Abstract
As annual minimum temperatures increase due to climate change, species once constrained by minimum temperatures are expanding poleward. Avicennia germinans (black mangrove), a freeze-intolerant tree, has been expanding northward into salt marsh-grass-dominated communities. Distribution and colonization dynamics of A. germinans are crucial for understanding changes in coastal habitats and ecosystem structure and function along the Gulf of Mexico Coast (USA). We transplanted A. germinans seedlings and propagules into salt marsh plots of S. alterniflora, half of which had the canopy removed, along a latitudinal gradient that spanned locations within and outside of A. germinans’ current range limits (29°7′20″N to 30°23′41″N). Plot microclimate and transplant survival and growth were monitored for 2 years. Canopy removal resulted in lower minimum temperatures and longer cumulative freeze duration. Seedling survival was greatest at the southernmost site; however, seedling growth was reduced in plots with the canopy intact, as hypothesized. Seedling survival at northern sites was limited to plots with the S. alterniflora canopy intact. Propagules survived and established at all sites in the second year, although there was no S. alterniflora canopy effect on propagule survival and establishment. Our results illustrate the complexity of ecological interactions between herbaceous marsh species and mangroves at the species’ range limit.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Mangroves are prominent coastal trees in tropical and subtropical latitudes, providing unique ecosystem services and functions, including high rates of primary productivity, atmospheric carbon sequestration, shoreline protection, and support for marine food webs (Duke et al., 2007). Climatic factors such as temperature can greatly influence plant species distribution, as well as the structure and function of these tidal ecosystems. Temperature is a global and regional factor contributing to the latitudinal distribution of mangrove species as mangroves are limited to tropical latitudes due to low freeze tolerance (Duke et al., 1998). In more temperate latitudes, mangrove forests are replaced by herbaceous coastal salt marsh species (Tomlinson, 1986; Kangas & Lugo, 1990; Yando et al., 2016). Feher et al. (2017) quantified positive linear relationships between temperature and aboveground productivity, as well as strong positive sigmoidal relationships between temperature and aboveground biomass and canopy height. As frequency of extreme cold events decreases with climate change, mangroves have been able to expand their ranges poleward (Cavanaugh et al., 2014).
One of the most widely distributed mangrove genera, Avicennia, has a global tropic and subtropical distribution from Australia (38°S, Avicennia marina (Forssk.) Vierh.) to Louisiana, USA (29°N, Avicennia germinans (L.) L.) (Tomlinson, 1986; Ellison & Farnsworth, 2001). In the Gulf of Mexico, A. germinans (black mangrove) grows at the northern latitudinal limit of its range in North America (Sherrod & McMillan, 1985). In parts of the northern Gulf of Mexico, the integration of mangroves into salt marsh is occurring, and the effect of mangrove range expansion into graminoid-dominated salt marsh could be substantial (Osland et al., 2013). Avicennia germinans, currently the only mangrove species found in Louisiana, has been expanding its range along the Louisiana salt marsh-dominated coast (Penfound & Hathaway, 1938; Sherrod & McMillan, 1985; Perry & Mendelssohn, 2009; Michot et al., 2010). Despite several severe freeze events in the past century, A. germinans has maintained its presence along the southern Louisiana coast (Perry & Mendelssohn, 2009). While mangroves and coastal salt marshes both provide numerous ecosystem services, they remain distinctly different in terms of ecosystem structure and habitats/species supported. Although it is well understood that minimum temperatures limit the distribution of mangroves into temperate latitudes, other ecological factors that facilitate or inhibit the expansion and the persistence of mangroves into new territories are not well understood. Achieving a greater understanding of the colonization dynamics of A. germinans, species interactions between mangroves and salt marsh species, and climatic drivers on this species, will increase our understanding of the replacement of herbaceous by woody plant species in temperate communities (Sharitz & McCormick, 1973; Osland et al., 2017).
The dispersal of mangrove propagules occurs in water (hydrochory) and is characterized by a period of floating, followed by a period of stranding, in which the propagules settle on sediment and the elongated hypocotyl and radicle orients the plant and secures it in the sediment (Rabinowitz, 1978a). Propagule and seedling mortality is high as young trees can be lost to fungus, herbivory, washing out of the system, or stranding in uninhabitable locations (Jimenez et al., 1985; Minchinton & Dalby-Ball, 2001; Clarke & Kerrigan, 2002). Mortality is higher in smaller sized propagules (Rabinowitz, 1978b). Surrounding vegetation can play a role in the establishment success (defined in this manuscript as the ability for propagules to survive to seedling stage) of mangrove propagules. For example, McKee et al. (2007) documented that the presence of Sesuvium portulacastrum (L.) L. and Distichlis spicata (L.) Greene, two species of herbaceous coastal plants, aids the establishment of red mangrove (Rhizophora mangle L.) propagules by increasing soil redox potential and decreasing soil temperature and salinity, thus ameliorating stressful environmental conditions. Further, greater light availability has been positively related to A. germinans seedling density at Twin Cays, Belize (McKee, 1995b), suggesting that light is an important resource for establishment across the range of A. germinans. However, the role of competition and/or facilitation of other marsh species on mangrove establishment at their latitudinal ranges is not adequately studied but remains important to understanding the species-colonization dynamics and predicting the future coastal wetlands with changing climate.
