Abstract
Because of the high growth rates often achieved by invasive alien macrophytes, their establishment in recipient ecosystems may alter the abundance and composition of litter entering detrital pathways, representing a significant—but often overlooked—ecological effect of these invasions. Crassula helmsii (Kirk) Cockayne (New Zealand pygmyweed) is an invasive alien macrophyte, notorious for its profuse growth in invaded waterbodies. C. helmsii is perennial and often forms dense stands, producing abundant detritus. To investigate whether some of C. helmsii’s impacts are mediated by this detritus, we conducted an 85-day litterbag experiment comparing decomposition of C. helmsii with that of Callitriche stagnalis Scop. (water-starwort), a commonly co-occurring native macrophyte. Macroinvertebrate assemblage composition was comparable between macrophyte species throughout the experiment, but shifted as plants decayed. Litterbags were initially dominated by the invasive shredder Crangonyx pseudogracilis Bousfield, 1958 and later by Euglesa casertana (Poli, 1791), an interstitial suspension feeder. C. helmsii litter decomposed more slowly, with proportionally less invertebrate-mediated breakdown, but was ultimately colonised by more abundant macroinvertebrates, including more C. pseudogracilis. Decomposition may be slowed by C. helmsii’s high carbon: nitrogen ratio. These results suggest that C. helmsii invasion may impact macroinvertebrate assemblages via the production of long-lasting and relatively unpalatable detritus.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Invasive species are proliferating worldwide, aided by human vectors of dispersal and anthropogenic change to recipient ecosystems (Seebens et al., 2017; IPBES, 2023). Establishing the factors which govern the ecological impacts of invasion is a key research goal in invasion biology and could enable problematic invasions to be pre-empted and acted against (Simberloff et al., 2005, 2013). Self-evidently, interactions between invasive species and recipient biota are key determinants of both ecosystem resilience to invasions, and the impacts of successful invasion (Maron & Vilà, 2001; Simberloff et al., 2013). Because of the foundational role of macrophytes (macroscopic green plants and macroalgae) in terrestrial, freshwater and marine systems, macrophyte invasions may cause far-reaching impacts on recipient ecosystems (Vilà et al., 2011; Maggi et al., 2015; Tasker et al., 2022). In freshwaters, much attention has been paid to determining the rules governing interactions between alien macrophytes and herbivores, mostly due to herbivory’s potential role in fostering ecosystem resilience through biotic resistance, wherein invasions may be suppressed by interactions with native biota (Parker & Hay, 2005; Morrison & Hay, 2011; Grutters et al., 2017; Oliveira et al., 2019). In contrast, relatively little research has focussed on interactions between alien macrophytes and detritivores (but see Cuassolo et al. 2020; Dekanová et al. 2021). Whilst detritivory clearly cannot play a direct role in biotic resistance, it may be instrumental to the wider ecosystem impacts of invasive macrophytes. Aquatic macrophytes are generally more frequently consumed as detritus than whilst alive, and macrophyte litter decomposition (conducted in part by detritivores) strongly influences freshwater nutrient cycling and energy flows (Newman, 1991; Shilla et al., 2006; Bakker et al., 2016; Dekanová et al., 2021; Thornhill et al., 2021). Impacts of a macrophyte invader on detritivores are therefore likely to be more significant than impacts on herbivores in determining the invasion’s impacts on the recipient ecosystem.
The freshwater decomposition of vascular plant detritus can be thought of as a 3-part process, consisting of (1) leaching; (2) microbial decomposition and (3) mechanical/invertebrate fragmentation (Webster & Benfield, 1986). Leaching of water-soluble compounds leads to considerable early mass loss (Gessner et al., 1999; Pope et al., 1999; Carvalho et al., 2015), concurrent with the beginnings of colonisation by microbes (e.g. bacteria, hyphomycete fungi) and fragmentation of detritus by invertebrate detritivores and/or mechanical action (e.g. by current or abrasives) (Webster & Benfield, 1986; Santonja et al., 2018). The latter two processes act in a positive feedback loop: microorganisms ‘condition’ the detritus, making it more palatable and nutritious for detritivores (Anderson et al., 2017). Resulting detritivore fragmentation increases the surface area of the detritus, promoting further microbial colonisation and decomposition (Newman, 1991; Longhi et al., 2008). The rate of decomposition is influenced by detritus traits (Cebrian & Lartigue, 2004), detritivore assemblage composition (Jonsson & Malmqvist, 2000; Gessner et al., 2010) and physical and chemical factors including temperature, pH, oxygen concentration and waterbody trophic state (Webster & Benfield, 1986).
Whilst the aquatic decomposition of invasive riparian, emergent and floating plant litter has been subject to some investigation (Chimney & Pietro, 2006; Saulino et al., 2018; Cuassolo et al., 2020; Dekanová et al., 2021), to our knowledge the decomposition of alien submerged plant litter has rarely been studied to date (Carpenter & Adams, 1979; Shilla et al., 2006). Given the importance of detritus to freshwater nutrient and energy flows, the availability and palatability of invasive macrophyte detritus is likely to strongly influence recipient ecosystems (Cebrian & Lartigue, 2004; Saulino et al., 2018). Detritus availability may vary according to macrophyte phenology and biomass production, and the rate of microbial decomposition. Palatability may vary according to nutrient concentrations, particularly nitrogen and phosphorus, and the retention of defensive chemicals, such as phenolic compounds, or structures, such as trichomes and sclerophylly (Webster & Benfield, 1986; Newman, 1991; Chimney & Pietro, 2006; Hanley et al., 2007). The overall rate of decomposition, and impacts on the invaded ecosystem, will be mediated by match/mismatch between these plant traits and the traits present in the recipient detritivore assemblage (Tiegs et al., 2013; Carvalho et al., 2015).
