Abstract
Dissolved oxygen (DO) level at the sediment–water interface is one key factor controlling redox-sensitive processes, such as nutrient cycling. Microcosm experiments with sediment collected from three reservoirs were performed to quantify the influences of water column oxygenation (oxic, anoxic, oxygen fluctuation), sediment characteristics (grain size distribution, total nitrogen and total phosphorus contents, microbial activities), and their interactions on nutrient fluxes from sediments to the water column. Algal growth bioassays were also performed using water from the microcosms to determine which conditions produced the most favorable growth conditions. Anoxic conditions increased the release of dissolved inorganic nitrogen (DIN), mainly as ammonium and phosphates, compared to the other DO conditions. Such effects were likely due to an inhibition of the nitrification–denitrification coupling process for DIN and a reductive dissolution of Fe (III) oxides for phosphates. Following this increased nutrient availability, algal growth in the bioassays was the highest in water collected from microcosms exposed to anoxic conditions. Under both oxic and anoxic conditions, the percentage of fine sediment particles led to decreasing DIN and phosphates fluxes by reducing the nutrient diffusion rate from sediments to the water column. Finally, both DO and sediment grain size controlled the contribution of sediments to reservoir eutrophication.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Excessive nutrient [nitrogen (N) and phosphorus (P)] loading in lakes and reservoirs classically results in the eutrophication of the water column and associated environmental problems, such as the excessive growth of toxin-producing cyanobacteria, oxygen depletion of the water column, or biodiversity loss (see Amorim & do Nascimento Moura, 2021 for a review). The nutrient enrichment of the water column is associated with external inputs (tributaries, soil run-off, groundwater supplies, atmospheric deposition) and sediment internal loadings (e.g., Meinikmann et al., 2015; Yang et al., 2020). Much effort has been made to reduce the external inputs of N and P to improve the water quality of eutrophic lakes and reservoirs, but this strategy is often not efficient enough when there is also sediment internal loading (Forsberg, 1989; Welch & Denniscooke, 2005; Nürnberg et al., 2012; Watson et al., 2016). For example, it has been shown for P (e.g., Søndergaard et al., 2003) and N (e.g., Jeppesen et al., 2005) that internal sources associated with a massive accumulation of nutrients in sediments can maintain eutrophication for more than 10 years after the reduction of external inputs. Assessing the internal nutrient loading is a main issue to mitigate eutrophication problems and efficiently restore the water quality of eutrophic water bodies (e.g., Li et al., 2023).
Dissolved oxygen (DO) concentration in the water column has been recognized as the main factor influencing the dynamics of dissolved inorganic nitrogen (DIN) and P at the sediment–water interface-SWI- (Boström et al., 1988; Moore et al., 1992; Wetzel, 2001; Beutel, 2006; Ekeroth et al., 2016; Rapin et al., 2019). Oxic/anoxic fluctuations may strongly influence N availability in the water column. For example, N–NH4+ produced by organic matter (OM) mineralization in sediments was nitrified into N–NO3− when DO was available to microorganisms, whereas N–NH4+ accumulated under anoxic conditions (Gautreau et al., 2020). Therefore, fluxes of inorganic N from the sediment to the water column were expected to be dominated by N–NO3− fluxes in oxic conditions and by N–NH4+ in anoxic conditions. Anoxic conditions do not allow nitrification and then nitrification–denitrification coupling that would favor N loss from the ecosystem (i.e., transformation of NO3− to gaseous N2). Consequently, anoxic conditions would limit N loss, favoring the accumulation of DIN in the water column of reservoir and lakes.
The reductive dissolution and oxidative precipitation of Fe/Mn oxyhydroxides are considered as essential to control P mobility at the oxic/anoxic interface (Rydin, 2000). Oxic conditions favor the formation of insoluble Fe(III) and Mn(IV) oxides with high PO43− sorption capacity and limited P availability in the water column (Bortleson & Lee, 1974; Giles et al., 2016). At the opposite, the dissolution of Fe/Mn oxyhydroxides under anoxic conditions induces the release of PO43− at the SWI (Søndergaard et al., 2003). Moreover, it has been reported that Fe content largely controlled P mobility at the sediment–water interface of lakes and reservoirs (e.g., Jensen et al., 1992; Beutel et al., 2008).
Factors other than oxic–anoxic conditions at the SWI may modulate the role of internal nutrient loadings on water column eutrophication (Rapin et al., 2019). First, molecular diffusion is classically considered as the main mechanism influencing nutrient fluxes from sediments to the water column (Berner, 1980; Anschutz et al., 2012). Sediment characteristics, such as particle size distribution, that determine efficient porosity are major for diffusion rates of dissolved solutes and nutrients from sediments to the water column according to the Fick’s First law (Lavery et al., 2001). Second, the contents and forms of nutrients accumulated in sediments determine their ability to be released to the water column (Ruban et al., 1999; Huang et al., 2018). For example, by comparing P release rates from sediments collected in four sites of Mona Lake, Steinman et al. (2009) showed that the sediment characterized by the highest P content produced the highest P release rates to the water column under oxic conditions. The mineralization of OM by microbial activities also contributes to the recycling of P and N accumulated under organic forms in sediments (Hupfer & Lewandowski, 2008). Therefore, both nutrient stocks and microbial activities in sediments would contribute to the production of DIN and PO43− that could be released to the water column (Rydin, 2000; Joshi et al., 2015).
The aim of this study was to quantify the influence of DO conditions on nutrient fluxes at the SWI in interaction with sediment characteristics (e.g., grain size distribution, concentrations of total N- TN- and total P-TP-, and microbial activities). A factorial approach using three DO conditions (oxic, anoxic, daily DO fluctuations) and sediments collected from three reservoirs that varied in grain size distribution, TN content, TP content, and microbial activities (Gautreau et al., 2023) was used to evaluate how sediment characteristics and DO may interact to determine fluxes of DIN and PO43− at the SWI and the resulting influence on algal growth. Nutrient fluxes were quantified using laboratory microcosms and their effects on algal growth were assessed using bioassays on water collected from the microcosms (as in Cymbola et al., 2008). As previously measured with one of the three reservoir sediments selected for the present study (Puyvalador sediments, Gautreau et al., 2020), anoxic conditions at the SWI were expected to increase NH4+ and PO43− releases in the water column in comparison with other DO conditions in all tested sediments. DIN concentrations (mainly as NH4+) should be the highest under anoxic conditions because nitrification of NH4+ into NO3− should be reduced/inhibited under low DO availability (Gilbert et al., 2016) compared to oxic and DO fluctuation conditions, which would favor NO3− releases from sediments. Under daily fluctuations of DO, nitrification was expected to start rapidly after oxygen re-introduction (Pett-Ridge et al., 2013) and denitrification should be stimulated during low oxygenated conditions, reducing the accumulation of DIN in the water column in comparison with the other treatments. Algal growth should be the highest in water collected from microcosms under anoxic conditions, because this DO treatment was expected to produce the highest nutrient enrichments of the water column.
Sediment characteristics were also expected to mediate DIN and PO43− at the SWI. First, the release of DIN and PO43− would be higher in coarse than in fine-grained sediments because the percentage of clay and silt (< 63 µm) would reduce the efficient porosity and then the diffusion of solutes and dissolved nutrients from sediments to water column (Huettel & Webster, 2001). Second, N content, P content, OM, and associated microbial activities were expected to increase DIN and PO43− releases in the interstitial water and subsequent releases to the water column (e.g., Kristensen, 2000; Glud, 2008). Highest DIN and PO43− fluxes were expected from sediments characterized by the highest nutrient contents and microbial activity.
Materials and methods
Sediment collection and preparation
Studied sediments were collected from Puyvalador (42°38′47.4″N, 2°07′28.9″E), Villerest (45°59′07.1″N, 4°02′44.3″E), and Grangent (45°27′58.2″N, 4°14′56.5″E) reservoirs to obtain contrasted sediment grain sizes, TN contents, TP contents, and microbial activities (as measured in Gautreau et al., 2023). For each reservoir, 15 l of sediments were collected with an Ekman grab and frozen (− 18°C) for several weeks to kill the ambient fauna that could influence nutrient fluxes by excretion and bioturbation (e.g., Fukuhara & Sakamoto, 1987). As freezing could alter microbial communities in sediments (Tolhurst et al., 2012), a subsample of fresh sediment was kept at 15°C to preserve the microbial activity and to be used as an inoculum for the experiment. This subsample was sieved at 80 μm to remove the ambient fauna and thoroughly mixed with defrosted sediments for microbial colonization for 15 days before microcosm preparation. This incubation time of 15 days followed by a stabilization period of 14 days in microcosms (see below and Fig. 1) was long enough to recover and stabilize sediment oxygen uptake rates (see Fig. 4 in Gautreau et al., 2020).