In Louisiana and Mississippi, the extent of Spartina alterniflora Loisel-dominated salt marsh includes freeze/frost-prone areas (Mississippi coast) and areas in which freezes are less common (southern Louisiana coast). Where cold temperature is an environmental stress, S. alterniflora can be considered stress-tolerant sensu Grime (1977) compared to A. germinans. The dynamic persistence of A. germinans at its northernmost range in the Gulf of Mexico may be related to the potential of salt marsh vegetation to facilitate early life-history stages of A. germinans. The Stress Gradient Hypothesis (SGH, Bertness & Callaway, 1994; Guo et al., 2013) predicts that facilitative interactions are more likely to occur in areas of high environmental stress, and competitive interactions are more likely in areas with low stress, and can be further influenced by not only the type of stress, but also by life-history stage and strategy (Miriti, 2006; Maestre et al., 2009). For example, short-stature A. germinans growing within salt marsh vegetation were observed to survive a severe freeze event in Florida (Lugo & Patterson, 1977) and Texas (Sherrod & McMillan, 1985). Additional experiments have demonstrated that A. germinans propagules could establish and survive in S. alterniflora-dominated areas when the effects of propagule redispersal and predation were eliminated using enclosures (Patterson et al., 1997; Hester et al., 2007). Furthermore, facilitative or competitive interactions between species drive species survival and zonation. Patterson et al. (1993) found that A. germinans seedlings could grow in permanently flooded soils, but growth was inhibited when S. alterniflora was present. As mangroves continue to be affected by climate change (Scavia et al., 2002; Alongi, 2008; Gilman et al., 2008), it becomes imperative to understand the cold temperature stress dynamics of mangrove range expansion at the mangrove-salt marsh ecotone (Osland et al., 2013).
In this study, we investigated the likelihood of facilitative interactions between S. alterniflora and A. germinans along a latitudinal range. We hypothesized that at the northern latitudinal range limit of A. germinans, where cold temperature stress would be greater due to more severe freeze events, S. alterniflora would facilitate A. germinans survival. Conversely, we predicted that negative interactions between S. alterniflora and A. germinans would be the dominant interaction in southern Louisiana coastal salt marsh where temperatures infrequently drop below freezing and both species commonly occur and compete for resources. In addition, we determined if propagules and seedlings of A. germinans could survive and establish beyond their current range limit via a transplant study. The goals of this research were to increase understanding of (1) the colonization and establishment dynamics of A. germinans; (2) ecological interactions between species along a stress gradient; and (3) inform predictions of coastal marsh plant community change under future climate scenarios.
Materials and methods
Study sites
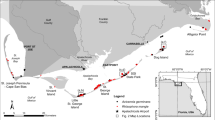
Beginning in November 2009, we established three salt marsh habitat transplant sites along the Louisiana and Mississippi coasts to represent a cold temperature gradient, spanning a latitudinal range from 29°7′20″N to 30°23′41″N (Fig. 1). Space-for-time substitution approaches, such as this, have been implemented in numerous ecological studies to predict system responses to changing climate and abiotic parameters (Pickett et al., 1989; Aronson et al., 1995; Twilley et al., 1999; Osland et al., 2013; Yando et al., 2016). Selected sites for this study spanned a geographic gradient from where A. germinans currently occurs in Southern Louisiana (Caminada, LA) to a northern site beyond the current range of A. germinans (Ocean Springs, MS). Three sites (Caminada, Rockefeller, and Ocean Springs) were established in the first year, and an additional mid-latitudinal site (St. Bernard) was established in the second year (2010) (Fig. 1). Although sites also varied longitudinally (88°W to 93°W), precipitation did not significantly differ across all our coastally located sites (NOAA, 2017). All sites were established in tall-form S. alterniflora-dominated marsh in close proximity (1.5–2 m) to a bayou or waterway on natural streamside berms, which were at marsh elevations conducive to promote A. germinans establishment (Alleman & Hester, 2011b).
Plot vegetation treatments
At each of the four transplant sites, we established ten 0.7 m2 plots. Each plot had one of two marsh vegetation treatments: the control treatment, in which the canopy was unaltered (canopy intact), and a clipping treatment (canopy removed) in which the canopy was removed at ground level. Plots also had A. germinans life-history stages treatment using two stages: propagule and seedling. The propagule treatment consisted of 25 live propagules dispersed randomly into the plot, whereas the seedling treatment consisted of 10 transplanted seedlings (10–20 cm in height) in the fall of each year. We established four replicate blocks at each site, each block was composed of four plots (one of each marsh vegetation and life-history stage treatment combination) for a total of 16 plots per site. In year 1 of the study (2009–2010), plots were established (consisting of the full experimental design) at each of the two sites (Caminada and Ocean Springs), and only seedling plots were established at the Rockefeller site. By the second year of the study (2010–2011), the full experimental design was established in all four sites (Caminada, Rockefeller, St. Bernard, and Ocean Springs), for a total of 64 plots. Aboveground biomass was collected from each plot the first time it was clipped for canopy-removal treatment, dried to a constant weight at 65°C in a drying oven, and weighed to estimate aboveground biomass at each site. Clipping was repeated in the spring and fall as well as after each freeze event. To prevent propagules from being redispersed by tidal action, propagule plots were enclosed within 1.2-m-tall enclosures constructed of plastic mesh (mesh openings approximately 2 × 2 cm), which were staked in place with 2.4 m long, 1.3 cm diameter PVC. In addition to propagule enclosures, shorter enclosures (0.6 m tall with the same size mesh) were placed around seedling plots at Ocean Springs in the second year of the study to prevent herbivory, likely by nutria, Myocastor coypus (Molina), which was observed in the first year.
Hydrology was determined from water-level gauges installed at three of the sites during the first year, except for the St. Bernard site, which was determined during the second year. Plots were flooded between 24 and 40% of the time (Fig. 1). In general, plots were not flooded during freeze events (data not shown) as strong northerly winds push the water out of the coastal marshes during the passage of cold fronts. Minimum water temperatures recorded by the water-level gauge remained well above freezing with the warmest minimum water temperature being recorded at the northernmost site, Ocean Springs (Fig. 1).
Plant collection and preparation
Seedlings (with approximately 10 leaves and no branching) and mature propagules of A. germinans were collected in the fall of each year from the Caminada Moreau Headland, Louisiana. Collected seedlings were placed in 235 mL plastic pots with drainage and transported to the University of Louisiana Ecology Center greenhouse facility, where they were maintained under ambient conditions in 20 ppt artificial saltwater (Spectrum Brands, Inc., Atlanta, GA, USA) until being removed from pots and transplanted in the field within 25 days. Prior to planting, seedling height and number of leaves were recorded, and seedlings randomly assigned to treatments. Collected propagules were kept in 18-L buckets of aerated 36 ppt artificial saltwater (replaced every other day) and maintained under ambient conditions at the Ecology Center until propagules were transferred to the experimental field plots within 17 days.
