may be a positive prognostic indicator for recovery and survival due to the combined therapy. Previous observations have demonstrated a protective role for IL-10 in animal models of septic inflammatory response and also found it to inhibit the production of ROS and reactive nitrogen intermediates when monocyte/macrophages are activated by IFN-γ [39]. Thus the suppressive effects of IL-10 on host responses might be predominantly mediated by antioxidant–antibiotic co-therapy. IL-12 is protective in several experimental models of bacterial infections [40]. In our study we also found elevated IL-12p70 at 3, 9 and 15 dpi when mice were treated with AZI followed by riboflavin after S. aureus infection, suggesting the protective effect of endogenously circulating IL-12, which happens to downregulate staphylococcal growth.
Our studies revealed that infection of mice with S. aureus caused the generation of severe oxidative stress in cardiac, hepatic, renal and splenic tissues (Figs. 4, 5, 6, 7). The level of oxidative stress was found to be high at all the time-points studied. The increased level of tissue lipid peroxidation (LPO) and reduction in the level of reduced glutathione (GSH), two important bio-markers, indicated generation of severe oxidative stress following infection. However, when the mice were co-treated with AZI, the antibiotic, and the B-vitamin, riboflavin, the level of LPO was found to be restored to almost near control values in all the tissues studied. Similarly, tissue reduced GSH level was also restored to control level following the co-treatment. In our studies, in mouse liver, kidney, heart and spleen, the antibiotic and the vitamin co-treatment seemed to stimulate glutathione biosynthesis. There were previous reports that antioxidant(s) stimulate glutathione biosynthesis in the mouse tissues [20]. The results indicate that the co-treatment has the ability to provide protection against oxidative stress in mouse tissues following infection with S. aureus. It is interesting to note, however, that neither AZI nor riboflavin alone is capable of completely ameliorating the oxidative stress following infection of the mice with the bacteria.
Generation of oxidative stress following infection of the mice with S. aureus was further confirmed by studying the activities of the two key antioxidant enzymes of heart, liver, kidneys and the spleen tissues. The activity of SOD (Cu–Zn-type) and catalase was found to be increased compared to control by more than twofold in all the tissues studied. The increase in SOD and catalase activity at all time-points studied may be an adaptive response of the tissue against the elevated levels of superoxide anion free radicals and hydrogen peroxide generated within the tissue following infection of the mice with S. aureus. However, when the mice were co-treated with azithromycin and riboflavin, the activity of SOD and catalase was found to be restored to near normal, indicating that the co-therapy with antibiotic and the vitamin was effective in combating oxidative stress following infection with the bacteria.
We also found similar results in our previous studies with different strains of S. aureus and different combination of antibiotic (ampicillin and gentamicin) and antioxidant (riboflavin and ascorbic acid) [20, 41]. In an ongoing project we are also doing experiments with a macrolide-resistant strain, which will give a clear idea about the effect of the antibiotic in the present results. The information obtained through our present studies relating to the efficacy of antibiotic-antioxidant co-therapeutic approach in ameliorating the oxidative stress induced due to infection of the mice with S. aureus may be important and useful in the treatment of human inflammatory conditions associated with bacterial infection, and needs further studies.
References
Anwar S, Prince LR, Foster SJ, Whyte MK, Sabroe I. The rise and rise of Staphylococcus aureus: laughing in the face of granulocytes. Clin Exp Immunol. 2009;157:216–24.
Canvin JM, Goutcher SC, Hagig M, Gemmell CG, Sturrock RD. Persistence of Staphylococcus aureus as detected by polymerase chain reaction in the synovial fluid of a patient with septic arthritis. Br J Rheumatol. 1997;36:203–6.
Mathews CJ, Weston VC, Jones A, Field M, Coakley G. Bacterial septic arthritis in adults. Lancet. 2010;375:846–55.
Abdelnour A, Bremell T, Holmdahl R, Tarkowski A. Role of T lymphocytes in experimental Staphylococcus aureus arthritis. Scand J Immunol. 1994;39:403–8.
Foster TJ. Immune evasion by staphylococci. Nat Rev Microbiol. 2005;3:948–58.
Darley ES, MacGowan AP. Antibiotic treatment of gram-positive bone and joint infections. J Antimicrob Chemother. 2004;53:928–35.