Crassula helmsii (New Zealand pygmyweed) is an alien aquatic plant, originally from Australasia, which has spread widely across small lentic waterbodies throughout NW Europe since its introduction in the mid-twentieth century (Smith & Buckley, 2020). Small waterbodies are particularly threatened by biological invasions due to their insular, island-like nature, with high endemism and species turnover between basins (Davies et al., 2008; Moorhouse & Macdonald, 2015). C. helmsii is notorious for the production of profuse biomass in the margins of these waterbodies, up to 1.5 kg m−2 (Dawson & Warman, 1987). As plants within these dense stands senesce, they can be expected to produce considerable volumes of litter (Carpenter & Lodge, 1986; Newman, 1991). Because C. helmsii is perennial and retains aboveground biomass throughout the year in many areas (Hussner, 2009; Smith & Buckley, 2020), this material is likely to be available almost permanently.
Prior field surveys (Tasker et al., 2024) revealed marked shifts in the taxonomic and functional composition of macroinvertebrate detritivores within C. helmsii-invaded sites compared to uninvaded waterbodies. In particular, alien detritivores were more abundant in sites invaded by C. helmsii, and traits associated with detritivory drove differences in functional assemblage composition between invaded and uninvaded sites. These results suggest that the impacts of C. helmsii invasion on detritivores are a major determinant of the plant’s impacts on ecosystem structure and function (Petchey & Gaston, 2006; Schmera et al., 2016), but C. helmsii decomposition has not been studied to date.
In order to investigate the mechanisms underpinning the impacts of C. helmsii on macroinvertebrate detritivores, we designed a field experiment to compare C. helmsii litter breakdown with that of an architecturally similar co-occurring native macrophyte. Litterbags were deployed in a C. helmsii- invaded pond in West Cornwall, UK, containing either C. helmsii or the widespread co-occurring native macrophyte, Callitriche stagnalis (common water-starwort). Through this experiment, we aimed to evaluate whether C. helmsii impacts recipient macroinvertebrate assemblages via its detritus, as suggested by Tasker et al. (2024).
We sought to address this question by assessing the decomposition rate of alien C. helmsii litter versus native C. stagnalis litter, and the composition of macroinvertebrate assemblages colonising C. helmsii vs. C. stagnalis during decomposition. We hypothesised that the rate of litter breakdown would differ between C. helmsii and C. stagnalis, as a result of their colonisation by distinct detritivore assemblages.
Methodology
Field experiment: detritus colonisation and breakdown
Experiment site
The field experiment was conducted in a circumneutral permanent pond with an area of 0.11 ha (pH 5.85, conductivity 141 µS/cm (May 2021)), surrounded by grassland and heathland in Sancreed, west Cornwall, United Kingdom (50° 06′ 18″ N, 005° 38′ 03″ W, altitude 180 m). The pond is artificial, having been dug in 1997, and has a mean depth of 1 m. It is fishless and well vegetated with submerged macrophytes throughout, including abundant Crassula helmsii amongst a mosaic of other macrophytes over a silt substratum. We did not record Callitriche stagnalis from the waterbody during our trial, although it occurs widely in similar habitats across the region, including at Sancreed (NBN Trust, 2023). Marginal areas are partly shaded by extensive riparian Salix L. The study was conducted between April and June 2023, during which time local monthly temperatures averaged 13.1 °C (mean daily max. 16.1 °C, min. 10.2 °C) (Met Office, 2023).
Experimental procedure
Crassula helmsii and Callitriche stagnalis were collected from Cadover Bridge, Dartmoor (50° 27′ 55″ N 4° 02′ 09″ W, March 2023), thoroughly rinsed to remove epiphytes and air-dried at a temperature of 26 ± 3 °C for 2 weeks. Once plants had attained constant mass, they were split into 5 ± 0.1 g portions and placed into 20 × 30 cm mesh litter bags (n = 42). Of these, 24 coarse mesh bags (n = 12 for each macrophyte species) had a 700 µm mesh base and 7 mm mesh on the upper side (adapted from Bedford, 2004), permitting access for macroinvertebrate detritivores. The remaining 18 fine mesh bags (n = 9 for each macrophyte species) were composed entirely of 700 µm mesh, for the quantification of microbial and meiofaunal decomposition in the absence of macroinvertebrates. Upon arrival at the experimental site, bags were weighted down with cleaned glass marbles, shut with cable ties (coloured to indicate the plant species within) and secured in groups to randomly distributed stakes in the margins of the waterbody (depth < 1 m). Fine and coarse mesh bags containing C. helmsii or C. stagnalis were distributed evenly across these stakes, so that for each retrieval date, an even number of bags of both macrophyte species were retrieved from each stake, negating potentially confounding variation in abiotic conditions across the waterbody. 14 bags (4 coarse, 3 fine for each macrophyte species) were extracted from the waterbody on each of 3 retrieval dates, after 10 (d10), 35 (d35) and 83 (d83) days. Retrieval dates were selected to encompass all stages of litter decay (rapid early mass loss through to slow breakdown of recalcitrant litter components), and collect macroinvertebrates associated with each stage of decomposition (Carvalho et al., 2015). Individual bags were retrieved using a large 500 µm mesh bag to prevent loss of plant material or invertebrates. Upon retrieval, litterbags were placed singly in 1 l pots containing 70% industrial denatured alcohol (for invertebrate fixation) and transferred to the laboratory for processing.
In the laboratory, macroinvertebrates were separated from plant material, which was then air-dried to constant mass and weighed (to the nearest milligramme: Adam Equipment PW254). Macroinvertebrates were then identified and counted. Where possible, specimens were identified to species level using a range of resources (Hammond et al., 1985; Elliott et al., 1988; Savage, 1989; Wallace et al., 1990; Edington & Hildrew, 1995; Nilsson, 1996; Foster & Friday, 2011; Dobson et al., 2012; Foster et al., 2014; Brochard et al., 2016; Smallshire & Swash, 2018; Rowson et al., 2021), with the exception of Bivalvia (species/genus), Diptera (subfamily) and Annelida (subclass).
Carbon: nitrogen analysis
To assess carbon:nitrogen (C:N) ratios, litter was freeze-dried, ground and passed through a 180 µm sieve. We weighed out ca. 5 mg of resulting powders into tin cups for C:N analysis in an elemental analyser (Elementar, Langensolbold, Germany, see Epstein et al., 2019). To minimise contamination by invertebrates and extraneous detritus, we included only litter from fine mesh bags in this analysis.