Continuous and post-freeze monitoring
Plot elevations were related to data collected from continuously recording Aqua TROLL-200 water level-conductivity gauges (In-Situ Inc., Ft. Collins, CO, USA) installed at each site to determine local site hydrology. Four HOBO Pro V2 water-temperature data loggers (Onset Computer Corporation, Cape Cod, MA, USA) were also installed at each site, one per block, at the approximate height of seedlings (10–20 cm above the soil) to measure temperature of air (or water temperature when flooded). Both seedlings and propagules were monitored for survival (presence of green photosynthetic tissue). Propagules were also monitored for establishment success which was indicated by the propagule extending its radicle into the soil and orienting itself as a seedling. Seedling leaf number and damage were recorded approximately 2 weeks following freeze events as this was the optimal time frame for determining freeze damage (Pickens & Hester, 2011). We recorded leaf number in the fall as an indicator of seedling growth because leaf number has previously been shown to indicate biomass effectively for seedlings (Schiffers & Tielborger, 2006). We quantified relative differences in marsh canopy between plots with ocular estimation of vegetative cover and relative light levels (percent photosynthetically active radiation (PAR) below versus above plot canopy) within plots using a LI-250 light meter (LI-COR Biosciences, Lincoln, NE, USA).
Soil physical and chemical variables were determined in fall and spring, and compared to expected values for Louisiana mangrove habitat (Patterson & Mendelssohn, 1991). Soil redox potential at 1 and 15 cm depths were determined using brightened platinum electrodes (Patrick et al., 1996), and two aliquots of soil pore-water were collected in the fall and spring using interstitial sippers as described by McKee et al. (1988). One aliquot of pore-water was immediately preserved onsite for total sulfide determination within 24 h using an Orion 9616BN Silver/Sulfide ion selective electrode (Thermo Fisher Scientific Inc., Waltham, MA, USA). A second aliquot of pore-water was used for the determination of pH and salinity within 15 min of sample collection using a Beckman Phi 200 millivolt meter (Beckman Coulter, Inc., Irving, TX, USA). Soil bulk density, percent moisture, and organic matter (loss on ignition) were determined annually as described in Sumner (2000).
Data analysis
For each year of the study, we analyzed the effect of site (Caminada, Rockefeller, St. Bernard, Ocean Springs) and canopy treatment (intact vs. removed) on microclimate metrics (minimum temperature, number of freeze events, cumulative hours below 0°C, and percent ambient light) using a two-way analysis of variance (ANOVA) in JMP 9 (SAS Institute Inc., Cary, NC, USA). Data from 2009 to 2010 were kept independent from 2010 to 2011 data because some transplants had to be replaced in the second year of the study. Significant treatment effects were determined using Tukey’s Honest Significant Differences (HSD) tests with alpha = 0.05. We analyzed the effects of site and canopy treatment on survival, establishment, growth, edaphic characteristics, and vegetative cover using a randomized block factorial ANOVA framework with the block effect nested within the site effect. Significant differences between levels of main effects were determined using Tukey’s Honest Significant Differences (HSD) tests with alpha = 0.05.
At the conclusion of the study, we used nonlinear regression analysis (Newton–Gauss method, JMP 9) to solve for the minimum temperature at which there was 50% seedling survival (lethal temperature 50%, LT50) using the following logistic function:
where y is the survival proportion of A. germinans seedling (excluding herbivory), LT50 is the lethal temperature at which 50% of seedlings died, and x is the minimum temperature. We also tested for a correlation between the minimum temperature and the duration of the freeze event (in hours) in which the minimum temperature was reached.
Results
Stress gradient and site characterization
Cold stress increased along sites from south to north, and was intensified by removal of the S. alterniflora canopy. In the winter of 2009–2010, minimum temperatures were lower in the northern site compared to mid-latitude and southern sites (site effect: F2,7 = 34.5 P < 0.01, Table 1). The number of distinct freeze events and the cumulative duration of freeze events (hours below 0°C) also increased from southern to northern sites (site effect: F2,7 = 218.7, P < 0.01, Table 1). Removal of the S. alterniflora canopy resulted in a colder (more stressful) microclimate within plots (canopy effect: F1, 7 = 6.0, P = 0.04, Table 1); plots with the canopy removed had minimum temperatures that on average were 1°C colder than those with the canopy intact (Table 1). Similar trends were observed in the second winter (2010–2011) as minimum temperatures again decreased from south to north (site effect: F3,11 = 72.2, P < 0.01, Tukey’s HSD, Table 1) and cumulative duration of freeze events increased from south to north (site effect: F3,11 = 87.3, P < 0.01, Tukey’s HSD, Table 1). Minimum temperature and cumulative duration of freeze events did not significantly differ between the two mid-latitude sites (Rockefeller and St. Bernard). The average effect of canopy removal at all sites in the second winter resulted in colder plot temperatures by 0.7°C (canopy effect: F1,11 = 12.7, P < 0.01, Table 1) and an average of 22 h longer exposure to freezing temperatures (canopy effect: F1,11 = 10.2, P < 0.01, Table 1). Light levels in plots with intact canopy treatments at all sites ranged between 12 and 24% of ambient above canopy light levels, which was less than light levels in plots with the canopy removed. Plots with canopy removed at Rockefeller showed 43% of above canopy light levels, and all other sites where canopy was removed showed light levels at 64–81% of ambient, above canopy, light (site × canopy effect: F3,44 = 8.2, P < 0.01, Table 1).