Microcosms and experimental set-up
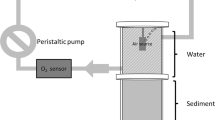
The experiment was performed in 36 microcosms consisting in Plexiglas cylinders with an internal diameter of 10 cm and a height of 20 cm (Fig. 1). For each reservoir sediment, 12 microcosms were filled with a layer of sediments (10 cm) overlaid with synthetic water (10 cm) [composition of synthetic water: 96-mg l−1 NaHCO3; 39.4-mg l−1 CaSO4·2H2O; 60-mg l−1 MgSO4·7H2O; 4-mg l−1 KCl; pH 7.5; (US EPA, 1991)] in a 15°C temperature-controlled room. Oxic conditions were maintained in the water column by bubbling water with atmospheric air. Microcosms were equipped with peristaltic pumps for a continuous recirculation of the water column at a gentle flow rate of 6 mL min−1 to avoid DO stratification but also sediment re-suspension due to a gentle flow rate. Oxygen probes were placed on the recirculation system of peristatic pumps to monitor DO concentrations in the water column of the microcosms (Fig. 1A).
After a 14-day period of stabilization under oxic conditions, the water column of each microcosm was gently removed and renewed, with minimum disturbance of sediment, by synthetic water to eliminate the initial flush of nutrients due to sediment manipulation. At the same time, 9 microcosms (3 microcosms per reservoir sediments) were used to determine initial sediment characteristics (Fig. 1B). The other 27 microcosms (9 microcosms per reservoir sediment) were used to quantify how DO concentrations and dynamics in the water column interacted with sediment characteristics to influence nutrient fluxes from the sediment to the water column. For each reservoir sediment, three DO treatments were applied in the water column of microcosms (3 microcosms per sediment, Fig. 1B): (1) oxic treatment with the water column saturated in DO, (2) DO fluctuation treatment with the water column of the microcosms experiencing DO supply followed by DO decrease every 12 h (aeration turn on and off every 12 h, according to Gautreau et al. 2020), and (3) anoxic treatment with a non-aerated water column experiencing anoxic conditions.
For the nine microcosms dedicated to the determination of initial conditions before the application of DO treatments (Fig. 1B), incubations were performed to evaluate initial oxygen uptake rates. Afterward, the central area of the nine microcosms were cored with cut-off syringes (i.e., 1 cm in diameter, 9 cm in height) and analyzed for measuring sediment grain size distributions, total organic carbon (TOC), total nitrogen (TN), and total phosphorus (TP) contents. As P binding in ferric (oxi)hydroxides play a key role in PO43− dynamics in sediments (Jensen et al., 1992), iron (Fe) content was also measured for each reservoir sediment. For the 27 microcosms used to evaluate the influences of DO conditions, sediment characteristics, and their interactions on nutrient dynamics, dissolved nutrients (NH4+, NO3−, NO2−, and PO43−) were measured at 7 times during the 15 days of the experiment: on day 0 (just before DO treatment application) and on days 3, 6, 8, 10, 13, and 15 after treatment application. At each sampling date, 30 ml of water was collected from the top of the water column, filtered through GF/F filters (0.7 µm, Whatman) and kept at 4°C until chemical analyses. During the experiment, the sampled volume of water for analyses (30 ml) was replaced with 30 ml of synthetic water to keep a constant volume of water column in each experiment unit. To prevent the oxygenation of anoxic microcosms during sampling, the water collected from the microcosms was replaced with synthetic water without introducing air into the microcosms. On day 15, the water column from each of the 27 microcosms was collected, filtered through GF/F filters (0.7 µm, Whatman) and kept at 4°C. This water served later as the medium for the algal growth bioassays (Fig. 1B).
Methods of analyses
Physical and chemical analyses on sediment samples
Grain size distribution was determined by laser diffractometry (Malvern Mastersizer 2000G, Malvern Products) on fresh sediments obtained by coring on day 0. The percentage of fine particles (i.e., silt and clay, < 63 μm) was calculated using package rysgran v2.1.0 (Ronael Gilbert et al., 2014) in R software v3.3.2. Sediment samples were dried (at 60°C for 72 h), ground in a mortar, and analyzed to determine their TOC, TN, and TP contents (in % per sediment dry mass) of TOC, TN, and TP. TOC and TN contents were determined by high-temperature in situ combustion on pre-acidified (i.e., HCL 2 mol l−1) dry samples using an elemental analyzer (FlashEA, Thermo Electron Corporation). The method of Murphy & Riley (1962) was used to determine TP. For each reservoir sediment, Fe content was measured per sediment dry mass by Inductively Coupled Plasma-Atomic Emission Spectroscopy (ICP-AES) after sediment mineralization with nitric acid and hydrochloric acid.
Oxygen uptake rates
Before core collections, measures of dissolved oxygen concentrations were made on the 9 microcosms (3 microcosms per sediment) used to assess sediment characteristics on day 0 of the experiment (Fig. 1B). These measurements were performed to evaluate oxygen uptakes from the 3 sediments. According to Gautreau et al. (2020), aeration of the water column of the microcosms was stopped and each unit was sealed to avoid contact with the atmosphere. Using the water recirculation system equipped with an oxygen probe (Fig. 1A) connected to a Pocket Oxygen Meter FireStingGO2 (Pyroscience GmbH), decrease in DO concentration was measured every hour for an incubation of 4 h in each microcosm. DO uptake rates in the microcosms were calculated from the linear decrease of DO over time and expressed as μmol of O2 consumed per hour and m2 of water–sediment interface.
Nutrient analyses on water samples and calculation of nutrient release rates
For all filtered water samples collected at 7 times during the experiment, N–NH4+, N–NO2−, N–NO3−, and P–PO43− concentrations were determined by standard colorimetric methods (Grasshoff et al., 1983) using a sequential analyzer (SmartChem200, AMS Alliance). Thereafter, nutrients were presented as DIN, N–NH4+ and N–NO3− (where N–NO3− corresponded to N–NO3− + N–NO2− because N–NO3− concentrations ≫ N–NO2− concentrations). For each microcosm and nutrient, release rate from sediment to the water column was estimated from the changes in nutrient concentration between the start (day 0) and the end (day 15) of the experiment. Nutrient release rates (mg day−1 m−2) are calculated using the following formula (Steinman et al., 2004):
where,
Cday 0 and Cday 15 are nutrient concentrations (mg l−1) in the water column at day 0 and day 15, respectively;
V is the volume of the water column in microcosms (0.785 l);
A is the sediment surface area in the microcosms (0.00785 m2);
15 is the time interval in days between day 0 and day 15.
Due to an analytical problem for the measurement of P–PO43− concentrations on day 15 in microcosms with Puyvalador sediments and experiencing anoxic conditions, PO43− release rates were calculated for these microcosms with PO43− concentrations measured on day 13 (i.e., Cday 15 was replaced by Cday 13 and the time interval of 15 was replaced by 13 in the formula).
Algal growth bioassays
According to the approach developed by Cymbola et al. (2008), algal bioassays were performed to evaluate the ability of the filtered water collected from each microcosm on day 15 (Fig. 1B) to sustain phytoplanktonic growth. The planktonic unicellular green alga Raphidocelis subcapitata (Korshikov) Nygaard et al., formerly known as Pseudokirchneriella subcapitata (Korshikov) Hindak and Selenastrum capricornutum Printz, was used for these bioassays due to its higher growth rates and greater sensitivity to various substances than other algae (e.g., Prata et al., 2022). This ubiquitous freshwater planktonic species was chosen because it is readily available from culture collections and easy to maintain under laboratory-controlled conditions (e.g., Venãncio et al., 2017; Suzuki et al., 2018). R. subcapitata specimens were acquired from the Culture Collection of the French National Natural History Museum and maintained in batch cultures in Chu growth medium (composition of Chu #10 medium: 40-mg l−1 Ca(NO3)2; 5-mg l−1 K2HPO4; 25-mg l−1 MgSO4∙7H2O; 20-mg l−1 Na2CO3; 25-mg l−1 Na2SiO3; 0.8-mg l−1 FeCl3 (Chu, 1942; Andersen, 2005). Cultures were maintained in a temperature-controlled room at 21°C with artificial day/night cycle of 16 h/8 h (60-µmol photons−1 m−2 s−1) and were continuously agitated at a speed sufficient to prevent sedimentation and cell aggregation. Algae were sub-cultured in Chu medium weekly for several weeks with a starter culture of 106 cell ml−1 to ensure a healthy and reproducible culture and maintain a supply of cells in the exponential growth phase.
