Abstract
Seedlings of two species of mangroves, Bruguiera gymnorrhiza and Rhizophora apiculata, were monitored for 8 years on the Pacific island of Kosrae, Federated States of Micronesia, to determine the effects of canopy cover and hydrogeomorphic zone on long-term survival and growth. Annual rainfall is high with little seasonality. Study plots with seedling subplots were in fringe, riverine, and interior hydrogeomorphic zones along four small rivers. The height of each seedling was measured each year and was designated each time as being under an open, partially closed, or closed canopy. Growth rates of both species were slow, and no seedling that was newly established during the study became a sapling (2 m). Many seedlings stopped growing for long periods of time. Bruguiera seedlings were more numerous than Rhizophora seedlings. They grew faster than Rhizophora seedlings except in riverine zones. Predicted growth rates of Bruguiera were fastest under an open canopy and slowest under a closed canopy, but Rhizophora was not affected by canopy status. Overall mortality rates for the two species were similar, averaging 36% year−1. El-Niño-Southern Oscillation events did not affect growth or mortality but may have caused some defoliation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mangrove forests around the world provide a plethora of goods and services to tropical and subtropical coastal communities, yet the life cycles of mangrove trees themselves are not well understood. Because these important forested wetlands are gradually disappearing (Friess et al., 2019; Murray et al., 2022), often due to human actions, it is essential to know how to reverse degradation where possible and restore forests with their full complement of species. This requires understanding seed dispersal, establishment, growth, and survival of trees in all stages of their life cycles and in the variety of hydrogeomorphic settings in which they are found.
The period from seedling establishment to the sapling stage of tropical and subtropical species of trees in general is referred to as the “black box” of tree population dynamics (Chang-Yang et al., 2021). Mangrove trees fit this description, although substantial advances in our understanding have been made in recent years (Krauss et al., 2008). We know that a freshly lodged mangrove propagule, i.e., partially buried in soil, must be able to establish a root system strong enough to hold the seedling erect and withstand the physical rigors of regular tides and currents as well as occasional storm tides (Balke et al., 2011). To expand our understanding of this process, we monitored growth and survival of established mangrove seedlings to determine critical elements of the regeneration process up to the sapling stage.
Hydrogeomorphic settings present a variety of challenges to freshly rooted mangrove seedlings. High and low intertidal zones, for instance, have frequently been used as the hydrogeomorphic zones of interest in studies of mangrove seedling growth and survival, because they usually represent extremes in degree of inundation, rate of water flow, range of salinity, and characteristics of the substrate. Lower zones are considered more stressful because of rapid water movement with tides and storms. Higher zones may be more protected and have greater nutrient availability, but they may also experience high salinity levels. Relative growth rates of mangrove seedlings over a 3-year period in Australia differed among some but not all of four species in the high versus low intertidal zones and in light gaps versus under the canopy; survival of all species was better in the high intertidal zone, regardless of light level (Smith, 1987). On the other hand, during the first year after establishment in a mangrove forest in Thailand, seven mangrove species were affected in different ways in both high and low intertidal zones over 1 year (Kitaya et al., 2002). Seedlings of Rhizophora mangle L. in Belize grew faster at mean and lowest flooding levels than at the highest flooding levels, whereas Avicennia germinans (L.) Stearn seedlings grew fastest at mean and highest flooding levels (Ellison & Farnsworth, 1993). Clearly, different species may respond differently to the same stresses and resources, providing a stochastic element to reforestation attempts involving different species and hydrogeomorphic settings. We examined the distributions of mangrove species as seedlings on a small Pacific island and the subsequent trajectory for two of the most common species from established seedling to sapling in three hydrogeomorphic zones.
We used a system of hydrogeomorphic settings that comprises three zones: fringe, riverine, and interior (sometimes called basin) (Ewel et al., 1998a; Woodroffe, 1992). Fringe forests experience the full force of diurnal tides at relatively constant salinity, and they generally have low tree species diversity and slow growth rates. Riverine mangrove forests experience diurnal tides but at lower salinities, as fresh river water is borne up to spread under the forest by the incoming tide. Both fringe and riverine zones may experience strong currents at times, but riverine forests may also receive dissolved nutrients and sediment deposits from upstream ecosystems. Interior mangrove forests experience less regular and less forceful flooding but in climates with lengthy dry seasons may be subjected to longer soil saturation and high salinity. Slow drainage may lower reducing conditions and lack of continuous flooding may allow salinity to increase as water is transpired. In some places, however, interior mangrove forests with shorter dry seasons and high rainfall may receive groundwater seepage and/or overland flow, with associated nutrient inputs, from terrestrial ecosystems higher in the watershed, and so may be more similar in average salinity levels to riverine forests. The basic characteristics of these zones can be easily learned by resource managers and other local users of a forest in deciding how to decrease degradation, increase the success of reforestation efforts, and manage wisely.
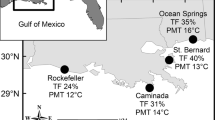
We monitored the establishment, survival, and growth of seedlings in three hydrogeomorphic zones along four rivers on the island state of Kosrae, Federated States of Micronesia (FSM), a high island approximately 1–2 million years old (Ur et al., 2013) (Fig. 1). For the last 2,000 years, sea level has been rising at the rate of 1–2 mm year−1, with mangrove peat now underlying much of the existing mangrove forest (Fujimoto et al., 1996). At the time of this study, air temperature averaged 27.2 °C with a mean low average of 25.1 °C and a mean high average of 30.1 °C; rainfall was abundant throughout the year (averaging 4–6 m year−1) and was higher in December and from March to June (Ling, 1995). A strong El Niño event in 1997–1998 caused a severe drought (and decreased sea levels) throughout Micronesia, including Kosrae (Pacific ENSO Update, 1998), and a moderate event occurred in 2002–2003 (Pacific ENSO Update, 2003; Ramsay et al., 2013; Fig. 2). La Niña events increased sea level 15–20 cm (and increased rainfall as well) in 1994–1996 and 1999–2001 (Pacific ENSO Update, 1998, 2003; Ramsay et al., 2013). Typhoons are very rare, although extreme wind events occasionally occur. Lightning is also rare.