Vegetative cover was variable at sites (46–68% cover), with the greatest cover occurring at Caminada the first year, and at St. Bernard and Rockefeller the second year (P < 0.05 for comparisons among sites over time, Table 2). Aboveground biomass was greatest at Rockefeller, and least at Ocean Springs (site effect: F3,16 = 27.2, P < 0.01, Table 2). The northernmost site, Ocean Springs, consistently had the greatest percent soil organic matter and percent moisture compared to the other sites (P < 0.01 for all comparisons between sites over time, Table 2). Removal of the S. alterniflora canopy did not significantly affect pH, salinity, soil redox potential, or sulfides (P > 0.05 for comparisons over time). Soil porewater pH was variable between sites during the study (Table 2), although the range of pH values from 6.6 to 7.6 is probably not ecologically significant. Salinity was approximately twice as high at Caminada and Rockefeller as the one at St. Bernard and Ocean Springs (P < 0.01 for all comparisons between sites over time, Table 2). Interstitial sulfides and soil reduction–oxidation potential at 1 and 15 cm depths were indicative of moderately reduced soils (Table 2).
Survival and growth
In the first year of the study (2009 cohort), propagules only survived at the southernmost site, Caminada (Fig. 2a). Survivorship was low overall, resulting in a lack of statistical difference in propagule survival between sites or canopy treatment. Seedlings performed better than propagules with greatest survival at the southernmost site; Caminada had 77.5% seedling survival on average, compared to 5% seedling survival at Rockefeller and no survival at Ocean Springs (site effect: F2,11 = 254.7, P < 0.01, Fig. 2b). By fall 2010, Caminada seedlings in plots with the canopy removed had 17 ± 1 leaves and seedlings in plots with the canopy intact had 14 ± 1 leaves, though the difference was not statistically significant (canopy effect: F1,6 = 5.6, P = 0.06).
Effect of site and S. alterniflora canopy treatment on percent survival (mean ± SE, N = 4) of A. germinans a propagules following the 2009–2010 winter at Caminada and Ocean Springs; b seedling at Caminada, Rockefeller, and Ocean Springs following the 2009–2010 winter; c propagules following the 2010–2011 winter at all sites; d seedling at all sites following the 2010–2011 winter. Letters indicate significant differences between sites
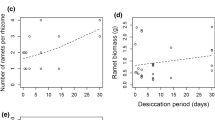
Propagule and seedling survival occurred at all sites in the second year (2010 cohort), and the effect of canopy removal was not significant (propagules, F1,15 = 3.4, P = 0.09; seedlings, F1,15 = 0.1, P = 0.82, Fig. 2c, d). Although seedling survival did not differ between canopy treatments, seedling survival was on average 49% greater at the two southern sites (Caminada and Rockefeller) compared to the northern sites (site effect: F3,15 = 32.6, P < 0.01, Tukey’s HSD, Fig. 2d). Herbivore damage to seedlings was noted at St. Bernard in the second year, particularly in plots with the canopy removed. Propagules survived and were able to establish at all sites, however; in the northern sites, only propagules in plots with the canopy removed survived (Fig. 3). A one-way ANOVA for each site separately revealed a significant effect of canopy removal on propagule establishment in Caminada (P = 0.02; Fig. 3). Importantly, seedling growth, represented by leaf number, was greater in plots with the canopy removed at the southernmost site (Caminada) and had more than twice the number of leaves compared to those with the canopy intact (site × canopy effect: F3,12 = 15.6, P < 0.01, Tukey’s HSD, Fig. 4).
The average temperature across sites at which there was 50% seedling survival (LT50) during a freeze event was − 4.6°C (Fig. 5). Minimum temperatures and duration recorded during freeze events ranged from − 1.6°C lasting 7.8 h to − 7.7°C lasting 15.5 h. Minimum temperature was highly correlated with the duration of the minimum temperature event (R2 = 0.92), therefore, a temperature of − 4.6°C corresponded with a freeze event that lasted approximately 12.2 h for this study.
Mean (± SE, N = 4) proportion A. germinans seedling survival as a logistic function of minimum temperature. The curved line represents the logistic function best fit, and the vertical line at − 4.6°C denotes the point of inflection, equal to the lethal temperature at which 50% survival is observed (LT50). The gray region represents the 95% confidence intervals around the LT50
Discussion
As expected, this study demonstrated greater A. germinans seedling survival (~ 40 to 80%) at lower latitudinal (southern) sites compared to survival rates (< ~ 20%) at higher latitude (northern) sites beyond the species’ current distribution. Although we hypothesized that the presence of S. alterniflora would facilitate survival of A. germinans at sites north of its current distribution, we did not find a significant effect of the S. alterniflora canopy on overall A. germinans propagule and seedling survival or propagule establishment at Ocean Springs (northernmost site). Interestingly, we did observe that seedling survival at the two northernmost sites (Ocean Springs and St. Bernard) only occurred in plots with the canopy intact, suggesting that the presence of surrounding vegetation may ameliorate harsh conditions associated with colder climates once A. germinans has reached the seedling life-history stage. Also interesting, although not statistically significant, at the northernmost sites propagule survival only occurred in plots where canopy was removed, suggesting that light availability may play a crucial role in plant survival at younger life-history stages. These observations suggest that the frequency of facilitative and competitive interactions between species not only shifts along a stress gradient, but changes throughout a species life history, as canopy seemingly differentially affected A. germinans depending on the plant’s life-history stage at the northernmost site. However, results were not statistically significant, and we believe the low survivorship (< 5%) of seedlings and propagules at the northernmost site (Ocean Springs) suggests that cold temperature stress likely overwhelmed any ameliorating or competitive effects of the canopy. Ocean Springs minimum temperatures recorded at seedling height in the second year were below the A. germinans LT50 calculated from this study (− 4.6°C), and were approaching the − 7.0°C minimum temperature threshold for mangrove dominance in saline wetland habitat (Osland et al., 2013).