Growth bioassays were performed in cotton-stoppered Nalgene Erlenmeyer flasks of 120 ml with 30 ml of medium and an inoculum of 106 cell ml−1 of algae harvested in culture medium on day 5 (i.e., during the exponential growth phase, Moreira-Santos et al., 2004). Bioassays were performed under the same conditions (light, temperature, and shaking) as cultures. A total of 30 growth tests were set up to measure the ability of surface waters collected in the 27 microcosms on day 15 (Fig. 1B) to promote algal growth in comparison with 3 positive control replicates that contained Chu medium with the algae R. subcapitata. The aim of this test was to evaluate which experimental treatments (9 treatments: 3 sediments * 3 oxygen conditions) could favor eutrophication in the water column. Based on preliminary tests (data not shown), a duration of 96 h commonly used for this type of bioassay (e.g., Ma et al., 2006; Paquet et al., 2019; Gebara et al., 2020) was selected to observe potential differences in algal growth rates among experimental treatments. Thus, after 96 h, samples were collected and fixed with glutaraldehyde (0.25% final concentration) and Pluronic® F- 68 0.01% final concentration). Algal cell concentrations were then determined by flow cytometry using an Attune NxT cytometer (Thermo Fisher) equipped with an air-cooled argon laser (488 nm, 50 mW). To optimize the individual counting of algal cells, samples were diluted (1/10000) in focusing fluid and analyses were performed at low flow speed (12.5 µl min−1). Algal populations were distinguished from cytometric and sample noises using light scattering (SSC) and red fluorescence from chlorophyll-a (550–735 nm, BL3). Algal concentrations measured in the water columns of microcosms were expressed in relationship with algal concentrations measured in the Chu medium used for algal cultivation to determine the significance of algal growth in the water columns compared to the culture medium. Therefore, proportions of algae concentration in the water column for each of the 27 microcosms (i.e., 3 sediments * 3 oxygen conditions * 3 replicates) over average algae concentration in positive control medium (i.e., 3 replicates with Chu medium) measured after 96 h of algal growth in bioassays were calculated.
Data analysis
The initial sediment characteristics (volumetric percentages of particles < 63 µm, TOC, TN, TP, and Fe contents, DO uptake rates) were compared among the three sediments using a 1-way ANOVAs. Tukey’s post hoc tests were performed when significant ANOVA results were obtained. For chemical (NH4+, NO3−, DIN, and PO43−) variables measured at different times, the effects of DO and sediments were tested by 2-way RM ANOVAs with time (days 3, 6, 10, 13, and 15) as the repeated factor and DO treatment (i.e., oxic, DO fluctuation and anoxic conditions) and sediment treatment (i.e., Puyvalador, Villerest, and Grangent sediments) as the fixed factors. For nutrient release rates, we performed 2-way ANOVA with DO treatment and sediment treatment as the fixed factors. Tukey’s post hoc tests were performed when significant ANOVA results were obtained. For all variables and before statistical analyses, the normality and homoscedasticity of the residues were verified using the Shapiro–Wilk’s test and the Bartlett’s test, respectively. Sphericity assumption for RM ANOVA was verified using Mauchly’s sphericity test.
For the proportions of algal concentrations obtained from algal growth bioassays, the effects of DO treatment (i.e., oxic, DO fluctuation and anoxic conditions) and sediment treatment (i.e., Puyvalador, Villerest, and Grangent sediments) were tested using a 2-way ANOVAs. Before analysis, proportions were logit transformed to normalize data (verified by the Shapiro–Wilk’s test). The homoscedasticity of the residues was also verified using the Bartlett’s test. When 2-way ANOVAs were significant (i.e., P < 0.05), Tukey’s post hoc tests were performed to determine which treatments (“oxygen * sediment” treatments) differed.
In addition, we used generalized linear models to evaluate the influence of sediment characteristics on DIN and P–PO43− release rates from sediment to the water column for oxic and anoxic conditions. Each model (one per released nutrient and DO treatment) focused on three factors that represented (1) the loading of the studied nutrients in sediments (TN for evaluating DIN released to the water column or TP for evaluating PO43− released to the water column), (2) the microbial activity in sediments (total oxygen uptake rate), and (3) the proportion of fine sediments (clay and silts < 63 µm). We checked for multi-collinearity among the three variables (TN or TP, total oxygen uptake rate, and the proportion of fine particles in sediments) using variance inflation factors (VIF, Zuur et al., 2010) and found them to be in acceptable range (all VIF < 12). As the three factors could influence release rates of nutrients, we performed a procedure of model selection based on information theory (Burnham & Anderson, 2002) and the ranking of models according to Akaike’s Information Criterion corrected for small sample size (AICc, “car” R package), starting from the most comprehensive model (including all factors). The adjusted McFadden’s pseudo-R2 (McFadden, 1974) was computed for all tested models to provide for a comparable estimation of the amount of explained variance among models with different number of parameters. All statistical analyses were done with the R v.3.3.2 software.
Results
Initial characteristics of the reservoir sediments
As expected, the sediments from the three reservoirs were characterized by different grain size distributions, chemical composition and microbial activities (Table 1). More precisely, the volumetric percentages of fine particles (i.e., silt and clay < 63 µm) in sediments were significantly different among reservoirs with percentages varying from 75.7% for Villerest sediments to 82.4% for Puyvalador sediments, respectively (1-way ANOVA, P < 0.001, Table 1). Moreover, Puyvalador sediments presented a unimodal distribution with a peak around 20 µm (Fig. 2A). Sediments of Villerest also presented a unimodal distribution with a peak around 20 µm but the distribution of sediment grain sizes around the mean was more dispersed than sediments of Puyvalador (Fig. 2B). Grangent sediments had a more heterogeneous distribution with two main modes: a first mode around 20 µm and a second mode around 300 µm (Fig. 2C).
TOC, TN, and TP contents were significantly different among sediments (Table 1, 1-way ANOVAs, P value < 0.05). For TOC and TN, an increasing gradient was observed from Grangent sediment (4.47% for TOC and 0.61% for TN) to Villerest sediment (5.90% for TOC and 0.76% for TN) with intermediate values for Puyvalador sediment (5.65% for TOC and 0.66% for TN). TP contents were significantly higher in Grangent (1.260 mg g−1 dry sediment) than in Puyvalador (0.896 µg g−1 dry sediment) sediments (Tukey’s post-hoc test, P < 0.05). Villerest sediments had TP concentrations that were not significantly different from those measured in the two other reservoir sediments (Table 1). Concentrations in Fe were significantly higher in Grangent sediments than in Puyvalador and Villerest sediments (Tukey’s post hoc tests, P < 0.05, Table 1). No significant difference in Fe concentration was found between Puyvalador and Villerest sediments (Tukey’s post hoc test, P > 0.05, Table 1). Microbial DO uptake rates were significantly contrasted among the three reservoir sediments (1-way ANOVAs, P value < 0.01, Table 1) with values increasing from Puyvalador and Villerest to Grangent sediments.
DO concentrations in the water column of the microcosms
The experimental design allowed us to generate contrasting DO conditions in the water column depending on the treatment. DO concentrations were maintained between 7 and 9 mg l−1 in the water column for the oxic treatment and were below 0.05 mg l−1 for the anoxic treatment during the whole experiment in all tested sediments. In the DO fluctuation treatment, daily variations of DO concentrations differed among sediment treatments with values comprised between 7 and 9 mg l−1 at the end of the aeration period and between 0.4 and 5 mg l−1 at the end of the no-oxygen supply period (Supplementary Material 1). After 12 h without oxygen supply, the water column of microcosms experienced DO concentrations of 0.44 mg l−1, 1.11 mg l−1, and 5 mg l−1 for Grangent, Villerest, and Puyvalador sediment treatments, respectively.
Influences of DO and sediment treatments on DIN dynamics
Under oxic and DO fluctuation conditions, N–NH4+ and N–NO3− concentrations in the water column of all microcosms varied during the first days of the experiment to reach relatively stable values after 8 days of experiment (Fig. 3, 2-way RM ANOVAs, “time” effect, P < 0.001 for N–NH4+ and N–NO3−, Supplementary Material 2 for detailed statistical results). Under anoxic conditions, N–NH4+ concentrations continuously increased during the experiment in all sediment treatments (Fig. 3). The DO treatment had a significant influence on the main forms of DIN measured in the water column (2-way RM ANOVAs, “DO” treatment, P < 0.001 for N–NH4+ and N–NO3−): N–NO3− was the dominant form in the microcosms experiencing the oxic treatment, whereas N–NH4+ was the dominant form in the microcosms under anoxic conditions (Fig. 3). The effects of the DO fluctuation treatment on DIN dynamics depended on the sediment treatment (2-way RM ANOVAs, interaction between “DO” and “sediment” treatments, P < 0.001 for DIN). N–NH4+ and N–NO3− concentrations in microcosms exposed to the DO fluctuation treatment were comparable to those measured in microcosms experiencing the oxic treatment with Puyvalador and Villerest sediments (Fig. 3). In contrast, N–NO3− concentrations measured in microcosms with Grangent sediments were significantly lower under the DO fluctuation conditions (0.81 mg l−1 on day 15) than under oxic conditions (3.7 mg l−1 on day 15). Grangent sediments produced the lowest DO concentrations (0.44 mg l−1) among the three sediments after stopping the aeration of the water column during 12 h under the DO fluctuation treatment (Supplementary Material 1). DIN enrichment in the water column of microcosms was significantly influenced by DO treatments (2-way RM ANOVAs, “DO” treatment, P < 0.001 for DIN). Higher concentrations of DIN were measured in the water column of microcosms under anoxic conditions than under oxic or DO fluctuation conditions for all tested sediments (Fig. 3). DIN concentrations measured on day 15 under anoxic conditions were 5.5-fold, 2.3-fold, and 1.9-fold higher compared to the oxic conditions for Puyvalador, Grangent and Villerest, sediments, respectively.