Data analysis
Decomposition rate was calculated based on the exponential decay model (Petersen & Cummins, 1974; Bärlocher, 2005; Thornhill et al., 2021), using the formula:
where DM0 is initial dry mass, DM1 is dry mass upon recovery and d is the number of days submersed. For convenience, −k is expressed positively hereafter.
Differences in mass loss and C:N ratio between C. helmsii and C. stagnalis litter were assessed using linear models. Microbial decomposition was assessed by analysing rates of decomposition in fine mesh bags, whilst invertebrate-mediated decomposition was assessed by calculating the difference in coarse mesh mass loss and average fine mesh mass loss per macrophyte species per retrieval date, and analysing these values (Dekanová et al., 2021). Differences in the taxon richness and abundance of macroinvertebrates associated with coarse litter bags were assessed using generalised linear models (packages lme4 (Bates et al., 2015) and MASS (Venables & Ripley, 2002)). Finding that the invasive Crangonyx pseudogracilis and native Euglesa casertana were by far the most abundant macroinvertebrates in our samples, we constructed additional generalised linear models to individually assess differences in the abundance of these species. Model assumptions were checked graphically, and generalised least squares fits (package nlme (Pinheiro et al., 2023)) used where issues with homogeneity of variance were evident. For all models, homogeneity of response (equivalence of breakdown slopes) was tested using Type III (simultaneous) ANCOVA (package car (Fox & Weisberg, 2018)). If no significant interaction between retrieval date and macrophyte species was observed, Type I (sequential) ANCOVA was used for significance testing of main effects, whereas results of Type III ANCOVA were reported where interactions were significant. Values of P < 0.05 were considered statistically significant.
Differences in taxonomic assemblage composition of macroinvertebrates associated with C. helmsii and C. stagnalis litter bags during breakdown were assessed using permutational multivariate analysis of variance (PERMANOVA) on a Bray–Curtis dissimilarity matrix (package vegan (Oksanen et al., 2022)). For this purpose, abundance data were square-root transformed to down-weight the influence of dominant taxa.
To assess differences in functional assemblage composition, we constructed a functional trait database using fuzzy-coded data (Tachet et al., 2010; Schmidt-Kloiber & Hering, 2015) encompassing 3 biological traits with 24 modalities: food, feeding type and maximal body size (Table 1). We then constructed a community weighted means (CWM) matrix by crossing our functional trait and taxon abundance databases (package ade4 (Thioulouse et al., 2018)), and used this to compute an ordination using fuzzy principal components analysis (FPCA) (Guareschi et al., 2021).
All analyses were conducted in the R computing environment (R Core Team, 2023).
Results
Litter decomposition
Mass loss
In our field trial, invertebrate-mediated decomposition was significantly greater in C. stagnalis litter than in litter of C. helmsii. Microbial decomposition was also significantly greater in C. stagnalis litter than C. helmsii litter (Table 2).
Averaged across the entire trial, rates of microbial (fine mesh) and invertebrate-mediated decomposition were approximately twice as high in C. stagnalis compared with C. helmsii (Table 3). After 83 days of decomposition, only 21.1% of C. stagnalis’ mass remained across coarse and fine mesh litter bags, whilst C. helmsii retained 49.1% of its original mass. (Fig. 1).
Carbon: nitrogen ratio
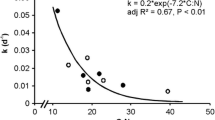
C. helmsii litter had a significantly higher carbon: nitrogen ratio than C. stagnalis litter, but litter carbon: nitrogen ratios did not change significantly throughout our experiment (Table 3, Fig. 2).
Macroinvertebrate colonisation
Abundance and taxonomic diversity
We observed a significant interaction effect of plant species and litterbag retrieval date on macroinvertebrate abundance, with C. helmsii litterbags containing fewer macroinvertebrates than C. stagnalis bags after 10 days, but more macroinvertebrates after 83 days (Fig. 3, Table 4).
Macroinvertebrate taxon richness did not differ significantly between C. helmsii and C. stagnalis, nor between litterbag retrieval dates. We found a significant interaction effect between plant and retrieval date on Crangonyx pseudogracilis abundance, with C. helmsii litterbags containing fewer C. pseudogracilis than C. stagnalis bags after 10 days, but more C. pseudogracilis after 83 days. Euglesa casertana abundance was significantly higher in litter of both macrophyte species at later retrieval dates (Fig. 3, Table 4).
Taxonomic and functional assemblage composition
Macroinvertebrate taxonomic composition differed significantly according to retrieval date (PERMANOVA: F1,18 = 4.750, P < 0.01), but not according to macrophyte species (PERMANOVA: F1,18 = 0.568, P > 0.05). The amphipod shredder C. pseudogracilis was the most abundant member of d10 and d35 macroinvertebrate assemblages, whilst the suspension feeding bivalve E. casertana was most abundant in d83 assemblages (Table 4, Fig. 3). Consequently, functional assemblage composition shifted during litter decomposition from trait space within our FPCA ordination associated with trait modalities food: dead plants (> 1 mm) and feeding mode: shredder towards space associated with the trait modalities food: detritus (< 1 mm) and feeding mode: filter feeder (Fig. 4). See Table S1 for a complete list of macroinvertebrate taxa recorded from litter bags.
A Fuzzy principal components analysis ordination, produced using a community weighted means matrix. First two axes account for 99.5% of the variation. Convex hulls represent the location in functional trait space of macroinvertebrate assemblages from d10, d35 and d83 litterbags. B Trait modalities most strongly driving assemblage functional composition. Green arrows: feeding type; brown: food; orange: maximal body size
Discussion
Given the major contribution made by detritus to aquatic energy flows and nutrient cycling (Webster & Benfield, 1986; Cebrian & Lartigue, 2004; Shurin et al., 2005), changes to the quantity and quality of detritus are likely to be a key factor determining the impacts of alien macrophyte invasion. In our field experiment, alien Crassula helmsii litter decomposed at a significantly slower rate than native Callitriche stagnalis litter, driven in part by reduced invertebrate-mediated decomposition throughout the trial. As we hypothesised, colonisation of litter by macroinvertebrates differed between macrophyte species. Initially, C. stagnalis litter supported more abundant macroinvertebrates, but after 83 days, C. helmsii litter supported higher macroinvertebrate abundance. However, taxonomic and functional composition did not differ significantly between macroinvertebrate assemblages colonising C. helmsii and C. stagnalis litter.