Many environmental factors affect the establishment of A. germinans, including temperature, light, nutrient availability, salinity, hydrology, and dispersal success (Krauss et al., 2008; Jones et al., 2016). Positive effects of S. alterniflora may occur in habitats where A. germinans survival and establishment is limited by herbivory. We observed that seedling herbivory damage at St. Bernard was more severe in plots with the canopy removed, resulting in live seedlings being found only in plots with an intact canopy. Regarding the ameliorating effect of S. alterniflora on cold temperatures for A. germinans propagules, positive interactions may be limited to scenarios where winter temperatures are low and propagules and seedlings are protected from freeze damage by the presence of dense vegetation (Lugo & Patterson, 1977; Sherrod & McMillan, 1985). Avicennia germinans propagules can survive exposure to an average temperature of − 6.5°C for up to 12 h (Pickens & Hester, 2011); only plots with the canopy removed at Ocean Springs were exposed to a minimum temperature lower than − 6.5°C in the second year of the study. At the southernmost site (Caminada), propagule establishment was significantly higher in plots where the canopy was removed (17% vs. ≤ 6%), suggesting that light availability is crucial in the growth and development of young A. germinans propagules into seedlings. In addition, the trend of low (< 10%) propagule survival and establishment success during this study is indicative of the stressful conditions for establishment of this viviparous species in coastal marshes (Alleman & Hester, 2011b). Our findings suggest that canopy, in sites where temperatures do not limit survival, may pose a greater limitation to propagule survival and establishment through decreasing light availability than cold temperatures.
Shade intolerance and competitive exclusion by other species has been well documented for A. germinans and Avicennia marina propagules at warmer, tropical latitudes (Rabinowitz, 1978b; Smith, 1987). Similarly, Guo et al. (2013) found that aboveground biomass of A. germinans transplanted in marsh along the southeastern Texas coast was greater when surrounding marsh vegetation was absent. In our study, the S. alterniflora canopy reduced light availability and temperature during the growing season. We found seedling growth at the southernmost site of this study (Caminada) was greater in plots with the canopy removed, with seedlings producing roughly twice as many leaves (Fig. 4). We believe the increased seedling growth in canopy removed plots at Caminada was likely due to the increase in light availability without lethal durations of freeze events. Our results are consistent with a study that demonstrated a reduced relative growth rate of A. germinans seedlings grown at a low light level, similar to that of a dense canopy, compared to a high light level indicative of a canopy gap (McKee, 1995a).
Our study provides evidence that A. germinans can establish and survive, for at least short periods of time, at locations north of its current range limit as propagules survived and established at all sites during the second year of the study. Our study suggests that the most influential factors on mangrove establishment and survival were temperature, light availability, and, although not the focus of this study, herbivory as well; however, it is important to recognize that factors outside of the scope of our research, such as edaphic characteristics, hydrology, and precipitation may also be highly influential on the establishment and survival of this species (Feher et al., 2017). The hydrologic and edaphic conditions of the four sites within this study displayed some variability, though not in a way that prohibited A. germinans establishment and growth. For example, the salinity range of plots (11–24 ppt) was well within the physiological limits of A. germinans seedlings (McMillan, 1971; Alleman & Hester, 2011a). During the second year of our study the two sites with greater salinity (24 ppt) also had greater survival than the sites with lower salinity (11–14 ppt). Both field and greenhouse studies have demonstrated that increased light availability promotes A. germinans survivorship and growth in low salinity environments (López-Hoffman et al., 2007). Furthermore, the flooding duration of our plots (24–40%) was within the hydrologic threshold for A. germinans as found in other studies (Rabinowitz, 1978a; Patterson et al., 1997; Alleman & Hester, 2011b). Average porewater temperatures at each site were greater than the temperature minimum (15°C) for A. germinans rooting while floating in water (McMillan, 1971). Previous research has suggested that propagule survival is sensitive to the immediate surroundings during cold temperature events, such as floating in water or being located under debris (Sherrod & McMillan, 1985; Pickens & Hester, 2011). Since propagules were placed directly on the soil surface, as occurs with natural stranding, the saturated soil likely buffered the colder air temperatures. Following this logic, cold temperature stress from south to north may not be as severe for the propagule life-history stage of A. germinans compared to seedlings.
Cold temperature stress creates a physiological limit to mangrove survival (Stuart et al., 2007), and future northward expansion of A.germinans in the southeastern United States will likely be modulated by winter climate (Osland et al., 2013). Sea-level rise and anthropogenic alterations to hydrology may also influence mangrove expansion by reducing previous barriers and allowing propagules to disperse further into marshes (Krauss et al., 2011; Raabe et al., 2012). Mangrove expansion is also largely influenced by the role of marsh vegetation structure to entrap propagules (Peterson & Bell, 2012), and as shown in this study, ameliorate stressful conditions associated with freeze events for seedlings. The results of our study suggest that the herbaceous canopy plays a dynamic role in altering the microclimate and growing conditions for A. germinans, by reducing the severity of minimum temperatures and cumulative duration of freeze events; however, at the cost of reducing light availability.
The role of herbivory in plant invasions, and possibly range expansions, has been debated (Maron & Vila, 2001), but may be important for A. germinans. It has been previously reported that herbivory (e.g., crab and insect) is a challenge to the survival, establishment, and growth of A. germinans early life-history stages (McKee, 1995b; Patterson et al., 1997; Delgado et al., 2001; McKee & Rooth, 2008). While not the focus of this study, herbivory should be recognized as an additional factor that may influence range expansion as herbivory was observed at St. Bernard in plots with the canopy removed. The role of herbivory in A. germinans range expansion into colder climates is still unknown, but our observations suggest that a dense vegetative canopy may help to prevent herbivory at the seedling life-history stage.
Conclusion
Freeze-intolerant Avicennia germinans trees are currently distributed throughout tropical and subtropical latitudes in the Americas and Africa (IUCN, 2010). With winter air temperatures becoming less extreme with climate change, coastal mangrove forests are expected to expand poleward into areas previously dominated by graminoid-dominated salt marsh (Osland et al., 2013; Saintilan et al., 2014; Yando et al., 2016). The ecotones at which these two habitat types meet consist of dynamic patches of salt marsh and newly established trees, and is largely controlled by the severity of freeze events (Osland et al., 2015, 2016), as well as precipitation regimes (Yando et al., 2016) and elevation (Rogers et al., 2005). The replacement of grass-dominated marshes by mangrove forests holds a variety of implications, notably changes in ecosystem services provided, including increased aboveground carbon pools (Doughty et al., 2016; Yando et al., 2016), changes in peat development and stability (Cahoon et al., 2013), and biological communities supported (Clarke & Hannon, 1967). Understanding interactions between A. germinans and other species, as well as colonization and dispersal dynamics, at its poleward limits as temperature averages change is essential to understanding coastal ecosystem response to climate change.