Concentration of N-NH4+ (A, B, C), N–NO3− as sum of N–NO3− and N–NO2− concentrations (D, E, F), and DIN (N–NH4+ + N–NO2− + N–NO3−) (G, H, I) in the water column during the experimentations for oxic, DO fluctuation and anoxic treatments of Puyvalador, Villerest, and Grangent sediments (mean ± standard error, N = 3)
For all DO treatments, microcosms filled with Villerest sediments exhibited significantly higher DIN in the water column than the two other sediments at the end of the experiment (2-way RM ANOVAs, “sediment” treatment, P < 0.001). For example, under oxic conditions, N–NO3− concentrations measured in the water column on day 15 were around two-fold and seven-fold higher in Villerest treatment (7.2 mg l−1) compared to Grangent (3.7 mg l−1) and Puyvalador (1.1 mg l−1) treatments, respectively (Fig. 3). In contrast, Puyvalador sediments produced the lowest concentrations of N–NO3− under oxic conditions and concentrations of N–NH4+ under anoxic conditions (Fig. 3). Consequently, DIN concentrations in the water column under both oxic and anoxic conditions increased from Puyvalador to Grangent and Villerest sediments (2-way RM ANOVAs, “sediment” treatment, P < 0.001 for DIN). No significant difference in DIN concentration was detected between Puyvalador (1.58 mg l−1) and Grangent (1.47 mg l−1) treatments under the DO fluctuation treatment (Tukey post hoc test, P > 0.05).
Calculations of DIN fluxes showed that N–NH4+ release rates were positive only in microcosms under anoxic conditions (Fig. 4A, 2-way ANOVA, “DO” treatment, P < 0.001). N–NH4+ release rates were significantly lower in Puyvalador microcosms (mean value: 3.98 mg day−1 m−2) than in Villerest (mean value: 8.38 mg day−1 m−2) or Grangent (mean value: 5.99 mg day−1 m−2) microcosms (2-way ANOVA, “sediment” treatment, P < 0.001). In contrast, only oxic and DO fluctuation conditions produced significant releases of N–NO3− into the water column (Fig. 4B, 2-way ANOVA, “DO” treatment, P < 0.001). Oxic and DO fluctuation treatments produced comparable N–NO3− release rates for Puyvalador and Villerest sediments, but N–NO3− release rate was lower under DO fluctuation conditions (0.40 mg day−1 m−2) than under oxic conditions (2.57 mg day−1 m−2) for microcosms filled with Grangent sediments (2-way ANOVA, interaction between “DO” and “sediment” treatments, P < 0.001). Significant differences in DIN release rates were found among sediment and DO treatments (Fig. 4C, 2-way ANOVA, interaction between “DO” and “sediment” treatments, P < 0.05). Release rates of DIN in microcosms filled with Villerest sediments were significantly higher for all DO conditions than release rates measured in microcosms with Puyvalador and Grangent sediments (Fig. 4C). Microcosms filled with Grangent sediments presented intermediate release rates of DIN between microcosms filled with Villerest and Puyvalador sediments for all DO treatments (Fig. 4C).
Release rates of N–NH4+ (A), N–NOx− (B), DIN (C), and P–PO43− (D) from the sediment to the water column for the three oxygen and sediment treatments (mean ± standard error, N = 3). For each panel, different symbols (#, § and ¤) show significant differences among treatments (see axis label, Tukey post hoc test, p < 0.05). Release rate of P-PO43− in the anoxic treatment was calculated at day 13 for Puyvalador under anoxic conditions due to an analytical problem for this measurement at day 15
Influences of DO and sediment treatments on P–PO 4 3− dynamics
P–PO43− concentration in the water column significantly varied during the course of the experiment in all conditions (2-way RM ANOVA, “time” effect, P < 0.001, Supplementary Material 2 for detailed statistical results) but in the oxic and DO fluctuation treatments they remained lower than 100 µg l−1 throughout the experiment (Fig. 5AB). In contrast, P–PO43− concentration in the water column of the anoxic treatment continuously increased to more than 1 mg l−1 at the end of the experiment (Fig. 5C). Anoxic conditions significantly increased P–PO43− concentrations in the water column in comparison with the other oxygen conditions (2-way RM ANOVA, “DO” treatment, P < 0.001). In the presence of Villerest sediments, P–PO43− concentrations reached 3.66 mg l−1 on day 15 in the anoxic treatment, which were higher by 66-fold (55.4 µg l−1) and 100-fold (36.2 µg l−1) compared to the oxic and DO fluctuation treatments, respectively. Significant differences among sediment treatments were also observed (2-way RM ANOVA, “sediment” treatment, P < 0.001) with Villerest sediments producing higher P–PO43− concentrations in the water column under anoxic conditions than the two other sediments (Fig. 5C). For example, P–PO43− concentrations measured on day 13 under anoxic conditions in microcosms with Villerest sediments (3.24 mg l−1) were more than 2.5-fold higher than those measured with Puyvalador (1.03 mg l−1) and Grangent sediments (1.17 mg l−1). Consequently, calculated release rates of P–PO43− were the highest in anoxic conditions, compared with the two other oxygen conditions (2-way ANOVA, “DO” treatment, P < 0.001; Fig. 4D). Under anoxic conditions, P–PO43− release rates from Villerest sediments (2.42 mg day−1 m−2) was significantly higher that rates measured with Puyvalador (0.69 mg day−1 m−2) and Grangent (0.95 mg day−1 m−2) sediments (2-way ANOVA, “sediment” treatment, P < 0.001).
Concentration in P–PO43− in water column during the experimentation for oxic (A), DO fluctuation (B), and anoxic (C) treatments for the three sediment treatments (Puyvalador, Villerest, and Grangent sediments) (mean ± standard error, N = 3). *Concentrations of P–PO43− in the anoxic treatment at day 15 for Puyvalador are lacking due to an analytical problem
Influence of sediment characteristics on DIN and P–PO 4 3− release rates from sediment to the water column
From the three sediment predictors (proportion of fine sediment particles, total nutrient content, and total oxygen uptake), generalized linear model selections showed that the proportion of fine particles in sediments (clay and silts < 63 µm) was the best predictor of nutrient release rates (both DIN and P–PO43− release rates) in both oxic and anoxic conditions (Table 2). Whatever the studied nutrient and oxygen conditions, nutrient release rates decreased with the proportion of fine particles (Fig. 6, generalized linear models, F(1,7) > 31, P < 0.001, adjusted R2 > 0.79 for the four relationships presented in Fig. 6).
Algal growth bioassays
Bioassays showed higher algal growth rates in the water collected from microcosms under anoxic treatment than in the water of microcosms that experienced oxic or DO fluctuation treatments (2-way ANOVA, “DO” treatment, P < 0.001). Algal growth rates were around two-fold higher in the water collected from microcosms filled with Puyvalador and Grangent sediments under anoxic conditions in comparison with the two other DO treatments (Fig. 7). This influence of the anoxic treatment on algal growth in bioassays was more marked with water column collected from Villerest microcosms (2-way ANOVA, interaction between “DO” and “sediment” treatments, P < 0.001): growth rates were increased by nine-fold and 4.5-fold in waters collected from the anoxic treatment compared with the waters from microcosms that experienced oxic or DO fluctuation treatments, respectively. Significant differences in algal growth rate were detected among sediment treatments (2-way ANOVA, “sediment” effect, P < 0.001). For microcosms that experienced anoxic conditions, algal growth rates in waters collected from Villerest microcosms were four- and three-fold higher than rates measured in waters collected from Puyvalador and Grangent microcosms, respectively. Algal growth measured in the water collected from Villerest microcosms under anoxic condition was the only treatment resulting in higher (× 1.9, Fig. 7) algal growth rate compared to the algal culture medium (i.e., in Chu medium used as positive control).