Nair SP, Williams RJ, Henderson B. Advances in our understanding of the bone and joint pathology caused by Staphylococcus aureus infection. Rheumatology (Oxford). 2000;39:821–34.
Liu GY. Molecular pathogenesis of Staphylococcus aureus infection. Pediatr Res. 2009;65:71R–7R.
Sakiniene E, Bremell T, Tarkowski A. Addition of corticosteroids to antibiotic treatment ameliorates the course of experimental Staphylococcus aureus arthritis. Arthritis Rheum. 1996;39:1596–605.
Nizet V. Understanding how leading bacterial pathogens subvert innate immunity to reveal novel therapeutic targets. J Allergy Clin Immunol. 2007;120:13–22.
Dormandy TL. Free-radical pathology and medicine. A review. J R Coll Physicians Lond. 1989;23:221–7.
Mirshafiey A, Mohsenzadegan M. The role of reactive oxygen species in immunopathogenesis of rheumatoid arthritis. Iran J Allergy Asthma Immunol. 2008;7:195–202.
Bodamyali T, Stevens CR, Billingham ME, Ohta S, Blake DR. Influence of hypoxia in inflammatory synovitis. Ann Rheum Dis. 1998;57:703–10.
Girard AE, Girard D, English AR, Gootz TD, Cimochowski CR, Faiella JA, Haskell SL, Retsema JA. Pharmacokinetic and in vivo studies with azithromycin (CP-62,993), a new macrolide with an extended half-life and excellent tissue distribution. Antimicrob Agents Chemother. 1987;31:1948–54.
Girard AE, Girard D, Retsema JA. Correlation of the extravascular pharmacokioetics of azithromycin with in vivo efficacy in models of localized infection. J Antimicrob Chemother. 1990;25:61–71.
Meyer AP, Bril-Bazuin C, Matfie H, Van Den Broek PJ. Uptake of azithromycin by human monocytes and enhanced intracellular antibacterial activity against Staphylococcus aureus. Antimicrob Agents Chemother. 1993;37:2318–22.
Cheung AL, Bayer AS, Zhang G, Gresham H, Xiong YQ. Regulation of virulence determinants in vitro and in vivo in Staphylococcus aureus. FEMS Immunol Med Microbiol. 2004;40:1–9.
Mazur-Bialy AI, Majka A, Wojtas L, Kolaczkowska E, Plytycz B. Strain-specific effects of riboflavin supplementation on zymosan-induced peritonitis in C57BL/6 J, BALB/c and CBA mice. Life Sci. 2011;88:265–71.
Martins SA, Combs JC, Noguera G, Camacho W, Wittmann P, Walther R, et al. Antimicrobial efficacy of riboflavin/UVA combination (365 nm) in vitro for bacterial and fungal isolates: a potential new treatment for infectious keratitis. Invest Ophthalmol Vis Sci. 2008;49:3402–8.
Mal P, Ghosh D, Bandyopadhyay D, Bishayi B. Ampicillin alone and in combination with riboflavin modulates Staphylococcus aureus infection induced septic arthritis in mice. Indian J Exp Biol. 2012. In press.
Majumdar S, Dutta K, Manna SK, Basu A, Bishayi B. Possible protective role of chloramphenicol in TSST-1 and coagulase-positive Staphylococcus aureus-induced septic arthritis with altered levels of inflammatory mediators. Inflammation. 2011;34:269–82.
Sen R, Das D, Bishayi B. Staphylococcal catalase regulates its virulence and induces arthritis in catalase deficient mice. Indian J Physiol Pharmacol. 2009;53:307–17.
Yao L, Berman JW, Factor SM, Lowy FD. Correlation of histopathologic and bacteriologic changes with cytokine expression in an experimental murine model of bacteremic Staphylococcus aureus infection. Infect Immun. 1997;65:3889–95.
Tissi L, von Hunolstein C, Mosci P, Campanelli C, Bistoni F, Orefici G. In vivo efficacy of azithromycin in treatment of systemic infection and septic arthritis induced by type IV group B Streptococcus strains in mice: comparative study with erythromycin and penicillin G. Antimicrob Agents Chemother. 1995;39:1938–47.
Verdrengh M, Tarkowski A. Riboflavin in innate and acquired immune responses. Inflamm Res. 2005;54:390–3.