Our research describes the impact of herbaceous marsh vegetation on winter conditions experienced by A. germinans early life-history stages at and beyond the limits of its current range. Specifically, the herbaceous canopy provides a microclimate that reduces the severity of freeze events (i.e., warmer temperatures and shorter durations of temperatures below 0°C). Given the narrow window of seedling survival of temperatures below freezing (Fig. 5 and Osland et al., 2013), the presence of the herbaceous marsh canopy may be a critical factor in the persistence and expansion of seedlings at the limit of their range. A warming climate may further increase opportunities for expansion through this facilitative mechanism. However, propagule establishment and survival may still be limited by other factors, such as light availability, at the edge of its range.
This study elucidates competitive and facilitative interactions between herbaceous marsh canopy and early life-history stages of A. germinans, particularly the modulation of the microclimate for seedlings during freeze events at and beyond the limit of their range. As annual average global temperatures continue to rise, temperature-limited A. germinans are able to expand their range poleward into salt marshes (Osland et al., 2013; Saintilan et al., 2014; Yando et al., 2016). The resulting changes in plant-community structure and marsh-to-woody shrub ecotone provide an opportunity to explore numerous ecological questions pertinent to land-management concerns. Restoration efforts in coastal wetlands of the northern Gulf of Mexico often rely on planting of mangrove seedlings or dispersal of propagules (Ellison, 2000; Kairo et al., 2001; Lewis, 2005). Typically, these plantings occur alongside manual plantings of S. alterniflora, or are geographically located adjacent to S. alterniflora marsh. The success of these restoration efforts, and our ability to predict future changes to coastal ecosystem structure and function, depends on our knowledge of the competitive and/or facultative interactions between these two important species under the constraining forces of abiotic parameters.
References
Alleman, L. K. & M. W. Hester, 2011a. Refinement of the fundamental niche of black mangrove (Avicennia germinans) seedlings in Louisiana: applications for restoration. Wetlands Ecology and Management 19: 47–60.
Alleman, L. K. & M. W. Hester, 2011b. Reproductive ecology of black mangrove (Avicennia germinans) along the Louisiana coast: propagule production cycles, dispersal limitations, and establishment elevations. Estuaries Coasts 34: 1068–1077.
Alongi, D. M., 2008. Mangrove forests: resilience, protection from tsunamis, and responses to global climate change. Estuarine, Coastal and Shelf Science 76: 1–13.
Aronson, J., S. Dhillion & E. Le Floch, 1995. On the need to select an ecosystem reference, however imperfect: a reply to Pickett and Parker. Restoration Ecology 3: 1–3.
Bertness, M. D. & R. Callaway, 1994. Positive interactions in communities. Trends in Ecology & Evolution 9: 191–193.
Cahoon, D. R., P. Hensel, J. Rybczyk, K. L. McKee, C. E. Proffitt & B. C. Perez, 2013. Mass tree mortality leads to mangrove peat collapse at Bay Islands, Honduras after Hurrican Mitch. Journal of Ecology 91(6): 1093–1105.
Cavanaugh, K. C., J. R. Kellner, A. J. Forde, D. S. Gruner, J. D. Parker, W. Rodriguez & I. C. Feller, 2014. Poleward expansion of mangroves is a threshold response to decreased frequency of extreme cold events. Proceedings of the National Academy of Sciences 111(2): 723–727.
Clarke, L. D. & N. J. Hannon, 1967. The mangrove swamp and salt marsh communities of the Sydney district: I. Vegetation, soils and climate. The Journal of Ecology 753–771
Clarke, P. J. & R. A. Kerrigan, 2002. The effects of seed predators on the recruitment of mangroves. Journal of Ecology 90(4): 728–736.
Delgado, P., P. F. Hensel, J. A. Jimenez & J. W. Day, 2001. The importance of propagule establishment and physical factors in mangrove distributional patterns in a Costa Rican estuary. Aquatic Botany 71: 157–178.
Doughty, C. L., J. A. Langley, W. S. Walker, I. C. Feller, R. Schaub & S. K. Chapman, 2016. Mangrove range expansion rapidly increases coastal wetland carbon storage. Estuaries and Coasts 39: 385–396.
Duke, N. C., M. C. Ball & J. C. Ellison, 1998. Factors influencing biodiversity and distributional gradients in mangroves. Global Ecology & Biogeography Letters 7: 27–47.
Duke, N. C., J. O. Meynecke, S. Dittmann, A. M. Ellison, K. Anger, U. Berger, S. Cannicci, K. Diele, K. C. Ewel, C. D. Field, N. Koedam, S. Y. Lee, C. Marchano, I. Nordhaus & F. Dahdouh-Guebas, 2007. A world without mangroves? Science Letters 317: 4.
Ellison, A. M., 2000. Mangrove restoration: do we know enough? Restoration Ecology 8: 219–229.
Ellison, A. M. & E. J. Farnsworth, 2001. Mangrove communities. In Bertness, M. D., S. D. Gaines & M. E. Hay (eds), Marine community ecology. Sinauer Associates Inc., Sunderland: 423–442.
Feher, L. C., M. J. Osland, K. T. Griffith, J. B. Grace, R. J. Howard, C. L. Stagg, N.M. Enwright, K.W. Krauss, C.A. Gabler, R.H. Day, & K. Rogers, 2017. Linear and nonlinear effects of temperature and precipitation on ecosystem properties in tidal saline wetlands. Ecosphere 8(10)
Gilman, E. L., J. Ellison, N. C. Duke & C. Field, 2008. Threats to mangroves from climate change and adaptation options: a review. Aquatic Botany 89: 237–250.