Ratios between algal growth in the water collected from the microcosms and algal growth in the positive control (Chu medium) for the three sediment treatments (i.e., Puyvalador, Villerest, and Grangent sediments) and the three oxygen treatments (i.e., oxic, DO fluctuation, and anoxic conditions) (mean ± standard error, N = 3)
Discussion
The prediction that anoxic conditions would produce higher concentrations of NH4+ and PO43− and lower concentrations of NO3− in the water column than oxic conditions was verified by the experimental results. We also validated that anoxic conditions would lead to higher total DIN concentrations in the water column compared with the oxic conditions. As classically observed (e.g., Carignan et al., 1985; Fenchel et al., 2012), NH4+ that was produced by OM mineralization via ammonification in the sediment environment diffused into the water column for all oxygen and sediment treatments. However, biogeochemical processes involved in the N cycling were different in oxic and anoxic conditions. Oxic conditions favored the nitrification process at the SWI (i.e., transformation of NH4+ into NO2− then NO3−), whereas nitrification was inhibited in anoxic conditions (Rysgaard et al., 1994; Malecki et al., 2004). Consequently, the accumulation of NH4+ in the water column was favored in anoxic conditions, whereas NO3− was the dominant form of DIN in the water column in oxic conditions. Release rates of NH4+ were the highest in anoxic conditions (between 3.9 and 8.4 mg day−1 m−2 for Puyvalador and Villerest, respectively) than in oxic conditions for all tested sediments. These results were comparable to release rates of NH4+ in meso/eutrophic lakes (between 5.5 and 10 mg day−1 m−2) measured under anoxic conditions, while they were negligible in oxic conditions (Beutel, 2006).
For P dynamics, PO43− concentrations in the water column were higher in anoxic than in oxic conditions (× 550 for Puyvalador, × 66 for Villerest, and × 40 for Grangent sediments). Anoxic conditions probably caused the reductive dissolution of Fe/Mn/Al oxides and the release of associated PO43− (e.g., Steinman et al., 2016). This reduction mechanism increased PO43− concentration into the interstitial water and consequently, the molecular diffusion of PO43− from the interstitial water to the water column (Mortimer, 1941; Boström et al., 1988; Boudreau, 1996; Rapin et al., 2019). The release rates of PO43− were also significantly higher in anoxic conditions than those measured under oxic conditions (between × 87 for Grangent and × 811 for Puyvalador sediments). These values were comparable to those reported in the review of Nürnberg (1988) for meso/eutrophic lakes (from 0.8 to maximum release rate of 32 mg day−1 m−2). DO availability modified PO43− dynamics through adsorption/desorption mechanisms with Fe/Mn/Al oxides, which was in accordance with the measured concentrations of Fe in sediments (Table 1) that might have adsorbed P in oxic conditions. Total iron to total phosphorus ratios (Fe:P by weight) in the three sediments (i.e., 34.7, 31.5, and 29.4 for Puyvalador, Villerest, and Grangent sediments, respectively) were largely higher than 15, which is the Fe:P ratio index (by weight) proposed by Jensen et al. (1992) above which Fe content is expected to control PO43− release from oxic sediments. Generalized linear models showed that the percentage of fine particles (< 63 µm) also appeared as a main factor influencing the release rate of PO43− (but also DIN) from sediments to the water column in both oxic and anoxic conditions. Differences in rates of diffusive transport of solutes at the SWI of control microcosms among sediment treatments were probably due to contrasting sediment properties (i.e., porosity and both pore connectivity and tortuosity) (Boudreau, 1996; Shen & Chen, 2007) associated with differences in particle grain size distribution among sediments. It seemed from the present experiment that the proportion of clay and silt particles in reservoir sediments reduced the release rates of PO43− by limiting molecular diffusion between sediment and surface water compartment. In contrast, the stock of phosphorus in sediments and the microbial mineralization of organic P had low influences on PO43− fluxes at the SWI. The generalized linear model selection did not indicate that total phosphorus and total oxygen uptake were the main factors influencing PO43− fluxes at the SWI in both oxic and anoxic conditions. However, this lack of link between TP and PO43− fluxes at the SWI was probably due to the narrow range of TP content of the reservoir sediments tested (from 0.89 to 1.26 mg of total phosphorus per g of dry sediment). Positive relationships between the concentration of TP in sediments and P release rates from sediments to the water column were classically observed when wider ranges of TP content were considered (e.g., Jensen et al., 1992).
While DIN forms measured in the water column depended on the oxygen treatment (NH4+ and NO3− in anoxic and oxic conditions, respectively), total DIN concentrations measured on day 15 in the anoxic conditions were 5.5-fold, 2.3-fold, and 1.9-fold higher compared to the oxic conditions for Puyvalador, Grangent, and Villerest sediments, respectively. These differences were probably linked to contrasted microbial activities involved in N cycles depending on oxygen availability. Under oxic conditions, NH4+ released in water column was successively nitrified (transformation of NH4+ to NO3−) and then denitrified (transformation of NO3− to gaseous N2) after diffusion of NO3− from oxic water column to anoxic layers of the sediment compartment. This denitrification process resulted in a net loss of N in all oxic treatments. Under anoxic conditions, NH4+ concentrations continuously increased in the water column, suggesting that NH4+ transformation into NO2− and NO3− was limited in the absence of oxygen. Consequently, denitrification process was inhibited under anoxic conditions, limiting the loss of DIN from water column by gaseous N2 production. No volatilization of NH3 associated with NH4–NH3 equilibrium could occur because pH (~ 7.2) was markedly lower than 10 in microcosms under anoxic treatment (Reddy & DeLaune, 2008). Moreover, NO2− concentrations measured in water column were very low (mean between 15 µg l−1 for Puyvalador and 26 µg l−1 for Villerest on day 15) in anoxic treatments, leading to no or very low anammox activity (transformation of N–NH4+ and N–NO2− to gaseous N2) (Mulder et al., 1995; Thamdrup & Dalsgaard, 2002). Differences in N loss by denitrification between anoxic (inhibition of denitrification) and oxic (nitrification of released NH4+ from sediment followed by a denitrification of the produced NO3−) treatments could explain the lower concentrations of DIN measured in water column of microcosms under oxic conditions than under anoxic conditions. As demonstrated by Jenkins & Kemp (1984) in estuarine sediments, oxygen availability had control over nitrification process and associated denitrification in the present study.
Under daily fluctuation of DO, we expected that nitrification and denitrification at the SWI would be stimulated alternately by high and low oxygen concentrations in the water column, reducing the accumulation of NO3− in the water column in comparison with the oxic treatment. This prediction has been validated for microcosms filled with Grangent sediments in which NO3− concentrations in water column were around three-fold lower in the DO fluctuation treatment than in the oxic treatment. The microbial activity and oxygen uptake rate measured in Grangent sediment were high enough to produce large DO fluctuations at the SWI that favored nitrification process during aeration (7–8 mg l−1 of DO) and denitrification process after the turn off of air source for 12 h (0.44 mg l−1 of DO). However, this stimulation of nitrification and denitrification alternately by low and high DO concentrations was not observed in the two other sediments characterized by lower oxygen uptake rates (− 40% for Puyvalador sediments and − 30% for Villerest sediments) than in Grangent sediments. Because of these lower respiration rates, DO concentrations never dropped below 1 mg l−1 under DO fluctuation treatments for Puyvalador (5.08 mg l−1) and Villerest (1.11 mg l−1) sediments. Consequently, DO fluctuations did not stimulate the nitrification–denitrification coupling and DIN dynamics were comparable in the DO fluctuation and oxic treatments for these two sediments. The DO fluctuation treatment showed that the microbial respiration rate in sediment might have regulated N cycling by determining oxic/anoxic conditions in the water column. This conclusion would be consistent with several works (Kemp et al., 2009; Howarth et al., 2011; Steinsberger et al., 2019) indicating that biogeochemical feedbacks associated with OM mineralization and oxygen uptake rates in sediments determined the availability of N in aquatic ecosystems. The selection of generalized linear models also showed that the percentage of fine particles (< 63 µm) was a main factor determining DIN release rate in both oxic and anoxic conditions. As observed for PO43−, DIN release rates decreased with the percentage of clay and silt particles, suggesting that fine particles significantly reduced the diffusion rate of dissolved nutrients from sediments to the water column. Nevertheless, it is worth noting that such physical effect of sediment characteristics on nutrient diffusion from sediments to the overlying water was observed in absence of bioturbating fauna, which could increase sediment porosity at the SWI (Berg et al., 2001). By creating biogenic structures in sediments, bioturbating fauna can increase the water and solute exchanges at the SWI in a wide range of sediments. Using the three reservoir sediments of the present experiment, Gautreau et al. (2023) demonstrated that the highest release rates of P–PO43− were measured in microcosms filled with Grangent sediments when the SWI was bioturbated by tubificid worms or chironomid larvae. The present study showed that, without bioturbation, the highest release rates of P–PO43− were measured in the water column of microcosms filled with Villerest sediments. So, the release rates measured in the present study cannot be directly transferred to reservoir sediments inhabited by bioturbating fauna (Mulsow et al., 1998).