Mean sea-level fluctuations between 1992 and 2012 for Kosrae showing effects of El Niño and La Niña events on sea levels. After Ramsay et al. (2013) with permission
Our analysis of seedling growth and survival focused on two of the most common species of mangrove trees on the island: Bruguiera gymnorrhiza and Rhizophora apiculata, hereafter called Bruguiera and Rhizophora, both in the Rhizophoraceae. (Authorities are listed in Table 1). We based a set of hypotheses on importance values of adult trees on the island (Table 2) and an earlier, short-term study of seedlings on Kosrae (Pinzón et al., 2003):
(H1)
Distributions of all mangrove seedlings reflect the density and distribution of adult trees of the same species.
(H2)
Bruguiera grows faster and survives better than Rhizophora. In particular,
(H2a)
Bruguiera survival rates are highest in riverine zones and lowest in fringe zones;
(H2b)
Rhizophora survival rates are highest in interior zones and lowest in riverine zones;
(H2c)
Growth and survival rates of both species are highest under open canopies in all zones.
(H3)
Neither growth nor mortality of either species is affected by ENSO events.
Both these species are widely distributed in the Indo-West Pacific, Bruguiera especially so. Understanding survival and growth patterns for these two common species in three hydrogeomorphic zones will be useful for designing management and reforestation projects throughout the region.
Materials and methods
Study site
Thirteen species of trees have been identified in Kosrae’s mangrove forests, of which only four species are found in all three zones (Table 1). Tree species richness is highest in interior zones, and trees are distinctly shorter in fringe zones (Ewel et al., 2003). Annual volume increment of mangrove trees throughout the FSM is 4.5 m3 ha−1 year−1, comparable to rates measured in Southeast Asian mangroves (Devoe & Cole, 1998).
These forests have been logged for many centuries. At the time of this study, Bruguiera and Rhizophora apiculata were commonly harvested for firewood. Seedling density is often high in tree gaps resulting from harvesting; these gaps are usually larger than 100 m2 (Fig. 3) (Pinzón et al., 2003) A survey conducted in 1999–2000 determined that 10% of the mangrove forest on Kosrae had been harvested over the previous 10 years (Hauff et al., 2006). Most (67%) harvest gaps were in interior mangroves, compared to 16% in riverine mangroves and 19% in fringe mangroves.
Natural gaps in Kosrae are caused primarily by the fall of a large branch, usually of Sonneratia alba, or the (often unexplained) death of a tree and are generally smaller than 50 m2 (Pinzón et al., 2003). Rhizophora apiculata and Bruguiera were by far the most common seedlings found in the understory when this study was conducted in 1995. Seedling densities in natural gaps in at that time were 0.9 m−2 and were higher in riverine zones, where densities in all gaps averaged 1.37 m−2; densities under the canopy were 0.54 m−2 (Pinzón et al., 2003).
Soil characteristics
There are no clear differences in soil characteristics among the three zones (Table 2). Levels of porewater salinity and soil redox potential show only trends, suggesting that high rainfall rates moderate any stress that trees might encounter in the rhizosphere, particularly in the interior zone. This is especially true for the Yela interior plot, which is hydrologically connected by groundwater to a contiguous extensive freshwater forested wetland (Drexler & DeCarlo, 2002).
Plot design
Permanent plots were established in 1995 in mangrove forests along four small rivers: the Utwe and Finkol Rivers on the south side of the island and the Yela and Okat Rivers on the west and northwest sides (Fig. 1). The fringe plot in the Finkol River was discontinued early in the study when it became obvious that it was more riverine than fringe. Eleven plots remained in the study.
The plots are 5-point cluster plots identical to forest inventory plots that were established in the 1980s throughout FSM and the Republic of Palau (Fig. 4). (See Online Resource Box S1 for a more detailed explanation of plot design.) Each plot covered an area of approximately 2.5 ha. Four permanent 1-m2 subplots were randomly located around each point in each plot, one in each quadrat, and none further than 10 m from the point, for a total of 20 per plot. This design enabled us to express seedling establishment on an areal basis and to associate background tree data with seedling data.
Seedling measurements
Numbered pigeon tags made of flexible plastic were affixed to each seedling in the subplots, and the seedling’s height from ground level was measured and then remeasured in subsequent years. The random placement of plots enabled construction of an unbiased picture of the distribution of species among zones and rivers. Up to five additional seedlings of Bruguiera and of Rhizophora that were approximately 50 cm tall (close to the minimum height for an established seedling) were located haphazardly (to avoid excessive time and trampling likely to be associated with a random sampling process) within 10 m of each point center and tagged and measured as above. When a seedling had died or could not be found, a new one as close to 50 cm in height as possible was selected to replace it. If a missing seedling was subsequently found, it was counted as alive again, but the missing height measurement remained as missing data for that year in growth regressions. Data on these additional seedlings increased the power of predictions of growth and survivorship rates.
Because of unforeseen circumstances, the study began in February 1996 with only measurements of the additional Bruguiera seedlings, whereas measurement of all seedlings in the subplots and the additional Rhizophora seedlings were measured annually starting in February 1997. Measurements of all seedlings continued in April 1998, June 1999, March 2000, January 2001, February 2002, February 2003, and January 2004. The additional Bruguiera seedlings were measured for 9 years and all the other seedlings for 8 years. Plants graduated out of the study when they exceeded 2 m and became saplings.