Grime, J. P., 1977. Evidence for existence of three primary strategies in plants and its relevance to ecological and evolutionary theory. The American Naturalist 111: 1169–1194.
Guo, H., Y. Zhang, Z. Lan & S. Pennings, 2013. Biotic interactions mediate the expansion of black mangrove (Avicennia germinans) into salt marshes under climate change. Global Change Biology 19: 2765–2774.
Hester, M. W., T. K. Henkel, J. M. Willis & P. Taylor, 2007. Enhancement of barrier island marsh creation through black mangrove propagule dispersal: A cost-effective alternative to planting seedlings. Final report. NOAA/CREST (Coastal Restoration Enhancement through Science and Technology) Lafayette
IUCN International Union for Conservation of Nature, 2010 Avicennia germinans. The IUCN Red List of Threatened Species, Version 2017-3.
Jimenez, J. A., A. E. Lugo & G. Cintron, 1985. Tree mortality in mangrove forests. Biotropica 177–185.
Jones, S. F., C. L. Stagg, K. W. Krauss & M. W. Hester, 2016. Tidal saline wetland regeneration of sentinel vegetation types in the Northern Gulf of Mexico: an overview. Estuarine, Coastal and Shelf Science 174: A1–A10.
Kairo, J. G., F. Dahdouh-Guebas, J. Bosire & N. Koedam, 2001. Restoration and management of mangrove systems – a lesson for and from the East African region. South African Journal of Botany 67: 383–389.
Kangas, P. C. & A. Lugo, 1990. The distribution of mangroves and saltmarsh in Florida. Tropical Ecology 31: 32–39.
Krauss, K. W., C. E. Lovelock, K. L. McKee, L. López-Hoffman, S. M. L. Ewe & W. P. Sousa, 2008. Environmental drivers in mangrove establishment and early development: a review. Aquatic Botany 89: 105–127.
Krauss, K. W., A. S. From, T. W. Doyle, T. J. Doyle & M. J. Barry, 2011. Sea-level rise and landscape change influence mangrove encroachment onto marsh in the Ten Thousand Islands region of Florida, USA. The Journal of Coastal Conservation 15: 629–638.
Lewis, R. R., 2005. Ecological engineerning for successful management and restoration of mangrove forests. Ecological Engineering 24: 403–418.
López-Hoffman, L., N. P. R. Anten, M. Martínez-Ramos & D. D. Ackerly, 2007. Salinity and light interactively affect neotropical mangrove seedlings at the leaf and whole plant levels. Oecologia 150: 545–556.
Lugo, A. & C. Z. Patterson, 1977. The impact of low temperature stress on mangrove structure and growth. Tropical Ecology 18: 149–161.
Maestre, F. T., R. M. Callaway, F. Valladares & C. J. Lortie, 2009. Refining the stress-gradient hypothesis for competition and facilitation in plant communities. Journal of Ecology 97: 199–205.
Maron, J. L. & M. Vila, 2001. When do herbivores affect plant invasion? Evidence for the natural enemies and biotic resistance hypotheses. Oikos 95: 361–373.
McKee, K. L., 1995a. Interspecific variation in growth, biomass partitioning, and defensive characteristics of neotropical mangrove seedlings – response to light and nutrient availability. American Journal of Botany 82: 299–307.
McKee, K. L., 1995b. Seedling recruitment patterns in a Belizean mangrove forest: effects of establishment ability and physico-chemical factors. Oecologia 101: 448–460.
McKee, K. L. & J. E. Rooth, 2008. Where temperate meets tropical: multi-factorial effects of elevated CO2, nitrogen enrichment, and competition on a mangrove-salt marsh community. Global Change Biology 14: 971–984.
McKee, K. L., I. A. Mendelssohn & M. W. Hester, 1988. Reexamination of pore water sulfide concentrations and redox potentials near the aerial roots of Rhizophora mangle and Avicennia germinans. American Journal of Botany 75: 1352–1359.
McKee, K. L., J. E. Rooth & I. C. Feller, 2007. Mangrove recruitment after forest disturbance is facilitated by herbaceous species in the Caribbean. Ecological Applications 17: 1678–1693.
McMillan, C., 1971. Environmental factors affecting seedling establishment of the black mangrove on the central Texas coast. Ecology 52: 927–930.
Michot, T. C., R. H. Day & C. J. Wells, 2010. Increase in black mangrove abundance in coastal Louisiana. Louisiana Natural Resource News 4–5.
Minchinton, T. E. & M. Dalby-Ball, 2001. Frugivory by insects on mangrove propagules: effects on the early life history of Avicennia marina. Oecologia 129(2): 243–252.
Miriti, M. N., 2006. Ontogenetic shift from facilitation to competition in a desert shrub. Journal of Ecology 94: 973–979.
NOAA, 2017. NOAA National Centers for Environmental Information. National Temperature and Precipitation Maps [available on internet at https://www.ncdc.noaa.gov/temp-and-precip/us-maps/1/201001?products[]=statewidepcpnrank#us-maps-select]. Accessed 19 Jan 2017
Osland, M. J., N. Enwright, R. H. Day & T. W. Doyle, 2013. Winter climate change and coastal wetland foundation species: salt marshes vs. mangrove forests in the southeastern United States. Global Change Biol 19: 1482–1494.
Osland, M. J., R. H. Day, A. S. From, M. L. McCoy, J. L. McLeod, & J. J. Kelleway, 2015. Life stage influences the resistance and resilience of black mangrove forests to winter climate extremes. Ecosphere, 6, art160.
Osland, M. J., N. M. Enwright, R. H. Day, C. A. Gabler, C. L. Stagg & J. B. Grace, 2016. Beyond just sea-level rise: considering macroclimatic drivers within coastal wetland vulnerability assessments to climate change. Global Change Biology 22: 1–11.
Osland, M. J., L. C. Feher, K. T. Griffith, K. C. Cavanaugh, N. M. Enwright, R. H. Day, C. L. Stagg, K. W. Krauss, R. J. Howard, J. B. Grace & K. Rogers, 2017. Climatic controls on the global distribution, abundance, and species richness of mangrove forests. Ecological Monographs 87(2): 341–359.