Algal growth bioassays showed that the highest algal growth rates were measured in the water column of microcosms containing the highest concentrations of PO43− (i.e., water collected from microcosms exposed to anoxic conditions). P availability for algae was higher in the water column of microcosms experiencing anoxic conditions than in other DO conditions. Following the Redfield ratio of 16 as the optimal molar N:P ratio for algal growth (Redfield, 1958; Goldman, 1980) and especially for the growth of Raphidocelis subcapitata (Silva et al., 2015), calculations of ratios from DIN and PO43− concentrations measured at the end of the experiment indicated that N:P ratios were not P-limited in waters collected from microcosms under anoxic conditions, whereas N:P ratios suggested P-limited conditions in water from microcosms exposed to DO fluctuation or oxic treatments. Ratios measured under anoxic conditions were 8.2, 14.2, and 14.1 for Villerest, Puyvalador, and Grangent sediments, respectively. In contrast, molar N:P ratios in oxic and DO fluctuation treatments were comprised between 65 and 1450, suggesting a strong P limitation and reduced algal growth rate (Guildford & Hecky, 2000). As observed in many eutrophic lakes during the summer and early autumn (Kowalczewska-Madura & Gołdyn, 2012; Søndergaard et al., 2017), our results confirmed that anoxic conditions at the SWI produced a comparatively higher release of PO43− than a release of DIN from sediments to water column, stimulating phytoplankton growth by relaxing P limitation. Under anoxic conditions, DIN was mainly as N–NH4+, which was likely more readily assimilated by Raphidocelis subcapitata than N–NO3− (Silva et al., 2015). This difference in nitrogen form availability could explain why the algal growth measured in the water collected from Villerest microcosms under anoxic condition was 1.9-fold higher than the growth rate measured in Chu medium characterized by nitrate as the sole source of inorganic nitrogen.
Conclusion
The first main conclusion of this study was that anoxic conditions at the SWI had a major influence on the release of DIN and PO43− from sediments to the water column. In all tested sediments, the anoxic treatment showed the largest release of DIN, as NH4+ and PO43−, compared to the oxic and DO fluctuation treatments. Sediment grain size distribution had also a major role in DIN and PO43− fluxes at the SWI in all DO conditions. By reducing the porosity, the percentage of silt and clay probably limited the diffusion rate of dissolved nutrients from sediment to water column. This physical effect on nutrient release rates is probably not dominant in sediments inhabited by bioturbating fauna but it should be in sediment systems characterized by anoxic conditions which are unfavorable for benthic fauna (e.g., Real & Prat, 1992). Under anoxic conditions, sediment grain size such as the percentage of fine particles could be used as a proxy to evaluate nutrient release rates at the SWI of reservoirs. These findings suggested to develop field works on a wide range of lakes and reservoirs to determine whether redox changes at the SWI associated with sediment characteristics could be used as early-warning indicators of algal blooms in eutrophic freshwater ecosystems.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Amorim, C. A. & A. do Nascimento Moura, 2021. Ecological impacts of freshwater algal blooms on water quality, plankton biodiversity, structure, and ecosystem functioning. Science of the Total Environment 758: 143605. https://doi.org/10.1016/j.scitotenv.2020.143605.
Andersen, R. A., 2005. Algal culturing techniques, Academic Press, Burlington:, 578.
Anschutz, P., A. Ciutat, P. Lecroart, M. Gérino & A. Boudou, 2012. Effects of tubificid worm bioturbation on freshwater sediment biogeochemistry. Aquatic Geochemistry 18: 475–497. https://doi.org/10.1007/s10498-012-9171-6.
Berg, P., S. Rysgaard, P. Funch & M. K. Sejr, 2001. Effects of bioturbation on solutes and solids in marine sediments. Aquatic Microbial Ecology 26: 81–94. https://doi.org/10.3354/ame026081.
Berner, R. A., 1980. Early diagenesis: A theoretical approach, Princeton University Press, Princeton:, 256.
Beutel, M. W., 2006. Inhibition of ammonia release from anoxic profundal sediments in lakes using hypolimnetic oxygenation. Ecological Engineering 28: 271–279. https://doi.org/10.1016/j.ecoleng.2006.05.009.
Beutel, M. W., T. M. Leonard, S. R. Dent & B. C. Moore, 2008. Effects of aerobic and anaerobic conditions on P, N, Fe, Mn, and Hg accumulation in waters overlaying profundal sediments of an oligo-mesotrophic lake. Water Research 42: 1953–1962. https://doi.org/10.1016/j.watres.2007.11.027.
Bortleson, G. C. & G. F. Lee, 1974. Phosphorus, iron, and manganese distribution in sediment cores of six Wisconsin lakes. Limnology and Oceanography 19: 794–801. https://doi.org/10.4319/lo.1974.19.5.0794.
Boström, B., J. M. Andersen, S. Fleischer & M. Jansson, 1988. Exchange of phosphorus across the sediment-water interface. In Persson, G. & M. Jansson (eds), Phosphorus in freshwater ecosystems Springer, Dordrecht: 229–244. https://doi.org/10.1007/978-94-009-3109-1_14.
Boudreau, B. P., 1996. The diffusive tortuosity of fine-grained unlithified sediments. Geochimica and Cosmochimica Acta 60: 3139–3142. https://doi.org/10.1016/0016-7037(96)00158-5.
Burnham, K. P. & D. R. Anderson, 2002. Model selection and multi-model inference: A practical information-theoretic approach, Springer, New York:, 488. https://doi.org/10.1007/b97636.
Carignan, R., F. Rapin & A. Tessier, 1985. Sediment porewater sampling for metal analysis: a comparison of techniques. Geochimica and Cosmochimica Acta 49: 2493–2497. https://doi.org/10.1016/0016-7037(85)90248-0.
Chu, S. P., 1942. The influence of the mineral composition of the medium on the growth of planktonic algae: Part I. Methods and culture media. Journal of Ecology 30: 284–325. https://doi.org/10.2307/2256574.
Cymbola, J., M. Ogdahl & A. D. Steinman, 2008. Phytoplankton response to light and internal phosphorus loading from sediment release. Freshwater Biology 53: 2530–2542.
Ekeroth, N., S. Blomqvist & P. Hall, 2016. Nutrient fluxes from reduced Baltic Sea sediment: effects of oxygenation and macrobenthos. Marine Ecology Progress Series 544: 77–92. https://doi.org/10.3354/meps11592.
Fenchel, T., T. H. Blackburn & G. M. King, 2012. Chapter 7: Aquatic sediments bacterial biogeochemistry: the ecophysiology of mineral cycling, Academic Press, London:, 121–142.
Forsberg, C., 1989. Importance of sediments in understanding nutrient cyclings in lakes. Hydrobiologia 176: 263–277. https://doi.org/10.1007/BF00026561.
Fukuhara, H. & M. Sakamoto, 1987. Enhancement of inorganic nitrogen and phosphate release from lake sediment by tubificid worms and chironomid larvae. Oikos 48: 312–320. https://doi.org/10.2307/3565519.
Gautreau, E., L. Volatier, G. Nogaro, E. Gouze, P. Marmonier & F. Mermillod-Blondin, 2020. The influence of bioturbation and water column oxygenation on nutrient recycling in reservoir sediments. Hydrobiologia 847: 1027–1040. https://doi.org/10.1007/s10750-019-04166-0.
Gautreau, E., L. Volatier, G. Nogaro, E. Gouze, P. Marmonier & F. Mermillod-Blondin, 2023. Interactions between microbial activity and bioturbation modes of benthic invertebrates determine nutrient releases from reservoir sediments. Freshwater Biology 68: 245–259. https://doi.org/10.1111/fwb.14021.
Gebara, R. C., L. D. O. G. Alho, G. S. Rocha, A. da Silva Mansano & M. D. G. G. Melão, 2020. Zinc and aluminum mixtures have synergic effects to the algae Raphidocelis subcapitata at environmental concentrations. Chemosphere 242: 125231. https://doi.org/10.1016/j.chemosphere.2019.125231.
Gilbert, F., S. Hulth, V. Grossi & R. C. Aller, 2016. Redox oscillation and benthic nitrogen mineralization within burrowed sediments: an experimental simulation at low frequency. Journal of Experimental Marine Biology and Ecology 482: 75–84.
Giles, C. D., P. D. Isles, T. Manley, Y. Xu, G. K. Druschel & A. W. Schroth, 2016. The mobility of phosphorus, iron, and manganese through the sediment–water continuum of a shallow eutrophic freshwater lake under stratified and mixed water-column conditions. Biogeochemistry 127: 15–34. https://doi.org/10.1007/s10533-015-0144-x.
Glud, R. N., 2008. Oxygen dynamics of marine sediments. Marine Biological Research 4: 243–289. https://doi.org/10.1080/17451000801888726.