Canopy cover
Starting in 1999, we recorded the level of canopy cover over each subplot and additional seedling as being open (0), partial (1), or complete (2). When a previously measured seedling was not found in 1 year but then was found in a later year, perhaps because of a compass orientation error the first time, its corresponding canopy cover level for the missing time was assigned based on the previous and current levels, as well as on levels of neighboring seedlings.
Statistical analysis
Because of the limited number of years available for determining response to ENSO events, permutation tests (Good, 2005) were performed to test for deviations from no relationship between growth rates (also mortality rates) and the previous year’s sea-level anomaly by species and by species and zone.
For each year and zone, the mean and associated 95% confidence interval of the recorded canopy cover levels (0, 1, and 2) were calculated irrespective of species, river, and sample unit (subplots and additional seedlings).
A single linear-mixed model of height growth that included zone, year, initial height, and years since initial measurement was used with the full data set to determine the rate of height growth for each of the two species. Repeated measures on the same seedling were accounted for as a random effect. Previous canopy cover level was added to the model to determine its effect on rate of height growth for the data set beginning in 1999. Analysis of variance (ANOVA) based on the models being adjusted for the varying times between measurements was used to determine significance of the factors.
A set of six linear-mixed models (Table 3) were used to calculate survival rates for both mangrove species, first over the entire study without including canopy cover levels and then including them with the data set beginning in 1999. The models included the previous height, river, and zone as factors. To determine the best model among them, the Akaike information criterion corrected for finite sample sizes (AICc) was calculated to rank the quality of the six models. Models 1 through 5 included separate intercepts for each river and zone combination. Model 1 added separate slopes for previous canopy cover and previous height. Model 2 added an interaction term for previous canopy cover and previous height for each river and zone combination. Models 3 and 4 added a linear effect for previous canopy cover and previous height, respectively. Model 5 incorporated linear effects for both previous canopy cover and previous height. Model 6 had a separate intercept, a linear effect for previous canopy cover, previous height, and the product of previous canopy cover previous height for each zone; river was ignored to see if it mattered.
These models were then used to calculate the probability of a seedling’s surviving from 1 year to the next using river, zone, and height at the previous measurement, given that the seedling was alive in the previous year. This was repeated on the shorter data set with canopy cover levels. We fit the logit transform of the probability of survival as a linear function of river and zone plus a separate random effect for each seedling to account for multiple observations on an individual seedling. We used SAS/STAT software Version 9 for the analysis (Littell et al., 2006) and R for generating figures (R Core Team, 2021). Several features of the data set such as discrete variables (e.g., river), small sample sizes, varying lengths of time between measurements, and different effects of a slope and intercept made tests of significance difficult to interpret.
The overall mortality rate for each species was the mean of the annual mortality rate estimates, which were based on the change in status of all seedlings measured in the associated year.
Results
Species distribution
Most mangrove seedling species were not distributed in location or abundance according to their adult trees. Only eight of the 13 tree species recorded in Kosrae’s mangrove forests appeared as seedlings in the subplots at all (Table 4). Only Bruguiera, by far the most common species of seedling, and Rhizophora apiculata were found in subplots in all three zones in our study. Mature Sonneratia alba trees are as common and widespread locally and regionally as Bruguiera and R. apiculata (Table 1), but only six S. alba seedlings were encountered in our seedling subplots over 9 years. Xylocarpus granatum seedlings were found in all four watersheds, as expected, mostly in interior plots and in one riverine plot as well. Seedlings of Barringtonia racemosa, a mangrove associate often found just upslope from mangrove forests, were abundant in one riverine plot as well as in an interior plot. Nypa fruticans, which has a relatively low salinity tolerance, and Rhizophora mucronata, which has very long propagules and so is seldom found far from water, occurred only in the Okat mangrove forest, the former in riverine and interior plots and the latter only in fringe plots. Cocos nucifera prefers sandy soils and so is not common in mangrove forests.
Over the entire study period, 471 Bruguiera seedlings and 123 R. apiculata seedlings appeared in the subplots (Table 4) for average establishment rates of 0.27 m−2 year−1 and 0.07 m−2 year−1, respectively. Including the other species in the subplots as well as 1,076 seedlings sampled outside the subplots (64% Bruguiera and 36% Rhizophora), 1,749 seedlings were measured over 9 years.
Characteristics of canopy cover
Canopy cover was highly consistent from year to year (Fig. 5). Overall, 67% of the measurements were the same level as the previous year. All estimates of the mean annual change in canopy cover were positive, suggesting a slight tendency for the canopy to close with time over all three zones. The only statistically significant change was for the interior zone, which had a slope of 0.084 canopy cover unit year−1 (SE = 0.02, P value = 0.016).
Of the 24 annual comparisons of river, zone, and river x zone interactions, there was only one significant difference in canopy cover levels among the three different zones and the four rivers (Online Resource Table S1). Among the 3,164 observations of canopy cover (at all the subplots as well as at additional seedlings selected in the surrounding area), 28% were level 0 (open canopy), 60% were level 1 (partial canopy), and 12% were level 2 (closed canopy).
Seedling growth rates
Seedlings of both Bruguiera and Rhizophora grew slowly most of the time. Over all zones and rivers throughout the study, at least 80% of all seedlings had growth rates of less than 15 cm year−1 (Fig. 6). The fastest rates for growth in height of Bruguiera ranged from 20 cm year−1 in the riverine zone to 65 cm year−1 in the interior zone, but for Rhizophora the fastest rates were uniformly 25–30 cm year−1.
The predicted rates of growth in height of both species were fastest in the interior zone and slowest in the riverine zone; predicted Bruguiera growth rates were consistently a little faster than Rhizophora growth rates (Fig. 7). They increased faster with age (years since initial measurement) and height gained. (Online Resource Table S2). In addition, the river x zone interaction was significant for Bruguiera. Initial height, age, and zone were significant factors for Rhizophora (Online Resource Table S2). The rate of growth for the fringe zone was not significantly different from the other two zones.