Our results suggest that C. helmsii detritus persists for longer, and ultimately hosts more abundant detritivores. Elevated invertebrate-mediated mass loss observed in C. stagnalis litter suggests that the percentage of detritus consumed by detritivores per unit time is higher in C. stagnalis than in C. helmsii, indicating that C. helmsii may be less palatable to macroinvertebrates. However, this result might also be explained by reduced mechanical breakdown in C. helmsii. The composition of macroinvertebrate assemblages colonising C. helmsii and C. stagnalis litterbags did not differ significantly, indicating that C. helmsii detritus is processed by similar macroinvertebrates to native macrophytes. Litterbag colonisation does not necessarily directly relate to consumption, however, and processes of macroinvertebrate colonisation might be influenced by the provision of comparable refugia due to the similar physical structure of C. helmsii and C. stagnalis (Tasker et al., 2022). Differences in mass loss between C. helmsii and C. stagnalis litter in fine mesh bags are likely driven by resistance of C. helmsii litter to microbial decomposition (Webster & Benfield, 1986; Santonja et al., 2018). Decomposition rates could be retarded by C. helmsii’s low nutritional quality, as revealed by its comparatively high carbon: nitrogen ratio (Li et al., 2012).
The breakdown rate we observed for C. helmsii (0.018 k d−1) is less than half the mean rate of 0.047 k d−1 calculated by Chimney and Pietro (2006) for submerged freshwater macrophytes, suggesting C. helmsii does indeed produce unusually recalcitrant detritus. Despite the lower percentage of C. helmsii detritus apparently consumed by macroinvertebrates in this trial, absolute consumption by detritivores may be higher than that of slower growing native macrophytes such as C. stagnalis because of the high biomass production (and consequently high detritus production) often attained by C. helmsii (Dawson & Warman, 1987; Cebrian & Lartigue, 2004). In addition, C. helmsii is a perennial, and tends to retain aboveground biomass in winter, so will yield varying quantities of detritus throughout much of the year, as opposed to the seasonal glut typical of most native macrophytes characteristic of the shallow fluctuating waters colonised by C. helmsii (Carpenter & Lodge, 1986; Hussner, 2009; Smith & Buckley, 2020). The reliable supply of abundant—but perhaps somewhat unpalatable—detritus produced by C. helmsii is likely to drive shifts in detritivore populations within recipient ecosystems.
Crassula helmsii may facilitate further alien invasions where promoted detritivores are non-native, resulting in additional indirect impacts on recipient ecosystems (Simberloff & Von Holle, 1999). Although we detected no significant overall difference in the abundance of C. pseudogracilis associated with C. helmsii vs. C. stagnalis litter bags, the alien amphipod was more abundant amongst C. helmsii detritus by the end of the experiment. The non-native bladder snail Physella acuta (Rowson et al., 2021) was also present in C. helmsii litterbags across all removal dates. In a recent field study, Tasker et al. (2024) found that non-native detritivores (and particularly C. pseudogracilis and P. acuta) were more abundant within C. helmsii-invaded waterbodies than in uninvaded waterbodies, perhaps demonstrating this effect in action.
The decomposition of aquatic vascular plant litter is understudied in comparison to the aquatic decomposition of allochthonous terrestrial plant material, particularly in lentic systems (Cummins et al., 1973; Gessner et al., 1999, 2010). Decomposition pathways of aquatic plant litter in lentic systems differ from better-studied processes of woody litter decomposition in lotic systems for several reasons. With the exception of some emergent species, aquatic plants typically have higher available nutrient concentrations than terrestrial plants, due largely to the absence of unpalatable structural components such as lignin, and detritus nutritional quality is strongly correlated with the percentage of detrital production which is consumed in freshwaters (Cebrian & Lartigue, 2004; Shilla et al., 2006; Bakker et al., 2016). Secondly, differing litter properties and detritivore species pools will mean that colonising detritivore assemblages will differ between habitats (Pope et al., 1999; Cebrian & Lartigue, 2004; Carvalho et al., 2015; Bakker et al., 2016). In addition, litter decomposition in small lentic waterbodies will proceed differently to decomposition in lotic systems (or larger lakes) due to the relative insignificance of mechanical breakdown by flow or wave action (Webster & Benfield, 1986; Santonja et al., 2018). The unanticipated colonisation of our litterbags by abundant E. casertana might represent one such divergence from better-studied processes of lotic woody litter decomposition (Cummins et al., 1973; Petersen & Cummins, 1974; Gessner et al., 2010). The fragmentation of microbially conditioned coarse particulate organic matter (CPOM) into fine particulate organic matter (FPOM) by macroinvertebrate shredders is a well-understood and near-ubiquitous component of litter decomposition in freshwaters (Cummins et al., 1973; Webster & Benfield, 1986; Pope et al., 1999; Santonja et al., 2018; Thornhill et al., 2021), but the role of macroinvertebrate collector-gatherers and suspension feeders in detritus processing has been less well studied. To our knowledge, the mass colonisation of litter by suspension feeding Pisidium/Euglesa spp. has not been reported from litter experiments to date (Cummins et al., 1973; Wallace & Webster, 1996; Pope et al., 1999; Carvalho et al., 2015; Dekanová et al., 2021). E. casertana is a small bivalve mollusc which is thought to primarily feed in the interstices of sediment, filtering dense suspended FPOM agitated into suspension by pumping water through the pedal aperture (Lopez & Holopainen, 1987). Colonisation of litterbags by abundant E. casertana, particularly in the last 6 weeks of our trial, suggests that Pisidium/Euglesa spp. may play an underappreciated role in assimilation of detrital carbon and nutrients into macrofaunal food webs within small lentic waterbodies. Alongside direct assimilation, suspension feeding by Pisidium/Euglesa spp. may increase detritus particle size via egestion of faecal pellets, enabling further uptake of detrital carbon and nutrients by collector-gatherers (Wallace & Webster, 1996). Without further study, it is difficult to determine the generalisability of these findings, however.