Goldman, J. C., 1980. Physiological processes, nutrient availability, and the concept of relative growth rate in marine phytoplankton ecology. In Falkowski, P. G. (ed), Primary productivity in the sea Springer, Boston: 179–194. https://doi.org/10.1007/978-1-4684-3890-1_10.
Grasshoff, K., M. Ehrhardt & K. Kremling, 1983. Methods of seawater analysis, 2nd ed. Verlag Chemie, Berlin:
Guildford, S. J. & R. E. Hecky, 2000. Total nitrogen, total phosphorus, and nutrient limitation in lakes and oceans: is there a common relationship? Limnology and Oceanography 45: 1213–1223. https://doi.org/10.4319/lo.2000.45.6.1213.
Howarth, R., F. Chan, D. J. Conley, J. Garnier, S. C. Doney, R. Marino & G. Billen, 2011. Coupled biogeochemical cycles: eutrophication and hypoxia in temperate estuaries and coastal marine ecosystems. Frontiers in Ecology and Environment 9: 18–26. https://doi.org/10.1890/100008.
Huang, C., L. Zhang, Y. Li, C. Lin, T. Huang, M. Zhang, A.-X. Zhu, H. Yang & X. Wang, 2018. Carbon and nitrogen burial in a plateau lake during eutrophication and phytoplankton blooms. Science of the Total Environment 616: 296–304. https://doi.org/10.1016/j.scitotenv.2017.10.320.
Huettel, M. & I. T. Webster, 2001. Porewater flow in permeable sediments. In Boudreau, B. P. & B. B. Jorgensen (eds), The benthic boundary layer: Transport processes and biogeochemistry Oxford University Press, New York: 144–177.
Hupfer, M. & J. Lewandowski, 2008. Oxygen controls the phosphorus release from lake sediments – a long-lasting paradigm in limnology. International Review of Hydrobiology 93: 415–432. https://doi.org/10.1002/iroh.200711054.
Jenkins, M. C. & W. M. Kemp, 1984. The coupling of nitrification and denitrification in two estuarine sediments. Limnology and Oceanography 29: 609–619. https://doi.org/10.4319/lo.1984.29.3.0609.
Jensen, H. S., P. Kristensen, E. Jeppesen & A. Skytthe, 1992. Iron: phosphorus ratio in surface sediment as an indicator of phosphate release from aerobic sediments in shallow lakes. Hydrobiologia 235(236): 731–743. https://doi.org/10.1007/978-94-011-2783-7_66.
Jeppesen, E., M. Sondergaard, J. P. Jensen, K. E. Havens, O. Anneville, L. Carvalho, M. F. Coveney, R. Deneke, M. T. Dokulil, B. O. Foy, D. Gerdeaux, S. E. Hampton, S. Hilt, K. Kangur, J. Köhler, E. Lammens, T. L. Lauridsen, M. Manca, M. Miracle, B. Moss, P. Noges, G. Persson, G. Phillips, R. Portielje, S. Romo, C. L. Schelske, D. Straile, I. Tatrai, E. Willen & M. Winder, 2005. Lake responses to reduced nutrient loading – an analysis of contemporary long-term data from 35 case studies. Freshwater Biology 50: 1747–1771. https://doi.org/10.1111/j.1365-2427.2005.01415.x.
Joshi, S., K. K. Ravi, J. B. David, E. B. Mark, L. S. Donald & P. J. Deb, 2015. Organic matter remineralization predominates phosphorus cycling in the mid-bay sediments in the Chesapeake Bay. Environmental Science and Technology 49: 5887–5896. https://doi.org/10.1021/es5059617.
Kemp, W. M., J. M. Testa, D. J. Conley, D. Gilbert & J. D. Hagy, 2009. Temporal responses of coastal hypoxia to nutrient loading and physical controls. Biogeosciences 6: 2985–3008. https://doi.org/10.5194/bg-6-2985-2009.
Kowalczewska-Madura, K. & R. Gołdyn, 2012. Spatial and seasonal variability of pore water phosphorus concentration in shallow Lake Swarzędzkie, Poland. Environmental Monitoring and Assessement 184: 1509–1516. https://doi.org/10.1007/s10661-011-2056-0.
Kristensen, E., 2000. Organic matter diagenesis at the oxic/anoxic interface in coastal marine sediments, with emphasis on the role of burrowing animals. Hydrobiologia 426: 1–24. https://doi.org/10.1007/978-94-011-4148-2_1.
Lavery, P. S., C. E. Oldham & M. Ghisalberti, 2001. The use of Fick’s First Law for predicting porewater nutrient fluxes under diffusive conditions. Hydrological Processes 15: 2435–2451. https://doi.org/10.1002/hyp.297.
Li, Y., Y. Liu, H. Wang, Z. Zuo, Z. Yan, L. Wang, D. Wang, C. Liu & D. Yu, 2023. In situ remediation mechanism of internal nitrogen and phosphorus regeneration and release in shallow eutrophic lakes by combining multiple remediation techniques. Water Research 229: 119394.
Ma, J., S. Wang, P. Wang, L. Ma, X. Chen & R. Xu, 2006. Toxicity assessment of 40 herbicides to the green alga Raphidocelis subcapitata. Ecotoxicology and Environmental Safety 63: 456–462. https://doi.org/10.1016/j.ecoenv.2004.12.001.
Malecki, L. M., J. R. White & K. R. Reddy, 2004. Nitrogen and phosphorus flux rates from sediment in the lower St. Johns River estuary. Journal of Environmental Quality 33: 1545–1555. https://doi.org/10.2134/jeq2004.1545.
McFadden, D., 1974. Conditional logit analysis of qualitative choice behaviour. In Zarembka, P. (ed), Frontiers in econometrics Academic Press, New York: 105–142.
Meinikmann, K., M. Hupfer & J. Lewandowski, 2015. Phosphorus in groundwater discharge – a potential source for lake eutrophication. Journal of Hydrology 524: 214–226. https://doi.org/10.1016/j.jhydrol.2015.02.031.
Moore, P., Jr., K. Reddy & D. Graetz, 1992. Nutrient transformations in sediments as influenced by oxygen supply. Journal of Environmental Quality 21: 387–393. https://doi.org/10.2134/jeq1992.00472425002100030014x.
Moreira-Santos, M., A. M. Soares & R. Ribeiro, 2004. An in situ bioassay for freshwater environments with the microalga Pseudokirchneriella subcapitata. Ecotoxicology and Environmental Safety 59: 164–173. https://doi.org/10.1016/j.ecoenv.2003.07.004.
Mortimer, C. H., 1941. The exchange of dissolved substances between mud and water in lakes. Journal of Ecology 29: 280–329. https://doi.org/10.2307/2256395.
Mulder, A., A. A. Graaf, L. A. Robertson & J. G. Kuenen, 1995. Anaerobic ammonium oxidation discovered in a denitrifying fluidized bed reactor. FEMS Microbiology Ecology 16: 177–184. https://doi.org/10.1111/j.1574-6941.1995.tb00281.x.
Mulsow, S., B. P. Boudreau & J. A. Smith, 1998. Bioturbation and porosity gradients. Limnology and Oceanography 43: 1–9. https://doi.org/10.4319/lo.1998.43.1.000I.
Murphy, J. & J. P. Riley, 1962. A modified single solution method for the determination of phosphate in natural waters. Analytica Chimica Acta 27: 31–36. https://doi.org/10.1016/S0003-2670(00)88444-5.
Nürnberg, G. K., M. Tarvainen, A. M. Ventelä & J. Sarvala, 2012. Internal phosphorus load estimation during biomanipulation in a large polymictic and mesotrophic lake. Inland Waters 2: 147e162. https://doi.org/10.5268/IW-2.3.469.
Paquet, N., N. Indiketi, C. Dalencourt, D. Larivière, S. Roberge, N. Gruyer, G. Triffault-Bouchet & C. Fortin, 2019. Toxicity of tailing leachates from a niobium mine toward three aquatic organisms. Ecotoxicology and Environmental Safety 176: 355–363. https://doi.org/10.1016/j.ecoenv.2019.03.065.
Pett-Ridge, J., D. G. Petersen, E. Nuccio & M. K. Firestone, 2013. Influence of oxic/anoxic fluctuations on ammonia oxidizers and nitrification potential in a wet tropical soil. FEMS Microbiology Ecology 85: 179–194. https://doi.org/10.1111/1574-6941.12111.
Prata, J. C., C. Venâncio, A. V. Girão, J. P. da Costa, I. Lopes, A. C. Duarte & T. Rocha-Santos, 2022. Effects of virgin and weathered polystyrene and polypropylene microplastics on Raphidocelis subcapitata and embryos of Danio rerio under environmental concentrations. Science of the Total Environment 816: 151642. https://doi.org/10.1016/j.scitotenv.2021.151642.