Patrick, W. H., R. P. Gambrell & S. P. Faulkner, 1996. Redox measurements of soils. In Sparks, D. L. (ed.), Methods of soil analysis, chemical methods. Soil Science Society of America Inc, Madison: 417–436.
Patterson, C. S. & I. A. Mendelssohn, 1991. A comparison of physicochemical variables across plant zones in a mangal/salt marsh community in Louisiana. Wetlands 11: 139–161.
Patterson, C. S., I. A. Mendelssohn & E. M. Swenson, 1993. Growth and survival of Avicennia germinans seedlings in a mangal/salt marsh community in Louisiana, USA. J Coast Res 9: 801–810.
Patterson, C. S., K. L. McKee & I. A. Mendelssohn, 1997. Effects of tidal inundation and predation in Avicennia germinans seedling establishment and survival in a sub- tropical mangal/salt marsh community. Mangroves Salt Marshes 1: 103–111.
Penfound, W. T. & E. S. Hathaway, 1938. Plant communities in the marshlands of southeastern Louisiana. Ecol Monogr 8: 1–56.
Perry, C. L. & I. A. Mendelssohn, 2009. Ecosystem effects of expanding populations of Avicennia germinans in a Louisiana salt marsh. Wetlands 29: 396–406.
Peterson, J. M. & S. S. Bell, 2012. Tidal events and salt-marsh structure influence black mangrove (Avicennia germinans) recruitment across ecotone. Ecology 93: 1648–1658.
Pickens, C. N. & M. W. Hester, 2011. Temperature tolerance of early life history stages of black mangrove Avicennia germinans: implications for range expansion. Estuaries Coasts 34: 824–830.
Pickett, S. T. A., J. Kolasa, J. J. Armesto & S. L. Collins, 1989. The ecological concept of disturbance and its expression at various hierarchical levels. Oikos 54: 128–136.
Raabe, E. A., L. C. Roy & C. C. McIvor, 2012. Tampa Bay coastal wetlands: nineteenth to twentieth century tidal marsh-to-mangrove conversion. Estuaries Coasts 35: 1145–1162.
Rabinowitz, D., 1978a. Dispersal properties of mangrove propagules. Biotropica 10: 47–57.
Rabinowitz, D., 1978b. Mortality and initial propagule size in mangrove seedlings in Panama. Journal of Ecology 66: 45–51.
Rogers, K., N. Saintilan & H. Heijnis, 2005. Mangrove encroachment of salt marsh in Western Port Bay, Victoria: the role of sedimentation, subsidence, and sea level rise. Estuaries and Coasts 28(4): 551–559.
Saintilan, N., N. Wilson, K. Rogers, A. Rajkaran & K. W. Krauss, 2014. Mangrove expansion and salt marsh decline at mangrove poleward limits. Global Change Biology 20: 147–157.
Scavia, D., J. C. Field, D. F. Boesch, R. W. Buddemeier, D. R. Cayan, M. Fogarty, M. A. Harwell, R. W. Howarth, C. Mason, D. J. Reed, T. C. Royer, A. H. Sallenger & J. G. Titus, 2002. Climate change impacts on us coastal and marine ecosystems. Estuaries 25: 149–164.
Schiffers, K. & K. Tielborger, 2006. Ontogenetic shifts in interactions among annual plants. Journal of Ecology 94: 336–341.
Sharitz, R. R. & J. F. McCormick, 1973. Population dynamics of two competing annual plant species. Ecology 54(4): 723–740.
Sherrod, C. L. & C. McMillan, 1985. The distributional history and ecology of mangrove vegetation along the northern Gulf of Mexico coastal region. Contributions in Marine Science 28: 129–140.
Smith, T. J., 1987. Effects of light and intertidal position on seedling survival and growth in tropical tidal forests. Journal of Experimental Marine Biology and Ecology 110: 133–146.
Stuart, S. A., B. Choat, K. C. Martin, N. M. Holbrook & M. C. Ball, 2007. The role of freezing in setting the latitudinal limits of mangrove forests. New Phytologist 173: 576–583.
Sumner, M. E., 2000. Handbook of soil science. CRC Press, LLC, Boca Raton.
Tomlinson, P. B., 1986. The botany of mangroves. Cambridge University Press, New York.
Twilley, R. R., V. H. Rivera-Monroy, R. Chen & L. Botero, 1999. Adapting an ecological mangrove model to simulate trajectories in restoration ecology. Marine Pollution Bulletin 37(8): 404–419.
Yando, E. S., M. J. Osland, J. M. Willis, R. H. Day, K. W. Krauss & M. W. Hester, 2016. Salt marsh-mangrove ecotones: using structural gradients to investigate the effects of woody plant encroachment on plant–soil interactions and ecosystem carbon pools. Journal of Ecology 104(4): 1020–1031.
Acknowledgements
We are grateful for the assistance of Michael Dupuis, Jonathan Willis, and Lauren Alleman in the field, and to Stacy Ortego and Chris Mabee for assistance in the lab. We appreciate the cooperation of the landowners and associated parties of the Edward Wisner Donation Trust (Cathy Norman and Robert Wiygul), Rockefeller State Wildlife Refuge (Jeb Linscombe and Tom Hess), Biloxi Marshlands Corporation (Eric Zollinger), and Gulf Coast Research Laboratory (Patrick Biber and William Hawkins), and facilities provided by the UL Lafayette Ecology Center. We also thank two anonymous reviewers for comments that improved this manuscript. Funding for this project was provided in part by a Society of Wetland Scientists Student Research Grant.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: K. W. Krauss
Rights and permissions
About this article
Cite this article
Pickens, C.N., Sloey, T.M. & Hester, M.W. Influence of salt marsh canopy on black mangrove (Avicennia germinans) survival and establishment at its northern latitudinal limit. Hydrobiologia 826, 195–208 (2019). https://doi.org/10.1007/s10750-018-3730-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-018-3730-9