The smaller data set for which canopy cover levels were available suggested that canopy cover had a positive influence on growth rates on Bruguiera but not Rhizophora (Fig. 8). Growth rates for Bruguiera in the interior zone would decrease as the canopy closed, whereas riverine rates were highest under an open canopy. Predicted growth rates for Rhizophora were consistently highest in the interior zone and lowest in the fringe zone, regardless of canopy cover. The ANOVA was similar for both species with significant effects of initial height and age for each species and interactions between age and river as well (Online Resource Table S3a and b).
Among the 1268 seedlings of Bruguiera and Rhizophora that survived in the study for at least 1 year, only 9% of Bruguiera seedlings and 2% of Rhizophora seedlings reached 2 m in height (Table 5). Only two of these (Bruguiera) were in a fringe zone. More seedlings of each species grew to at least 1.5 m, but there were still no Rhizophora seedlings this tall in the fringe zone.
Of the 352 seedlings with at least 5 measurements, 47 grew to at least 2 m in height (40 for Bruguiera and 7 for Rhizophora, Fig. 9). Because final data could be either the measured height or just the status of being over 2 m, calculations for estimating annual growth rates required at least two actual height measurements. That reduced the number of graduated Bruguiera seedlings used for calculating growth rate to 27 and Rhizophora seedlings to 4 as shown in Fig. 9. Growth rates of the tallest seedlings during the previous 5 years ranged from 4 to 46 cm year−1.
Seedling survival
During the 8 years of the subplot measurements alone, 18 Bruguiera seedlings and 6 Rhizophora seedlings grew to 2 m in the 220 seedling subplots. None of these were in the fringe zone. Of the two species, Bruguiera represented 79% of the individuals established in the subplots and 21% were Rhizophora. Nearly the same proportion became saplings: 75% were Bruguiera and 25% were Rhizophora. Average annual mortality rates for the seedlings in the subplots were 37% for Bruguiera and 35% for Rhizophora.
The best model for Bruguiera (Model 2 in Table 3) with a separate intercept and slope for each river x zone x canopy cover level combination) contained the interaction term between previous canopy cover level and previous height, but the best model for Rhizophora did not. Both models were clearly best as the differences in AIC units from the best to the next best models were greater than 10 AICc units for both species (Online Resource Table S4). However, the interaction terms were highly significant, with some river x zone x canopy cover level combinations taking extreme values, making interpretation difficult.
As expected, annual seedling survival increased with size (Fig. 10; Online Resource Table S5a and b). By the time seedlings attained a height of 1 m in interior and riverine zones, their probability of survival to a height of 2 m in the next year was roughly 90% for both species. Bruguiera seedlings in the fringe zone never exceeded 70% probability of survival to 2 m.
The smaller data set used to evaluate the effect of canopy cover on survival did not include enough observations with both previous height and previous canopy cover level to evaluate the effect of canopy cover in each combination of river and zone. The available data suggest that probability of survival of both species may be best under an open or partially open canopy, especially in the fringe zone (Fig. 11).
Predicted probabilities of survival of Bruguiera and Rhizophora seedlings for each zone and previous canopy cover level averaged over rivers. For both species in the fringe zone only the data from the Utwe River were adequate for the estimation. For Bruguiera the interior zone data from the Finkol River were insufficient for estimation and therefore not included. The remaining estimates were averages over all four rivers
None of the permutation tests showed that an ENSO event had a significant effect on growth or mortality of either species at the 5% level.
Discussion
Kosrae was an appropriate site for this study because of relatively easy access to replicate watersheds, similarity of its species composition with mangrove stands around the western Pacific rim and a recent forest inventory database. The physical and chemical differences among zones on Kosrae are damped by the island’s normally high rainfall and by groundwater inflow into at least one of the interior mangrove swamps (Ewel et al., 1998a, b; Drexler & Ewel, 2001). Seedlings therefore encounter a relatively benign environment in terms of salinity. The trajectories we have described for the two most common species may represent response to the best of conditions when comparisons are made with other sites.
Kosrae’s normally high rainfall mitigates against dramatic changes such as were caused by ENSO-related events in Australia in 2015 (Duke et al., 2017). In Kosrae, groundwater measurements along transects from mangroves to upslope freshwater forested wetlands showed salinity increasing to 14 to 21 PSU in the landward edge of the Yela interior mangrove forest during the 1997–1998 El Niño event (Drexler & Ewel, 2001), but salinities at these levels are not considered stressful to mangroves. The same El Niño event decreased surface elevations in our Yela and Utwe plots; elevations increased afterward at the riverine and interior zones but not so much in the fringe zones (Krauss et al., 2010).
Because there were too few ENSO events during our study to allow us to detect significant effects on mangrove seedlings, it is worth noting that the degree of canopy opening at the beginning of our study, closing gradually over the next several years, particularly in the interior zone (Fig. 4) may have been a legacy of the same 1997–1998 event. Defoliation is an early sign of damage due to drought in other wetland tree species (e.g., Taxodium distichum L. Rich. (bald cypress) (Brown, 1981)) and was implicated as fractional canopy coverage in the massive mangrove dieback in Australia in 2015. (Abhik et al., 2021). The long drought during the El Niño may have caused defoliation that was more pronounced in the interior zones.
Increased rainfall and higher tides associated with La Niña events have not affected other mangrove forests either (e.g., Galeano et al., 2017; Hickey et al., 2021). Deeper and faster-moving water during especially high tides in La Niña events may hinder early establishment of seedlings, but neither longer inundation nor increased rainfall is likely to have as much effect. Indeed, seedlings planted in a neotropical mangrove forest with average rainfall of 7–11 m year−1 grew slowly but suffered low mortality over a year that included a major El Niño event followed by a La Niña event (Riascos et al., 2018). Seedlings in basin (interior) sites in that study grew faster than in riverine or fringe sites.