In general, additional research on aquatic plant decomposition is much needed, given the key role of autochthonous detrital pathways in energy and nutrient flow through many freshwater ecosystems (Cebrian & Lartigue, 2004; Bakker et al., 2016), and possible contributions to carbon burial (Taylor et al., 2019). To our knowledge, whilst the role of emergent plant invasions in altering litter supply has been highlighted in previous studies (Cuassolo et al., 2020; Dekanová et al., 2021), this field experiment represents a first attempt to assess the impacts of submerged plant invasion on detritivorous macroinvertebrates. These impacts are inevitably context- and taxon-specific, so it is difficult to draw any generalisations from this experiment. In future, general trends (and a predictive framework) might be elucidated via similar litter experiments using multiple alien macrophytes alongside a suite of native comparators, with trait information incorporated into analyses (Grutters et al., 2017). These could indicate, for instance, whether facilitation of non-native detritivores—as observed in our litter experiment—is a predictable consequence of submerged alien macrophyte invasion.
Conclusion
Our field experiment indicates that the impacts of C. helmsii may indeed be mediated by detritus. C. helmsii detritus is colonised by a taxonomically and functionally similar macroinvertebrate assemblage to native macrophyte detritus, but breaks down slower, with a lower rate of invertebrate-mediated decomposition. At later stages of decomposition, C. helmsii may support more abundant detritivores than native macrophytes. Where present in the species pool, C. helmsii may facilitate the invasive amphipod C. pseudogracilis. Given the dense stands typically formed by C. helmsii (Dawson & Warman, 1987), its perennial growth (Smith & Buckley, 2020) and the recalcitrance of its litter demonstrated here, C. helmsii is likely to produce copious, long-lasting detritus throughout the year, driving considerable impacts on the detritivore assemblage of invaded waterbodies, and consequently upon wider ecosystem structure and functioning. Parenthetically, the colonisation of our litter bags by abundant E. casertana may indicate an underappreciated contribution of pea clams (Pisidium/Euglesa spp.) to detritus processing within these small lentic waterbodies.
Data/code availability
The dataset and R script associated with this study are available from the University of Plymouth’s PEARL repository (https://pearl.plymouth.ac.uk).
References
Anderson, T. R., D. W. Pond & D. J. Mayor, 2017. The role of microbes in the nutrition of detritivorous invertebrates: a stoichiometric analysis. Frontiers in Microbiology 7: 2113. https://doi.org/10.3389/fmicb.2016.02113.
Bakker, E. S., K. A. Wood, J. F. Pagès, G. F. Veen, M. J. A. Christianen, L. Santamaría, B. A. Nolet & S. Hilt, 2016. Herbivory on freshwater and marine macrophytes: a review and perspective. Aquatic Botany 135: 18–36. https://doi.org/10.1016/j.aquabot.2016.04.008.
Bärlocher, F., 2005. Leaf Mass Loss Estimated by Litter Bag Technique. Methods to Study Litter Decomposition: A Practical Guide. Springer, Berlin: 37–42. https://doi.org/10.1007/1-4020-3466-0_6.
Bates, D., M. Mächler, B. M. Bolker & S. C. Walker, 2015. Fitting linear mixed-effects models using lme4. Journal of Statistical Software 67: 1–48. https://doi.org/10.18637/jss.v067.i01.
Bedford, A. P., 2004. A modified litter bag design for use in lentic habitats. Hydrobiologia 529: 187–193. https://doi.org/10.1007/S10750-004-6409-3.
Brochard, C., D. Groenendijk, E. van der Ploeg & T. Termaat, 2016. Fotogids Larvenhuidjes van Libellen. KNNV Uitgeverij, Zeist.
Carpenter, S. R. & M. S. Adams, 1979. Effects of nutrients and temperature on decomposition of Myriophyllum spicatum L. in a hard-water eutrophic lake. Limnology and Oceanography 24: 520–528. https://doi.org/10.4319/LO.1979.24.3.0520.
Carpenter, S. R. & D. M. Lodge, 1986. Effects of submersed macrophytes on ecosystem processes. Aquatic Botany 26: 341–370. https://doi.org/10.1016/0304-3770(86)90031-8.
Carvalho, C., L. U. Hepp, C. Palma-Silva & E. F. Albertoni, 2015. Decomposition of macrophytes in a shallow subtropical lake. Limnologica 53: 1–9. https://doi.org/10.1016/J.LIMNO.2015.04.003.
Cebrian, J. & J. Lartigue, 2004. Patterns of herbivory and decomposition in aquatic and terrestrial ecosystems. Ecological Monographs 74: 237–259. https://doi.org/10.1890/03-4019.
Chimney, M. J. & K. C. Pietro, 2006. Decomposition of macrophyte litter in a subtropical constructed wetland in south Florida (USA). Ecological Engineering 27: 301–321. https://doi.org/10.1016/j.ecoleng.2006.05.016.
Cuassolo, F., V. Díaz Villanueva & B. Modenutti, 2020. Litter decomposition of the invasive Potentilla anserina in an invaded and non-invaded freshwater environment of North Patagonia. Biological Invasions 22: 1055–1065. https://doi.org/10.1007/s10530-019-02155-x.
Cummins, K. W., R. C. Petersen, F. O. Howard, J. C. Wuycheck & V. I. Holt, 1973. The utilization of leaf litter by stream detritivores. Ecology 54: 336–345. https://doi.org/10.2307/1934341.
Davies, B., J. Biggs, P. Williams, M. Whitfield, P. Nicolet, D. Sear, S. Bray & S. Maund, 2008. Comparative biodiversity of aquatic habitats in the European agricultural landscape. Agriculture, Ecosystems and Environment 125: 1–8. https://doi.org/10.1016/j.agee.2007.10.006.