Burchill MA, Nardelli DT, England DM, DeCoster DJ, Christopherson JA, Callister SM, et al. Inhibition of interleukin-17 prevents the development of arthritis in vaccinated mice challenged with Borrelia burgdorferi. Infect Immun. 2003;71:3437–42.
Dixon RA. Curative effects of tobramycin or gentamicin therapy on mouse arthritis caused by Mycoplasma pulmonis. Antimicrob Agents Chemother. 1981;20:321–6.
Chattopadhyay A, Biswas S, Bandyopadhyay D, Sarkar C, Datta AG. Effect of isoproterenol on lipid peroxidation and antioxidant enzymes of myocardial tissue of mice and protection by quinidine. Mol Cell Biochem. 2003;245:43–9.
Sedlak J, Lindsay RH. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellmanʼs reagent. Anal Biochem. 1968;25:192–205.
Martin JP, Dailey M, Sugarman E. Negative and positive assays of superoxide dismutase based on hematoxylin autoxidation. Arch Biochem Biophys. 1987;255:329–36.
Beers RF Jr, Sizer IW. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem. 1952;195:133–40.
Dutta K, Mishra MK, Nazmi A, Kumawat KL, Basu A. Minocycline differentially modulates macrophage mediated peripheral immune response following Japanese encephalitis virus infection. Immunobiology. 2010;215:884–93.
Johnson WM, Tyler SD, Ewan EP, Ashton FE, Pollard DR, Rozee KR. Detection of genes for enterotoxins, exfoliative toxins, and toxic shock syndrome toxin 1 in Staphylococcus aureus by the polymerase chain reaction. J Clin Microbiol. 1991;29:426–30.
Becker K, Roth R, Peters G. Rapid and specific detection of toxigenic Staphylococcus aureus: use of two multiplex PCR enzyme immunoassays for amplification and hybridization of staphylococcal enterotoxin genes, exfoliative toxin genes, and toxic shock syndrome toxin 1 gene. J Clin Microbiol. 1998;36:2548–53.
Das D, Das A. Analysis of variance. In: Das D, editor. Statistics in biology and psychology. Calcutta: Academic; 2005. pp. 280–93.
Bremell T, Lange S, Yacoub A, Ryden C, Tarkowski A. Experimental Staphylococcus aureus arthritis in mice. Infect Immun. 1991;59:2615–23.
Sasaki H, Hou L, Belani A, Wang CY, Uchiyama T, Muller R, et al. IL-10, but not IL-4, suppresses infection-stimulated bone resorption in vivo. J Immunol. 2000;165:3626–30.
Yoshii T, Magara S, Miyai D, Nishimura H, Kuroki E, Furudoi S, et al. Local levels of interleukin-1beta, -4, -6 and tumor necrosis factor alpha in an experimental model of murine osteomyelitis due to staphylococcus aureus. Cytokine. 2002;19:59–65.
Moore KW, O’Garra A, de Waal Malefyt R, Vieira P, Mosmann TR. Interleukin-10. Annu Rev Immunol. 1993;11:165–90.
Hultgren OH, Stenson M, Tarkowski A. Role of IL-12 in Staphylococcus aureus-triggered arthritis and sepsis. Arthritis Res. 2001;3:41–7.
Mal P, Dutta S, Bandyopadhyay D, Dutta K, Basu A, Bishayi B. Gentamicin in combination with ascorbic acid regulates the severity of Staphylococcus aureus infection induced septic arthritis in mice. Scand J Immunol. 2012. In press.
Acknowledgments
The corresponding author (Biswadev Bishayi) thanks the Council of Scientific and Industrial Research (CSIR) Ministry of Human Resource Development, Government of India, New Delhi, India for funding this project. The author (BB) is indebted to Dr. Sunil Kumar Manna, Scientist and Head, Immunology Division, Centre for DNA Fingerprinting and Diagnostics, Hyderabad, India for providing us with the primers for TSST-1 and coagulase. KD is recipient of Research Associateship in Biotechnology and Life Sciences from Department of Biotechnology, Govt. of India.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: John Di Battista.
Rights and permissions
About this article
Cite this article
Mal, P., Dutta, K., Bandyopadhyay, D. et al. Azithromycin in combination with riboflavin decreases the severity of Staphylococcus aureus infection induced septic arthritis by modulating the production of free radicals and endogenous cytokines. Inflamm. Res. 62, 259–273 (2013). https://doi.org/10.1007/s00011-012-0574-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-012-0574-z