Species distribution
The overall densities of Bruguiera and Rhizophora in the seedling subplots reflect the high importance value (which includes a measure of basal area as well as density and frequency) of mature trees in Kosrae’s mangrove forest. However, whereas the highest importance value for Bruguiera trees was in the riverine zone and the lowest in the fringe zone, seedlings were most numerous in the interior zone and least in the riverine zone. Importance values for Rhizophora trees, on the other hand, were highest in the interior zone and lowest in the riverine zone, whereas seedlings were most numerous in the riverine zone and least in the fringe zone.
The distribution and density of the other four most common species of seedlings on Kosrae do not reflect the current population of trees either. Most notable is the near absence of Sonneratia alba regeneration in the subplots. The fruit is a buoyant berry and should spread easily with the tide. An earlier survey on Kosrae (Pinzón et al., 2003) found abundant S. alba regeneration on a newly emerged mud flat seaward from the forest. Over the years, as sea level has risen (0.8–1.8 mm year−1in the last several decades: Krauss et al., 2010), the island has been eroding, with sediments accumulating in some mangrove stands. Over a 6.5-year period, surface elevation decreased in two of our plots but increased 1.3 mm year−1 in the interior zones at Yela and Utwe, 1.2 mm year−1 at the Utwe interior zone, and 6.3 at the Utwe riverine site. Adult trees can continue to thrive in these changing conditions, but successful regeneration is shifting seaward. As young trees lodge in mud flats, the growing stems may help to trap sediments, generating a new fringe zone in spite of sea level rise (Some of S. alba’s persistence in riverine and interior zones may also be due to vegetative regeneration by branch layering (Holbrook & Putz, 1982)).
The four other common species each has buoyant propagules that could reach any zone. Nypa fruticans germination is limited to brackish and fresh waters, but is widespread in our plots. Xylocarpus granatum germinates most successfully in upper intertidal zones. Problems in establishing roots quickly enough to withstand repeated flooding may be a cause of seedling failure; seedling predation may also be implicated (Allen et al., 2003). Lumnitzera littorea, a valuable timber tree, is now found only in a few places on the island; its limited distribution may reflect the effects of logging during a time when a small sawmill was operating on the island. On Pohnpei, it tends to be near the upper end of the tidal range (Ellison et al., 2022). Individuals found in fringe zones may be the remains of a population that is no longer successfully regenerating because of rising sea levels.
Growth and mortality of seedlings
The fastest growth rates were recorded for Bruguiera in the interior zone (except for one notable sample in the fringe zone), whereas rates for Rhizophora were similar among all three zones (Fig. 6). There were significant interactions among rivers in some cases. Many long-surviving seedlings of both Bruguiera and Rhizophora in all three zones simply stopped growing, remaining shorter than 150 cm; some kept on growing but slowly, so that only a few graduated into saplings, especially in the riverine zone (Fig. 9), and some underwent spurts of growth.
Predictive growth rate models without considering canopy cover suggested that both species would grow fastest in the interior zone and slowest in the riverine zone (Fig. 7). The two species responded similarly when canopy cover was included in the analysis, although Bruguiera was predicted to grow faster than Rhizophora under an open canopy in early stages of growth in both interior and riverine zones, as well as under a partially closed canopy in the interior zone. (Fig. 8). Canopy cover appeared to affect Bruguiera’s predicted growth rates, which decreased under increasing cover (Fig. 8). Rhizophora’s predicted growth rates were similar among all three zones regardless of canopy cover.
Annual mortality rates of 35–37% for Rhizophora and Bruguiera, respectively, assuage earlier concerns that Bruguiera might be replacing Rhizophora in Kosrae (Pinzón et al., 2003). Probability of survival tended to be lowest in fringe zones, but surprisingly similar in the other two zones for both species. Development of prop roots as a seedling grows taller may explain the more rapid increase in survival for Rhizophora. Survival of Rhizophora may be much enhanced under an open canopy.
Bruguiera maintained a strong presence in all zones. It is considered shade tolerant in its earliest seedling stages (e.g., Krauss and Allen) and was predicted to respond only slightly in height growth to open and partially open canopies in this analysis. Predicted survival as it neared the sapling stage responded to an open or partial canopy at interior sites and especially at fringe sites (Fig. 11). Similar results were seen in Australia, when Bruguiera seedlings planted in high and low intertidal sites and in a light gap and under a closed canopy over 8 years survived better in the high intertidal in light gaps but showed no difference in growth rates among the four treatments (Smith, 1987). As our seedlings grew taller and were no longer so dependent on propagule reserves, the two species’ predicted growth rates tended to converge (Fig. 7). Bruguiera seedlings in our study grew fastest in interior zones, whereas growth rates for Rhizophora ranged most widely in riverine zones. Predicted growth rate of Bruguiera seedlings responded more in early stages to an open canopy in both riverine and interior zones and to a partial canopy, than Rhizophora. Rhizophora was predicted to increase its likelihood of survival earlier than Bruguiera, particularly under an open canopy, but to be less successful under a closed canopy. Bruguiera was predicted to grow faster in the riverine zone than in the fringe zone, but only under an open canopy. Under an open or partially open canopy, seedlings were predicted to reach high probabilities of surviving by the time they had reached 100 cm, but that may have taken several years.
Maintenance of a mangrove canopy in Kosrae depends on continual growth into the canopy from very few of the many seedlings that establish there. Most seedlings grow very slowly, some remaining less than 1.5 m tall for years; a few undergo spurts and grow taller, and only a few of those become saplings (Figs. 5, 8). Statistical analysis of these predictions shows significant interactions without clear patterns to help us understand this process any better. In both cases, what increases growth increases survival, and the probability of survival increases with age. Predicted growth rates under ideal conditions suggest that the average Bruguiera seedling could grow to 100 cm in 6 years; Rhizophora could require 7 years (Fig. 7).