Dawson, F. H. & E. A. Warman, 1987. Crassula helmsii (T. Kirk) cockayne: is it an aggressive alien aquatic plant in Britain? Biological Conservation 42: 247–272. https://doi.org/10.1016/0006-3207(87)90071-1.
Dekanová, V., I. Svitková, M. Novikmec & M. Svitok, 2021. Litter breakdown of invasive alien plant species in a pond environment: rapid decomposition of Solidago canadensis may alter resource dynamics. Limnologica 90: 125911. https://doi.org/10.1016/J.LIMNO.2021.125911.
Dobson, M., S. Pawley, M. Fletcher & A. Powell, 2012. Guide to freshwater Invertebrates. Scientific Publication No 68. Freshwater Biological Association, Cumbria.
Edington, J. M. & A. G. Hildrew, 1995. Caseless Caddis Larvae of the British Isles. Freshwater Biological Association, Cumbria.
Elliott, J. M., U. H. Humpesch & T. T. Macan, 1988. Larvae of the British Ephemeroptera. Freshwater Biological Association, Cumbria.
Epstein, G., A. Foggo & D. A. Smale, 2019. Inconspicuous impacts: widespread marine invader causes subtle but significant changes in native macroalgal assemblages. Ecosphere 10: e02814. https://doi.org/10.1002/ECS2.2814.
Foster, G. N. & L. E. Friday, 2011. Keys to Adults of the Water Beetles of Britain and Ireland (Part 1). Field Studies Council for the Royal Entomological Society, Shrewsbury.
Foster, G. N., D. T. Bilton & L. E. Friday, 2014. Keys to Adults of the Water Beetles of Britain and Ireland (Part 2). Field Studies Council for the Royal Entomological Society, Shrewsbury.
Fox, J. & S. Weisberg, 2018. An R Companion to Applied Regression. SAGE Publications, Thousand Oaks.
Gessner, M. O., E. Chauvet & M. Dobson, 1999. A Perspective on leaf litter breakdown in streams. Oikos 85: 377. https://doi.org/10.2307/3546505.
Gessner, M. O., C. M. Swan, C. K. Dang, B. G. McKie, R. D. Bardgett, D. H. Wall & S. Hättenschwiler, 2010. Diversity meets decomposition. Trends in Ecology & Evolution 25: 372–380. https://doi.org/10.1016/J.TREE.2010.01.010.
Grutters, B. M. C., Y. O. A. Roijendijk, W. C. E. P. Verberk & E. S. Bakker, 2017. Plant traits and plant biogeography control the biotic resistance provided by generalist herbivores. Functional Ecology 31: 1184–1192. https://doi.org/10.1111/1365-2435.12835.
Guareschi, S., A. Laini, J. England, T. Johns, M. Winter & P. J. Wood, 2021. Invasive species influence macroinvertebrate biomonitoring tools and functional diversity in British rivers. Journal of Applied Ecology 58: 135–147. https://doi.org/10.1111/1365-2664.13795.
Hammond, C., R. Merritt & A. E. Gardner, 1985. The Dragonflies of Great Britain and Ireland. Revised Harley Books, Colchester.
Hanley, M. E., B. B. Lamont, M. M. Fairbanks & C. M. Rafferty, 2007. Plant structural traits and their role in anti-herbivore defence. Perspectives in Plant Ecology, Evolution and Systematics 8: 157–178. https://doi.org/10.1016/J.PPEES.2007.01.001.
Hussner, A., 2009. Growth and photosynthesis of four invasive aquatic plant species in Europe. Weed Research 49: 506–515. https://doi.org/10.1111/j.1365-3180.2009.00721.x.
IPBES, 2023. Thematic Assessment Report on Invasive Alien Species and their Control of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services. IPBES Secretariat, Bonn.
Jonsson, M. & B. Malmqvist, 2000. Ecosystem process rate increases with animal species richness: evidence from leaf-eating, aquatic insects. Oikos 89: 519–523. https://doi.org/10.1034/J.1600-0706.2000.890311.X.
Li, X., B. Cui, Q. Yang, H. Tian, Y. Lan, T. Wang & Z. Han, 2012. Detritus quality controls macrophyte decomposition under different nutrient concentrations in a eutrophic shallow lake, North China. PLoS ONE 7: 42042. https://doi.org/10.1371/JOURNAL.PONE.0042042.
Longhi, D., M. Bartoli & P. Viaroli, 2008. Decomposition of four macrophytes in wetland sediments: organic matter and nutrient decay and associated benthic processes. Aquatic Botany 89: 303–310. https://doi.org/10.1016/J.AQUABOT.2008.03.004.
Lopez, G. R. & I. J. Holopainen, 1987. Interstitial suspension feeding by Pisidium spp. (Pisidiidae: Bivalvia): a new guild in the lentic benthos? American Malacological Bulletin 5: 21–29.
Maggi, E., L. Benedetti-Cecchi, A. Castelli, E. Chatzinikolaou, T. P. Crowe, G. Ghedini, J. Kotta, D. A. Lyons, C. Ravaglioli, G. Rilov, L. Rindi & F. Bulleri, 2015. Ecological impacts of invading seaweeds: a meta-analysis of their effects at different trophic levels. Diversity and Distributions 21: 1–12. https://doi.org/10.1111/DDI.12264.
Maron, J. L. & M. Vilà, 2001. When do herbivores affect plant invasion? Evidence for the natural enemies and biotic resistance hypotheses. Oikos 95: 361–373. https://doi.org/10.1034/J.1600-0706.2001.950301.X.
Met Office, 2023. Historic station data—Camborne, Cornwall. Retrieved from https://www.metoffice.gov.uk/research/climate/maps-and-data/historic-station-data.
Moorhouse, T. & D. Macdonald, 2015. Are invasives worse in freshwater than terrestrial ecosystems? Wiley Interdisciplinary Reviews: Water 2: 1–8. https://doi.org/10.1002/wat2.1059.
Morrison, W. E. & M. E. Hay, 2011. Herbivore preference for native vs exotic plants: generalist herbivores from multiple continents prefer exotic plants that are evolutionarily Naïve. PLoS ONE 6: 17227. https://doi.org/10.1371/journal.pone.0017227.