Rapin, A., M. Grybos, M. Rabiet, B. Mourier & V. Deluchat, 2019. Phosphorus mobility in dam reservoir affected by redox oscillations: an experimental study. Journal of Environmental Science 77: 250–263. https://doi.org/10.1016/j.jes.2018.07.016.
Real, M. & N. Prat, 1992. Factors influencing the distribution of chironomids and oligochaetes in profundal areas of Spanish reservoirs. Netherland Journal of Aquatic Ecology 26: 405–410. https://doi.org/10.1007/BF02255269.
Reddy, K. R. & R. D. DeLaune, 2008. Biogeochemistry of wetlands: science and applications, CRC Press, Boca Raton:
Redfield, A. C., 1958. The biological control of chemical factors in the environment. American Scientist 46: 205–221.
Ronael Gilbert, E., M. Garcia de Camargo & L. Sandrini-Neto, 2014. Package rysgran. https://www.rdocumentation.org/packages/rysgran/versions/2.1.0.
Ruban, V., S. Brigault, D. Demare & A. M. Philippe, 1999. An investigation of the origin and mobility of phosphorus in freshwater sediments from Bort-Les-Orgues Reservoir, France. Journal of Environmental Monitoring 1: 403–407. https://doi.org/10.1039/A902269D.
Rydin, E., 2000. Potentially mobile phosphorus in Lake Erken sediment. Water Research 34: 2037–2042. https://doi.org/10.1016/S0043-1354(99)00375-9.
Rysgaard, S., N. Risgaard-Petersen, S. Niels Peter, J. Kim & N. Lars Peter, 1994. Oxygen regulation of nitrification and denitrification in sediments. Limnology and Oceanography 39: 1643–1652. https://doi.org/10.4319/lo.1994.39.7.1643.
Shen, L. & Z. Chen, 2007. Critical review of the impact of tortuosity on diffusion. Chemical Engineering Science 62: 3748–3755. https://doi.org/10.1016/j.ces.2007.03.041.
Silva, N. F. P., A. L. Gonçalves, F. C. Moreira, T. F. C. V. Silva, F. G. Martins, M. C. M. Alvim-Ferraz, R. A. R. Boaventura, V. J. P. Vilar & J. C. M. Pires, 2015. Towards sustainable microalgal biomass production by phycoremediation of a synthetic wastewater: a kinetic study. Algal Research 11: 350–358. https://doi.org/10.1016/j.algal.2015.07.014.
Søndergaard, M., J. P. Jensen & E. Jeppesen, 2003. Role of sediment and internal loading of phosphorus in shallow lakes. Hydrobiologia 506: 135–145. https://doi.org/10.1023/B:HYDR.0000008611.12704.dd.
Søndergaard, M., T. L. Lauridsen, L. S. Johansson & E. Jeppesen, 2017. Nitrogen or phosphorus limitation in lakes and its impact on phytoplankton biomass and submerged macrophyte cover. Hydrobiologia 795: 35–48. https://doi.org/10.1007/s10750-017-3110-x.
Steinman, A., R. Rediske & K. R. Reddy, 2004. The reduction of internal phosphorus loading using alum in Spring Lake, Michigan. Journal of Environmental Quality 33: 2040–2048. https://doi.org/10.2134/jeq2004.2040.
Steinman, A. D., M. Ogdahl & X. Chu, 2009. Spatial and temporal variability of internal and external phosphorus loads in an urbanizing watershed. Aquatic Ecology 43: 1–18. https://doi.org/10.1007/s10452-007-9147-6.
Steinman, A., M. Abdimalik, M. E. Ogdahl & M. Oudsema, 2016. Understanding planktonic vs. benthic algal response to manipulation of nutrients and light in a eutrophic lake. Lake and Reservoir Management 32: 402–409. https://doi.org/10.1080/10402381.2016.1235065.
Steinsberger, T., B. Müller, C. Gerber, B. Shafei & M. Schmid, 2019. Modeling sediment oxygen demand in a highly productive lake under various trophic scenarios. PLoS ONE 14: e0222318. https://doi.org/10.1371/journal.pone.0222318.
Suzuki, S., H. Yamaguchi, N. Nakajima & M. Kawachi, 2018. Raphidocelis subcapitata (= Pseudokirchneriella subcapitata) provides an insight into genome evolution and environmental adaptations in the Sphaeropleales. Scientific Reports 8: 8058. https://doi.org/10.1038/s41598-018-26331-6.
Thamdrup, B. & T. Dalsgaard, 2002. Production of N2 through anaerobic ammonium oxidation coupled to nitrate reduction in marine sediments. Applied and Environmental Microbiology 68: 1312–1318. https://doi.org/10.1128/AEM.68.3.1312-1318.2002.
Tolhurst, T. J., M. G. Chapman, A. J. Underwood & J. J. Cruz, 2012. Technical note: the effects of five different defaunation methods on biogeochemical properties of intertidal sediment. Biogeosciences 9: 3647–3661. https://doi.org/10.5194/bg-9-3647-2012.
US EPA, 1991. Methods for measuring the acute toxicity of effluents and receiving waters to freshwater and marine organisms. EPA/600/4-90/027. https://19january2017snapshot.epa.gov/sites/production/files/2015-08/documents/acute-freshwater-and-marine-wet-manual_2002.pdf
Venâncio, C., E. Anselmo, A. Soares & I. Lopes, 2017. Does increased salinity influence the competitive outcome of two producer species? Environmental Science and Pollution Research 24: 5888–5897. https://doi.org/10.1007/s11356-016-8346-x.
Watson, S. B., C. Miller, G. Arhonditsis, G. L. Boyer, W. Carmichael, M. N. Charlton, R. Confesor, D. C. Depew, T. O. Hook, S. A. Ludsin, G. Matisoff, S. P. McElmurry, M. W. Murray, R. P. Richards, Y. R. Rao, M. M. Steffen & S. W. Wilhelm, 2016. The re-eutrophication of Lake Erie: harmful algal blooms and hypoxia. Harmful Algae 56: 44–66. https://doi.org/10.1016/j.hal.2016.04.010.
Welch, E. & G. Denniscooke, 2005. Internal phosphorus loading in shallow lakes: importance and control. Lake and Reservoir Management 21: 209e217. https://doi.org/10.1080/07438140509354430.
Wetzel, R. G., 2001. The phosphorus cycle. Limnology: lake and river ecosystems, Academic Press, San Diego: https://doi.org/10.1016/B978-0-08-057439-4.50017-4.
Yang, C., P. Yang, J. Geng, H. Yin & K. Chen, 2020. Sediment internal nutrient loading in the most polluted area of a shallow eutrophic lake (Lake Chaohu, China) and its contribution to lake eutrophication. Environmental Pollution 262: 114292. https://doi.org/10.1016/j.envpol.2020.114292.
Zuur, A. F., E. N. Ieno & C. S. Elphick, 2010. A protocol for data exploration to avoid common statistical problems. Methods in Ecology and Evolution 1: 3–14. https://doi.org/10.1111/j.2041-210X.2009.00001.x.
Acknowledgements
The authors thank the ANRT (Association Nationale Recherche Technologie) and EDF (Electricité de France) for Edwige Gautreau’s Ph.D. Grant (convention bourse CIFRE-EDF: No. 0733/2016). This study was funded by a partnership between EDF and CNRS (CNRS No. 149389) and by a Contrat Plan Etat Région (Lyon Metropole/Auvergne-Rhône-Alpes Region/French Government) within the framework of Sedaqua+ Platform (FR 3728 Biodiversité, Eau, Ville, Santé–BioEEnViS-). This work was performed within the framework of the EUR H2O’Lyon (ANR-17-EURE-0018) of Université de Lyon (UdL), within the program ‘‘Investissements d’Avenir’’ operated by the French National Research Agency (ANR). We also thank Athos Environnement (Antoine Thouvenot, David Foltier, and Benjamin Legrand), Pierre Rossignol, Laurent Simon, Pierre Marmonier, Simon Navel, Yohan Lebon, Colin Issartel, and Antoine Gosset for their support and advices during field sampling and laboratory works. The manuscript greatly benefited from insightful comments raised by two anonymous reviewers.
Funding
This work received support from CIFRE-EDF (Grant No. 0733/2016), Électricité de France (Grant No. 149389), Contrat Plan Etat Région (Auvergne-Rhône-Alpes) (Grant No. Sedaqua).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing interests to declare that are relevant to the content of this article.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Additional information
Handling editor: Andrew Dzialowski
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mermillod-Blondin, F., Gautreau, E., Pinasseau, L. et al. Interactions between sediment characteristics and oxygen conditions at the sediment–water interface of reservoirs: influences on nutrient dynamics and eutrophication. Hydrobiologia 851, 3433–3452 (2024). https://doi.org/10.1007/s10750-024-05508-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-024-05508-3