If no mortality had occurred after “graduation” of the seedlings, the density of saplings in the seedling plots would have been 818 ha−1 and 273 ha−1. Among the 16 randomly selected forest survey plots on Kosrae (including the 12 established for this study), density of Bruguiera trees is 30 ha−1 and 2 ha−1 for Rhizophora trees (Cole et al., 1999). Subsequent mortality must reduce the sapling density to this level. It is not yet possible to estimate how long it would take to reach the sapling stage. The only individuals that did so were already established at the beginning of this study; the period from establishment to sapling is likely to be longer than 8 years, and there are no estimates on how much time is necessary for a tree to take its place as a co-dominant in the canopy. In a mangrove forest in Malaysia dominated by Rhizophora apiculata, the time required for a gap caused by a lightning strike to experience canopy closure is 25.5 ± 6.9 years (Amir, 2012). This forest is at roughly the same latitude as Kosrae but receives less rainfall in a more seasonal pattern. With no significant dry season but without the benefits that a light gap brings to growth and survival, Kosrae’s seedlings may take at least as long as 25 years to reach canopy height.
What kills the seedlings? Kosrae has a substantial crab population, and I (KCE) have seen crabs in the canopy of mangrove trees at night, but I have not seen evidence of extensive herbivory on live or standing dead seedlings. Predation rates of 23% were recorded for seedlings of X. granatum (Allen et al., 2003), but there were no signs of predation on either Bruguiera or Rhizophora. High turnover of terrestrial tropical tree seedlings during succession is common, often caused by pathogens that cause damping-off disease (Augspurger & Kelly, 1984). Seedlings growing at high densities or competing for limiting resources such as light are particularly susceptible to a range of diseases caused by fungi, oomycetes, and viruses that can damage leaves, stems, and roots (Gilbert, 2002). This source of mortality should be a component in calculations of net primary productivity and sediment accretion.
The role of hydrogeomorphic zones during the growth of a mangrove seedling has become a little more complex. Slow actual and predicted growth rates for both species in the riverine zone, sometimes similar to or less than rates in the fringe zone were unexpected. Higher-scale geomorphic features may be implicated A more regional geomorphologic system classifies the mangrove stands in the four rivers we studied as occupying estuaries/deltas, lagoons/backmarshes behind land barriers, or coral reefs (Fujimoto et al., 1996; Thom, 1984). The mangrove forests along the Yela and Okat Rivers are designated as estuary/delta type, meaning that river water flows beyond the mangroves before encountering a protective feature, such as a reef. The mangroves along the Utwe and Finkol Rivers are barrier/backmarsh types, where river water encounters protective landforms such as beach ridges closer to the outflow. This feature was probably the cause of our abandoning the Finkol River fringe plot and perhaps was responsible for the lack of significance among zones in some of our analyses as well. The quantity and quality of sediments being deposited in the mangrove forests may also have differed between the two sets of rivers. Indeed, comparison of growth and survival of mangrove seedlings in fringe zones among corresponding geomorphologic types on other islands might be useful in increasing our understanding of growth and survival of mangrove seedlings.
Management recommendations
Reforestation of mangroves by planting seedlings is a popular recovery strategy around the world, but most efforts are doomed, often because of the failure to rectify significant changes in freshwater inputs from both groundwater seepage and river flow to the mangrove forest (Lewis, 2005; Lee et al., 2019). Reforestation of fringe sites may seem attractive for restoration of a tree barrier to destructive waves that might affect interior forests and coastal infrastructure, but even though seedling establishment rate might be satisfactory, the growth and survival rates recorded in this study do not support this. Where planting appears to be appropriate, managers must be prepared for significant turnover, and replanting several times may be necessary, especially in fringe and riverine zones. One way of detecting this has been used for several species in the Rhizophoraceae, which produce a regular number of leaf scars per year on the main stem before branching occurs; it is therefore possible to estimate growth rate (or time since canopy opening) from the density of leaf scars on the stem (Duke & Pinzón, 1992; Hauff et al., 2006). Managing tree removal such as by concentrating harvesting in interior zones where seedling establishment and survival are more likely would be more productive.
Conclusion
Bruguiera and Rhizophora dominated mangrove reproduction on Kosrae FSM, a small tropical island with high rainfall and little seasonality. Bruguiera was more numerous than Rhizophora among both seedlings and mature trees. Contrary to our expectation, however, neither species was most dense in the hydrogeomorphic zone where adult trees had the highest importance value. Of particular note is that Sonneratia alba, which was also widely distributed on the island, was poorly represented among seedlings.
Although both target species experienced slow growth rates most of the time, Bruguiera seedlings were likely to grow faster in height than Rhizophora except in riverine zones. Mean annual mortality rates were ca. 36% for both species, but Bruguiera may not have survived as well in fringe zones as Rhizophora. Lack of sufficient data, especially for Rhizophora, make further comparisons in survivorship between the two species difficult.
Predicted growth rates suggested that Bruguiera would grow faster than Rhizophora under an open canopy in early stages of growth in both interior and riverine zones. Under a closed canopy, the two species did not appear to differ. The few data available for estimating probability of survival suggest that both species would do similarly well under open and partially closed canopies. Rhizophora may survive as well as Bruguiera under a closed canopy except in the fringe zone.
We could not detect any ENSO effects on growth or mortality of either species, although it is possible that a strong El Niño in 1997 may have caused some defoliation of the canopy.