NBN Trust, 2023. Callitriche stagnalis map on the NBN Atlas. Retrieved from https://species.nbnatlas.org/species/NBNSYS0000143455.
Newman, R. M., 1991. Herbivory and detritivory on freshwater macrophytes by invertebrates: a review. Journal of the North American Benthological Society 10: 89–114. https://doi.org/10.2307/1467571.
Nilsson, A. N., 1996. Aquatic Insects of North Europe: A Taxonomic Handbook. Apollo Book, Colchester.
Oksanen, J., G. Simpson, F. Blanchet, R. Kindt, P. Legendre, R. O’Hara, P. H. Solymos, M. Barbour, M. Chirico, M. R. De Caceres, M. Friendly, D. McGlinn, M. Ouellette, C. Ter Braak, & J. Weedon, 2022. vegan: Community Ecology. Retrieved from https://cran.r-project.org/web/packages/vegan
Oliveira, M. V. C., M. S. Dainez-Filho, A. P. S. Bertoncin, C. M. Muniz, T. Meurer, B. R. S. Figueiredo, S. M. Thomaz, S. L. Fávaro & R. P. Mormul, 2019. Native snails choose an invasive macrophyte over a native macrophyte as a food resource. Canadian Journal of Zoology 97: 362–367. https://doi.org/10.1139/cjz-2018-0116.
Parker, J. D. J. D. & M. E. M. E. Hay, 2005. Biotic resistance to plant invasions? Native herbivores prefer non-native plants. Ecology Letters 8: 959–967. https://doi.org/10.1111/j.1461-0248.2005.00799.x.
Petchey, O. L. & K. J. Gaston, 2006. Functional diversity: back to basics and looking forward. Ecology Letters 9: 741–758. https://doi.org/10.1111/J.1461-0248.2006.00924.X.
Petersen, R. C. & K. W. Cummins, 1974. Leaf processing in a woodland stream. Freshwater Biology 4: 343–368. https://doi.org/10.1111/j.1365-2427.1974.tb00103.x.
Pinheiro, J., D. Bates, & R Core Team, 2023. nlme: Linear and Nonlinear Mixed Effects Models. R package version 3.1-162. Retrieved from https://cran.r-project.org/package=nlme.
Pope, R. J., A. M. Gordon & N. K. Kaushik, 1999. Leaf litter colonization by invertebrates in the littoral zone of a small oligotrophic lake. Hydrobiologia 392: 99–112. https://doi.org/10.1023/A:1003537232319.
R Core Team, 2023. R: a Language and Environment for Statistical Computing. R Core Team, Vienna.
Rowson, B., H. Powell, M. Willing, M. Dobson & H. Shaw, 2021. Freshwater Snails of Britain and Ireland. Field Studies Council, National Museum of Wales, Shrewsbury.
Santonja, M., L. Pellan & C. Piscart, 2018. Macroinvertebrate identity mediates the effects of litter quality and microbial conditioning on leaf litter recycling in temperate streams. Ecology and Evolution 8: 2542. https://doi.org/10.1002/ECE3.3790.
Saulino, H. H. L., R. M. Thompson & S. Trivinho-Strxino, 2018. Herbivore functional traits and macroinvertebrate food webs have different responses to leaf chemical compounds of two macrophyte species in a tropical lake’s littoral zone. Aquatic Ecology 52: 165–176. https://doi.org/10.1007/s10452-018-9652-9.
Savage, A. A., 1989. Adults of the British Aquatic Hemiptera Heteroptera. Freshwater Biological Association, Cumbria.
Schmera, D., J. Heino, J. Podani, T. Erős & S. Dolédec, 2016. Functional diversity: a review of methodology and current knowledge in freshwater macroinvertebrate research. Hydrobiologia 787(1): 27–44. https://doi.org/10.1007/S10750-016-2974-5.
Schmidt-Kloiber, A. & D. Hering, 2015. www.freshwaterecology.info—an online tool that unifies, standardises and codifies more than 20,000 European freshwater organisms and their ecological preferences. Ecological Indicators 53: 271–282. https://doi.org/10.1016/J.ECOLIND.2015.02.007.
Seebens, H., T. M. Blackburn, E. E. Dyer, P. Genovesi, P. E. Hulme, J. M. Jeschke, S. Pagad, P. Pyšek, M. Winter, M. Arianoutsou, S. Bacher, B. Blasius, G. Brundu, C. Capinha, L. Celesti-Grapow, W. Dawson, S. Dullinger, N. Fuentes, H. Jäger, J. Kartesz, M. Kenis, H. Kreft, I. Kühn, B. Lenzner, A. Liebhold, A. Mosena, D. Moser, M. Nishino, D. Pearman, J. Pergl, W. Rabitsch, J. Rojas-Sandoval, A. Roques, S. Rorke, S. Rossinelli, H. E. Roy, R. Scalera, S. Schindler, K. Štajerová, B. Tokarska-Guzik, M. Van Kleunen, K. Walker, P. Weigelt, T. Yamanaka & F. Essl, 2017. No saturation in the accumulation of alien species worldwide. Nature Communications 8: 1–9. https://doi.org/10.1038/ncomms14435.
Shilla, D., T. Asaeda, T. Fujino & B. Sanderson, 2006. Decomposition of dominant submerged macrophytes: implications for nutrient release in Myall Lake, NSW, Australia. Wetlands Ecology and Management 14: 427–433. https://doi.org/10.1007/s11273-006-6294-9.
Shurin, J. B., D. S. Gruner & H. Hillebrand, 2005. All wet or dried up? Real differences between aquatic and terrestrial food webs. Proceedings of the Royal Society b: Biological Sciences 273: 1–9. https://doi.org/10.1098/RSPB.2005.3377.
Simberloff, D. & B. Von Holle, 1999. Positive interactions of nonindigenous species: invasional meltdown? Biological Invasions 1: 21–32. https://doi.org/10.1023/A:1010086329619.
Simberloff, D., I. M. Parker & P. N. Windle, 2005. Introduced species policy, management, and future research needs. Frontiers in Ecology and the Environment 3: 12–20. https://doi.org/10.1890/1540-9295(2005)003[0012:ispmaf]2.0.co;2.
Simberloff, D., J. L. Martin, P. Genovesi, V. Maris, D. A. Wardle, J. Aronson, F. Courchamp, B. Galil, E. García-Berthou, M. Pascal, P. Pyšek, R. Sousa, E. Tabacchi & M. Vilà, 2013. Impacts of biological invasions: what’s what and the way forward. Trends in Ecology and Evolution 28: 58–66. https://doi.org/10.1016/j.tree.2012.07.013.
Smallshire, D. & A. Swash, 2018. Britain’s Dragonflies. Princeton University Press, Princeton.
Smith, T. & P. Buckley, 2020. Biological flora of the British isles: Crassula helmsii. Journal of Ecology 108: 797–813. https://doi.org/10.1111/1365-2745.13336.
Tachet, H., M. Bournaud, P. Richoux & P. Usseglio-Polatera, 2010. Invertébrés d’eau douce—systématique, biologie, écologie. CNRS Editions, Paris.
Tasker, S. J. L., A. Foggo & D. T. Bilton, 2022. Quantifying the ecological impacts of alien aquatic macrophytes: a global meta-analysis of effects on fish, macroinvertebrate and macrophyte assemblages. Freshwater Biology 67: 1847–1860. https://doi.org/10.1111/FWB.13985.
Tasker, S. J. L., A. Foggo, K. Scheers, J. van der Loop, S. Giordano & D. T. Bilton, 2024. Nuanced impacts of the invasive aquatic plant Crassula helmsii on Northwest European freshwater macroinvertebrate assemblages. Science of the Total Environment 913: 169667. https://doi.org/10.1016/J.SCITOTENV.2023.169667.
Taylor, S., P. J. Gilbert, D. A. Cooke, M. E. Deary & M. J. Jeffries, 2019. High carbon burial rates by small ponds in the landscape. Frontiers in Ecology and the Environment 17: 25–31. https://doi.org/10.1002/FEE.1988.
Thioulouse, J., A. B. Dufour, T. Jombart, S. Dray, A. Siberchicot & S. Pavoine, 2018. Multivariate Analysis of Ecological Data with ade4. Multivariate Analysis of Ecological Data with ade4. Springer, New York. https://doi.org/10.1007/978-1-4939-8850-1.
Thornhill, I., N. Friberg, L. Batty, V. Thamia & M. E. Ledger, 2021. Leaf breakdown rates as a functional indicator were influenced by an invasive non-native invertebrate in urban ponds. Ecological Indicators 124: 107360. https://doi.org/10.1016/J.ECOLIND.2021.107360.
Tiegs, S. D., S. A. Entrekin, G. H. Reeves, D. Kuntzsch & R. W. Merritt, 2013. Litter decomposition, and associated invertebrate communities, in wetland ponds of the Copper River delta, Alaska (USA). Wetlands 33: 1151–1163. https://doi.org/10.1007/S13157-013-0470-5.
Venables, W. N. & B. D. Ripley, 2002. Modern Applied Statistics with S. Springer, New York. https://doi.org/10.1007/978-0-387-21706-2.
Vilà, M., J. L. Espinar, M. Hejda, P. E. Hulme, V. Jarošík, J. L. Maron, J. Pergl, U. Schaffner, Y. Sun & P. Pyšek, 2011. Ecological impacts of invasive alien plants: a meta-analysis of their effects on species, communities and ecosystems. Ecology Letters 14: 702–708. https://doi.org/10.1111/j.1461-0248.2011.01628.x.
Wallace, J. B. & J. R. Webster, 1996. The role of macroinvertebrates in stream ecosystem function. Annual Review of Entomology 41: 115–139. https://doi.org/10.1146/annurev.en.41.010196.000555.
Wallace, I. D., B. Wallace & G. N. Philipson, 1990. A Key to the Case-Bearing Caddis Larvae of Britain and Ireland. Freshwater Biological Association, Cumbria.
Webster, J. R. & E. F. Benfield, 1986. Vascular plant breakdown in freshwater ecosystems. Annual Review of Ecology and Systematics 17: 567–594. https://doi.org/10.1146/annurev.es.17.110186.003031.
Acknowledgements
This research was conducted whilst in receipt of a PhD studentship funded by the University of Plymouth. We are grateful to Bruce Wotton McTurk and Alma Hathway for providing access to Caer Bran nature reserve. We also thank Alex Fraser for constructing the litterbags used in this trial.
Funding
Funding was provided by Plymouth University.
Author information
Authors and Affiliations
Contributions
Samuel Tasker contributed towards conceptualisation, methodology, investigation, data curation, formal analysis, visualisation, writing—original draft, and writing—review & editing. Andrew Foggo contributed towards methodology, formal analysis (supporting), writing—review & editing, and supervision. David Bilton contributed towards conceptualisation, methodology, writing—review & editing, supervision, and funding acquisition.
Corresponding author
Ethics declarations
Competing interests
This research was conducted whilst in receipt of a PhD studentship funded by the University of Plymouth. The authors have no relevant financial or non-financial interests to declare.
Ethical approval
No approval of research ethics committees was required to accomplish the goals of this study because experimental work was conducted with unregulated invertebrate species.
Additional information
Handling editor: Margarita Patricia Florencio Díaz
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tasker, S.J.L., Foggo, A. & Bilton, D.T. Are impacts of the invasive alien plant Crassula helmsii mediated by detritus? A litter experiment in a temperate pond. Hydrobiologia 851, 4135–4148 (2024). https://doi.org/10.1007/s10750-024-05571-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-024-05571-w




 ) and Callitriche stagnalis (
) and Callitriche stagnalis ( ) over 83 days. Dotted bars = fine mesh; dashed bars = coarse mesh
) over 83 days. Dotted bars = fine mesh; dashed bars = coarse mesh
 ) and Callitriche stagnalis (
) and Callitriche stagnalis ( ) litter over 83 days in fine mesh bags. Error bars (SE) too small to be visible, so omitted
) litter over 83 days in fine mesh bags. Error bars (SE) too small to be visible, so omitted