Data availability
Data archive: Ewel, Katherine C. 2022. Mangrove seedling measurements at Kosrae, Federated States of Micronesia. Fort Collins, CO: Forest Service Research Data Archive. https://doi.org/10.2737/RDS-2022-0069
References
Abhik, S., P. Hope, H. H. Hendon, L. B. Hutley, S. Johnson, W. Drosdowsky, J. R. Brown & N. C. Duke, 2021. Influence of the 2015–2016 El Niño on the record-breaking mangrove dieback along northern Australia coast. Scientific Reports 11: 1–12.
Allen, J. A., K. W. Krauss & R. D. Hauff, 2003. Factors limiting the intertidal distribution of the mangrove species Xylocarpus granatum. Oecologia 135: 110–121.
Amir, A. A., 2012. Canopy gaps and the natural regeneration of Matang mangroves. Forest Ecology and Management 269: 60–67.
Augspurger, C. K. & C. K. Kelly, 1984. Pathogen mortality of tropical tree seedlings: experimental studies of the effects of dispersal distance, seedling density, and light conditions. Oecologia 61: 211–217.
Balke, T., T. J. Bouma, E. M. Horstman, E. L. Webb, P. L. A. Erftemeijer & P. M. J. Herman, 2011. Windows of opportunity: thresholds to mangrove seedling establishment on tidal flats. Marine Ecology Progress Series 440: 1–9.
Brown, S., 1981. A comparison of the structure, primary productivity, and transpiration of cypress ecosystems in Florida. Ecological Monographs 5: 403–427.
Chang-Yang, C.-H., J. Needham, C.-L. Lu, C.-F. Hsieh, I.-F. Sun & S. M. McMahon, 2021. Closing the life cycle of forest trees: the difficult dynamics of seedling-to-sapling transitions in a subtropical rainforest. Journal of Ecology 109: 2705–2716.
Cole, T. G., K. C. Ewel & N. N. Devoe, 1999. Structure of mangrove trees and forests in Micronesia. Forest Ecology and Management 117: 95–109.
Curtis, J. T. & R. P. McIntosh, 1951. An upland forest continuum in the prairie-forest border region of Wisconsin. Ecology 32: 476–496.
Devoe, N. N. & T. G. Cole, 1998. Growth and yield in mangrove forests of the Federated States of Micronesia. Forest Ecology and Management 103: 33–48.
Drexler, J. Z. & E. W. DeCarlo, 2002. Source water partitioning as a means of characterizing hydrologic function in mangroves. Wetlands Ecology and Management 10: 103–111.
Drexler, J. Z. & K. C. Ewel, 2001. Effects of ENSO-related drought on hydrology and salinity in a Micronesian wetland complex. Estuaries 24: 347–356.
Duke, N. C. & Z. S. Pinzón, 1992. Aging Rhizophora seedlings from leaf scar notes: a technique for studying recruitment and growth in mangrove forests. Biotropica 24: 173–186.
Duke, N. C., J. M. Kovacs, A. D. Griffiths, L. Preece, D. J. E. Hill, P. van Oosterzee, J. Mackenzie, H. S. Morning & D. Burrows, 2017. Large-scale dieback of mangroves in Australia’s Gulf of Carpenteria: a severe ecosystem response, coincidental with an unusually extreme weather event. Marine and Freshwater Research 68: 1816–1829.
Ellison, A. M. & E. J. Farnsworth, 1993. Seedling survivorship, growth, and response to disturbance in Belizean mangal. American Journal of Botany 80: 1137–1145.
Ellison, J. C., K. J. Buffington, K. M. Thorne, D. Gesch, J. Irwin & J. Danielson, 2022. Elevation of mangrove forests of Pohnpei, Micronesia. Estuarine, Coastal and Shelf Science 268: 107780.
Ewel, K. C., J. A. Bourgeois, T. G. Cole & S. Zheng, 1998a. Variation in environmental characteristics and vegetation in high-rainfall mangrove forests in Kosrae, Micronesia. Global Ecology and Biogeography Letters 7: 49–56.
Ewel, K. C., S. Zheng, Z. S. Pinzón & J. A. Bourgeois, 1998b. Environmental effects of canopy gap formation in high-rainfall mangrove forests. Biotropica 30: 510–518.
Ewel, K. C., R. D. Hauff & T. G. Cole, 2003. Analyzing mangrove forest structure and species distribution on a Pacific island. Phytocoenologia 33: 251–266.
Friess, D. A., K. Rogers, C. E. Lovelock, K. W. Krauss, S. E. Hamilton, S. Y. Lee, R. Lucas, J. Primavera, A. Rajkaran & S. Shi, 2019. The state of the world’s mangrove forests: past, present, and future. Annual Review of Environment and Resources 44: 89–115.
Fujimoto, K., T. Miyagi, T. Kikuchi & T. Kawana, 1996. Mangrove habitat formation and response to Holocene sea-level changes on Kosrae Island, Micronesia. Mangroves and Salt Marshes 1: 47–57.
Galeano, A., L. E. Urrego, V. Botero & G. Bernal, 2017. Mangrove resilience to climate extreme events in a Colombian Caribbean island. Wetlands Ecology and Management 25: 743–760.
Gilbert, G. S., 2002. Evolutionary ecology of plant diseases in natural ecosystems. Annual Review of Phytopathology 40: 13–43.
Good, P., 2005. Testing hypotheses. In: Permutation, Parametric and Bootstrap Tests of Hypotheses. Springer Series in Statistics. Springer, New York, NY. https://doi.org/10.1007/0-387-27158-9_3
Hauff, R. D., K. C. Ewel & J. Jack, 2006. Tracking human disturbance in mangroves: estimating harvest rates on a Micronesian island. Wetlands Ecology and Management 14: 95–105.
Hickey, S. M., B. Radford, J. N. Callow, S. R. Phinn, C. M. Duarte, & C. E. Lovelock, 2021. ENSO feedback drives variations in dieback at a marginal mangrove site. Scientific Reports 11: Article 8130.
Holbrook, N. M. & F. E. Putz, 1982. Vegetative seaward expansion of Sonneratia alba trees in a Malaysian mangrove forest. The Malaysian Forester 45: 278–281.
Kitaya, Y., V. Jintana, S. Piriyayotha, D. Jaijing, K. Yabuki, S. Izutani, A. Nishimiya & M. Iwasaki, 2002. Early growth of seven mangrove species planted at different elevations in a Thai estuary. Trees 16: 150–154.
Krauss, K. W. & J. A. Allen, 2003. Factors affecting the regeneration of the mangrove Bruguiera gymnorrhiza (L.) Lamk) on a tropical Pacific island. Forest Ecology and Management 176: 49–60.
Krauss, K. W., C. E. Lovelock, K. L. McKee, L. López-Hoffman, S. M. L. Ewe & W. P. Sousa, 2008. Environmental drivers in mangrove establishment and early development: a review. Aquatic Botany 89: 105–127.
Krauss, K. W., D. R. Cahoon, J. A. Allen, K. C. Ewel, J. C. Lynch & N. Cormier, 2010. Surface elevation change and susceptibility of different mangrove zones to sea-level rise on Pacific high islands of Micronesia. Ecosystems 13: 129–143.
Lee, S. Y., S. Hamilton, E. B. Barbier, J. Primavera & R. R. Lewis III., 2019. Better restoration policies are needed to conserve mangrove ecosystems. Nature Ecology & Evolution 3: 870–872.
Lewis, R. R. III, 2005. Ecological engineering for successful management and restoration of mangrove forests. Ecological Engineering 24: 403–418.
Ling, D. L, 1995. www.comfsm.fm/~dleeling/weather/kosrain.html
Littell, R. C., G. A. Milliken, W. W. Stroup, R. D. Wolfinger, & O. Schabenberber, 2006. SAS for Mixed Models, Second Edition, SAS Publishing
Murray, N. J., T. A. Worthington, P. Bunting, S. Duce, V. Hagger, C. E. Lovelock, R. Lucas, M. I. Saunders, M. Sheaves, M. Spalding, N. J. Waltham & M. B. Lyons, 2022. High-resolution mapping of losses and gains of Earth’s tidal wetlands. Science 376: 744–749.
Pacific ENSO Update, 1998. Vol 4(2).
Pacific ENSO Update, 2003. Vol. 9(3).
Pinzón, Z. S., K. C. Ewel & F. E. Putz, 2003. Gap formation and seedling regeneration in mangrove forests in Micronesia. Journal of Tropical Ecology 19: 143–153.
R Core Team, 2021. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/.
Ramsay, D., A. Webb, S. Abraham, R. Jackson, & B. Charley, 2013. Kosrae Shoreline Management Plan. National Institute of Water and Atmospheric Research, New Zealand. Fig. D-2, p. 89.
Riascos, J. M., J. R. Cantera & J. F. Blanco-Libreros, 2018. Growth and mortality of mangrove seedlings in the wettest neotropical mangrove forests during ENSO: implications for vulnerability to climate change. Aquatic Botany 147: 34–42.
Smith, T. J. III, 1987. Effects of light and intertidal position on seedling survival and growth in tropical tidal forests. Journal of Experimental Marine Biology and Ecology 110: 133–146.
Thom, B. G., 1984. Coastal landforms and geomorphic processes. In Snedaker, S. C. & J. G. Snedaker (eds), The Mangrove Ecosystem: Research Methods UNESCO, Paris: 3–17.
Ur, R. H., N. Hideo & K. Kei, 2013. Geologic origin of the volcanic islands of the Caroline group in the Federated States of Micronesia, Western Pacific. South Pacific Studies 33: 101–118.
Woodroffe, C., 1992. Mangrove sediments and geomorphology. In Robertson, A. I. & D. M. Alongi (eds), Tropical Mangrove Ecosystems: Coastal and Estuarine Studies 41 American Geophysical Union, Washington DC: 7–41.
Acknowledgements
This paper is dedicated to the late Erick Waguk, Kosrae State Forester, who participated enthusiastically in this project throughout its duration. He regularly communicated research progress to his fellow Kosraeans and understood the long-term value of protecting the research sites. An earlier study by Z. Pinzón provided the inspiration for this project. G. Cornelius, S. Abraham, and N. Nena provided administrative support at many stages. T. Cole and E. Waguk assisted in plot establishment and data collecting for many years; T. Cole oversaw data management and prepared Figs. 1 and 2. Also field assistance was provided by J. Allen, K. Bonine, J. Bourgeois, P. Brooks, N. Cormier, J. Hariguchi, R. Hauff, K. Isisaki, J. Jack, K. Krauss, R. MacKenzie, M. Nena, M. Palik, T. Tara, and S. Zheng. We thank W. Cropper, Jr., J. Allen, J. Ewel, G. Gilbert, and three anonymous reviewers for helpful comments on earlier versions of this manuscript.
Funding
This study was funded by the USDA Forest Service.
Author information
Authors and Affiliations
Contributions
KCE conceived of and carried out the study. JB cleaned the data set and designed, conducted, and interpreted the statistical analysis. KCE wrote most of the text.
Corresponding author
Ethics declarations
Competing interest
The authors have no conflicts of interest. No external funds were used in this research.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Guest editors: Andre A. Padial, Dennis F. Whigham, Ken W. Krauss, & Emily M. Dangremond / Emerging Studies in Aquatic Sciences – an Homage to Dr. Brij Gopal’s Legacy
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ewel, K.C., Baldwin, J. Long-term patterns of growth and survival of mangrove seedlings in Micronesia. Hydrobiologia 851, 1679–1696 (2024). https://doi.org/10.1007/s10750-022-05041-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-022-05041-1